Abstract
Staphylococcus aureus (S. aureus) can cause a broad range of potentially fatal inflammatory complications (e.g. sepsis, endocarditis). Its emerging antibiotic resistance and formidable immune evasion arsenal have emphasized the need for more effective antimicrobial approaches. Complement is an innate immune sensor that rapidly responds to bacterial infection eliciting C3-mediated opsonophagocytic and immunomodulatory responses. Extracellular Fibrinogen-binding Protein (Efb) is a key immune evasion protein of S. aureus that intercepts complement at the level of C3. To date, Efb has not been explored as a target for monoclonal antibody (mAb)-based antimicrobial therapeutics. Herein we have isolated donor-derived anti-Efb IgGs that attenuate S. aureus survival through enhanced neutrophil killing. A phage library screen yielded mAbs (miniAbs) that selectively inhibit the interaction of Efb with C3 partly by disrupting contacts essential for complex formation. Surface Plasmon Resonance-based kinetic analysis enabled the selection of miniAbs with favorable Efb-binding profiles as therapeutic leads. MiniAb-mediated blockade of Efb attenuated S aureus survival in a whole blood model of bacteremia. This neutralizing effect was associated with enhanced neutrophil-mediated killing of S. aureus, increased C5a release and modulation of IL-6 secretion. Finally, these miniAbs afforded protection from S. aureus-induced bacteremia in a murine renal abscess model, attenuating bacterial inflammation in kidneys. Overall, these findings are anticipated to pave the way towards novel antibody-based therapeutics for S. aureus-related diseases.
Keywords: Complement, Staphylococcus aureus, Efb, mini-antibodies, passive immunization, bacteremia, antimicrobial therapy
Introduction
Staphylococcus aureus is a human pathogen that has an increasingly negative impact on human health (1). It is a leading cause of hospital and community-acquired infections ranging from skin or soft tissue abscesses, to severe necrotizing infections with a highly invasive phenotype (2). Contraction of S. aureus can lead to potentially fatal conditions (i.e., endocarditis, septicemia), and persistent inflammatory complications associated with implanted medical devices (i.e., intravascular catheters, pacemakers) (1).
The massive consumption of antibiotics has led to the emergence of drug-resistant strains, designated MRSA for methicillin–resistant S. aureus (3,4). This pronounced antibiotic resistance, paired with the formidable immune evasion arsenal that is deployed by S. aureus to thwart the host immune response at multiple levels, has led to an alarmingly high mortality rate (11-45% of invasive MRSA-related diseases) in the U.S (CDC Threat Report 2013, http://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf). This daunting clinical problem calls for the design of novel therapeutic approaches to boost conventional antimicrobial treatment. Thus far, S. aureus-targeted vaccines relying on traditional immunization strategies have not produced successful outcomes in clinical trials (5,6). Furthermore, S. aureus is an endemic microbe in the human population whose pathogenesis can be triggered by subtle changes in the host's immune surveillance landscape. This aspect renders the design of effective vaccines even more challenging.
To circumvent these problems, antimicrobial therapeutic design is now being directed towards antibody-based vaccines for passive immunization (7). Human mAb-based therapies offer multiple advantages over conventional antimicrobial treatments (8,9). They display high selectivity for pathogen-specific structures, lack adverse complications as opposed to plasma-derived products (e.g. intravenous immunoglobulin, IVIG) and more importantly, they cannot easily be exploited by the pathogen for developing resistance to therapy (10). A prime concern of mAb-based therapeutic design, however, is the optimal selection of S. aureus targets that are instrumental in promoting the pathogen's virulence (6,11,12).
Complement is a crucial innate immune sentinel which protects from bacterial infection by triggering a cascade of protein-protein interactions that leads to C3-mediated pathogen opsonophagocytosis (13). S. aureus has evolved several strategies to subvert complement by secreting molecules that selectively inhibit distinct components and activation pathways (14-16). Among an array of secreted proteins, S. aureus encodes Efb (Extracellular Fibrinogen-binding protein), a key immune evasion protein that selectively targets the complement component C3 (17). Efb is a 16-kDa protein which consists of two structurally and functionally distinct domains; an N-terminal domain (1-64 aa) which binds to fibrinogen (Fg) (18) and the C-terminus domain (Efb-C, 65-165 aa) which binds to C3 and its fragments C3b and C3d (17). Efb blocks complement activation by allosterically inhibiting formation of C3b-containing C3 and C5 convertases (17,19,20). In vivo studies using mutant S. aureus strains lacking Efb have revealed its virulence-promoting role in S. aureus pathogenesis (21-23).
Exploiting the potent complement-modulatory activity of Efb, and its crucial role in promoting S. aureus’ infectivity, we have developed and characterized recombinant human mAbs (termed henceforth, miniAbs) that neutralize the function of Efb both in vitro and in vivo by blocking its interaction with complement C3. These targeted antimicrobial agents potently attenuate the survival of S. aureus in models of bacteremia, and show promise for further development as Ab-based vaccine candidates for passive immunization.
Materials and Methods
Proteins/ Reagents
MiniAbs against Efb-C were generated by screening a HuCal Ab library (Bio-Rad AbD Serotec GmbH, Puchheim, Germany) as described previously (24). A miniAb consists of a dimeric Fab fragment linked together by an oligomerization domain flanked by two epitope tags (24). C3 and recombinant C3d were purified based on previous protocols (17,25,26). Recombinant Efb, Efb-C, Efb-C-RENE, Ehp and SCIN-A proteins were expressed and purified as described previously (27). S. aureus, strain Newman was purchased from ATCC (25904).
Human plasma samples
Routine clinical blood specimens along with clinical information were obtained from patients admitted at the General Hospital “Evangelismos”, (Athens, Greece) under an approved human subject's protocol. Patients were characterized as S. aureus-positive (i.e., presenting infections of the blood, lung, or skin) based on microbiology laboratory reports. Blood was obtained from healthy individuals who had previously been identified as S. aureus-negative (i.e., no active infection at the time of blood sampling).
Whole blood model of bacteremia
Experiments with blood from healthy donors were performed as described previously (28). The whole blood model was applied in two distinct formats: i) the “endogenous” format: Briefly, S. aureus cells (1× 108 CFU/ml ) were incubated with 50% whole blood in the presence of miniAbs for 4 h at 37°C. ii) the “exogenous” format: S. aureus cells (2.5× 106 CFU/ml) were incubated with 40% whole blood in the presence of 1.5 μM recombinant Efb-C for 2 h at 37°C after the addition of miniAbs. After incubation, a small volume of the samples was plated for calculation of S. aureus survival rate. The remaining volume of the samples was centrifuged for plasma collection.
Neutrophil killing assay
To assess neutrophil killing, S. aureus cells (Newman strain) at 5× 106 CFU/ml were pre-opsonized for 15 min with 10% human serum, followed by incubation with 1× 106 neutrophils for 30 min at 37°C. 50nM of miniAbs or human donor-derived IgGs were added to S. aureus suspensions during the preopsonization step, in the presence of 50nM Efb-C. S. aureus killing was analyzed on TSB agar plates. The percentage of cell killing was calculated based on the formula (T0-T30/T0 × 100).
Isolation of anti-Efb-C IgG from human plasma
Human plasma verified by ELISA for the presence of anti-Efb-C IgG (see below) was applied to a CNBr-activated Sepharose-4B column (GE Healthcare) where Efb-C had been immobilized. After extensive washing with PBS, IgG bound to Efb-C were eluted with 0.1M glycine, pH 2.5. Eluted fractions were immediately neutralized by addition of Tris-HCl, pH 8.0 and dialyzed O/N against PBS at 4°C. As a negative control for all subsequent studies, plasma was passed through the same column and the unbound material was collected. Next, IgG were isolated from the unbound fraction and lack of anti-Efb-C IgG was confirmed by ELISA.
Surface Plasmon Resonance (SPR)
i) Selectivity profile of miniAbs
The interaction between the miniAbs and S. aureus proteins was measured using a Biacore 3000 (GE Healthcare) at 25 °C. HBS-EP (0.01M HEPES (pH 7.4), 150 mM NaCl, 3 mM EDTA, 0.005% (v/v) Tween-20) was used as the mobile phase throughout the experiment. Anti-human Fab antibodies (Fab binder Kit, GE Healthcare) were immobilized at a density of 10,000 resonance units (RU) on a CM5 sensor chip according to the manufacturer's instructions. MiniAbs were captured on individual flow cells of the Fab-binding sensor chip at a density of ~600 RU. S. aureus proteins were diluted to a concentration of 100 nM in HBS-EP and injected for 1 min at a 20 μl/min flow rate with a dissociation phase of 2 min. At the end of each screening cycle, the miniAb's were stripped off the surface by injecting glycine buffer pH 2.1 twice for 1 min each to allow for capturing of a new miniAb set. Signals from an empty Fab-binder flow cell and from an ensemble of buffer blank injections were used to perform double referencing of samples responses. Data processing and evaluation was performed using Scrubber (v2.0; BioLogic Software Pty Ltd, Campbell, Australia).
ii) Kinetic analysis of the MiniAb/Efb-C interaction
For the kinetic studies, miniAbs were captured at a density of 500 RU on individual flow cells as described above. A single cycle kinetic titration model was employed (29), in which five concentrations of Efb-C spanning a fivefold dilution series (3.3 - 270 nM) were injected for 2 min each without intermittent regeneration. At the end of each cycle, the dissociation was observed for 5 min and miniAb's were stripped off the surface. Three buffer blanks were included in each analysis and their average signals were subtracted from the processed sample responses (double referencing). Each data set was fitted to a Langmuir 1:1 interaction model (single cycle method kindly provided by GE Healthcare) and the equilibrium dissociation constant (KD) was calculated.
Immunoblotting
S. aureus strains Newman, ΔEfb (kindly provided by Dr. Suzan Rooijakkers, University Medical Center Utrecht, The Netherlands) and ΔEhp were grown in TSB medium for 20 hours at 37 °C. Following cells sedimentation culture supernatants were concentrated with 13% TCA. Samples were ran on a SDS-PAGE gel and transferred onto a nitrocellulose membrane. After blocking of the membrane with 5% milk /PBS/Tween-20 (0.05%) for 1 hour, the polyclonal antibody anti-Efb-C or the miniAbs were added at final dilution 1/100 or 1 μg/ml respectively, and incubated for 1 hour. Next, the membrane was washed twice with PBS/Tween-20 followed by incubation with the anti-rabbit-HRP (Bio-Rad, Puchheim, Germany) or anti-mouse-HRP at 1/2000 dilution. Immunoreactive bands were visualized by chemiluminescence (ECL kit, GE Healthcare) according to the manufacturer's instructions on a Fujifilm LAS-4000 luminescent image analyzer (Fujifilm Manufacturing USA Inc, Greenwood, SC, USA).
ELISAs
Anti-Efb IgG detection in human plasma
For the detection of anti-Efb IgG in plasma from S. aureus positive and negative human donors, 96-well ELISA plates were coated with 2μg/ml Efb-C O/N at 4°C. After blocking the wells with 1% BSA / PBS for 1 hour at room temperature, plasma samples were serially diluted and incubated for 30 minutes. Following washing of the wells with PBS/Tween-20, the polyclonal Ab anti-human IgG-HRP was added at 1/10000. Samples were incubated for 30 minutes and after extensive washing, immune complexes were detected at 405nm.
Competitive ELISA for measuring antibody-mediated inhibition of binding of C3 fragments to Efb-C
i) Inhibition by donor-derived IgG
ELISA plates were coated with 5μg/ml Efb-C as above. After blocking with 5% BSA/ PBS/Tween-20 for 1 hour at room temperature, donor IgGs were serially diluted starting from a concentration of 10 μg/ml. Following incubation for 45 minutes, 1 μg/ml of C3 was added to the wells and the mixture of the proteins was further incubated for 30 minutes. Following washing, the anti-C3 (clone 755, HyCult) was added at 2 μg/ml for 30 minutes. Following the addition of the anti-mouse-IgG-HRP (1/2000) the plate was washed, and OD was measured at 405nm. The results were expressed as percentage of inhibition of Efb-C/C3 binding, using as negative control (0% inhibition), O.D. values obtained from samples without donor-derived IgGs.
ii) Inhibition by miniAbs
ELISA plates were coated and blocked as described above. MiniAbs were serially diluted and incubated for 45 minutes followed by addition of 1 μg/ml of C3/C3b/C3d and further incubation for 30 minutes. Following washing, the monoclonal anti-C3 (clone 755, HyCult), anti-C3b (clone I3/15, HyCult), 1H8 (anti-human C3d) (30) were added at 2 μg/ml for 30 minutes. Following the addition of the anti-mouse-IgG-HRP (1/2000), the plate was washed, and immune complexes were detected as above. The results were expressed as percentage of inhibition of Efb-C -C3/C3b/C3d binding, using as negative control (0% inhibition) values obtained from samples without miniAbs.
Efb detection in whole blood
Lepirudin-anticoagulated whole blood was incubated with S. aureus, Newman strain (1× 107 CFU/ml) and samples were collected at various time intervals (0-12 h). Plasma was recovered and analyzed for the presence of Efb according to the following ELISA scheme (31): ELISA plates were coated with 1 μg/ml fibrinogen (Sigma) as above. After blocking with 2% BSA/ PBS, plasma samples were serially diluted and incubated for 1 h at 37 °C. Following washing, a polyclonal anti-Efb-C Ab was added at 1/100, followed by an anti-rabbit IgG-HRP at 1/2000 for 30 minutes. After washing, immune complexes were detected at 405nm. The results were expressed in arbitrary units (AU) corresponding to the levels of Efb.
ELISA for C5a detection
ELISA plates were coated with the anti-human C5a mAb (R&D Systems, Inc., Minneapolis, MN) as described above. After blocking, plasma samples were serially diluted and incubated for 30 minutes. Following washing, a rabbit polyclonal anti-human C5a (CompTech, Tyler, Texas, USA) was added at a dilution of 1/2000 for 30 minutes. Following washing, anti-rabbit-HRP IgG was added at 1/2000 for 30 minutes and immune complexes were measured at 405nm. A standard curve of recombinant human C5a was used to quantify C5a levels in plasma (ng/ml).
ELISA for IL-6 detection
The presence of IL-6 in human plasma recovered from whole blood exposed to S. aureus and miniAbs was measured using an ELISA kit according to the manufacturer's instructions (ImmunoTools, GmbH, Germany).
Mouse renal abscess model
All animal protocols and procedures were approved by the NCSRD Institutional Review Board and Ethics Committee (Permit Number: 240/CED/10.10) and fully complied with National and International guidelines on Ethics on Animal Use. Female Balb/c mice, 6-8 weeks old, were provided by the Hellenic Pasteur Institute (Athens, Greece).
S. aureus (Newman strain) was grown O/N in TSB medium and diluted 1/100 in fresh media the following day. Cultures were grown to an OD660 of 0.7, centrifuged and resuspended in sterile PBS. The inoculum was verified by plating and enumeration on TSB plates. For the renal abscess model mice received intraperitoneally a single injection of miniAb-A1 (1 mg/kg) or PBS (vehicle injection) (n≥6) 2 h prior to infection. Subsequently, the mice were injected via the tail vein either with control-PBS (n ≥ 4) or with 1 ×107 CFU of S. aureus cells (n ≥ 12). Mice were monitored daily for weight changes and other pathological signs (e.g. ruffled fur, reduced cage activity). On day 10, all mice were sacrificed by CO2 inhalation. Blood was collected by exsanguination and various organs were excised (e.g. kidneys, lungs, liver). Kidneys were homogenized and plated for bacterial load enumeration. A section of the kidneys was processed for histology. H&E stained sections were scanned, in a NanoZoomer 2.0-HT (Hamamatsu) digital slide scanner and images were processed using the Aperio ImageScope v11.1.2.760 software. Inflamed regions as well as the whole kidney section surface were defined, and the percentage of parenchymal inflammation was calculated using the formula: [(sum of all inflamed areas) / (whole kidney section area)] ×100%.
Results
Human donors possess neutralizing anti-Efb IgGs that attenuate bacterial survival
To assess the antibody response against Efb and compare the IgG profiles of healthy donors (S. aureus-negative) with those of individuals presenting with S. aureus infections of the skin, lung etc. (S. aureus-positive) we initially screened by ELISA a large number of serum samples (N=104 per group) for the presence of anti-Efb IgG. This antibody profiling identified higher titers of anti-Efb IgG in S. aureus-negative donors as compared to donors positive for S. aureus infections (P<0.05) (Fig. 1A). Prompted by this observation we sought to determine whether such donor-derived anti-Efb antibodies would be capable of blocking the complement modulatory function of Efb which has previously been assigned to the C-terminus region of the molecule (Efb-C) (17).
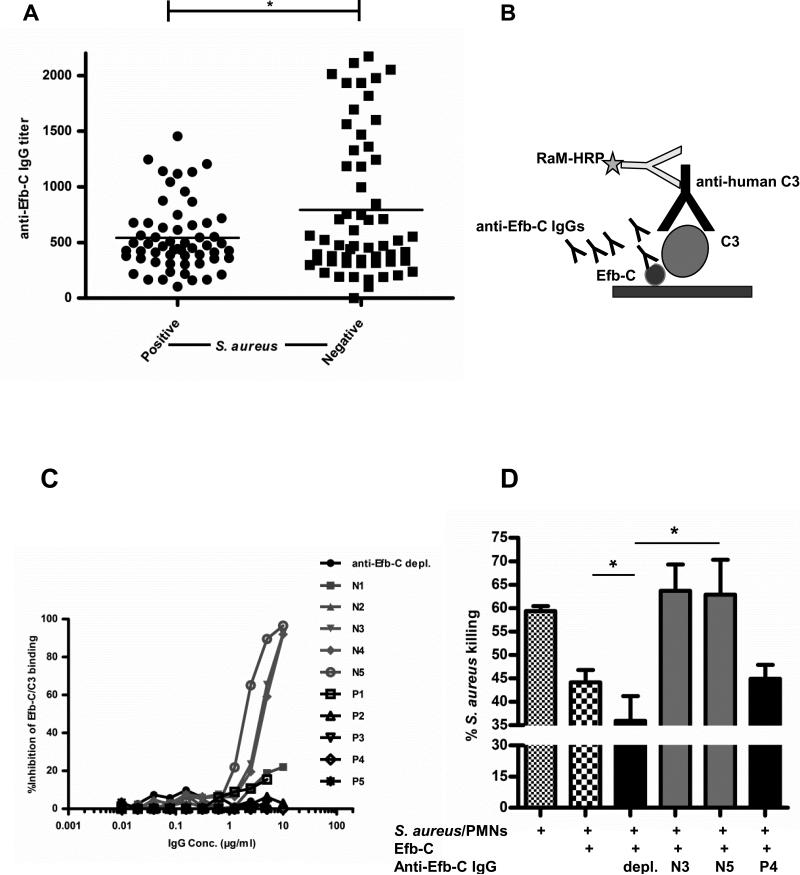
Figure 1. Human donor-derived IgGs against Efb-C attenuate S. aureus survival.
(A) Anti-Efb-C IgG titers in plasma of S. aureus-positive and negative human donors. Each symbol represents an individual person's value; horizontal line represents the mean value of each group; *, P<0.05. (B) Schematic outline of the competitive ELISA employed to determine the ability of anti-Efb-C-specific human IgGs to inhibit the Efb-C/C3 interaction.. The polyclonal nature of the human IgGs is denoted by the presence of multiple antibody molecules. (C) Anti-Efb-C IgGs isolated from individual donors (P, S. aureus-positive; N, S. aureus-negative) inhibit the binding of C3 to Efb-C in a dose-dependent manner. A human IgG preparation that was depleted of its Efb-C-specific antibodies was used as a negative control (anti-Efb-C depl.). (D) Human IgGs displaying pronounced inhibitory effects in the Efb-C/C3 interaction potentiate the PMN-mediated killing of S. aureus cells. Briefly, PMNs were incubated with pre-opsonized S. aureus in the presence of recombinant Efb-C and miniAbs. The percentage (%) of cell killing was calculated following bacterial colony counting. Anti-Efb-C depleted IgG (see above) was used as a negative control (depl.). Data represent mean ± st.dev. of three separate experiments. *, P<0.05
To this end, we set up a competitive ELISA that would allow us to assess the effect of these antibodies on the interaction of Efb-C with C3 (Fig. 1B). Our ELISA studies revealed a panel of affinity-purified anti-Efb human IgG that blocked the Efb-C/C3 interaction, reaching almost 100% inhibition in most cases (Fig. 1C). Strikingly, these blocking antibodies had been isolated from donors that were classified as S. aureus-negative (N3, N4, N5), whereas antibodies isolated from S. aureus-positive donors did not show any blocking effect (P1-P5).
Complement activation leads to enhanced microbial killing, in part via recruitment and activation of potent phagocytic cells, such as neutrophils (32). In this respect, we next asked whether these blocking antibodies could potentiate the neutrophil-mediated killing of S. aureus through their inhibitory effect on complement. While recombinant Efb-C significantly reduced S. aureus cell killing (P<0.05), addition of the blocking antibodies N3 and N5, effectively restored S. aureus killing to the levels observed in the control sample lacking Efb-C (Fig.1D).
Discovery of highly selective Efb-C-targeting miniAbs through a phage library screen
Our initial observations on the presence of neutralizing anti-Efb antibodies in human donors and their capacity to promote bacterial cell killing led us to hypothesize that such antibodies might have therapeutic value as highly selective antimicrobial agents. However, due to the limited availability of human donor-derived IgG and the potential adverse effects associated with the use of human plasma products in therapeutic applications (10), we focused our effort on generating human monoclonal anti-Efb-C antibodies that would display similar protective antimicrobial properties.
A human miniAb library comprising bivalent human F(ab)2 was screened against the target protein Efb-C and the panning resulted in twelve miniAbs with unique primary amino acid sequences. SDS-PAGE analysis revealed highly pure miniAb preparations consisting of two distinct bands at the apparent molecular weights of 34 kDa and 26 kDa under reducing conditions (data not shown).
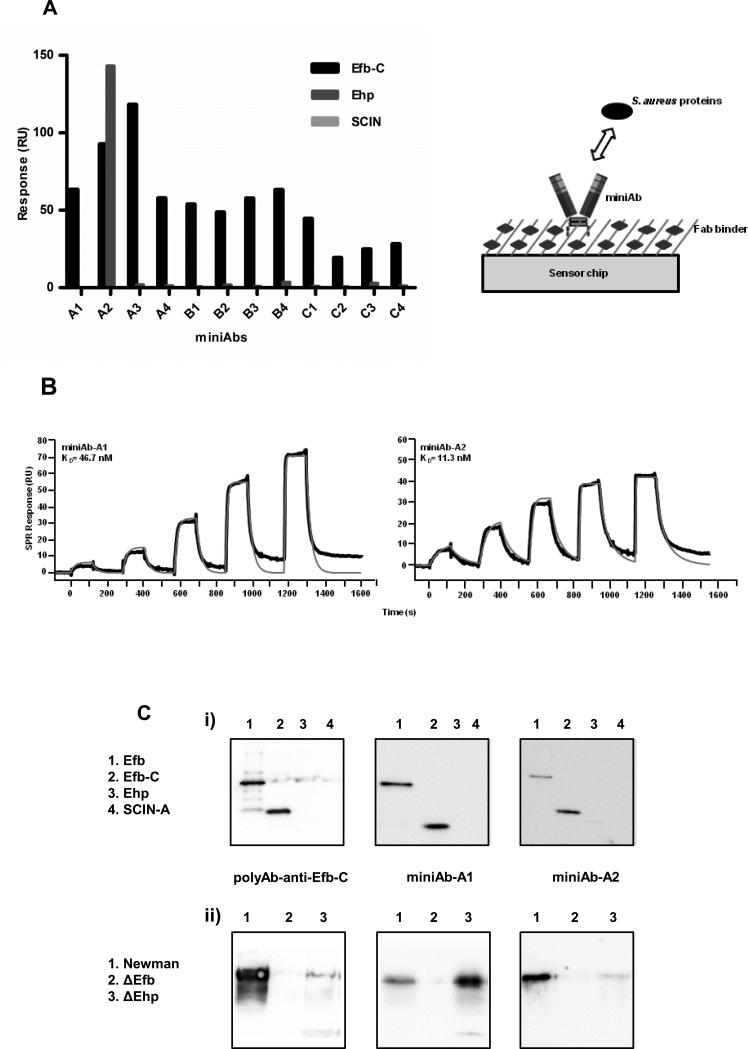
To test the specificity of the generated miniAbs for Efb-C we employed a surface plasmon resonance (SPR)-based approach (see schematic representation, Figure 2A, right panel). To obtain a comprehensive selectivity profile we also tested the binding of the miniAbs to the S. aureus-encoded complement modulatory proteins, Ehp (33) and SCIN-A (34) which share 44% and 12% sequence identity with Efb-C, respectively. Most miniAbs were highly selective for Efb-C, since no binding was recorded for Ehp and SCIN-A (Fig. 2A). The only exception was miniAb-A2, which exhibited a unique selectivity profile binding both Efb-C and its homologous protein Ehp (33).
Figure 2. Selectivity and binding profiles of the anti-Efb-C miniAbs generated from a human F(ab)2 library screening.
(A) Left Panel. Relative binding activities of the miniAbs towards selected complement modulatory proteins of S. aureus as determined by SPR analysis. RU, resonance units. Right Panel. Schematic outline of the SPR-based experimental setup employed to determine the selectivity of the various miniAbs. (B) Kinetic analysis of the interaction between selected miniAbs and Efb-C. The processed binding curves (black lines) were fitted to kinetic models (red lines). The KD values corresponding to each miniAb are shown in the respective sensogram. Data are representative of three independent experiments. (C) Immunoreactivity of selected miniAbs with i) a group of selected S. aureus recombinant proteins or ii) culture supernatants from different S. aureus strains (Newman, ΔEfb, ΔEhp). Immunoblot analysis using a polyclonal antiserum raised against Efb-C was performed in parallel to ensure protein presence and integrity.
All twelve miniAbs were analyzed for their binding affinity to Efb-C. The binding kinetics of those miniAbs exhibiting the highest binding scores from our initial SPR-based selectivity analysis are shown in Fig. 2B. We observed that the binding profiles of all miniAbs generally followed a Langmuir 1:1 interaction model. Deviations observed in the kinetic fits of miniAb-A1 may have been caused by heterogeneity in the protein preparation or target rebinding effects.
Evaluation of the epitope specificity of the miniAbs for the recombinant proteins Efb, Efb-C, Ehp and SCIN-A, revealed that miniAbs A1-A2 likely recognize linear epitopes on proteins Efb and Efb-C (Fig. 2C). No reactivity was observed with the proteins Ehp and SCIN-A, confirming the high selectivity attested to these antibodies by our SPR analysis, with the exception of miniAb-A2 which yielded faint reactivity with the Ehp protein. Attempts to determine whether the miniAbs also recognize conformational epitopes on Efb using native electrophoresis failed to produce conclusive results due to the high pI values of the proteins.
To further discern the epitope specificity of the miniAbs, culture supernatants from wild-type (wt) Newman and ΔEfb and ΔEhp strains were analyzed by immunoblotting (Fig. 2C). MiniAbs A1-A2 recognized epitopes on the native Efb protein, which is secreted by the S. aureus strains Newman and ΔEhp.
MiniAbs interfere with the binding of Efb-C to C3 and its activated fragments C3b and C3d by disrupting contacts essential for complex formation
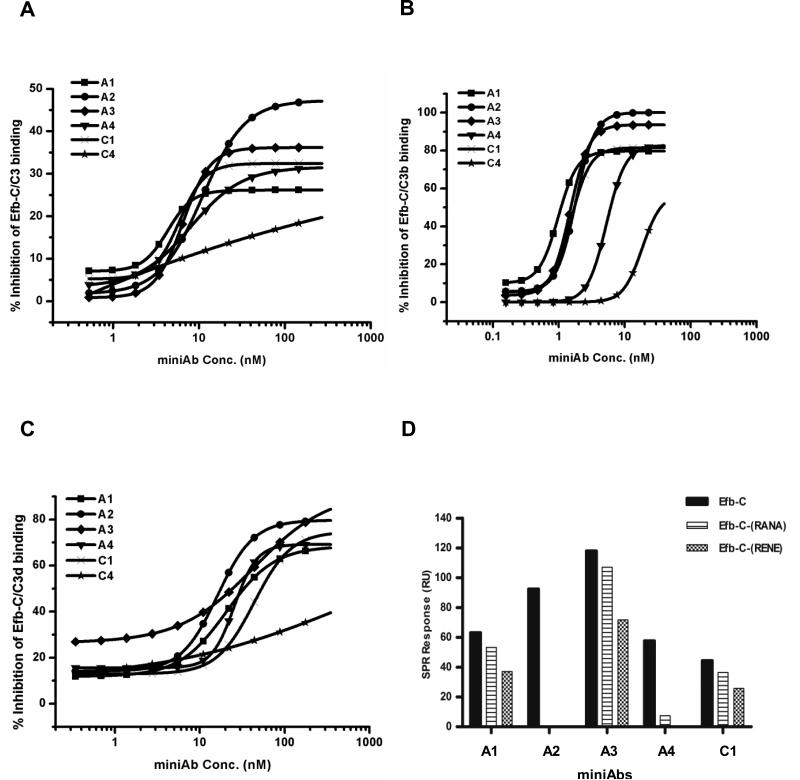
The generation of highly selective miniAbs recognizing Efb-C prompted us to investigate whether these antibodies can exert neutralizing effects by interfering with the binding of Efb-C to its target protein C3. It has previously been shown that Efb-C binds with differential affinity to all thioester-containing C3 fragments besides native C3 (i.e., C3b and C3d) (17). To test if the miniAbs block the binding of Efb-C to these C3-derived molecules we performed a competitive ELISA, as described in Figure 1B. MiniAbs A1, A2, A3 and A4 displayed the highest inhibitory effects against all C3 fragments tested (C3, C3b and C3d) (Fig. 3A-C). Interestingly, the same miniAbs were also selected for having the highest binding affinities for Efb-C (as shown above). On the contrary miniAb-C4, which showed negligible binding to Efb-C, did not significantly inhibit the interaction of Efb-C with all the C3 fragments. Previous studies have shown that Efb-C binds C3d with higher affinity than C3 or C3b (17). Confirming this observation, the miniAb's inhibitory dose required for competing out the binding of Efb-C to C3d was considerably higher when compared to C3 and C3b, which was clearly reflected by the calculated IC50 values in each case (Table I).
Figure 3. MiniAbs impair the interaction of Efb-C with native C3 and its TED-containing fragments.
(A-C) The effect of the selected miniAbs on blocking the formation of (A) the Efb-C/C3, (B) Efb-C/C3b, and (C) Efb-C/C3d complex was determined by a competitive ELISA. Each graph is representative of three independent experiments. (D) SPR-based differential analysis of miniAb binding to wild-type Efb-C and a set of nonfunctional Efb-C mutants. The miniAbs displaying the highest inhibitory profiles (A1-4) show reduced binding to the nonfunctional mutants Efb-C-RANA and Efb-C-RENE, compared to wild-type Efb-C.
Table I.
MiniAbs interfere with the binding of C3 and its activated fragments C3b and C3d to Efb-C.
| IC50 (nM) | |||
|---|---|---|---|
| miniAb | C3 | C3b | C3d |
| A1 | 4.34 | 9.5 | 20.7 |
| A2 | 11 | 15.12 | 16.1 |
| A3 | 6 | 12.7 | 43.5 |
| A4 | 8 | 56.9 | 25.5 |
| B1 | 11.2 | 8.15 | 47.9 |
| B2 | 7.1 | 9.66 | 43 |
| B3 | 9 | 12.76 | 31.4 |
| B4 | 14.3 | 3.48 | 31.5 |
| C1 | 13.2 | 13.28 | 47.3 |
| C2 | 7.1 | 0.9 | 43 |
| C3 | N/A | 3.94 | 42.1 |
| C4 | N/A | 2.38 | N/A |
The IC50 values of all miniAbs are shown with respect to each Efb-C ligand tested. N/A, non-applicable.
As stated above, one of the miniAbs isolated from the phage library screening (i.e., miniAb-A2) showed comparable binding to both Efb-C and Ehp proteins. However, when tested in the same assay, miniAb-A2 did not inhibit the interaction of C3d with Ehp (Fig. S1). This result can partly be explained by the fact that Ehp has two distinct binding sites on human C3d (33), while miniAb-A2 is likely to interfere with only one of these sites on C3d.
Guided by structural insights of the C3d/Efb-C complex that have revealed the crucial role of specific Efb-C residues in mediating the contact with C3d (17,35) we next asked whether these miniAbs recognize epitopes on Efb-C that might contain key structural elements for complex formation. To test this hypothesis, we determined the binding of the inhibitory miniAbs A1-A4 to the loss-of-function Efb-C mutant proteins, Efb-C-(RENE) and Efb-C-(RANA), which have been shown to abolish the binding of Efb-C to C3d (17). As illustrated in Figure 3D, reduced binding of all miniAbs to the mutant Efb-C proteins was observed, consistent with our hypothesis about the miniAb-mediated interference of complex formation. More specifically, A2 appears to have a particularly high sensitivity for the essential amino acids. In the case of A1, A3 and C1, these effects seem much less pronounced.
MiniAbs attenuate S. aureus survival in a whole blood model of bacteremia
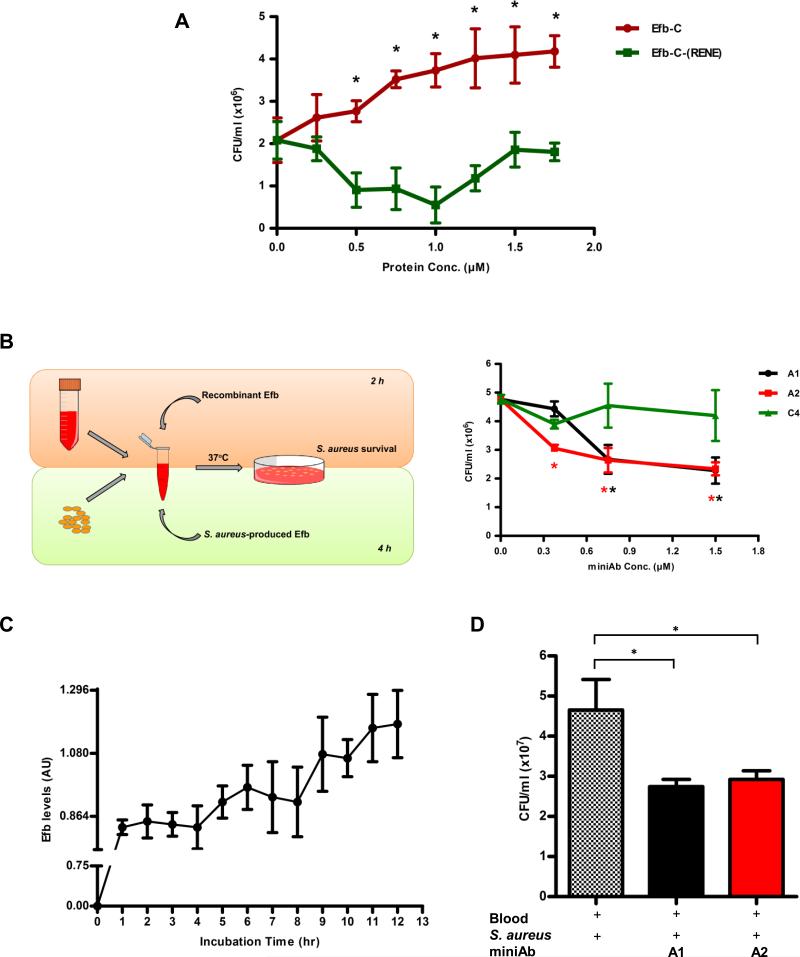
Our initial studies demonstrated that the miniAbs block the interaction of Efb-C with C3/C3b, thereby interfering with a key immune evasion mechanism of S. aureus. Based on these findings, we sought to determine whether these miniAbs can exert a protective effect by decreasing S. aureus survival in an ex vivo model that closely simulates human bacteremia. To this end we employed a whole blood model of S. aureus infection (28). Since the miniAbs specifically target the C-terminus region of Efb (Efb-C) we initially assessed the activity of recombinant Efb-C in this model to validate its key role in pathogen survival (Figure 4A). Exogenously added Efb-C had a dose-dependent positive effect on S. aureus survival, compared to its inactive counterpart (Efb-C-RENE) (P<0.05).
Figure 4. Attenuation of S. aureus growth in whole blood by Efb-C-neutralizing miniAbs.
(A) Recombinant Efb-C promotes the growth S. aureus (CFU/ml) in whole blood, in a dose-dependent fashion, as compared to inactive control protein. Data represent mean ± st.dev. of three separate experiments. *, P<0.05 with reference to the 0μM Efb-C value. (B) Left panel. Schematic overview of the whole blood model of S. aureus-induced bacteremia. Our experimental setup included two distinct but also complementary approaches to assess the efficacy of the miniAbs in blocking S. aureus survival: i) The “exogenous” approach was based on the addition of recombinant Efb to blood mixed with live S. aureus cells while, ii) the “endogenous” approach relied on the production of native Efb by S. aureus cells. Right panel. Efb-C-neutralizing miniAbs cause a dose-dependent decrease of S. aureus growth (CFU/ml) in whole blood (“exogenous” approach). Data represent mean ± st.dev. of three separate experiments. *, P<0.05, with reference to the 0μM miniAb value. (C) Efb secretion profile of S. aureus cells in whole blood. During a 12 h-incubation plasma was collected at various time points and levels of Efb were measured by sandwich ELISA. AU, arbitrary units. (D) MiniAb treatment attenuates S. aureus growth (CFU/ml) in whole blood, as determined by the “endogenous” approach. The ‘+’ symbol denotes the addition of each component in the reaction mixture. Data represent mean ± st.dev. of three separate experiments. *, P<0.05.
Having verified the survival-promoting effect of Efb-C in our system, we then tested the ability of selected inhibitory miniAbs (A1, A2) to neutralize this effect, thereby restoring S. aureus growth to basal levels. Both miniAbs-A1 and A2 effectively reversed the Efb-C-dependent effect on S. aureus growth, attenuating bacterial survival in a dose dependent manner (P<0.05), (Fig. 4B, right panel).
To translate these findings in a more clinically relevant context, we evaluated the effect of the miniAbs in a whole blood model of S. aureus bacteremia targeting the native Efb protein that is secreted by S. aureus cells (Figure 4B, left panel). Despite having the same complement-modulatory activity (17), full length Efb and Efb-C have not been compared side by side in a whole blood model for their growth-promoting activity on S. aureus. A direct comparison of these proteins in our system revealed a similar effect on S. aureus growth (Fig. S2). The slightly more pronounced effect of Efb may be explained with the fibrinogen-bridging effect that was recently suggested (36).
To establish the time course of Efb secretion in whole blood inoculated with S. aureus (1× 107 CFU/ml) we first assayed its production at several time points following S. aureus inoculation. As shown in Fig. 4C, S. aureus-secreted Efb could be detected by ELISA as early as 2-3 h following inoculation and consistently increases for the duration of the experiment (12 h).
Having established that native Efb is secreted in detectable amounts in blood, we then evaluated the neutralizing effect of the miniAbs A1-A2 in the whole blood model. Consistent with our findings in the exogenous model, we observed a significant attenuation of S. aureus survival in the presence of these antibodies, as compared to control (Fig. 4D).
MiniAbs potentiate the neutrophil-mediated killing of S. aureus via complement modulation
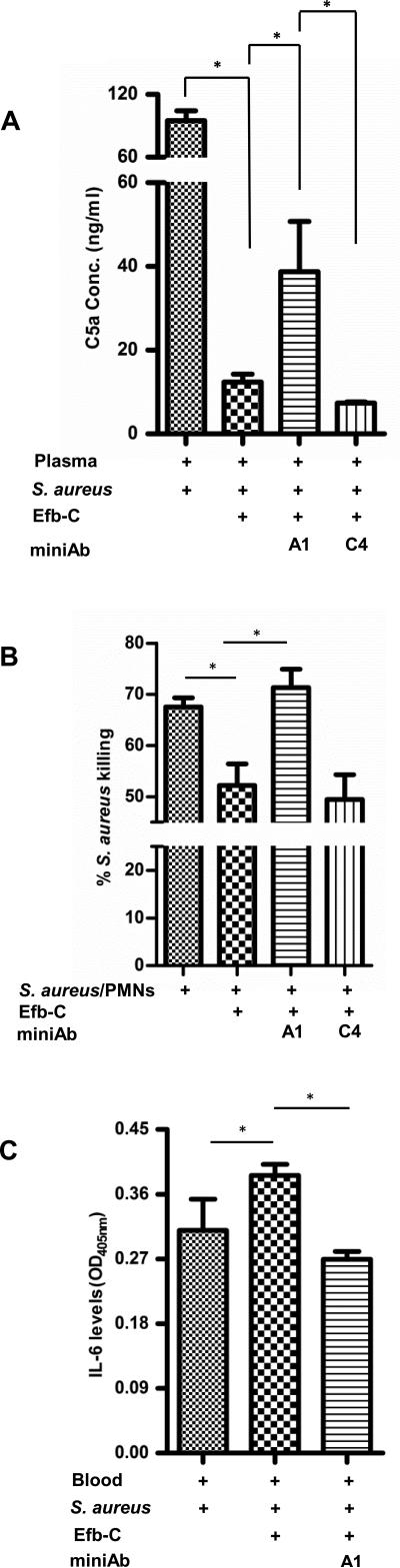
To gain further insight into the mechanism by which the miniAbs impact S. aureus survival, we determined whether they could restore levels of complement activation counteracting the function of Efb. While the addition of Efb-C significantly reduced complement activation in S. aureus-infected blood, as evidenced by lower C5a levels (Fig. 5A), the blocking miniAb-A1 could restore C5a generation to a significant extent (25% increase over Efb-C alone).
Figure 5. Interception of Efb by neutralizing miniAbs restores C5a levels, leading to enhanced neutrophil killing and reduction of bacterially-induced IL-6.
(A) Neutralizing miniAb-A1 restores C5a generation in S. aureus-infected plasma. C5a was measured by sandwich ELISA in plasma incubated with S. aureus, in the presence of recombinant Efb-C (1,5 μM), with or without miniAbs. The ‘+’ symbol denotes the addition of a specific reagent to the reaction; Data represent mean ± st.dev. of three separate experiments. *, P<0.05. (B) Neutralizing miniAbs enhance the neutrophil-mediated killing of S. aureus cells. Data represent mean ± st.dev. of three separate experiments. *, P<0.05. (C) MiniAb-mediated blockage of Efb leads to a reduction of IL-6 reflecting the decrease of bacterial burden. Briefly, plasma samples were incubated with S. aureus in the presence of equimolar amounts of Efb-C and miniAb-A1. IL-6 levels were measured by a sandwich ELISA. Data represent mean ± st.dev. of three separate experiments. *, P<0.05.
This observation prompted us to investigate whether this miniAb-mediated increase in C5a generation could reflect a greater neutrophil killing activity toward S. aureus cells. Indeed, as shown in Fig. 5B, the blocking miniAb-A1 abrogated the inhibitory effect of Efb-C on S. aureus killing (28% inhibition), significantly restoring the bactericidal activity of neutrophils (PMNs) to approximately basal levels (approx. 70%).
IL-6 is a key proinflammatory cytokine that is elevated during bacterial infection (37). To determine whether miniAb-mediated blockade of Efb interferes with cytokine release, thereby attenuating the inflammatory complications of bacteremia, we measured IL-6 levels in S. aureus-infected blood treated with blocking miniAbs. Addition of recombinant Efb-C caused an increase in IL-6 levels (P<0.05), which was reciprocal to the increase of bacterial burden (data not shown). On the contrary, the blocking miniAb-A1 caused a significant reduction in IL-6 levels (Fig. 5C), corresponding to the lower bacterial burden observed in the presence of the miniAb (see also Figure 4B).
MiniAb-mediated blockade of Efb confers protection from S. aureus-induced inflammation in a murine renal abscess model
To evaluate the therapeutic efficacy of the most promising blocking miniAb in vivo we employed the S. aureus-induced, sublethal renal abscess model (38).
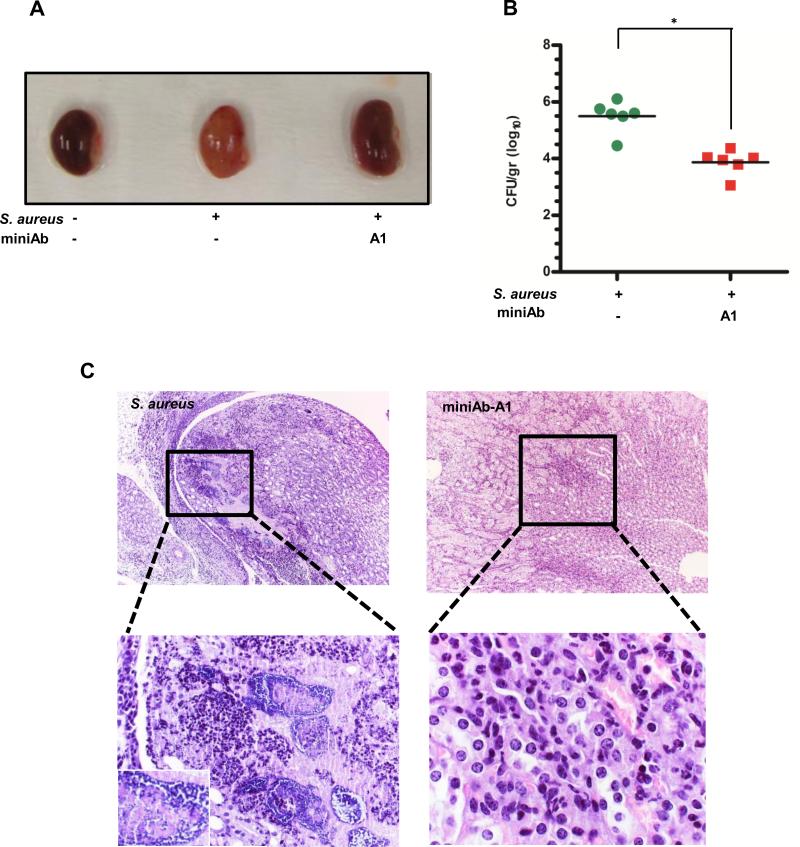
Mice injected with a single I.P. dose of the blocking miniAb-A1 (1mg/kg) 2 h prior to staphylococcal infection exhibited a significantly more active behavior and a clinical score that closely resembled that of non-infected control animals (i.e., greater movement, less ruffled fur and a notable hind limb reflex) throughout the duration of the experiment (until day 10 post infection). This observation was substantiated by the gross morphology of the kidneys harvested from miniAb-injected animals on Day 10 (Fig. 6A). While kidneys from non-treated control (S. aureus-infected) animals displayed large areas of dense inflammatory infiltrates and extensive tissue damage and presence of abscesses with Staphylococcal communities, kidneys from miniAb-treated mice showed clear signs of recovery underscored by fewer, less diffuse inflammatory infiltrates and smaller areas of tissue damage, with lower number of detectable Staphylococcal communities (inflammatory score: 6.39%+1.95, p=0.00014) (Fig. 6C, right panel). Conversely, kidney sections from control animals (S. aureus-infected, no miniAb) presented larger areas of inflammation and tissue necrosis (inflammatory score: 25.7%±4.93, p=0.00014), with greater numbers of staphylococal communities (Fig. 6C, left panel). These findings were further corroborated by colony enumeration experiments in which kidney homogenates from miniAb-treated mice showed a significantly lower bacterial burden (log10CFU: 3.87±0.44), compared to kidneys from control animals (log10CFU 5.50±0.55, p=0.002) (Fig. 6B, see also Table II).
Figure 6. MiniAbs attenuate kidney abscess formation in S. aureus-infected mice.
(A) Representative images of kidneys harvested from control (PBS-injected), S. aureus-infected, and infected mice pretreated with the neutralizing miniAb-A1. Treatment with miniAb-A1 significantly reversed the gross morphological deterioration of the organ, closely resembling the control (PBS-injected) kidneys. In contrast, the S. aureus-infected kidney shows prominent signs of bacterial inflammation and abscess formation, represented by yellow spots on the surface of the kidney parenchyma. (B) MiniAb treatment attenuates bacterial colonization in S. aureus-infected kidneys. Bacterial titers were measured by plating kidney homogenates. *, P<0.05. (C) MiniAb-A1 affords protection from S. aureus-induced inflammation and attenuates kidney tissue damage. Top panels represent low- (×10) and bottom panels represent high-power (×40) magnification of photomicrographs from H/E-stained, paraffin-embedded kidney sections. The kidney papilla parenchyma from an S. aureus-infected mouse presents prominent signs of bacterial inflammation as evidenced by abscess formation and presence of bacterial communities (see lower left panel and inset, ×100). Conversely, treatment of mice with miniAb-A1 leads to a significant restoration of kidney histology as evidenced by the reduced extent of tissue damage and inflammation (upper right panel) and the significantly reduced infiltrates containing fewer staphylococci throughout the tissue (lower right panel). One representative image per group (N=6) is shown here. *, P<0.05.
Table II.
MiniAb-A1 prevents renal abscess formation in S. aureus-infected mice
| Staphylococcal load and abscess formation in renal tissue | |||||
|---|---|---|---|---|---|
| Treatment | Log10CFU g−1a | P-valueb | Reduction (Log10CFU g−1) | % Inflamed area | P-value |
| Mock | 5.50 ± 0.55 | N/A | N/A | 25.7±4.93 | N/A |
| miniAb-A1 | 3.87± 0.44 | 0.002 | 1.63 | 6.39±1.95 | 0.00014 |
Means ± st.dev. of staphylococcal load as Log10CFU g−1 in homogenized renal tissues 10 days after infection in cohorts of 6 BALB/c mice per group. A representative of two independent animal experiments is shown.
Statistical significance was calculated with the unpaired two-tailed Students t test and P-values were recorded.
*, P<0.05.
c Reduction in bacterial load calculated as Log10CFU g−1.
Prompted by the observation that a single dose of the miniAb-A1 elicited a prolonged protective effect in S. aureus-challenged mice, we assayed the stability of this antibody preparation in mouse serum for an extended time period (10 days). As shown in Fig. S4, miniAb-A1 showed a remarkable stability profile and no signs of proteolytic degradation in mouse serum.
Overall, the significant protection afforded by the Efb-C-targeted miniAb in S. aureus-challenged mice, as reflected by the diminished bacterial burden and significantly lower inflammatory scores in the kidneys, along with the prolonged therapeutic effect of a single-dose regimen in the renal abscess model all point to the further clinical exploitation of these Ab-based antimicrobial agents as leads for passive vaccination against staphylococcal infections.
Discussion
The clinical management of S. aureus-associated diseases remains challenging, particularly in view of the emerging antibiotic resistance of this pathogen, and the lack of targeted therapeutics that can abrogate its virulence (39) (11). Here we report the isolation and characterization of human miniAbs that neutralize the function of the S. aureus-encoded complement inhibitory protein Efb by blocking its key interaction with complement C3 (40) (16). To the best of our knowledge, this is the first report of fully human Efb-targeting antibodies that can be further exploited as vaccine leads for S. aureus-related diseases.
The rationale behind the selection of Efb as a target for human antibody development resonates well with its central role within the immune evasion arsenal of S. aureus. Efb inhibits the formation of C3b-containing convertases, thereby abrogating crucial C3-dependent effector functions that promote pathogen opsonic tagging (16). Furthermore, it appears to shield the microbe from phagocytes through the interaction of its N-terminal domain with fibrinogen and the formation of an impenetrable ‘net’ over its surface (36). Thus, its broad inhibitory impact on innate immunity renders Efb an attractive target for the development of ‘first-line’ therapeutic antibodies that could contain bacterial growth along with antibiotics, while also affording the lymphoid compartment enough time to mount antigen-specific effector responses for the subsequent elimination of the microbe.
Applying a whole blood model of S. aureus-induced bacteremia we demonstrated that Efb-C increases bacterial survival to a similar extent as the full-length protein (Efb). The absence of a similar growth-promoting effect from its non-functional mutant Efb-C-RENE underscored the specificity of this action and also implied that it relies on complement interception through formation of C3/Efb-C complexes.
Previous studies had attempted to measure anti-Efb antibody titers in S. aureus carriers (41,42). However, due to the limited number of clinical samples, a comprehensive profile of Efb's immunogenicity remains elusive. Our effort to systematically probe the Efb-targeted antibody profile of a large cohort of donors revealed higher antibody titers in donors classified as negative for S. aureus, compared to donors having active S. aureus infections. This somewhat counterintuitive result reflects an ongoing debate as to whether IgG responses to staphylococcal antigens such as Efb, tightly correlate with acute infections or with commensal colonization and long-term non-symptomatic carriage in the population (41,42). Furthermore there are disparate reports suggesting that S. aureus-evoked humoral responses display variability depending on frequency of exposures, strain antigenicity and preferable site of colonization (41). Moreover, our S. aureus-‘negative’ cohort might include a fair proportion of non-symptomatic carriers that have previously been reported to present high-titer antibodies. Therefore the lack of patient stratification and long-term monitoring of anti-S. aureus humoral responses in our study make the interpretation of these results rather speculative. Notably however, previous studies have suggested that high-titer antibodies are stable for years in healthy individuals, thus likely conferring protective immunity against S. aureus (42). This latter observation prompted us to interrogate whether these S. aureus-negative donors carry neutralizing antibodies that can control S. aureus infections. Notably, anti-Efb antibodies isolated from ‘S. aureus-negative’ donors were capable of blocking the C3d-Efb interaction. More importantly these antibodies potentiated the neutrophil-mediated killing of S. aureus, suggesting that their blocking effect on the C3d-Efb interaction significantly attenuated complement activation, compromising downstream effector functions (i.e. neutrophil recruitment and phagocytosis). To circumvent several limitations and potential adverse effects (e.g. safety risks) associated with the use of IVIG in passive vaccination protocols (10), we adopted a high-throughput screening approach to generate human monoclonal antibodies against Efb-C, while preserving both the diversity and magnitude of scale (e.g. 109) of the human antibody repertoire (24).
The high selectivity of the resulting anti-Efb-C mini-antibodies makes them promising candidates for therapeutic modulation of S. aureus’ virulence. Furthermore, the lack of an Fc portion from the miniAb structure confers more benefits such as the bypass of FcγR-mediated effector pathways that may lead to faster clearance through the hepatosplenic phagocytic system, undesirable immune activation, or higher effective concentrations due to elimination of non-specific binding to Fc receptors (9,43). SPR-based analysis allowed the initial selection of the antibodies with higher affinities towards Efb-C and the fitting of the binding kinetics revealed a single-site mode of interaction with their ligand. However, due to their dimeric nature, each miniAb is expected to bind and neutralize up to two Efb molecules in solution.
Previous studies have elucidated the structural determinants of the Efb-C/C3 interaction, revealing distinct binding affinities of Efb-C for C3 and its TED-containing fragments(17) (35), and implying a binding mode that is influenced by differential accessibility to the target proteins (19). Our study revealed a panel of miniAbs that potently inhibit the C3/Efb-C interaction. Structural studies have indicated that Efb-C residues R131 and N138, located within the binding interface of Efb-C/C3d, contribute significantly to this interaction. In search of a plausible explanation for the miniAb-mediated blockade of the C3d/Efb-C interaction, we hypothesized that the epitopes recognized by these antibodies lie within the binding interface of the two molecules, encompassing the crucial Efb-C residues R131 and N138. Supporting this hypothesis, SPR-based analysis revealed that the blocking miniAbs-A2 and A4 most likely recognize epitopes within the binding interface, as both antibodies failed to recognize the mutant Efb-C proteins. These findings strongly suggest that the inhibitory effect of the miniAbs is largely attributed to disruption of the Efb-C/C3 complex. They also provided the basis for the subsequent evaluation of the antimicrobial activity of these miniAbs in models of S. aureus-associated bacteremia.
To ascertain the neutralizing potential of these miniAbs in a setting that closely resembles acute bacterial infection, the lead miniAbs-A1 and A2 were evaluated in a whole blood model of S. aureus-induced bacteremia (28). To ensure that this model represents a reliable approach for assessing the neutralizing ability of the antibodies we first tested the antibodies A1 and A2, adding recombinant Efb to the blood-bacteria mixture. Corroborating our hypothesis, both antibodies significantly attenuated bacterial survival in a dose-dependent manner.
To date, the pattern of Efb expression in S. aureus-infected blood has not been determined. An early secretion profile was observed in our whole blood model, consistent with a putative role of Efb in the initial stages of bacterial infection. This early Efb secretion can be attributed to the immediate sensing of the pathogen by complement and the effective immune evasion mechanism that is deployed to counter its detrimental effect on bacterial survival. This strategy allowed for a closer simulation of the human condition, in which S. aureus enters the bloodstream in significantly lower titers and gradually begins to secrete its own evasion proteins under the pressure of diverse blood cell populations. Having established the time frame of Efb secretion in blood, we then employed miniAb blockade in targeting only the endogenously produced Efb protein. The growth-attenuating effect of miniAbs A1 and A2 in this setting is a first indication that these miniAbs might have therapeutic potential as a ‘rescue’ treatment option for acute septicemia, alongside conventional antibiotics.
Previous studies have suggested that C3b-containing convertases define major ‘hot spots’ for modulation by Efb (20). Efb blocks the formation of the AP C3 proconvertase (C3bB) and also exerts a pronounced inhibitory effect on the function of the AP C5 convertase (C3bBb3b) (20). Other studies have underscored the ability of Efb to prevent C5a generation thereby downregulating the activation and phagocytic activity of neutrophils (44,45). It is therefore conceivable that Efb might exert a more pronounced inhibitory effect on AP C5 convertases in our blood system due to the additional binding afforded by the presence of a second C3b moiety in this complex, as compared to the AP C3 convertase (20). In line with this mechanistic perspective, miniAb-A1 significantly increased C5a levels in our whole blood model by blocking the effect of recombinant Efb-C on complement activation. This finding is in good agreement with previous studies indicating that the inhibitory effect of Efb-C on C3b-containing convertases also extends to C5 convertases leading to potent inhibition of effector generation (i.e. C5a release).
A hallmark of acute septicemia is the derailment of the innate immune response to bacterial challenge (32). IL-6 is among the key proinflammatory cytokines that become elevated in circulation signifying the presence of an increased bacterial burden (37). Notably, complement activation products (e.g. C5a) also appear to influence this response (32). C5a can promote the recruitment of phagocytes (i.e. neutrophils and macrophages) in the bloodstream. As a consequence of this, bacterial cell killing would be expected to be increased in our bacteremia model. In such circumstances, IL-6 production by immune effector cells, as a direct correlate of bacterial burden in the circulation, would be significantly attenuated. Indeed, this negative feedback loop was verified in our model, as treatment with miniAb-A1 effectively restored C5a generation and potentiated bacterial cell killing, both of which were eventually reflected by the significantly lower IL-6 levels.
Having tested the neutralizing capacity of the miniAbs in vitro, we next validated their therapeutic potential in the clinically relevant renal abscess model. Administration of miniAb-A1 to S. aureus-infected mice resulted in significant protection of the kidneys from inflammatory lesions (i.e., bacterial abscesses with prominent infiltrates) and in significant preservation of organ morphology. This was also reflected by the organ's anatomical integrity in the Ab-treated animals, which closely resembled that of the control cohort.
The lower bacterial titers in the renal tissue of Ab-treated animals corroborated the protective effect of the miniAbs correlating with the presence of fewer inflammatory foci within the renal tissue, which contained scarce bacterial cells. The mechanism by which these miniAbs exert their therapeutic effect might rely on their ability to restore complement activation, thereby increasing C5a generation and promoting the chemotactic recruitment of phagocytic cells (see Figure 7). Interestingly, competitive binding studies revealed that miniAb-A1 may exert a broader neutralizing effect by modulating the Efb/fibrinogen interaction, possibly via steric interference with the N-terminal domain (Fig. S3). Notably, the pronounced therapeutic efficacy of miniAb-A1 in our mouse model likely reflects its dual capacity to block the complement inhibitory function of Efb and also impair its ability to recruit fibrinogen to the pathogen's surface (36). Furthermore, the lack of an Fc region raises the possibility that such miniAbs might display a slower clearance rate, partly due to the absence of FcR-mediated interactions. Such a prolonged plasma residence coupled to a more effective diffusion within the renal tissue would likely explain their sustained therapeutic efficacy in the renal abscess model. Further studies are warranted however to discern the biodistribution and pharmacokinetic profile of these neutralizing miniAbs in models of S. aureus-induced bacteremia.
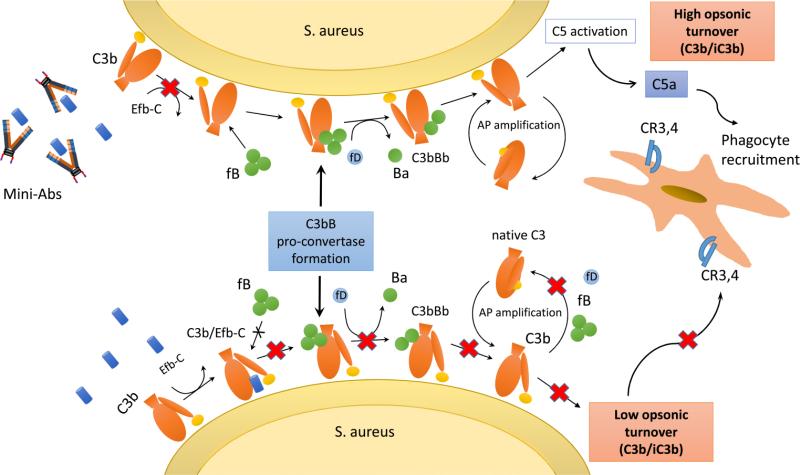
Figure 7. Schematic outline of the proposed mechanism by which Efb-targeting miniAbs contribute to the phagocytic clearance of S. aureus.
During the course of an S. aureus infection, secretion of Efb leads to reduced complement activation partly due to impaired AP C3 pro-convertase formation. This impairment results in lower C5a generation and attenuated activation of phagocytic cells. Conversely, in the presence of Efb-neutralizing miniAbs complement activation is restored, leading to increased opsonic tagging of the pathogen, enhanced C5a generation and subsequently to its phagocytic clearance by complement receptor-bearing phagocytes (e.g. macrophages, neutrophils).
AP, Alternative Pathway; fB, factor B; fD, factor D; CR3, complement receptor type 3; CR4, complement receptor type 4.
In conclusion, our studies have identified novel Ab-based antimicrobial agents that exploit the complement-inhibitory protein Efb as a target for the treatment of S. aureus-induced bacteremia. Furthermore, these studies provide a rational framework for the functional refinement (i.e., affinity maturation) and comprehensive evaluation of these mini-antibodies as novel immunotherapeutics in various clinically relevant models of bacterial infection.
Supplementary Material
Acknowledgements
The authors wish to thank Ms Georgia Avgerou, Department of Pathology, Locus Medicus S.A., for her excellent technical assistance in histopathology and mouse tissue processing. The authors also thank Dr. Edimara S. Reis for critically reading the manuscript.
This work was supported by a “Marie Curie” IRG grant (FP7-PEOPLE-2010-RG: No. 268291 to G.S. and D.C.M.), a Research Grant by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID 2012) to G.S., by NIH grants AI071028 and AI113552 to B.V.G. and AI068730, AI030040, and AI097805 to J.D.L. and D.R. D.C.M and G.S. acknowledge funding from the Greek General Secretariat for Research and Technology and the European Regional Development Fund under the Action “Development Grants For Research Institutions–KRIPIS” of OPCE II.
References
- 1.Lowy FD. Staphylococcus aureus infections. N. Engl. J. Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 2.Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Zell ER, Fosheim GE, McDougal LK, Carey RB, Fridkin SK. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 3.Chambers HF, Deleo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat. Rev. Microbiol. 2009;7:629–641. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diep BA, Gill SR, Chang RF, Phan TH, Chen JH, Davidson MG, Lin F, Lin J, Carleton HA, Mongodin EF, Sensabaugh GF, Perdreau-Remington F. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet. 2006;367:731–739. doi: 10.1016/S0140-6736(06)68231-7. [DOI] [PubMed] [Google Scholar]
- 5.Fowler VG, Jr., Proctor RA. Where does a Staphylococcus aureus vaccine stand? Clin. Microbiol. Infect. 20 Suppl. 2014;5:66–75. doi: 10.1111/1469-0691.12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeDent A, Kim HK, Missiakas D, Schneewind O. Exploring Staphylococcus aureus pathways to disease for vaccine development. Semin. Immunopathol. 2012;34:317–333. doi: 10.1007/s00281-011-0299-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohapatra S, Juan HS. Designer monoclonal antibodies as drugs: the state of the art. Expert. Rev. Clin. Immunol. 2008;4:305–307. doi: 10.1586/1744666X.4.3.305. [DOI] [PubMed] [Google Scholar]
- 8.Presta LG. Molecular engineering and design of therapeutic antibodies. Curr. Opin. Immunol. 2008;20:460–470. doi: 10.1016/j.coi.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 9.Nelson AL, Dhimolea E, Reichert JM. Development trends for human monoclonal antibody therapeutics. Nat. Rev. Drug Discov. 2010;9:767–774. doi: 10.1038/nrd3229. [DOI] [PubMed] [Google Scholar]
- 10.Ballow M. Safety of IGIV therapy and infusion-related adverse events. Immunol. Res. 2007;38:122–132. doi: 10.1007/s12026-007-0003-5. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi SD, Deleo FR. A MRSA-terious enemy among us: boosting MRSA vaccines. Nat. Med. 2011;17:168–169. doi: 10.1038/nm0211-168. [DOI] [PubMed] [Google Scholar]
- 12.Chavakis T, Preissner KT, Herrmann M. The anti-inflammatory activities of Staphylococcus aureus. Trends Immunol. 2007;28:408–418. doi: 10.1016/j.it.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat. Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foster TJ. Immune evasion by staphylococci. Nat. Rev. Microbiol. 2005;3:948–958. doi: 10.1038/nrmicro1289. [DOI] [PubMed] [Google Scholar]
- 15.Kim HK, Thammavongsa V, Schneewind O, Missiakas D. Recurrent infections and immune evasion strategies of Staphylococcus aureus. Curr. Opin. Microbiol. 2012;15:92–99. doi: 10.1016/j.mib.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia BL, Ramyar KX, Ricklin D, Lambris JD, Geisbrecht BV. Advances in understanding the structure, function, and mechanism of the SCIN and Efb families of Staphylococcal immune evasion proteins. Adv. Exp. Med. Biol. 2012;946:113–133. doi: 10.1007/978-1-4614-0106-3_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hammel M, Sfyroera G, Ricklin D, Magotti P, Lambris JD, Geisbrecht BV. A structural basis for complement inhibition by Staphylococcus aureus. Nat. Immunol. 2007;8:430–437. doi: 10.1038/ni1450. [DOI] [PubMed] [Google Scholar]
- 18.Palma M, Wade D, Flock M, Flock JI. Multiple binding sites in the interaction between an extracellular fibrinogen-binding protein from Staphylococcus aureus and fibrinogen. J. Biol. Chem. 1998;273:13177–13181. doi: 10.1074/jbc.273.21.13177. [DOI] [PubMed] [Google Scholar]
- 19.Chen H, Ricklin D, Hammel M, Garcia BL, McWhorter WJ, Sfyroera G, Wu YQ, Tzekou A, Li S, Geisbrecht BV, Woods VL, Jr., Lambris JD. Allosteric inhibition of complement function by a staphylococcal immune evasion protein. Proc. Natl. Acad. Sci. U. S. A. 2010;107:17621–17626. doi: 10.1073/pnas.1003750107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jongerius I, Kohl J, Pandey MK, Ruyken M, van Kessel KP, van Strijp JA, Rooijakkers SH. Staphylococcal complement evasion by various convertase-blocking molecules. J. Exp. Med. 2007;204:2461–2471. doi: 10.1084/jem.20070818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rooijakkers SH, van Kessel KP, van Strijp JA. Staphylococcal innate immune evasion. Trends Microbiol. 2005;13:596–601. doi: 10.1016/j.tim.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Palma M, Nozohoor S, Schennings T, Heimdahl A, Flock JI. Lack of the extracellular 19-kilodalton fibrinogen-binding protein from Staphylococcus aureus decreases virulence in experimental wound infection. Infect. Immun. 1996;64:5284–5289. doi: 10.1128/iai.64.12.5284-5289.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jongerius I, von Kockritz-Blickwede M, Horsburgh MJ, Ruyken M, Nizet V, Rooijakkers SH. Staphylococcus aureus virulence is enhanced by secreted factors that block innate immune defenses. J. Innate. Immun. 2012;4:301–311. doi: 10.1159/000334604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knappik A, Ge L, Honegger A, Pack P, Fischer M, Wellnhofer G, Hoess A, Wolle J, Pluckthun A, Virnekas B. Fully synthetic human combinatorial antibody libraries (HuCAL) based on modular consensus frameworks and CDRs randomized with trinucleotides. J. Mol. Biol. 2000;296:57–86. doi: 10.1006/jmbi.1999.3444. [DOI] [PubMed] [Google Scholar]
- 25.Basta M, Hammer CH. A rapid FPLC method for purification of the third component of human and guinea pig complement. J. Immunol. Methods. 1991;142:39–44. doi: 10.1016/0022-1759(91)90290-v. [DOI] [PubMed] [Google Scholar]
- 26.Guthridge JM, Rakstang JK, Young KA, Hinshelwood J, Aslam M, Robertson A, Gipson MG, Sarrias MR, Moore WT, Meagher M, Karp D, Lambris JD, Perkins SJ, Holers VM. Structural studies in solution of the recombinant N-terminal pair of short consensus/complement repeat domains of complement receptor type 2 (CR2/CD21) and interactions with its ligand C3dg. Biochemistry. 2001;40:5931–5941. doi: 10.1021/bi0101749. [DOI] [PubMed] [Google Scholar]
- 27.Geisbrecht BV, Bouyain S, Pop M. An optimized system for expression and purification of secreted bacterial proteins. Protein Expr. Purif. 2006;46:23–32. doi: 10.1016/j.pep.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Mollnes TE, Brekke OL, Fung M, Fure H, Christiansen D, Bergseth G, Videm V, Lappegard KT, Kohl J, Lambris JD. Essential role of the C5a receptor in E coli-induced oxidative burst and phagocytosis revealed by a novel lepirudin-based human whole blood model of inflammation. Blood. 2002;100:1869–1877. [PubMed] [Google Scholar]
- 29.Karlsson R, Katsamba PS, Nordin H, Pol E, Myszka DG. Analyzing a kinetic titration series using affinity biosensors. Anal. Biochem. 2006;349:136–147. doi: 10.1016/j.ab.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 30.Aguado MT, Lambris JD, Tsokos GC, Burger R, Bitter-Suermann D, Tamerius JD, Dixon FJ, Theofilopoulos AN. Monoclonal antibodies against complement 3 neoantigens for detection of immune complexes and complement activation. Relationship between immune complex levels, state of C3, and numbers of receptors for C3b. J. Clin. Invest. 1985;76:1418–1426. doi: 10.1172/JCI112119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ko YP, Liang X, Smith CW, Degen JL, Hook M. Binding of Efb from Staphylococcus aureus to fibrinogen blocks neutrophil adherence. J. Biol. Chem. 2011;286:9865–9874. doi: 10.1074/jbc.M110.199687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo RF, Ward PA. Role of C5a in inflammatory responses. Annu. Rev. Immunol. 2005;23:821–852. doi: 10.1146/annurev.immunol.23.021704.115835. [DOI] [PubMed] [Google Scholar]
- 33.Hammel M, Sfyroera G, Pyrpassopoulos S, Ricklin D, Ramyar KX, Pop M, Jin Z, Lambris JD, Geisbrecht BV. Characterization of Ehp, a secreted complement inhibitory protein from Staphylococcus aureus. J. Biol. Chem. 2007;282:30051–30061. doi: 10.1074/jbc.M704247200. [DOI] [PubMed] [Google Scholar]
- 34.Rooijakkers SH, Ruyken M, Roos A, Daha MR, Presanis JS, Sim RB, van Wamel WJ, van Kessel KP, van Strijp JA. Immune evasion by a staphylococcal complement inhibitor that acts on C3 convertases. Nat. Immunol. 2005;6:920–927. doi: 10.1038/ni1235. [DOI] [PubMed] [Google Scholar]
- 35.Chen H, Schuster MC, Sfyroera G, Geisbrecht BV, Lambris JD. Solution insights into the structure of the Efb/C3 complement inhibitory complex as revealed by lysine acetylation and mass spectrometry. J. Am. Soc. Mass Spectrom. 2008;19:55–65. doi: 10.1016/j.jasms.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ko YP, Kuipers A, Freitag CM, Jongerius I, Medina E, van Rooijen WJ, Spaan AN, van Kessel KP, Hook M, Rooijakkers SH. Phagocytosis escape by a Staphylococcus aureus protein that connects complement and coagulation proteins at the bacterial surface. PLoS. Pathog. 2013;9:e1003816. doi: 10.1371/journal.ppat.1003816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bosmann M, Ward PA. The inflammatory response in sepsis. Trends Immunol. 2013;34:129–136. doi: 10.1016/j.it.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng AG, Kim HK, Burts ML, Krausz T, Schneewind O, Missiakas DM. Genetic requirements for Staphylococcus aureus abscess formation and persistence in host tissues. FASEB J. 2009;23:3393–3404. doi: 10.1096/fj.09-135467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schaffer AC, Lee JC. Vaccination and passive immunisation against Staphylococcus aureus. Int. J. Antimicrob. Agents. 2008;32(Suppl 1):S71–S78. doi: 10.1016/j.ijantimicag.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 40.Hammel M, Sfyroera G, Ricklin D, Magotti P, Lambris JD, Geisbrecht BV. A structural basis for complement inhibition by Staphylococcus aureus. Nat. Immunol. 2007;8:430–437. doi: 10.1038/ni1450. [DOI] [PubMed] [Google Scholar]
- 41.Verkaik NJ, de Vogel CP, Boelens HA, Grumann D, Hoogenboezem T, Vink C, Hooijkaas H, Foster TJ, Verbrugh HA, van BA, van Wamel WJ. Anti-staphylococcal humoral immune response in persistent nasal carriers and noncarriers of Staphylococcus aureus. J. Infect. Dis. 2009;199:625–632. doi: 10.1086/596743. [DOI] [PubMed] [Google Scholar]
- 42.Dryla A, Prustomersky S, Gelbmann D, Hanner M, Bettinger E, Kocsis B, Kustos T, Henics T, Meinke A, Nagy E. Comparison of antibody repertoires against Staphylococcus aureus in healthy individuals and in acutely infected patients. Clin. Diagn. Lab Immunol. 2005;12:387–398. doi: 10.1128/CDLI.12.3.387-398.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Congy-Jolivet N, Probst A, Watier H, Thibault G. Recombinant therapeutic monoclonal antibodies: mechanisms of action in relation to structural and functional duality. Crit Rev. Oncol. Hematol. 2007;64:226–233. doi: 10.1016/j.critrevonc.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 44.van Kessel KP, Bestebroer J, van Strijp JA. Neutrophil-Mediated Phagocytosis of Staphylococcus aureus. Front Immunol. 2014;5:467. doi: 10.3389/fimmu.2014.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Serruto D, Rappuoli R, Scarselli M, Gros P, van Strijp JA. Molecular mechanisms of complement evasion: learning from staphylococci and meningococci. Nat. Rev. Microbiol. 2010;8:393–399. doi: 10.1038/nrmicro2366. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.