Abstract
PU.1 is an ETS family transcription factor important for the development of multiple hematopoietic cell lineages. Previous work demonstrated a critical role for PU.1 in promoting Th9 development, and in limiting Th2 cytokine production. Whether PU.1 has functions in other T helper lineages is not clear. In this report we examined the effects of ectopic expression of PU.1 in CD4+T cells and observed decreased expression of genes involved with the function of T follicular helper (Tfh) cells, including Il21 and Tnfsf5 (encoding CD40L). T cells from conditional mutant mice that lack expression of PU.1 in T cells (Sfpi1lck−/−) demonstrated increased production of CD40L and IL-21 in vitro. Following adjuvant-dependent or adjuvant-independent immunization, we observed that Sfpi1lck−/− mice had increased numbers of Tfh cells, increased germinal center B cells, and increased antibody production in vivo. This correlated with increased expression of IL-21 and CD40L in Tfh cells from Sfpi1lck−/− mice, compared to control mice. Finally, although blockade of IL-21 did not affect germinal center B cells in Sfpi1lck−/− mice, anti-CD40L treatment of immunized Sfpi1lck−/− mice decreased germinal center B cell numbers and antigen-specific immunoglobulin concentrations. Together, these data indicate an inhibitory role of PU.1 in the function of T follicular helper cells, germinal centers, and Tfh-dependent humoral immunity.
Introduction
Transcription factors are essential components of cellular development and differentiation. Typically, networks of transcription factors work together within the nucleus of a cell orchestrating the establishment of characteristics that define the phenotype of a cell. The CD4+ T helper (Th) subsets, Th1 and Th2 cells, require the transcription factors T-bet and GATA3 for differentiation. RORγt promotes the differentiation of Th17 cells, Foxp3 functions as a master transcription factor in T-regulatory cells, and there is a requirement for Bcl6 in T follicular helper (Tfh) cell differentiation (1-3). PU.1 is a transcription factor that belongs to the E-twenty six (ETS) family of proteins, and we have shown that PU.1 is crucial for the differentiation of the IL-9-producing, Th9 subset, in humans and mice (4, 5). Although, these transcription factors are of great importance in their respective cell types, the activity of T-bet alone in Th1 cells for example, is insufficient for induction of the complete Th1 phenotype (6). Additional factors are necessary to induce and regulate the phenotype and functions of the different Th subsets. Since the discovery of Bcl6 as the master transcription factor in Tfh cells, extensive investigation has focused on identifying additional factors that positively or negatively regulate the development of Tfh cells.
Tfh cells were first proposed as a Th subset more than a decade ago (7, 8). Tfh cells have the unique function of providing help to B cells that bind the same antigen and this interaction occurs in specialized areas termed germinal centers. In addition to Bcl6, a variety of transcription factors, cytokines and receptor-ligand interactions also play a crucial role in establishment of the Tfh phenotype and germinal center activity. BLIMP1 is a transcription factor that negatively regulates Tfh cell development by antagonizing Bcl6 activity (1). IRF4, c-MAF, and STAT3 positively regulate Tfh cell development, and the absence of these transcription factors drastically impairs Tfh differentiation and thus germinal center development (9-15). IL-21 is a cytokine that is expressed by Tfh cells that facilitates the ability of Tfh cells to help germinal center B (GCB) cells survive and undergo class-switching recombination. In the absence of IL-21, differentiation of GCB cells is impaired, although Tfh cells are only modestly impacted (16-18). The necessity of IL-21 within the germinal center parallels the requirement of CD40L for proper germinal center development. Interaction of CD40L with its cognate partner CD40 expressed on GCB cells promotes the survival and proliferation of GCB cells. The interaction between CD40 and CD40L is essential for initiation and progression of germinal center activity (19, 20). However, the transcriptional regulation of IL-21 and CD40L in Tfh cells is not well understood.
PU.1 is expressed in thymic precursors, but expression is extinguished during thymic T cell development at the DN2 stage, regulation that is necessary for T cells to develop (21). However, PU.1 is expressed in mature peripheral T cells and expression is induced by T cell activation and culture of T cells with specific cytokines, including the cytokines that promote Treg, Th9 and Th2 development (4, 22-25). When mice with a conditional allele of PU.1 (Sfpi1) are mated to an Lck-Cre transgenic, the gene is deleted at the DN3-DN4 stage of thymic development, stages when deletion would not be expected to have an effect (26). Despite deletion, thymic and peripheral T cell populations are normal. PU.1-deficient T cells have a lower activation threshold under limiting TCR stimulation, owing to increases in TCR expression (22). Although Th1, Th17 and Treg development appears normal, PU.1 promotes Th9 development and limits heterogeneity in Th2 populations (4, 22, 23). Whether PU.1 has functions in additional Th lineages is not known. In this report we define a role for PU.1 in limiting Tfh development, IL-21 and CD40L expression in Tfh cells, and in the germinal center response.
Materials and Methods
Mice
C57BL/6 mice (WT) were purchased from Harlan Bioscience. PU.1 conditional mutant mice were generated by crossing Sfpi1 fl/fl mice on the C57BL/6 background with mice containing the Cre recombinase transgene under the control of the Lck promoter (4, 27). Mice were kept in a pathogen-free environment, and all studies were approved by the Animal Care and Use Committee of the Indiana University School of Medicine.
T helper Cell Differentiation
Naïve CD4+CD62L+ T cells were isolated from spleen and lymph nodes by magnetic separation using kits that employ negative selection (Miltenyi Biotech). Naïve cells were cultured in complete RPMI-1640 medium (supplemented with 10% (vol/vol) FBS (Atlanta Biologicals), 1mM glutamine (BioWhittaker), 100 U/mL penicillin (BioWhittaker), 100 μg/mL of streptomycin (BioWhittaker), 10mM HEPES, pH 7.3 (BioWhittaker), 1 mM sodium pyruvate (BioWhittaker) and 50 μM 2-mercaptoethanol) on α-CD3 (2μg/mL; 145-2C11; BioXcell) coated plates in the presence of soluble α-CD28 (1-2μg/mL) under Th1 (5ng/mL IL-12; 50 U/mL IL-2 and 10 μg/mL anti-IL-4, 11B11), Th2 (10ng/mL IL-4; and 10μg/mL anti-IFN-γ, XMG), Th9 (10 ng/mL IL-4; 2ng/mL TGF-β; and 10μg/mL anti-IFN-γ, XMG) , Th17 (100ng/mL IL-6; 10 ng/mL IL-1β; 2ng/mL TGF-β; 10μg/mL anti-IFN-γ, XMG; 10μg/mL and anti-IL-4, 11B11) and T regulatory cell conditions (2ng/mL TGF-β;10μg/mL anti-IFN-γ, XMG; 10μg/mL and anti-IL-4, 11B11). Cells were expanded after three days with fresh media and cytokines for Th1 (media only), Th2 (media only), Th17 (50ng/mL IL-6; 5ng/mL IL-1β; and 20U/mL of IL-2) Th9 (10ng/mL IL-4; 2ng/mL TGF-β; and 50U/mL IL-2), and T-regulatory cells (50U/mL IL-2). After 5 days, cells were restimulated on α-CD3 coated plates for 24 hours, and supernatants were collected for ELISA. For CD40L staining, naïve CD4+ T cells were stimulated with PMA (50ng/mL) and Ionomycin (500ng/mL) for 2 hours. Cells were either stained for surface CD4 (RM4-5) and CD40L expression or permeabalized for intracellular CD40L staining. For Tfh-like cell culturing, naïve cells were cultured in complete RPMI-1640 medium on anti-CD3 (10 μg/mL; 145-2C11; BioXcell) and anti-CD28 (10 μg/mL) coated plates under Tfh–like cell conditions (100 ng/mL IL-6; 50 ng/mL IL-21; 10 μg/mL anti-IL-2, anti-IFN-γ, anti-IL-4, and anti-TGF-β).
Retroviral transduction
Bicistronic retroviral expression vectors expressing either eGFP (MIEG), or hCD4 in combination with the mouse gene for PU.1, Sfpi1 (MIEG- Sfpi1), were described previously (23). Th cells cultured in Th17 conditions were transduced with retroviral supernatant, MIEG or MIEG- Sfpi1, 2 days after culturing in the presence of 8 μg/mL polybrene. Cells were also given IL-2 and expanded 3 days after culturing. After 5 days, cells were sorted based on GFP expression and stimulated on α-CD3 coated plates for 24 hours. Supernatants were collected for ELISA.
Chromatin Immunoprecipitation
ChIP assay was performed as previously described (25). Immunoprecipitations were performed with rabbit polyclonal antibodies (control IgG or PU.1 [T-21])(Santa Cruz Biotechnology Inc.). Quantification of binding DNA was performed with SYBR Green Fast PCR Master Mix using the ABI 7500 Fast Real-time PCR System (both from Applied Biosystems). Tnfsf5 primers (364 bp upstream from TSS) were as follows: (forward) 5′ AAC-TGG-TGA-ACC-CCA-AAC-TTT-A 3′ and (reverse) 5′ CAC-CCA-TAT-CAT-TCA-CTT-CCA-G 3′. Pdcd1 primers (1168 bp upstream from TSS) were as follows: (forward) 5′ TAA-TGT-TTC-CTT-CCC-CAC-CA 3′ and (reverse) 5′CTG-GGG-CAT-TCT-GAT-GAT-TT 3′. Il21 primers (437 bp upstream from TSS) were as follows: (forward) 5′ TGC-CGC-TGC-TTT-ACT-CAT-TG 3′ and (reverse) 5′ GCA-CCG-TCA-GCT-TTC-AGA-GA 3′. To quantify immunoprecipitated DNA, a standard curve was generated from serial dilutions of input DNA. To calculate ChIP results as a percentage of input, the amount of the immunoprecipiated DNA from the IgG control was subtracted from the amount of the immunoprecipitated DNA from the PU.1 antibody, followed by normalizing against the amount of the input DNA.
MOG35-55 peptide and SRBC immunizations
Mice were immunized with 100-150 μg of MOG35-55 peptide (Genemed Synthesis) subcutaneously (s.c.) with in an emulsion of complete Freud’s Adjuvant (CFA) containing 1mg/mL of heat killed H37RA strain of Mycobacterium tuberculosis (Sigma-Aldrich) in the hind leg region. Pertussis toxin (List Biological Laboratories, Inc) in PBS was injected intraperitoneally (i.p.) at a dose of 100-250 μg on the day of immunization and again 2 days after. sRBC (VWR Intl.) immunizations were done with 1 × 109 sRBC injected i.p. After 7 days, mice were sacrificed and splenocytes stained with Tfh and GC B cell markers.
Surface and Intracellular Staining
Splenocytes were treated with Fc-block for 5 minutes at RT and stained with Tfh markers CXCR5 (SPRCL5, Biolegend), CD4 (RM4-5, Biolegend), PD-1 (J43, Biolegend), and ICOS (C398.4A, eBioscience). CXCR5 staining was carried out at RT for 45 minutes and washed. Antibodies for CD4, PD-1, and ICOS were subsequently added. GCB cells were stained with Fas at 40 for 45 minutes, washed, and stained for B220 and GL-7. Cells were stimulated for 2 or 4 hours in the presence of PMA and Ionomycin for CD40L (MR1) and IL-21 staining, respectively. After 1 hour and 2 hours, for CD40L and IL-21 staining, respectively, cells were treated with 3μM monensin. After stimulation cells were surface stained for Tfh markers, and stained for IL-21. IL-21 staining was conducted using the IL-21R-human IgG chimera (R&D systems) with PE-anti-Human Fc gamma (eBiosciences) as the secondary antibody as described previously (28).
Tfh Gene Expression
Wild-type and PU.1lck−/− mice were given one injection of 1 × 109 SRBCs i.p. Seven days after immunization mice were sacrificed and splenocytes were stained with CXCR5, CD4, and PD-1 antibodies. CD4+CXCR5HighPD-1High (Tfh) and CD4+CXCR5−PD-1− (non-Tfh) cells were sorted by flow cytometry. RNA from sorted cells was isolated with Trizol to generate cDNA. Quantitative PCR was conducted to measure gene expression and results are relative to expression of β2-microglobulin as an internal control.
CD40L Blocking Experiments
Wild type and PU.1lck−/− mice were given one injection of 1 × 109 sRBCs i.p. CD40L blocking antibody (MR1, BioXcell) or control antibody (hamster IgG, BioXcell) were given i.p. on days 5 and 6 at concentration of 250μg/mL in PBS on each day. Mice were sacrificed on day 7, serum was collected to determine antibody titer and splenocytes were stained for Tfh and GCB cell markers.
Statistical analysis
The Student’s two-tailed t-test was used for pairwise statistical comparison or ANOVA for comparisons of multiple groups. p values of 0.05 or less were considered as significant.
Results
PU.1 regulates the expression of CD40L and IL-21
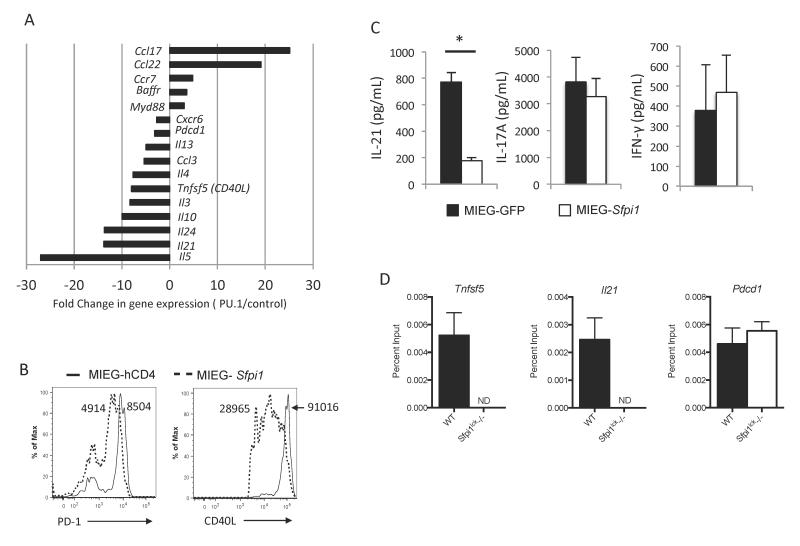
Although PU.1 induces IL-9 production in Th9 cells, whether it has functions in other Th subsets is not known. Results from a study comparing Th2 cells with ectopically expressed PU.1 to control cells demonstrate that PU.1 can activate and repress gene expression in T cells (Figure 1A). Consistent with our previous reports (4, 22, 23) we observed repression of Th2 cytokine genes and induction of chemokines associated with type 2 inflammation (Figure 1A). Among other PU.1-repressed genes we observed genes encoding CD40L (Tnfsf5) and IL-21. To determine if ectopic expression of PU.1 regulates the protein expression of a subset of these genes, we compared the expression of CD40L and PD-1 (Pdcd1) on the surface of control and PU.1 transduced T cells cultured under Tfh-like conditions. We observed PU.1 repressed expression of both surface receptors (Figure 1B). We performed similar experiments ectopically expressing PU.1 in naïve CD4+ T cells isolated from mice with a conditional allele of Sfpi1 that encodes PU.1 crossed to an Lck-Cre transgenic strain (termed Sfpi1lck−/− mice) cultured under Th17 conditions. Retroviral expression of PU.1 in Sfpi1lck−/− cells decreased IL-21 production, but did not affect production of IL-17A or IFNγ Figure 1C). To determine if PU.1-dependent regulation of these genes was potentially through a direct mechanism, we performed ChIP assays for PU.1 and assessed binding to regulatory elements in Tnfsf5, Il21, and Pdcd1. Comparing binding in wild type and Sfpi1lck−/− T cells, we observed significant binding of PU.1 at the Tnfsf5 and Il21 promoters but not at the Pdcd1 gene (Figure 1D).
Figure 1.
PU.1-dependent gene regulation in CD4+ T-cells. A, Naïve wild type CD4 T cells were cultured under Th2 conditions and transduced with control retrovirus or retrovirus expressing PU.1. Changes in expression of genes displayed as the ratio of expression of genes in CD4+ T-cells ectopically expressing PU.1 relative to expression in control vector transduced cells. B, Naïve wild type CD4 T cells were cultured under Tfh-like conditions and transduced with control retrovirus or retrovirus expressing PU.1. After five days of culture, transduced cells were stimulated with PMA and Ionomycin for 5 hours and stained for PD-1 and CD40L. Histograms indicate PD-1 and CD40L expression. Numbers indicate MFI. C, Naïve cells from Sfpi1lck−/− mice were cultured under Th17conditions and retrovirally transduced with an empty control vector (MIEG-GFP), or a vector expressing the PU.1 gene, MIEG-Sfpi1. After 5 days of culture, cells were sorted by GFP expression and restimulated with anti-CD3. Supernatants were collected and IL-21, IL-17a, and IFN-γ secretion was measured by ELISA. Data are representative of 3-5 mice/group. D, Chromatin immunoprecipitation was performed from wild type and Sfpi1lck−/− activated T cells cultured for three days. Chromatin was immunoprecipitated with anti-PU.1 and isolated DNA was identified with quantitative PCR for promoter regions of the indicated genes. Statistical significance was determined with a two-tailed t test, *, p<0.05.
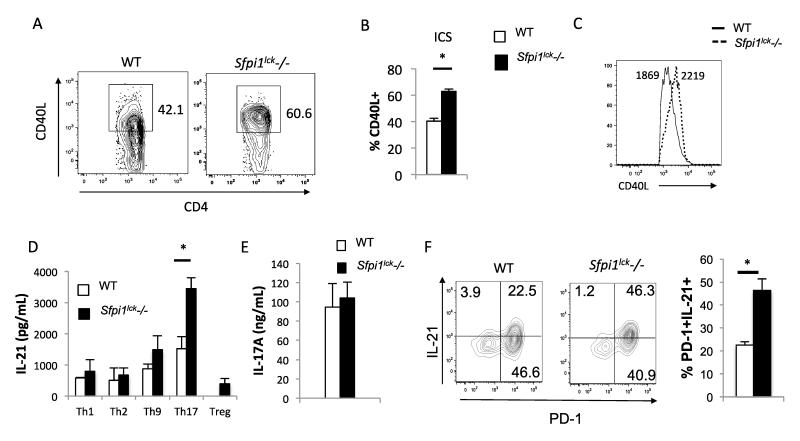
To investigate whether endogenous PU.1 regulates the expression of CD40L in CD4+T cells, CD4+ T cells were isolated from wild type and Sfpi1lck−/− mice and stimulated with PMA and Ionomycin for 2 hours. After 2 hours of stimulation, surface and intracellular CD40L expression was determined by flow cytometry. An increase in the percentage of CD4+ cells that stained positive for CD40L was observed when stimulated CD4+ cells from Sfpi1lck−/− mice were compared to cells from wild type mice (Figure 2A-B). The CD40L mean fluorescence intensity (MFI) of cells that were CD40L+ was also increased in stimulated CD4+ cells from Sfpi1lck−/− mice (Figure 2C). These data demonstrate that PU.1 limits the expression of CD40L in CD4+ T cells.
Figure 2.
CD4+ T-cells from Sfpi1lck−/− mice show increased CD40L and IL-21 expression in vitro. Total CD4+ T-cells from Sfpi1lck−/− and WT mice were stimulated with PMA + Ionomycin for 2 hours. A, Flow cytometric analysis of intracellular staining for CD40L. The average percent of CD4+CD40L+ cells (B) and histograms of the MFI of CD40L+ cells (C) are indicated. D, Naïve cells CD4+CD62L+ were isolated from WT and Sfpi1lck−/− mice and cultured under Th1, Th2, Th9, Th17, and T-regulatory conditions. Concentrations of IL-21 (D) produced by the indicated Th subsets, and IL-17A (E) produced by Th17 cells was measured by ELISA. F, Naïve cells from wild type and Sfpi1lck−/− mice were cultured under Tfh conditions. After 3 days of culture cells were restimulated with PMA and Ionomycin for 5 hours and surface and intracellular cytokine staining was conducted. Contour plots indicate PD-1 and IL-21 staining and graphs indicate the average of values from 3 mice. Data are representative of 3-5 mice/experiments. Statistical significance was determined with a two-tailed t test, *, p<0.05.
Many Th subsets express IL-21 (28-31). We differentiated Th1, Th2, Th9, Th17 and T-regulatory cells in vitro for 5 days, restimulated equal numbers of cells on anti-CD3 coated plates for an additional 24 hours, and collected supernatants to measure IL-21 using ELISA. Th17 cells derived from Sfpi1lck−/− mice produced significantly more IL-21 compared to wild type T cells (Figure 2D). However, IL-17A production in Th17 cultures was similar between both groups (Figure 2E). In parallel experiments, naïve T cells from wild type and Sfpi1lck−/− mice were cultured under conditions to generate Tfh-like cells and we observed a greater percentage of IL-21-positive PD-1-positive cells in cultures that lacked PU.1 expression (Figure 2F). Thus, PU.1 regulates the expression of CD40L and IL-21.
MOG35-55 immunized Sfpi1lck−/− mice show decreased resolution of germinal center activity
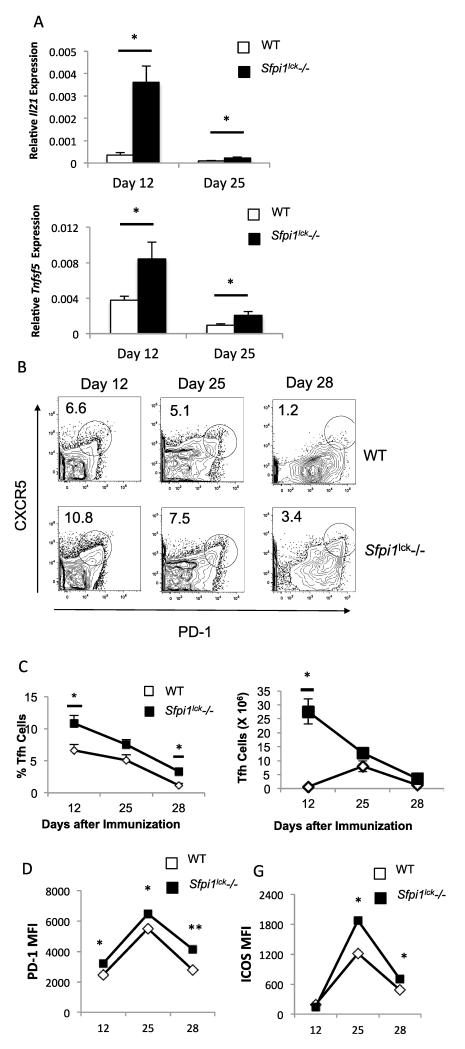
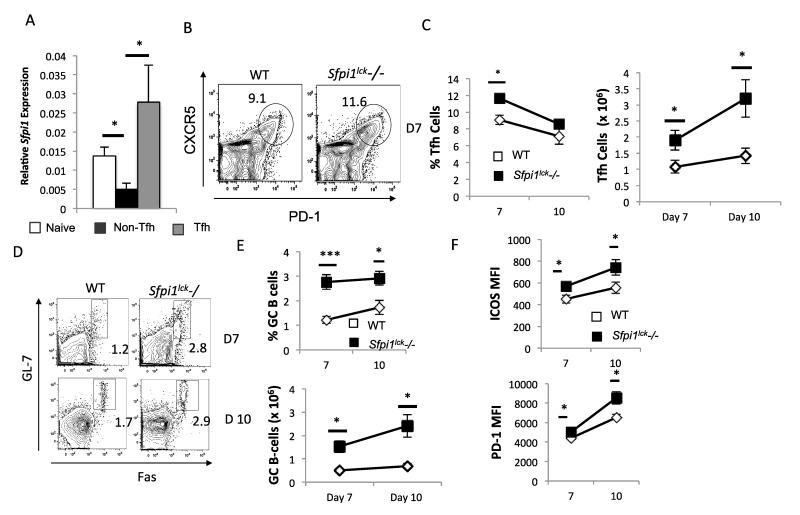
CD40L and IL-21 promote germinal center activity and germinal center B-cell differentiation (16, 17, 19, 20, 32). The cognate interaction between CD40-expressing GCB cells and CD40L-expressing Tfh cells is important for germinal center B cell survival, proliferation, and maturation. Lack of CD40L expression in humans and mice impairs proper germinal center formation and function (19, 33-37). IL-21 also contributes, though is not essential for the differentiation and expansion of GCB cells (32). The findings that PU.1 can regulate CD40L and IL-21 expression led us to hypothesize that PU.1 may be important in Tfh and germinal center activity. To investigate if PU.1 has any impact on Tfh development, wild type and Sfpi1lck−/− mice were immunized with an emulsion containing myelin oligodendricyte glycoprotein (MOG) peptide in CFA. At several time points after immunization the spleens from wild type and Sfpi1lck−/− mice were analyzed for expression of Il21 and Tnfsf5 by qPCR. Il21 expression in splenocytes from immunized Sfpi1lck−/− mice was significantly increased at 12 and 25 days after immunization, compared to splenocytes from wild type mice (Figure 3A). Tnfsf5 expression was also higher compared to wild type mice (Figure 3A). We then examined splenic Tfh cells using flow cytometry. Sfpi1lck−/− mice showed increased percentages and absolute numbers of Tfh cells (CD4+CXCR5+PD-1+) 12 days after immunization compared to wild type mice, with no significant differences in total splenic cellularity (Figure 3B-C). The increase in the percentage of Tfh cells in Sfpi1lck−/− mice persisted on Days 25 and 28, although absolute numbers of Tfh cells were not significantly different at the later time points (Figure 3B-C). The MFI of PD-1 and ICOS expressed on the surface of Tfh cells was greater in Sfpi1lck−/− compared to wild type mice (Figure 3D).
Figure 3.
Sfpi1lck−/− mice have increased Tfh cells after immunization with MOG35-55. WT and Sfpi1lck−/− mice were immunized with MOG35-55 and sacrificed 12, 25, and 28 days after initial immunization. A, Spleens from immunized mice were harvested from wildtype and Sfpi1lck−/− mice for mRNA analysis. mRNA levels of the indicated genes 12 and 25 days after immunization are shown. B, Splenocytes from immunized mice were stained for Tfh markers and analyzed by flow cytometry. C, Percent of Tfh and number of Tfh cells in wild type and Sfpi1lck−/− mice 12, 25 and 28 days after immunization are shown. D, PD-1 and ICOS expression by WT and Sfpi1lck−/− Tfh cells were measured by flow cytometry. Data are representative of 2-3 experiments with 3-6 mice per group (A-D). Statistical significance was determined with a two-tailed t test, *, p<0.05; **, p<0.005.
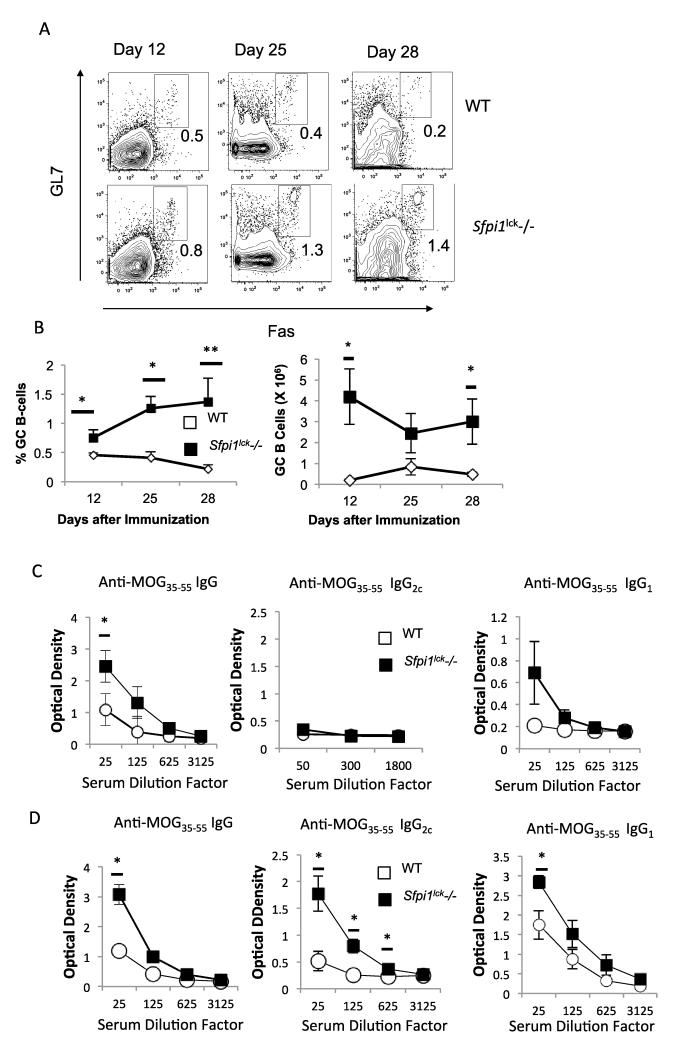
To determine if increased Tfh numbers and function resulted in increased germinal center B (GCB) cells, we examined GCB populations using flow cytometry and antigen-specific antibody production in the serum. We observed increased GCB cells in Sfpi1lck−/− compared to wild type mice beginning on day 12 (Figure 4A-B). The percent of GCB in Sfpi1lck−/− mice increased over the 28 day period examined and was significantly greater than observed in wild type mice at all time points (Figure 4A-B). The absolute number of GCB cells was also significantly increased at day 12 and 28 (Figure 4B). In agreement with the increase in GCB cells, we also observed increased MOG35-55-specific IgG titers at day 12 (Figure 4C) and increased MOG35-55-specific IgG, IgG2c and IgG1 titers in Sfpi1lck−/− mice compared to wild type mice on day 25 (Figure 4D). The enhanced germinal center activity in Sfpi1lck−/− mice suggests that PU.1 is a negative regulator of Tfh cells and indirectly GCB cell formation and without PU.1 expression in Tfh cells, the resolution of germinal center activity is attenuated.
Figure 4.
Sfpi1lck−/− mice have enhanced germinal center B-cell development after MOG35-55 immunization. WT and Sfpi1lck−/− mice were immunized with MOG35-55 and analyzed 12, 25, and 28 days after immunization. A, Splenocytes were stained with germinal center B-cell markers and analyzed flow cytometry. B, Percent of GC B-cells and number of GC B-cells in wild type and Sfpi1lck−/− mice are indicated. C-D, Serum IgG, IgG2c, and IgG1 titers were measured on days 12 (C) and 25 (D). Data are representative of 2-3 experiments with 3-6 mice per group (A-C). Statistical significance was determined with a two-tailed t test, *, p<0.05; **, p<0.005.
Increased germinal center formation in the absence of PU.1 following SRBC immunization
The MOG35-55-immunization experiments were performed in the context of examining EAE in mice that lack PU.1 expression in T cells. We observed that Sfpi1lck−/− mice had greater disease severity through an as yet undetermined mechanism (data not shown). To further define the effects of PU.1-deficiency on Tfh development in a model that does not have an ongoing inflammatory disease and in an adjuvant-independent manner, we used immunization with sheep red blood cells (SRBCs). SRBCs are highly immunogenic, resulting in a robust GC activity, allowing for the study of greater numbers of Tfh cells.
We first wanted to examine PU.1 expression in a Tfh population from immunized mice. We sorted the Tfh (CD4+CXCR5+PD-1+), non-Tfh (CD4+CXCR5−PD-1-low) and naïve T (CD4+ CD62L+) cell populations from wild type mice and analyzed PU.1 expression by qPCR. PU.1 expression was higher in Tfh cells compared to non-Tfh cells, but was not significantly different from PU.1 expression in naïve CD4 T cells (Figure 5A).
Figure 5.
Tfh and GC B cell analysis in WT and Sfpi1lck−/− mice after SRBC immunization. WT and Sfpi1lck−/− mice were immunized with SRBC for analysis of germinal center activity. A, Naïve, CD4+CXCR5+PD-1+Tfh cells, and CD4+CXCR5−PD-1− Non-Tfh cells were sorted from wild type spleen and mRNA was isolated for analysis of gene expression. B, Splenocytes from SRBC immunized mice were stained for Tfh cell analysis by flow cytometry. C, The average frequency and number of Tfh cells 7 and 10 days after immunization are indicated. D, Splenocytes from SRBC immunized mice were stained for GC B cells. E, The average frequency and number of GCB cells 7 and 10 days after SRBC immunization are shown. F, PD-1 and ICOS expression on Tfh cells 7 and 10 days after immunization are shown. Data are representative of 2 experiments with 6 mice per group (A) and 2 experiments (B-D) with 8 mice per group. Statistical significance was determined with a two-tailed t test, *,p<0.05; ***, p<0.0001.
We then examined Tfh and GCB cells seven and ten days after SRBC immunization. Sfpi1lck−/− mice showed higher percentages and absolute numbers of Tfh and GCB cells compared to wild type mice seven days after immunization, with no significant differences in total splenic cellularity (Figure 5B-E). On day 10, the percentage of Tfh cells in Sfpi1lck−/− mice and wild type mice was similar, however, the absolute numbers of Tfh cells, and the percentages and absolute numbers of GCB cells in Sfpi1lck−/− mice was significantly higher than in wild type mice (Figure 5B-E). Tfh cells from Sfpi1lck−/− mice also showed significantly higher levels of PD-1 and ICOS on both day 7 and day 10 (Figure 5F).
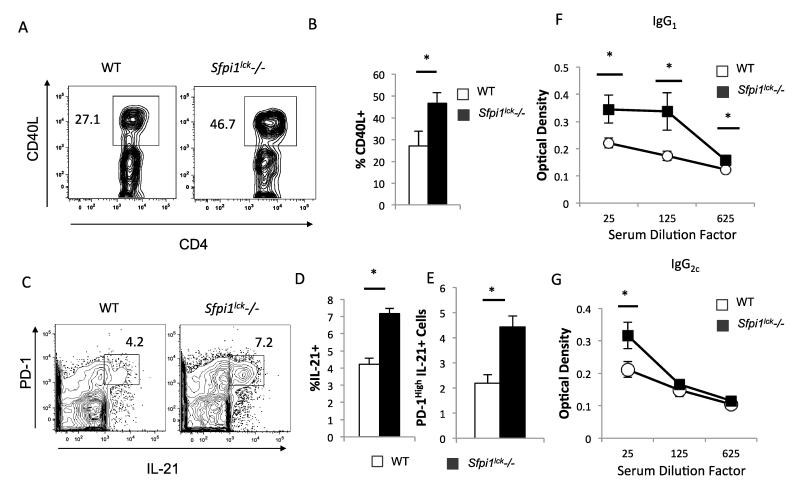
To further understand what factors may be contributing to the increase in GCB cells in Sfpi1lck−/− mice, we looked at the expression of CD40L and IL-21 in Tfh cells from Sfpi1lck−/− and wild type mice. Tfh cells from Sfpi1lck−/− mice had increased expression of intracellular CD40L compared to wild type mice (Figure 6A-B). Sfpi1lck−/− Tfh cells also had a higher percentage of IL-21-positive Tfh cells with a higher MFI compared to wild type mice (Figure 6C-D and data not shown). The number of IL-21-positive cells within the spleen of Sfpi1lck−/− mice was two-fold larger than the number seen in wild type mice (Figure 6E). Apart from the increased CD40L and IL-21 expression by Sfpi1lck−/− Tfh cells the increase in GCB cells in immunized Sfpi1lck−/− could also be due to decreased follicular regulatory cells. However, the percentage of T follicular regulatory cells within the spleens of Sfpi1lck−/− and wild type mice were similar (data not shown). SRBC protein specific IgG1 and IgG2c antibody titers were also significantly elevated in Sfpi1lck−/− mice compared to wild type mice (Figure 6F-G).
Figure 6.
Gene expression and antibody production in WT and Sfpi1lck−/− mice after SRBC immunization. WT and Sfpi1lck−/− mice were immunized with SRBC and stained for CD40L and IL-21 expression in Tfh cells. CD40L (A,B) and IL-21 (C,D) expression by WT and Sfpi1lck−/− Tfh cells was determined by flow cytometry. (E) The percentage of CD4+CXCR5+ PD-1High cells that are IL-21+ was determined by flow cytometry. Serum from SRBC immunized WT and Sfpi1lck−/− mice were collected and IgG1 (F) and IgG2c (G) titers for SRBC protein specific antibodies were determined by ELISA. Data are representative of 2 experiments with 5-6 mice per group (A-D) and 2 experiments with 6 mice per group (C-G). Statistical significance was determined with a two-tailed t test. *, p<0.05.
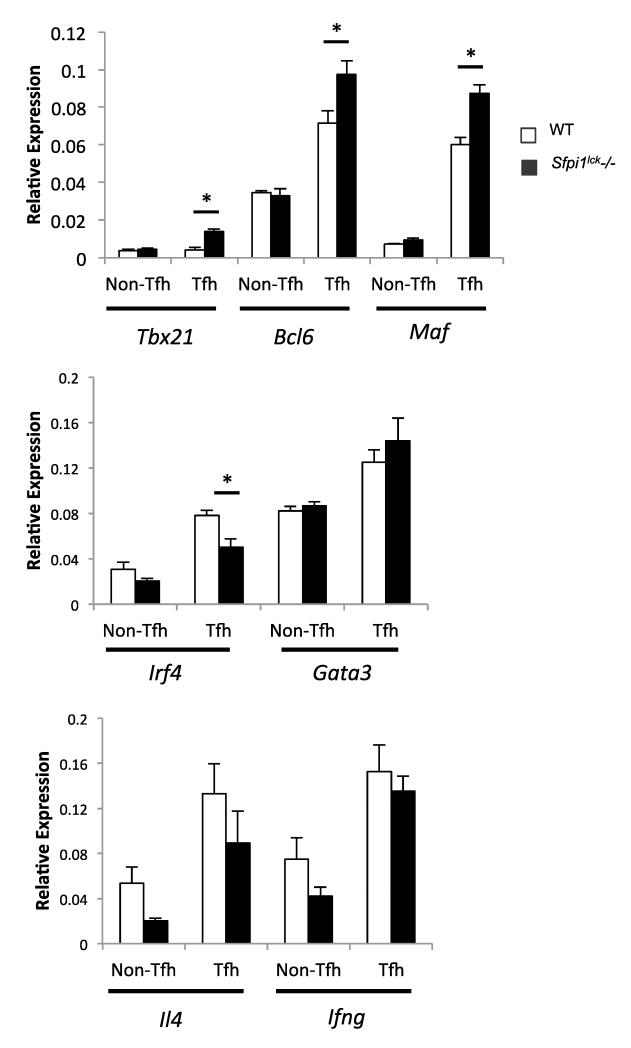
We next determined if there were alterations in the expression of other Tfh-associated genes caused by the absence of PU.1, we sorted Tfh cells (CD4+CXCR5+PD-1+) from Sfpi1lck−/− and wild type mice 7 days after SRBC immunization. Sfpi1lck−/− Tfh cells showed a significant increase in Tbx21 mRNA expression (Figure 7), consistent with previous reports of increased T-bet expression in IL-21-secreting Tfh cells (38) . We also observed that Sfpi1lck−/− Tfh cells showed a significant increase in Bcl6 and Maf expression (Figure 7). Sfpi1lck−/− Tfh cells also showed a decrease in Irf4 mRNA expression (Figure 7). Although this decrease is significant, the requirement for IRF4 in Tfh cell development suggests the decrease has only a modest biological effect (39). We did not observe any difference in Gata3, Ifng, or Il4 expression by wild type and Sfpi1lck−/− Tfh cells (Figure 7). Together, these data indicate an important requirement for PU.1 in limiting Tfh development and regulating multiple genes within the Tfh population.
Figure 7.
Gene expression analysis of sorted Tfh cells from SRBC immunized WT and Sfpi1lck−/− mice. Tfh and Non-Tfh cells were sorted from SRBC immunized WT and Sfpi1lck−/− mice and expression of the indicated genes was determined by qPCR. Data are representative of 2 experiments with 6 mice per group. Statistical significance was determined with a two-tailed t test. *, p<0.05.
Blocking CD40L in Sfpi1lck−/− mice decreases Germinal Center B cells and restores normal Immunoglobulin levels
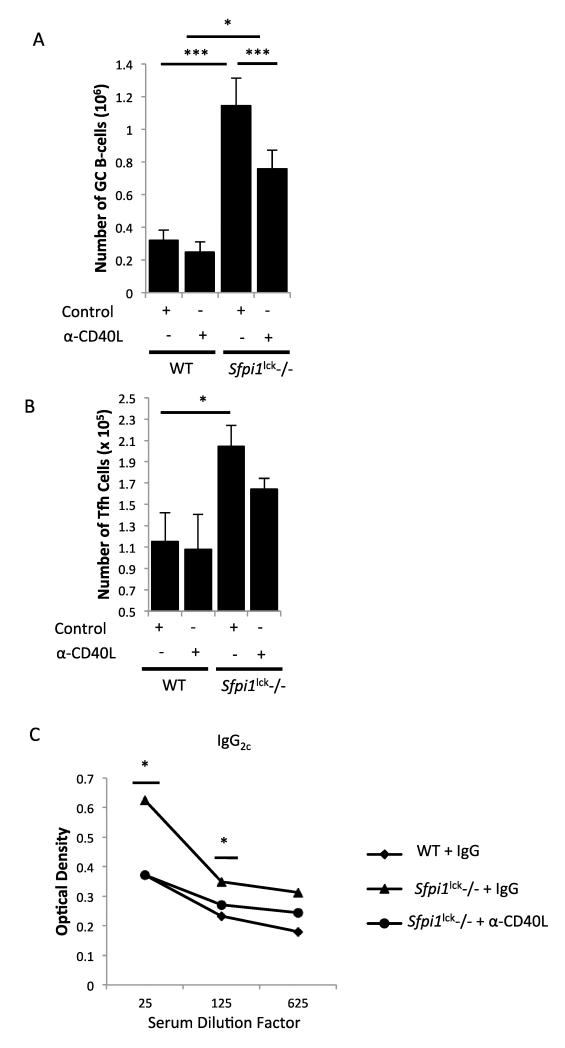
CD40L and IL-21 both play an important role in the expansion of GCB cells. However, the absence of CD40L expression in humans and mice appears to have a more dramatic impact on GC development and subsequently B-cell function (19, 20, 29, 40-44). We used blocking antibodies against IL-21 or CD40L to determine if either factor was contributing to the enhanced development of GCB cells seen in Sfpi1lck−/− mice. We immunized Sfpi1lck−/− mice with SRBC and injected mice with IL-21 blocking antibodies on days 2, 4, and 6. On day 7 mice were sacrificed and GC Tfh and B cells were assessed. We found that blocking IL-21 did not change the number of GCB cells in wild type or Sfpi1lck−/− mice (data not shown). We next blocked CD40L activity after SRBC immunization by treating Sfpi1lck−/− and wild type mice with control antibody or antibodies against CD40L. Blocking CD40L significantly decreased the number of GCB cells in Sfpi1lck−/− (Figure 8A). Yet, the dose used in these experiments had only a modest effect on wild type GCB cell development, suggesting that GCB cells in Sfpi1lck−/− mice were more sensitive to this treatment (Figure 8A). Despite the reduction in GCB cells in Sfpi1lck−/− mice that received CD40L blocking antibody, there were still elevated GCB cell numbers in Sfpi1lck−/− mice compared to wild type mice that received control antibody. We observed a slight but not statistically significant decrease in Tfh cells in Sfpi1lck−/− mice after CD40L antibody treatment (Figure 8B). To determine if the CD40L-mediated reduction in GCB cells was reflected in function, we analyzed the titers of IgG2c in mice that received control and CD40L blocking antibodies. Anti-CD40L treatment attenuated the increase in IgG2c compared to Sfpi1lck−/− mice that received control antibody (Figure 8C). The titers of IgG2c observed in Sfpi1lck−/− mice that received anti-CD40L antibody was indistinguishable from titers observed in wild type mice (Figure 8C). Thus, PU.1 negatively regulates Tfh cell development and controls GCB cell numbers by a mechanism that is at least partly dependent upon CD40L.
Figure 8.
Blocking of CD40L attenuates germinal center B-cell increases in Sfpi1lck−/− mice. WT and Sfpi1lck−/− mice were immunized with SRBC and treated with control antibody or CD40L blocking antibody on days 5 and 6 post-immunization. On day 7 mice were sacrificed and splenocytes analyzed for Tfh cells and GCB cells by flow cytometry. The total number of GCB cells (A) and Tfh cells (B) are indicated. (C) SRBC protein specific IgG2c titers were analyzed by ELISA. Data are representative of 2 experiments with 4-8 mice per group (A-C). Statistical significance was determined with one way ANOVA (A) and two-tailed t test (B-C), *, p<0.05; ***, p<0.0001.
Discussion
Mounting a lasting immune response against invading organisms is a key component of the adaptive immune response. Tfh cells and GCB cells are vital factors in the production of high affinity antibodies during an initial infection and later during subsequent infections. A balance of initiation and resolution of the germinal center response is required for prevention of recurrent or persistent infections or on the other side of the spectrum, autoimmunity. Therefore, a comprehensive understanding of the factors that positively or negatively regulate germinal center activity is vital. Tfh cells express the surface proteins that facilitate cognate B cell interactions within the germinal center. Among the proteins that facilitate these interactions, CD40L and IL-21 are principal orchestrators within germinal centers that enable Tfh cells to provide help to B cells. Yet, how CD40L and IL-21 are regulated in Tfh cells is not completely understood.
The observations in this report suggest that PU.1 is a regulator of Tfh activity, at least partially through regulation of CD40L. We observed that ectopic expression of PU.1 in CD4 T cells represses Tnfsf5 mRNA and CD40L surface expression. PU.1 bound directly to the Tnfsf5 promoter, and T cells that lack PU.1 expression have increased CD40L expression. In two different models of antibody production, both adjuvant-dependent and –independent, mice with PU.1-deficient T cells had increased frequency and number of Tfh cells and GCB cells, and increased IgG production. Importantly, antibodies to CD40L diminished GCB cell numbers and antibody production, suggesting a sensitivity to blockade of this surface ligand. Preliminary experiments with in vitro-derived Tfh-like cells demonstrated that PU.1-deficient Tfh-like cells have an increased ability to induce IgG2c production during an in vitro culture assay where cell numbers are normalized (data not shown). Thus, at least some of the effects we observe on in vivo Tfh function appear to be intrinsic to the population. However, some of the increased function of PU.1-deficient Tfh cells might be due to increased numbers of cells.
It is still not entirely clear how PU.1 mediates the expansion of the Tfh population. It is possible that PU.1 regulates one of the surface receptors studied in this report, which impact GCB cell expansion that subsequently feeds back to promote Tfh expansion. We noted that although CD40L blockade decreased GCB cell expansion and IgG production, it did not significantly inhibit Tfh numbers. This could be due to an insufficient concentration of anti-CD40L to completely block the effects of the interaction, but might also indicate another mechanism. We have previously shown that the absence of PU.1 in CD4+ T cells leads to increased TCR expression and increased T cell activation (22). Although T-cell receptor (TCR) signaling is necessary for multiple stages of Tfh and germinal center development (45, 46), we also observed that increased stimulation of PU.1-deficient CD4 T cells results in increased IL-2 production, a cytokine known to inhibit Tfh development (39). Thus, it seems unlikely that the TCR regulatory function of PU.1 contributes to the Tfh phenotype in vivo.
We present evidence that shows PU.1 is important for regulating IL-21 expression by Tfh cells. Although IL-21 did not appear to be responsible for the increased Tfh activity in the absence of PU.1-expressing T cells, PU.1 bound the Il21 promoter, and PU.1-dependent regulation of IL-21 production was observed in vitro and in vivo. IL-21 expression by Tfh cells has been shown to be downstream of ICOS and c-maf (9). Ectopic expression of PU.1 in CD4+ T cells leads to decreased Il21 and Maf mRNA, but not ICOS. Our in vivo studies show increased expression of IL-21 protein and Maf mRNA in Sfpi1lck−/− Tfh cells, compared to controls. These reports, coupled with the data that PU.1 binds the Il21 promoter, suggests that PU.1-dependent regulation could be both direct, and indirect, through c-Maf. PD-1 expression was also decreased by ectopic PU.1 expression and Tfh cells in Sfpi1lck−/− mice express greater amounts of PD-1. However, we did not detect PU.1 binding to the Pdcd1 promoter, suggesting that either PU.1 binds to a separate regulatory region, or that PD-1 is regulated indirectly, possibly through regulation of BCL6 (47).
Our findings also suggest that changes in the expression of PU.1 in T cells might play a role in autoimmunity. Gene association studies have linked increased PU.1 expression in CD4+ T cells to systemic lupus erythematosus (48) and the ability of PU.1 to alter Tfh functions might be a potential mechanism for increased autoantibody production. Moreover, the ETS DNA recognition motif is enriched in sequences near enhancer regions associated with human Tfh cell gene regulation (49). These data would suggest that altered PU.1 expression might contribute to autoantibody production in patient populations.
This report provides greater evidence for the involvement of PU.1 in germinal center B-cell development and antibody production. We show that PU.1 has intrinsic functions within the Tfh population and that PU.1-deficiency results in altered expression of CD40L, IL-21 and transcription factors including Maf and Irf4. This might parallel the ability of PU.1 to recruit Bcl6 and repress genes in GCB cells (50). Moreover, the effects of PU.1 deficiency in T cells impact GCB cell development, expansion and antibody production. Whether the ability of PU.1 to regulate antibody production leads to altered risk of autoimmunity is not clear and will be an important question for the future.
Acknowledgments
This work was supported by Public Health Service grants R01 AI057459 to MHK, and R03 AI110987 and R21 AI113523 to ALD. OA was supported by PHS F31 Al100542, DP was supported by PHS T32HL007910, and MMH was supported by PHS T32 AI060519. Support provided by the HB Wells Center was in part from the Riley Children’s Foundation.
2. Abbreviations
- GC
germinal center
- GCB cell
germinal center B cell
- SRBC
sheep red blood cells
- Tfh
T follicular helper cell
- Th
T helper
References
- 1.Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, Dent AL, Craft J, Crotty S. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, Wang Y-H, Dong C. Bcl6 Mediates the Development of T Follicular Helper Cells. Science. 2009;325:1001. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu D, Rao S, Tsai LM, Lee SK, He Y, Sutcliffe EL, Srivastava M, Linterman M, Zheng L, Simpson N, Ellyard JI, Parish IA, Ma CS, Li QJ, Parish CR, Mackay CR, Vinuesa CG. The Transcriptional Repressor Bcl-6 Directs T Follicular Helper Cell Lineage Commitment. Immunity. 2009;31:457. doi: 10.1016/j.immuni.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Chang HC, Sehra S, Goswami R, Yao W, Yu Q, Stritesky GL, Jabeen R, McKinley C, Ahyi AN, Han L, Nguyen ET, Robertson MJ, Perumal NB, Tepper RS, Nutt SL, Kaplan MH. The transcription factor PU.1 is required for the development of IL-9-producing T cells and allergic inflammation. Nat Immunol. 2010;11:527. doi: 10.1038/ni.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramming A, Druzd D, Leipe J, Schulze-Koops H, Skapenko A. Maturation-related histone modifications in the PU.1 promoter regulate Th9-cell development. Blood. 2012;119:4665. doi: 10.1182/blood-2011-11-392589. [DOI] [PubMed] [Google Scholar]
- 6.Thieu VT, Yu Q, Chang H-C, Yeh N, Nguyen ET, Sehra S, Kaplan MH. Signal Transducer and Activator of Transcription 4 Is Required for the Transcription Factor T-bet to Promote T Helper 1 Cell-Fate Determination. Immunity. 29:679. doi: 10.1016/j.immuni.2008.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breitfeld D, Ohl L, Kremmer E, Ellwart J, Sallusto F, Lipp M, Förster R. Follicular B Helper T Cells Express Cxc Chemokine Receptor 5, Localize to B Cell Follicles, and Support Immunoglobulin Production. The Journal of Experimental Medicine. 2000;192:1545. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. Cxc Chemokine Receptor 5 Expression Defines Follicular Homing T Cells with B Cell Helper Function. The Journal of Experimental Medicine. 2000;192:1553. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bauquet AT, Jin H, Paterson AM, Mitsdoerffer M, Ho IC, Sharpe AH, Kuchroo VK. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH -17 cells. Nature Immunology. 2009;10:167. doi: 10.1038/ni.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bollig N, Brus̈tle A, Kellner K, Ackermann W, Abass E, Raifer H, Camara B, Brendel C, Giel G, Bothur E, Huber M, Paul C, Elli A, Kroczek RA, Nurieva R, Dong C, Jacob R, Mak TW, Lohoff M. Transcription factor IRF4 determines germinal center formation through follicular T-helper cell differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:8664. doi: 10.1073/pnas.1205834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hiramatsu Y, Suto A, Kashiwakuma D, Kanari H, Kagami SI, Ikeda K, Hirose K, Watanabe N, Grusby MJ, Iwamoto I, Nakajima H. C-Maf activates the promoter and enhancer of the IL-21 gene, and TGF-β inhibits c-Maf-induced IL-21 production in CD4+ T cells. Journal of Leukocyte Biology. 2010;87:703. doi: 10.1189/jlb.0909639. [DOI] [PubMed] [Google Scholar]
- 12.Ise W, Kohyama M, Schraml BU, Zhang T, Schwer B, Basu U, Alt FW, Tang J, Oltz EM, Murphy TL, Murphy KM. The transcription factor BATF controls the global regulators of class-switch recombination in both B cells and T cells. Nature Immunology. 2011;12:536. doi: 10.1038/ni.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kroenke MA, Eto D, Locci M, Cho M, Davidson T, Haddad EK, Crotty S. Bcl6 and Maf cooperate to instruct human follicular helper CD4 T cell differentiation. Journal of Immunology. 2012;188:3734. doi: 10.4049/jimmunol.1103246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma CS, Avery DT, Chan A, Batten M, Bustamante J, Boisson-Dupuis S, Arkwright PD, Kreins AY, Averbuch D, Engelhard D, Magdorf K, Kilic SS, Minegishi Y, Nonoyama S, French MA, Choo S, Smart JM, Peake J, Wong M, Gray P, Cook MC, Fulcher DA, Casanova JL, Deenick EK, Tangye SG. Functional STAT3 deficiency compromises the generation of human T follicular helper cells. Blood. 2012;119:3997. doi: 10.1182/blood-2011-11-392985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ray JP, Marshall HD, Laidlaw BJ, Staron MM, Kaech SM, Craft J. Transcription factor STAT3 and type I interferons are corepressive insulators for differentiation of follicular helper and T helper 1 cells. Immunity. 2014;40:367. doi: 10.1016/j.immuni.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Linterman MA, Beaton L, Yu D, Ramiscal RR, Srivastava M, Hogan JJ, Verma NK, Smyth MJ, Rigby RJ, Vinuesa CG. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. The Journal of Experimental Medicine. 2010;207:353. doi: 10.1084/jem.20091738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zotos D, Coquet JM, Zhang Y, Light A, D’Costa K, Kallies A, Corcoran LM, Godfrey DI, Toellner K-M, Smyth MJ, Nutt SL, Tarlinton DM. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell–intrinsic mechanism. The Journal of Experimental Medicine. 2010;207:365. doi: 10.1084/jem.20091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poholek AC, Hansen K, Hernandez SG, Eto D, Chandele A, Weinstein JS, Dong X, Odegard JM, Kaech SM, Dent AL, Crotty S, Craft J. In Vivo Regulation of Bcl6 and T Follicular Helper Cell Development. The Journal of Immunology. 2010;185:313. doi: 10.4049/jimmunol.0904023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foy TM, Laman JD, Ledbetter JA, Aruffo A, Claassen E, Noelle RJ. gp39-CD40 interactions are essential for germinal center formation and the development of B cell memory. The Journal of Experimental Medicine. 1994;180:157. doi: 10.1084/jem.180.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han S, Hathcock K, Zheng B, Kepler TB, Hodes R, Kelsoe G. Cellular interaction in germinal centers. Roles of CD40 ligand and B7-2 in established germinal centers. The Journal of Immunology. 1995;155:556. [PubMed] [Google Scholar]
- 21.Anderson MK, Weiss AH, Hernandez-Hoyos G, Dionne CJ, Rothenberg EV. Constitutive expression of PU.1 in fetal hematopoietic progenitors blocks T cell development at the pro-T cell stage. Immunity. 2002;16:285. doi: 10.1016/s1074-7613(02)00277-7. [DOI] [PubMed] [Google Scholar]
- 22.Chang H-C, Han L, Jabeen R, Carotta S, Nutt SL, Kaplan MH. PU.1 Regulates TCR Expression by Modulating GATA-3 Activity. The Journal of Immunology. 2009;183:4887. doi: 10.4049/jimmunol.0900363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang H-C, Zhang S, Thieu VT, Slee RB, Bruns HA, Laribee RN, Klemsz MJ, Kaplan MH. PU.1 Expression Delineates Heterogeneity in Primary Th2 Cells. Immunity. 2005;22:693. doi: 10.1016/j.immuni.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 24.Goswami R, Jabeen R, Yagi R, Pham D, Zhu J, Goenka S, Kaplan MH. STAT6-dependent regulation of Th9 development. J Immunol. 2012;188:968. doi: 10.4049/jimmunol.1102840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goswami R, Kaplan MH. Gcn5 is required for PU.1-dependent IL-9 induction in Th9 cells. J Immunol. 2012;189:3026. doi: 10.4049/jimmunol.1201496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jabeen R, Chang HC, Goswami R, Nutt SL, Kaplan MH. The transcription factor PU.1 regulates gammadelta T cell homeostasis. PLoS One. 2011;6:e22189. doi: 10.1371/journal.pone.0022189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dakic A, Metcalf D, Di Rago L, Mifsud S, Wu L, Nutt SL. PU.1 regulates the commitment of adult hematopoietic progenitors and restricts granulopoiesis. The Journal of Experimental Medicine. 2005;201:1487. doi: 10.1084/jem.20050075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suto A, Kashiwakuma D, Kagami S.-i., Hirose K, Watanabe N, Yokote K, Saito Y, Nakayama T, Grusby MJ, Iwamoto I, Nakajima H. Development and characterization of IL-21–producing CD4+ T cells. The Journal of Experimental Medicine. 2008;205:1369. doi: 10.1084/jem.20072057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eto D, Lao C, DiToro D, Barnett B, Escobar TC, Kageyama R, Yusuf I, Crotty S. IL-21 and IL-6 Are Critical for Different Aspects of B Cell Immunity and Redundantly Induce Optimal Follicular Helper CD4 T Cell (Tfh) Differentiation. PLoS ONE. 2011;6:e17739. doi: 10.1371/journal.pone.0017739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 31.Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory TH17 cells. Nature. 2007;448:484. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ozaki K, Spolski R, Feng CG, Qi C-F, Cheng J, Sher A, Morse HC, Liu C, Schwartzberg PL, Leonard WJ. A Critical Role for IL-21 in Regulating Immunoglobulin Production. Science. 2002;298:1630. doi: 10.1126/science.1077002. [DOI] [PubMed] [Google Scholar]
- 33.Allen R, Armitage R, Conley M, Rosenblatt H, Jenkins N, Copeland N, Bedell M, Edelhoff S, Disteche C, Simoneaux D. CD40 ligand gene defects responsible for X-linked hyper-IgM syndrome. Science. 1993;259:990. doi: 10.1126/science.7679801. a. et. [DOI] [PubMed] [Google Scholar]
- 34.Aruffo A, Farrington M, Hollenbaugh D, Li X, Milatovich A, Nonoyama S, Bajorath J, Grosmaire LS, Stenkamp R, Neubauer M, Roberts RL, Noelle RJ, Ledbetter JA, Francke U, Ochs HD. The CD40 ligand, gp39, is defective in activated T cells from patients with X-linked hyper-IgM syndrome. Cell. 1993;72:291. doi: 10.1016/0092-8674(93)90668-g. [DOI] [PubMed] [Google Scholar]
- 35.Korthauer U, Graf D, Mages HW, Briere F, Padayachee M, Malcolm S, Ugazio AG, Notarangelo LD, Levinsky RJ, Kroczek RA. Defective expression of T-cell CD40 ligand causes X-linked immunodeficiency with hyper-IgM. Nature. 1993;361:539. doi: 10.1038/361539a0. [DOI] [PubMed] [Google Scholar]
- 36.DiSanto JP, Bonnefoy JY, Gauchatt JF, Fischer A, Saint Basile G. d. CD40 ligand mutations in X-linked immunodeficiency with hyper-IgM. Nature. 1993;361:541. doi: 10.1038/361541a0. [DOI] [PubMed] [Google Scholar]
- 37.Fuleihan R, Ramesh N, Loh R, Jabara H, Rosen RS, Chatila T, Fu SM, Stamenkovic I, Geha RS. Defective expression of the CD40 ligand in X chromosome-linked immunoglobulin deficiency with normal or elevated IgM. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:2170. doi: 10.1073/pnas.90.6.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luthje K, Kallies A, Shimohakamada Y, Belz GT, Light A, Tarlinton DM, Nutt SL. The development and fate of follicular helper T cells defined by an IL-21 reporter mouse. Nat Immunol. 2012;13:491. doi: 10.1038/ni.2261. [DOI] [PubMed] [Google Scholar]
- 39.Crotty S. T Follicular Helper Cell Differentiation, Function, and Roles in Disease. Immunity. 2014;41:529. doi: 10.1016/j.immuni.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bryant VL, Ma CS, Avery DT, Li Y, Good KL, Corcoran LM, de Waal Malefyt R, Tangye SG. Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells: predominant role of IL-21 produced by CXCR5+ T follicular helper cells. J Immunol. 2007;179:8180. doi: 10.4049/jimmunol.179.12.8180. [DOI] [PubMed] [Google Scholar]
- 41.Deenick EK, Avery DT, Chan A, Berglund LJ, Ives ML, Moens L, Stoddard JL, Bustamante J, Boisson-Dupuis S, Tsumura M, Kobayashi M, Arkwright PD, Averbuch D, Engelhard D, Roesler J, Peake J, Wong M, Adelstein S, Choo S, Smart JM, French MA, Fulcher DA, Cook MC, Picard C, Durandy A, Klein C, Holland SM, Uzel G, Casanova JL, Ma CS, Tangye SG. Naive and memory human B cells have distinct requirements for STAT3 activation to differentiate into antibody-secreting plasma cells. J Exp Med. 2013;210:2739. doi: 10.1084/jem.20130323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kotlarz D, Zietara N, Uzel G, Weidemann T, Braun CJ, Diestelhorst J, Krawitz PM, Robinson PN, Hecht J, Puchalka J, Gertz EM, Schaffer AA, Lawrence MG, Kardava L, Pfeifer D, Baumann U, Pfister ED, Hanson EP, Schambach A, Jacobs R, Kreipe H, Moir S, Milner JD, Schwille P, Mundlos S, Klein C. Loss-of-function mutations in the IL-21 receptor gene cause a primary immunodeficiency syndrome. J Exp Med. 2013;210:433. doi: 10.1084/jem.20111229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levy J, Espanol-Boren T, Thomas C, Fischer A, Tovo P, Bordigoni P, Resnick I, Fasth A, Baer M, Gomez L, Sanders EA, Tabone MD, Plantaz D, Etzioni A, Monafo V, Abinun M, Hammarstrom L, Abrahamsen T, Jones A, Finn A, Klemola T, DeVries E, Sanal O, Peitsch MC, Notarangelo LD. Clinical spectrum of X-linked hyper-IgM syndrome. J Pediatr. 1997;131:47. doi: 10.1016/s0022-3476(97)70123-9. [DOI] [PubMed] [Google Scholar]
- 44.Qamar N, Fuleihan RL. The hyper IgM syndromes. Clin Rev Allergy Immunol. 2014;46:120. doi: 10.1007/s12016-013-8378-7. [DOI] [PubMed] [Google Scholar]
- 45.Tubo NJ, Pagán AJ, Taylor JJ, Nelson RW, Linehan JL, Ertelt JM, Huseby ES, Way SS, Jenkins MK. Single naive CD4+ T cells from a diverse repertoire produce different effector cell types during infection. Cell. 2013;153:785. doi: 10.1016/j.cell.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fazilleau N, McHeyzer-Williams LJ, Rosen H, McHeyzer-Williams MG. The function of follicular helper T cells is regulated by the strength of T cell antigen receptor binding. Nat Immunol. 2009;10:375. doi: 10.1038/ni.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hollister K, Kusam S, Wu H, Clegg N, Mondal A, Sawant DV, Dent AL. Insights into the role of Bcl6 in follicular Th cells using a new conditional mutant mouse model. J Immunol. 2013;191:3705. doi: 10.4049/jimmunol.1300378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hikami K, Kawasaki A, Ito I, Koga M, Ito S, Hayashi T, Matsumoto I, Tsutsumi A, Kusaoi M, Takasaki Y, Hashimoto H, Arinami T, Sumida T, Tsuchiya N. Association of a functional polymorphism in the 3′ - untranslated region of SPI1 with systemic lupus erythematosus. Arthritis & Rheumatism. 2011;63:755. doi: 10.1002/art.30188. [DOI] [PubMed] [Google Scholar]
- 49.Weinstein JS, Lezon-Geyda K, Maksimova Y, Craft S, Zhang Y, Su M, Schulz VP, Craft J, Gallagher PG. Global transcriptome analysis and enhancer landscape of human primary T follicular helper and T effector lymphocytes. Blood. 2014;124:3719. doi: 10.1182/blood-2014-06-582700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wei F, Zaprazna K, Wang J, Atchison ML. PU.1 Can Recruit BCL6 to DNA To Repress Gene Expression in Germinal Center B Cells. Molecular and Cellular Biology. 2009;29:4612. doi: 10.1128/MCB.00234-09. [DOI] [PMC free article] [PubMed] [Google Scholar]