Abstract
Lumichrome is a photodegradation product of riboflavin and is available as a photosensitizer and fluorescent dye. To develop new efficient methods of lumichrome production, we isolated bacterial strains with high lumichrome productivity from soil. The strain with highest productivity was identified as Microbacterium sp. strain TPU 3598. Since this strain inductively produced lumichrome when cultivated with riboflavin, we developed two different methods, a cultivation method and a resting cell method, for the production of large amounts of lumichrome using the strain. In the cultivation method, 2.4 g (9.9 mmol) of lumichrome was produced from 3.8 g (10.1 mmol) of riboflavin at the 500-ml scale (98% yield). The strain also produced 4.7 g (19.4 mmol) of lumichrome from 7.6 g (20.2 mmol) of riboflavin (96% yield) by addition of riboflavin during cultivation at the 500-ml scale. In the resting cell method, 20 g of cells (wet weight) in 100 ml of potassium phosphate buffer, pH 7.0, produced 2.4 g of lumichrome from 3.8 g of riboflavin (98% yield). Since the lumichrome production by these methods was carried out in suspension, the resulting lumichrome was easily purified from the cultivation medium or reaction mixture by centrifugation and crystallization. Thus, the biochemical methods we describe here are a significant improvement in terms of simplicity and yield over the existing chemical, photolytic, and other biochemical methods of lumichrome production.
INTRODUCTION
Lumichrome (7,8-dimethylalloxazine), the main photodegradation product of riboflavin in neutral or acidic conditions (Fig. 1) (1, 2), is known as an effective photosensitizer (3, 4) and a fluorescent dye with different UV absorbance and fluorescence spectra depending on pH (5, 6) and solvent (7–9). Therefore, lumichrome has many industrial applications, including its use as a fluorescence probe (10), as a metal ion sensor (11), for the inactivation of biological contaminant (12, 13), and in memory and semiconductor devices (14).
FIG 1.
Production of lumichrome from riboflavin.
Lumichrome production methods developed to date include the photolysis of riboflavin (15) or chemical synthesis (16, 17). In the photolysis method, lumichrome was obtained in 72% yield from riboflavin (10 mg; 26.6 μmol in 300 ml water) by irradiation for 100 h under a fluorescent lamp and purification by column chromatography (15). Chemical methods for lumichrome production have been described by some groups (16, 17), and the most effective method is synthesis of lumichrome from riboflavin via formylmethylflavin (16). Formylmethylflavin (2.16 g; 7.59 mmol), produced by treating riboflavin with NaIO4, was heated with 220 ml of a 50% acetic acid solution to be converted to lumichrome (1.12 g; 4.64 mmol) in 61% yield. However, these production methods are insufficient for industrial lumichrome production in the yield and scale-up manufacturing.
Biochemical production methods also have been reported using Devosia riboflavina (formerly Pseudomonas riboflavina) ATCC 9526 (18) and some Gram-positive bacteria (19). D. riboflavina produced 64.5 mg of lumichrome from 100 mg of riboflavin by cultivation at 30°C for 4 days in 100 ml medium (18). A Gram-positive bacterium produced 26.3 mg of lumichrome from 42 mg of riboflavin in an 82% yield by cultivation at 37°C for 10 days in 500 ml medium (19). These biological methods using lumichrome-producing microorganisms are superior to the chemical methods in terms of simplicity, but they have low productivity and are slow.
Therefore, we screened new bacterial strains for high levels of lumichrome production and developed novel lumichrome production methods using the isolated strain.
MATERIALS AND METHODS
Chemicals and bacterial strain.
Lumichrome was purchased from Tokyo Chemical Industry (Tokyo, Japan). Riboflavin and dimethyl sulfoxide (DMSO) were purchased from Wako Chemical Industries (Osaka, Japan). Tryptone, Phytone peptone, and yeast extract were purchased from Becton Dickinson (Franklin Lanes, NJ). All other chemicals were commercially sourced and used without further purification. Devosia riboflavina ATCC 9526 (18) was purchased from the NITE Biological Resource Center.
Screening for lumichrome-producing microorganisms.
Eighteen soil samples were suspended in distilled water and incubated at 30°C for 2 to 4 days on a gellan gum plate consisting of 0.05% riboflavin and a basal medium (0.2% K2HPO4, 0.05% MgSO4 · 7H2O, 0.002% MnCl2 · 4H2O, 0.003% FeSO4 · 7H2O, and 0.05% yeast extract, pH 7.0). Colonies that formed a clear zone on this plate were subcultured onto a tryptone glucose yeast extract (TGY) agar (0.5% tryptone, 0.5% yeast extract, 0.1% glucose, 0.1% K2HPO4, and 1.5% agar, pH 7.0) plate to obtain a pure strain. Each isolated strain then was cultivated in 5 ml of nutrient medium (0.2% glucose, 0.3% Phytone peptone, 0.3% yeast extract, and 0.1% K2HPO4, pH 7.0) containing 0.5 μmol riboflavin at 30°C for 24 h with shaking (300 strokes/min). Cells were harvested by centrifugation (22,300 × g, 3 min, 25°C), and the supernatant was assayed by high-performance liquid chromatography (HPLC) to confirm lumichrome production.
Identification of the selected strain.
The identification of the newly isolated lumichrome-producing bacterial strain Microbacterium sp. strain TPU 3598 was performed by TechnoSuruga Laboratory Co., Ltd. (Shizuoka, Japan).
Optimization of lumichrome production by the cultivation method.
Microbacterium sp. strain TPU 3598 was cultivated at 30°C for 10 h in 10 ml of nutrient medium, pH 7.0, containing 1.0 μmol riboflavin. The effect of cultivation temperature on lumichrome production under the above-described conditions was investigated by varying the cultivation temperature to 16°C, 25°C, 30°C, and 37°C. To determine the optimal pH for lumichrome production, the above-described cultivation conditions were carried out under a pH range of 5.0 to 9.0. In these investigations, the amounts of lumichrome and riboflavin in the culture supernatant were quantified by HPLC analysis.
The optimal riboflavin amount in the medium was investigated by varying the riboflavin amount within the range of 50 to 250 μmol. After the cultivation period, the culture was centrifuged (22,300 × g, 5 min, 4°C) to separate the supernatant and precipitate. Lumichrome and riboflavin in the supernatant were analyzed by HPLC. The precipitate was dissolved in DMSO, and the cells were removed by centrifugation (22,300 × g, 3 min, 25°C). This supernatant, containing lumichrome and riboflavin in DMSO, was diluted with 10 mM potassium phosphate buffer (KPB), pH 7.0, and analyzed by HPLC.
Production of lumichrome under optimal conditions of the cultivation method.
A 10-ml starter culture of Microbacterium sp. strain TPU 3598 in nutrient medium was cultivated at 30°C for 16 h. This culture then was inoculated into 500 ml of fresh nutrient medium, pH 7.0, containing 3.8 g (10.1 mmol) of riboflavin and cultivated at 30°C for 24 h.
Standard conditions of the resting cell reaction.
Microbacterium sp. strain TPU 3598 was cultivated at 30°C for 16 h in 10 ml of nutrient medium, pH 7.0, containing 1.0 μmol riboflavin. Two hundred milligrams of cells (wet weight) was harvested by centrifugation (8,000 × g, 5 min, 4°C), washed with 10 mM KPB, pH 7.0, and resuspended in 10 ml of the same buffer. Ten milliliters of cell suspension was incubated with 50 μmol riboflavin at 30°C for 8 h, 0.1 ml of the reaction mixture was mixed with 0.9 ml DMSO, and insoluble material was discarded by centrifugation (22,300 × g, 3 min, 25°C). The resulting supernatant was diluted 10-fold with 10 mM KPB, pH 7.0, and the amounts of lumichrome and riboflavin were analyzed by HPLC.
Optimization of lumichrome production by the resting cell reaction.
We sought to optimize the conditions of the resting cell reaction method of lumichrome production. The effect of reaction temperature was investigated under the above-described standard conditions, except that the incubation temperature was varied to 16°C, 25°C, 30°C, and 37°C. The optimal reaction pH was investigated under the above-described standard conditions, except that the reaction pH was varied in the range of pH 4.5 to 9.0 using 10 mM acetate buffer, pH 4.5 to 5.5, 10 mM KPB, pH 6.0 to 8.0, 10 mM Tris-HCl, pH 8.0 to 9.0, and 10 mM glycine-NaOH, pH 8.6 to 9.0. Maximum riboflavin amounts were investigated by incubation of 50 to 250 μmol riboflavin with 200 mg, 400 mg, and 600 mg of cells (wet weight) at 30°C in 10 ml of 10 mM KPB, pH 7.0.
Production of lumichrome under the optimal conditions of the resting cell method.
A starter culture of Microbacterium sp. strain TPU 3598 was cultivated at 30°C for 16 h in 10 ml of nutrient medium, pH 7.0. This culture then was inoculated into 1,000 ml of fresh nutrient medium containing 100 μmol riboflavin and cultivated at 30°C for 16 h. The cells were harvested by centrifugation and washed with 10 mM KPB, pH 7.0. Twenty grams of cells then was incubated with riboflavin (3.8 g, 10.1 mmol) at 30°C for 24 h in 500 ml of 10 mM KPB, pH 7.0, or for 30 h in 100 ml of 10 mM KPB, pH 7.0.
Purification of lumichrome.
Lumichrome produced by both the cultivation and resting cell methods was purified as follows. Following the cultivation or resting cell method at the 500-ml scale as described above, the insoluble material, including lumichrome, was separated from cells by low-speed centrifugation (220 × g, 20 min, 4°C). The lumichrome in the insoluble material then was dissolved with 300 ml of DMSO at 80°C, and the remaining material was removed by centrifugation (22,300 × g, 5 min, 25°C). Lumichrome in the soluble state was crystallized by the addition of water (900 ml) and dried in vacuo.
Analysis of lumichrome and riboflavin.
Lumichrome and riboflavin were analyzed by an HPLC apparatus (Shimadzu LC-20AP; Shimadzu, Kyoto, Japan) equipped with a Cosmosil 5C18MS-II column maintained at 50°C (2.0 by 150 mm; Nacalai Tesque, Kyoto, Japan) using 25% methanol in 10 mM acetate buffer, pH 4.5, at a flow rate of 0.4 ml/min. Lumichrome production was detected at 254 nm and confirmed by comparison of retention time with an authentic standard.
The 1H and 13C nuclear magnetic resonance (NMR) spectra of lumichrome were recorded on a Bruker Biospin Avance II 400 spectrometer (Brucher Biospin; Rheinstetten, Germany) in a pyridine-d5 solution. The 1H NMR chemical shift was referenced to residual C5HD4N (δ = 8.71, 7.55, 7.19) signals. The 13C NMR chemical shift was referenced to the solvent C5D5N (δ = 149.9, 135.5, 123.5).
Localization of enzyme.
The isolated strain was cultivated in 10 ml of nutrient medium, pH 7.0, containing 1.0 μmol riboflavin at 30°C for 24 h. Cells (200 mg [wet weight]) were harvested by centrifugation (8,000 × g, 5 min, 4°C), washed with 10 ml of 10 mM KPB, pH 7.0, and resuspended in the same buffer. The cells were disrupted with Multi-beads Shocker (Yasui Kikai, Osaka, Japan) (2,700 rpm, 60-s on time, 60-s off time, 6 cycles, 0.1-mm YGB01 glass bead, 4°C) and centrifuged (22,300 × g, 20 min, 4°C) to separate the cell extract and cell debris. The cell extract and debris were incubated in 10 mM KPB, pH 7.0, containing 1.0 μmol riboflavin at 30°C for 15 min.
RESULTS
Isolation and identification of lumichrome-producing microorganisms.
Two microorganisms, which were designated TPU 3598 and TPU 3599, were isolated from 18 soil samples by the formation of a clear zone on a gellan gum plate containing riboflavin. Both strains then were cultivated at 30°C for 24 h in 10 ml of nutrient medium, pH 7.0, containing 1.0 μmol riboflavin, and their lumichrome-producing activity from riboflavin was assayed by HPLC analysis. The isolated strains, TPU 3598 and TPU 3599, converted 1.0 μmol riboflavin into lumichrome completely after 10 h and 12 h of cultivation, respectively. Due to its faster conversion time, we selected the TPU 3598 strain for subsequent analysis.
The 16S rRNA gene sequence of the TPU 3598 strain (see Fig. S1 in the supplemental material) was 99.9% identical to those of Microbacterium oxydans and Microbacterium maritypicum. Strain TPU 3598 was Gram positive, non-spore forming, motile, and rod shaped (0.6 to 0.7 by 1.0 to 1.2 μm). The strain grew at 30°C and 37°C but not at 42°C. The strain was catalase positive, β-galactosidase positive, β-glucosidase positive, pyrazinamidase positive, amylase negative, oxidase negative, and urease negative. d-Glucose, d-ribose, d-mannitol, and gelatin were digested by this strain, but not d-xylose, d-lactose, and starch. Colonies of the strain were convex, smooth, and yellow after 48 h of incubation at 30°C on nutrient agar (Oxoid; Basingstoke, England). These taxonomical and biological characteristics determined that the TPU 3598 strain belonged to Microbacterium. This strain was differentiated from the closely related M. oxydans because it was negative for H2S production (20) and from M. maritypicum because it was lipase activity negative and Voges-Proskauer test positive (21). On the basis of these results, we identified the isolated strain as Microbacterium sp. strain TPU 3598. It was deposited at the NITE Biological Resource Center with the number NITE P-01973.
Time course of cell growth and lumichrome production by Microbacterium sp. strain TPU 3598.
Production of lumichrome by Microbacterium sp. strain TPU 3598 was investigated by culturing at 30°C for 24 h in 10 ml of nutrient medium, pH 7.0, containing 1.0 μmol riboflavin. Cell growth was detected after 2 h of inoculation and reached a maximum after 10 h of cultivation. Lumichrome production began after 4 h of cultivation and reached a maximum after 10 h (0.99 μmol lumichrome was produced from 1.0 μmol riboflavin). Lumichrome production occurred slightly later than cell growth, and the produced lumichrome was stable in the culture without further degradation even after 24 h of cultivation (Fig. 2).
FIG 2.
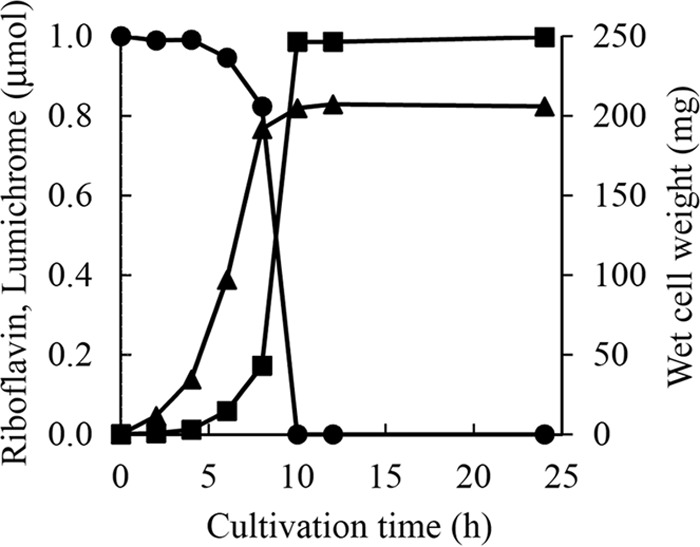
Time course of cell growth and lumichrome production by Microbacterium sp. strain TPU 3598. Microbacterium sp. strain TPU 3598 was cultivated at 30°C in 10 ml of nutrient medium, pH 7.0, containing 1.0 μmol riboflavin. ●, riboflavin; ■, lumichrome; ▲, cell weight (wet weight). Results represent means obtained from three determinations. Standard deviations are less than 0.01.
Effect of temperature on lumichrome production by the cultivation method.
The optimal cultivation temperature was determined by culturing at 16°C, 25°C, 30°C, and 37°C for 10 h as described in Materials and Methods. The levels of cell growth and lumichrome production at 16°C and 37°C were much lower than those at the other temperatures (0.4% and 4.9% yield, respectively). The highest cell growth and lumichrome production were obtained by cultivation at 30°C (98.5% yield) (Fig. 3A). Thus, we selected 30°C as the optimal cultivation temperature for production of lumichrome by this method.
FIG 3.
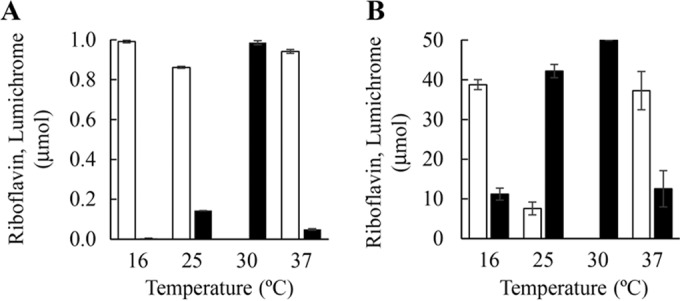
Effect of temperature on lumichrome production. (A) Cultivation method. The isolated strain was cultivated for 10 h in 10 ml of nutrient medium, pH 7.0, containing 1.0 μmol riboflavin. (B) Resting cell method. Lumichrome production was carried out with 200 mg of cells for 8 h in 10 ml of 10 mM KPB, pH 7.0, containing 50 μmol riboflavin. Black and white bars correspond to the amounts of lumichrome and riboflavin, respectively. Error bars indicate the standard deviations obtained from three replicates.
Effect of pH on lumichrome production by the cultivation method.
The optimal pH of the cultivation method was determined by testing a pH range of 5.0 to 9.0 as described in Materials and Methods. Cell growth and lumichrome production at pH 5.0 were much lower than those at the other pH values (0.4% yield). The strain grew well in the pH range from 6.0 to 9.0, and levels of lumichrome production between pH 7.0 and 9.0 were similar to each other, while the production level at pH 6.0 was lower than that at pH 7.0 to 9.0 (Fig. 4A). Therefore, we selected pH 7.0 as the optimal pH for the production of lumichrome by the cultivation method.
FIG 4.
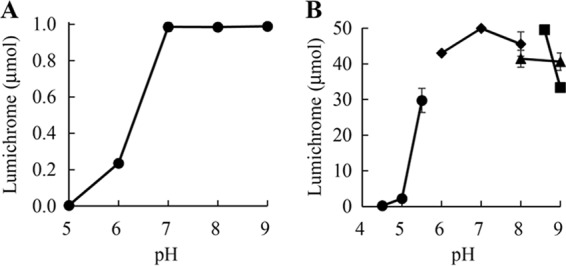
Effect of pH on lumichrome production. (A) Cultivation method. The isolated strain was cultivated at 30°C for 10 h in 10 ml of nutrient medium containing 1.0 μmol riboflavin. (B) Resting cell method. Lumichrome production was carried out with 200 mg of cells at 30°C for 8 h in 10 ml of buffer containing 50 μmol riboflavin. ●, acetate buffer; ◆, KPB; ▲, Tris-HCl buffer; ■, Gly-NaOH buffer. Error bars indicate the standard deviations from three replicates.
Maximum riboflavin amount in the cultivation method.
The maximum riboflavin amount for efficient production of lumichrome by the cultivation method was determined by varying the riboflavin amount between 50 μmol and 250 μmol in 10 ml of nutrient medium, pH 7.0, as described in Materials and Methods. The strain completely converted riboflavin up to 200 μmol to lumichrome within 24 h (Fig. 5A). When the cultivation time was prolonged to 65 h, 250 μmol riboflavin also was completely converted into lumichrome. Since a remarkably long cultivation time was required for complete conversion of 250 μmol riboflavin, we used 200 μmol riboflavin per 10 ml medium for subsequent studies.
FIG 5.
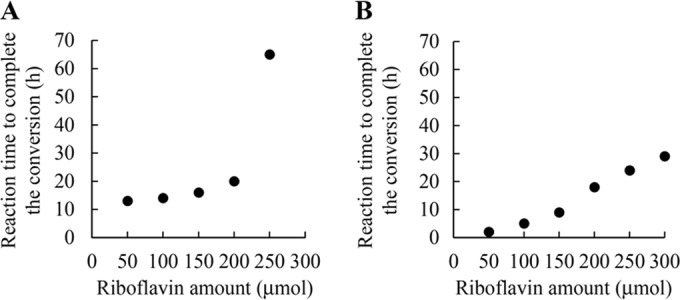
Reaction time for complete conversion of riboflavin to lumichrome. (A) Cultivation method. The isolated strain was cultivated at 30°C to utilize 50 to 250 μmol riboflavin completely in 10 ml of nutrient medium, pH 7.0. (B) Resting cell method. A total of 600 mg of cells was incubated with 50 to 300 μmol riboflavin at 30°C in 10 ml of 10 mM KPB, pH 7.0. Results represent means obtained from three determinations. Standard deviations are less than 1.0.
Production of lumichrome under the optimal conditions of the cultivation method.
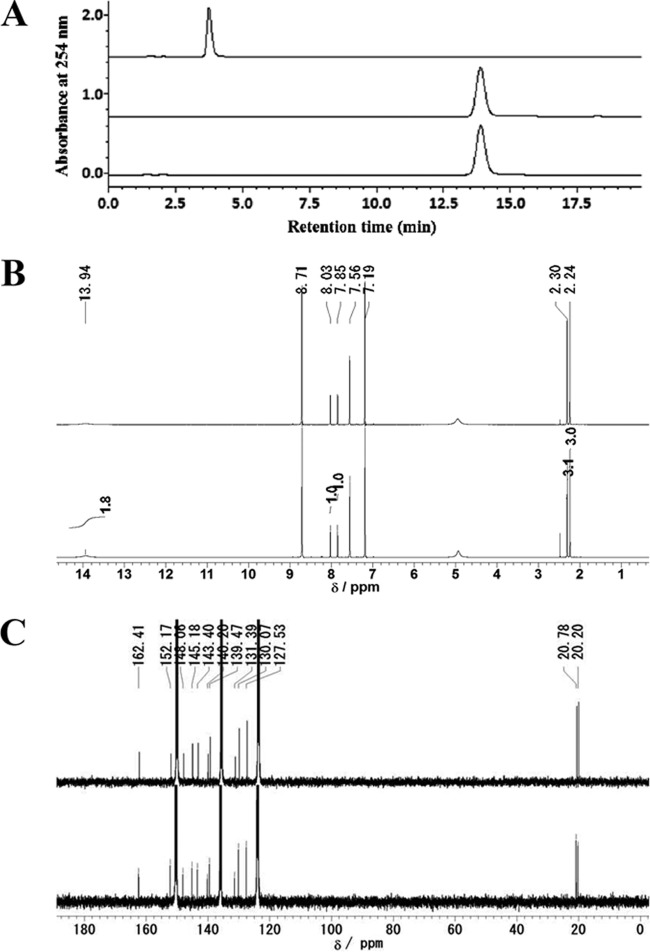
As mentioned above, we demonstrated that cultivation at 30°C and pH 7.0 was optimal for lumichrome production by the cultivation method, and 200 μmol riboflavin in 10 ml of the medium was completely converted to lumichrome within 24 h under these optimal conditions. To confirm these results on a large scale, the cultivation method was carried out in 500 ml of nutrient medium, pH 7.0, containing 3.8 g (10.1 mmol) of riboflavin. After cultivation at 30°C for 24 h, the resulting lumichrome was purified according to the procedures described in Materials and Methods. The cells were easily separated from insoluble materials, including lumichrome, by low-speed centrifugation. After solubilizing lumichrome in DMSO, the remaining bacterial cells and insoluble contaminants were discarded by high-speed centrifugation. The lumichrome then was crystallized by the addition of water, and the crystallized reaction product was analyzed by HPLC and NMR. The product was identified as lumichrome by the retention time of HPLC (Fig. 6A) and based on NMR spectra (Fig. 6B and C), and no other product was detected in the crystallized product. Thus, 2.4 g (9.9 mmol) of highly purified lumichrome was obtained from 3.8 g (10.1 mmol) of riboflavin in 98% yield by the cultivation method at the 500-ml scale.
FIG 6.
Identification of the purified product. (A) HPLC chromatogram of the purified product. Shown are the authentic standard of riboflavin (upper), authentic standard of lumichrome (middle), and purified product (lower). (B) 1H NMR spectrum of the purified product. Shown are the authentic standard of lumichrome (upper) and the reaction product (lower). 1H NMR (400 MHz, pyridine-d5) δ 2.26 and 2.33 (each 3H, s), 7.89 and 8.06 (1H, s), 13.86 (2H, br). (C) 13C NMR spectrum of the purified product. Shown are the authentic standard of lumichrome (upper) and the reaction product (lower). 13C NMR (100 MHz, pyridine-d5) δ 20.20, 20.77, 127.53, 130.07, 131.39, 139.46, 140.19, 143.40, 145.18, 148.06, 152.16, 162.41.
Production of lumichrome by the improved cultivation method.
As described above, 200 μmol riboflavin in 10 ml medium, which corresponds to 3.8 g of riboflavin in 500 ml medium, was completely converted to lumichrome within 24 h of cultivation under optimal conditions. However, 65 h of cultivation was required for complete conversion of 250 μmol riboflavin in 10 ml medium. To produce larger amounts of lumichrome, we improved this cultivation method. At the 500-ml scale, nutrient medium, pH 7.0, containing 3.8 g (10.1 mmol) of riboflavin was first inoculated with the isolated strain and cultivated for 24 h under the same conditions as those described above. After the cultivation, more riboflavin (3.8 g) was added into the culture, and it was further cultivated at 30°C for 30 h. The product then was purified by following the procedure described above. Using this modified method, 4.7 g (19.4 mmol) of lumichrome was obtained from 7.6 g (20.2 mmol) of riboflavin in 96% yield. Thus, adding additional riboflavin during cultivation increased the amount of lumichrome production by the cultivation method.
Effect of riboflavin amount added in the cultivation step on lumichrome production by the resting cell method.
Next, the possibility of using resting cells for the conversion was investigated. Since lumichrome productivity by the resting cell reaction was induced by cells cultivated with small amounts of riboflavin, the effect of riboflavin on lumichrome productivity was investigated by the addition of 0 to 2.0 μmol riboflavin in 10 ml of nutrient medium, pH 7.0. When cells cultivated without riboflavin were used for the resting cell reaction, only 3.3 μmol lumichrome was produced from 50 μmol riboflavin following incubation at 30°C for 8 h. When cells cultivated in medium containing 0.5 μmol or 1.0 μmol riboflavin were used in the resting cell reaction, 19.4 μmol or 49.7 μmol lumichrome, respectively, was produced from 50 μmol riboflavin. Cells cultivated in the medium containing 2.0 μmol riboflavin also converted 50 μmol riboflavin to lumichrome completely (Fig. 7A). We used the cells cultivated in 10 ml of nutrient medium containing 1.0 μmol riboflavin for the production of lumichrome by the resting cell method.
FIG 7.
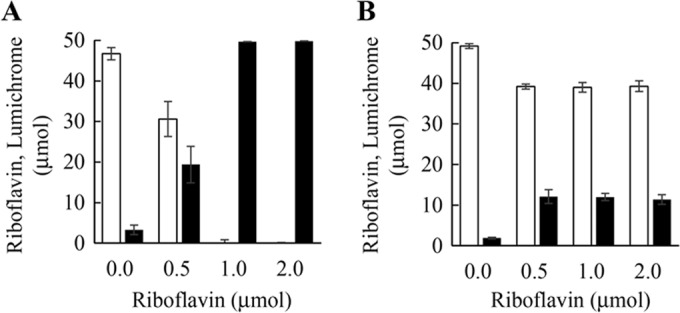
Effect of riboflavin amount in cultivation step on lumichrome production by resting cell reaction. (A) Microbacterium sp. strain TPU 3598. (B) Devosia riboflavina ATCC 9526. Cells were cultivated in 10 ml of the nutrient medium at 30°C for 16 h. Lumichrome production by resting cell reaction was carried out with 200 mg of cells for 8 h in 10 ml of 10 mM KPB, pH 7.0, containing 50 μmol riboflavin. Black and white bars correspond to the amounts of lumichrome and riboflavin, respectively. Error bars indicate the standard deviations obtained from three replicates.
For comparison with the isolated strain, we performed the resting cell reaction using Devosia riboflavina ATCC 9526 under the same conditions as those described above. As shown in Fig. 7B, D. riboflavina cultivated in the medium without riboflavin produced 1.9 μmol lumichrome from 50 μmol riboflavin. When cells cultivated in medium containing 0.5 μmol, 1.0 μmol, and 2.0 μmol were used in the resting cell reaction, 12.0 μmol lumichrome was produced. Thus, lumichrome productivity of our isolated strain was four times higher than that of D. riboflavina.
Effect of reaction temperature on lumichrome production by the resting cell method.
The optimal reaction temperature of lumichrome production was determined by incubation at 16°C, 25°C, 30°C, and 37°C for 8 h using the cells obtained by cultivation with 1.0 μmol riboflavin. The resting cell reaction at 16°C and 37°C converted 50 μmol riboflavin to 11.2 μmol and 12.6 μmol lumichrome, respectively, in 10 ml of 10 mM KPB, pH 7.0. The reaction at 25°C produced 42.2 μmol lumichrome after 8 h of incubation. When the reaction was carried out at 30°C, 49.9 μmol lumichrome was produced after 8 h of incubation (Fig. 3B). These results indicated that incubation at 30°C is optimal for production of lumichrome by the resting cell method.
Effect of reaction pH on lumichrome production by the resting cell method.
The optimal pH of the resting cell method was determined by incubation at 30°C for 8 h in the pH range of 4.5 to 9.0 as described in Materials and Methods. Lumichrome production at acidic pHs was lower than that at neutral and alkaline pHs. The reaction at pHs 6.0 to 8.5 exhibited greater lumichrome production, more than 80% yield, and the highest yield was obtained by incubation with KPB, pH 7.0 (Fig. 4B). A similar yield was obtained by incubation with glycine-NaOH buffer at pH 8.5, but the yield decreased remarkably using glycine-NaOH buffer at pH 9.0. Therefore, we selected KPB, pH 7.0, as the optimal buffer for the production of lumichrome by the resting cell method.
Maximum riboflavin amounts for lumichrome production by the resting cell method.
Since complete conversion of riboflavin to lumichrome is preferred for the production of highly pure lumichrome, the maximum riboflavin amounts able to be converted completely to lumichrome was investigated by varying the added riboflavin amount between 50 and 300 μmol in 10 ml of 10 mM KPB, pH 7.0. When 100 μmol riboflavin was incubated with 200 mg, 400 mg, and 600 mg of cells (wet weight), it was completely converted to lumichrome after 30 h, 10 h, and 5 h of incubation, respectively (data not shown). When 200 μmol riboflavin was incubated with 600 mg of cells (wet weight), it was completely converted to lumichrome after 18 h of incubation (Fig. 5B). The riboflavin amount converted to lumichrome completely within 30 h of incubation was investigated at pH 7.0 and 30°C using 600 mg of cells (wet weight) in 10 ml of the resting cell reaction. In this reaction, 300 μmol riboflavin was completely converted to lumichrome within 30 h of incubation. These results indicated that 100 mg of cells (wet weight) can completely convert 50 μmol riboflavin to lumichrome within 30 h of incubation in 10 ml of reaction mixture.
Production of lumichrome under the optimal conditions of the resting cell method.
We obtained results indicating that lumichrome could be produced from riboflavin by the resting cell method and that 50 μmol riboflavin was completely converted to lumichrome by 100 mg of cells (wet weight) in 10 ml of the reaction mixture under optimal conditions. To confirm these results on a larger scale, the resting cell method was carried out in 500 ml of reaction mixture using 3.8 g (10.1 mmol) of riboflavin and the cells obtained from 1,000 ml culture (20 g of cells [wet weight]). In this resting cell reaction, riboflavin was completely converted to lumichrome within 24 h of incubation. The lumichrome then was purified by centrifugation and crystallization according to the procedure described in Materials and Methods, and we obtained 2.4 g (9.9 mmol) of purified lumichrome at 98% yield.
To scale down the resting cell reaction volume, the cells obtained from 1,000 ml culture were incubated with 3.8 g of riboflavin at 30°C for 30 h in 100 ml of 10 mM KPB, pH 7.0. Under these conditions, riboflavin also was completely converted to lumichrome even in 100-ml reaction volumes with the same conversion yield (98%). Thus, the resting cell reaction could be performed in a 10% volume of the culture volume.
Storage stability of Microbacterium sp. strain TPU 3598.
When the cells with high lumichrome productivity are stable, lumichrome can be produced anytime by the resting cell method. Therefore, the cells cultivated in 10 ml of nutrient medium containing 1.0 μmol riboflavin were harvested and stored at −20°C for 4 weeks. The frozen cells then were thawed and used for the resting cell method. The resting cell reaction with 200 mg of cells at 30°C in 10 ml of 10 mM KPB, pH 7.0, gave 49.8 μmol lumichrome within 8 h of incubation. Thus, the lumichrome production activity of the cells was maintained following 4 weeks of storage at −20°C (Table 1).
TABLE 1.
Effect of storage on lumichrome productiona
| Storage period (days) | Lumichrome production |
|
|---|---|---|
| Amt (μmol) | Yield (%) | |
| 0 | 50.0 ± 0.2 | 100.0 |
| 7 | 50.0 ± 0.02 | 100.0 |
| 28 | 49.8 ± 0.54 | 99.6 |
The cells were stored at −20°C. Lumichrome production was carried out with 200 mg of cells at 30°C for 8 h in 10 ml of buffer containing 50 μmol riboflavin. The indicated values represent means and standard deviations obtained from three determinations.
Localization of enzyme.
To analyze the enzyme localization, the cells were disrupted by glass beads as described in Materials and Methods and separated into cell extract and cell debris by centrifugation. When cell extract was incubated with 1.0 μmol riboflavin at 30°C for 15 min in 1 ml of 10 mM KPB, pH 7.0, production of lumichrome was not recognized (see Fig. S2 in the supplemental material). On the other hand, the cell debris, which was precipitated by centrifugation after cell disruption, produced 0.96 μmol lumichrome after 12.5 min of incubation.
DISCUSSION
Lumichrome is a useful chemical with potential applications in a wide range of fields (10–14). Several lumichrome production methods have been developed to date, including photolysis of riboflavin (15), chemical synthesis (16, 17), and biochemical methods (18, 19). However, these production methods are insufficient for industrial use owing to several disadvantages, such as low yields, slow reaction rates, and complicated extraction and/or purification requirements by column chromatography. To solve these deficiencies, we isolated a new bacterial strain, Microbacterium sp. strain TPU 3598, as a high producer of lumichrome from riboflavin. The strain grew well in the medium containing riboflavin and exhibited higher lumichrome productivity than D. riboflavina (Fig. 7), which was reported to be a lumichrome-producing bacterium (18), although lumichrome is used as an antibacterial agent to generate singlet oxygen (12, 13). These results indicate that the isolated strain is a lumichrome-resistant bacterium and that the enzyme for the production of lumichrome is strongly induced by riboflavin. Therefore, we developed two different methods for lumichrome production using the newly isolated strain. One is a cultivation method, and the other is a resting cell method. The two methods showed the same characteristics, in that the suspended lumichrome is produced from the suspended riboflavin with high yield. The water solubility of riboflavin is around 220 μM (0.0825 mg/ml) (22), whereas that of lumichrome is only 20 μM (0.0048 mg/ml) (23). Since the water solubility of lumichrome is lower than that of riboflavin, the lumichrome produced from the soluble riboflavin quickly changes to an insoluble state during both the cultivation and resting cell method. Because the above-described reaction and solubility state change occur continuously, high levels of lumichrome are accumulated in the solid state. We confirmed that in both methods, the majority of the produced lumichrome existed in the insoluble form (99 μmol insoluble compared to 0.9 μmol soluble) when 100 μmol riboflavin was used as the starting substrate at the 10-ml scale. This indicates that most of the produced lumichrome is obtained in the solid state due to the low water solubility (Fig. 8) and that the amount of soluble lumichrome does not affect the total lumichrome yield.
FIG 8.
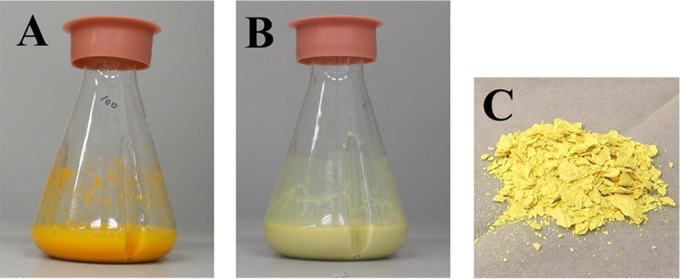
Accumulation of the produced lumichrome as a solid. Twenty grams of cells was incubated with 3.8 g of riboflavin at 30°C for 30 h in 100 ml of 10 mM KPB, pH 7.0. (A) Before incubation; (B) after 30 h of incubation; (C) purified lumichrome.
The two methods also showed some different characteristics. Although both methods exhibited maximal lumichrome yields at 30°C, lumichrome production of the cultivation method at 25°C was only 14.3% of that obtained at 30°C (Fig. 3A). In contrast, the yield of the resting cell reaction at 25°C was 84.7% of that obtained at 30°C (Fig. 3B). The optimal pH of the cultivation method was 7.0 to 9.0, and lumichrome production at pH 6.0 was only 23.6% of that at pH 7.0 (Fig. 4A). The resting cell reaction also exhibited an optimum at pH 7.0, but lumichrome production at pH 6.0 was 80% of the maximal amount (Fig. 4B). In addition to cultivation temperature and pH, lumichrome production with the cultivation method also was affected by the riboflavin content in the medium. For example, the cultivation method gave 200 μmol/10 ml lumichrome within 20 h of cultivation (Fig. 5A). The strain also could grow in 250 μmol/10 ml riboflavin, but the growth rate was reduced, and 65 h of cultivation was required to achieve complete conversion to lumichrome (Fig. 5A). In the case of the resting cell reaction, 300 μmol/10 ml riboflavin was completely converted to lumichrome within 29 h of incubation by 600 mg of cells (wet weight) (Fig. 5B). These results indicate that the cultivation method is strongly affected by the growth rate of the cells but that a high conversion yield is obtained under the optimal conditions: 2.4 g (9.9 mmol) of lumichrome was produced from 3.8 g (10.1 mmol) of riboflavin at the 500-ml scale after 24 h of cultivation. The method was improved by adding additional riboflavin to the culture (19.4 mmol lumichrome was produced at the 500-ml scale after 54 h of cultivation in 96% yield). These product amounts and yields obtained with the cultivation method are superior to those of the previously described methods (15–19).
The resting cell method reported here is the first of its kind, and the product amounts and yields obtained also are superior to those of previously described methods (15–19). For example, 2.4 g (9.9 mmol) of lumichrome was produced from 3.8 g (10.1 mmol) of riboflavin by incubation for 24 h. In addition, the resting cell method has several useful characteristics. First, lumichrome productivity can be induced by cells cultivated with low concentrations of riboflavin (Fig. 7A). Second, the high lumichrome productivity of these cells is stably maintained even after prolonged storage by freezing (Table 1). Finally, this method also can be performed with small volumes at only 10% of the culture volume. Thus, our biochemical methods allow for greater efficiency in the production of lumichrome with high yield.
The production methods described here have additional advantages with respect to the purification step. Highly purified lumichrome is easily obtained through a simple purification procedure of centrifugation and crystallization. The bacterial cells are separated from the produced lumichrome by low-speed centrifugation. Lumichrome then is solubilized with DMSO, and further impurities are removed by high-speed centrifugation. The lumichrome solubilized in DMSO is easily crystallized by the addition of water, so that highly purified lumichrome can be obtained by these methods without the need for column chromatography.
Riboflavin is a water-soluble vitamin (24) and is produced industrially with the use of microorganisms. For example, more than 10 g/liter of riboflavin is produced by fermentation with Candida famata (25), which is obtained as a solid form. This suggests that lumichrome can be easily produced by combining our cultivation methods for lumichrome production with the riboflavin fermentation method.
Some groups (26, 27) have investigated the purification of riboflavin hydrolase, which catalyzes the degradation of riboflavin, from D. riboflavina. However, these attempts have not yet been successful. Here, we revealed that the enzyme was not easily solubilized by disruption of the cells. Thus, our results indicate that riboflavin hydrolase strongly binds to the cells and reacts to riboflavin (see Fig. S2 in the supplemental material). We are now carrying out studies on the purification of the enzyme, because both the enzyme and gene would be useful for further improvement of the present biochemical methods of lumichrome production.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Exploratory Research for Advanced Technology (ERATO) program of the Japan Science and Technology Agency (JST).
We appreciate Daisuke Matsui and Kimiyasu Isobe for useful advice and discussions.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.02166-15.
REFERENCES
- 1.Huang R, Kim JH, Min BD. 2006. Photosensitizing effect of riboflavin, lumiflavin, and lumichrome on the generation of volatiles in soy milk. J Agric Food Chem 54:2359–2364. doi: 10.1021/jf052448v. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad I, Fashihullah Q, Vaid HMF. 2004. A study of simultaneous photolysis and photoaddition reactions of riboflavin in aqueous solution. J Photochem Photobiol B 75:13–20. doi: 10.1016/j.jphotobiol.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Remucal KC, McNeil K. 2011. Photosensitized amino acid degradation in the presence of riboflavin and its derivatives. Environ Sci Technol 45:5223–5229. doi: 10.1021/es2003414. [DOI] [PubMed] [Google Scholar]
- 4.Sikorska E, Khmelinskii VI, Prukała W, Williams L, Patel M, Worrall RD, Bourdelande LJ, Koput J, Sikorski M. 2004. Spectroscopy and photophysics of lumiflavins and lumichromes. J Phys Chem A 108:1501–1508. doi: 10.1021/jp037048u. [DOI] [Google Scholar]
- 5.Prukała D, Sikorska E, Koput J, Khmelinskii I, Karolczak J, Gierszewski M, Sikorski M. 2012. Acid–base equilibriums of lumichrome and its 1-methyl, 3-methyl, and 1,3-dimethyl derivatives. J Phys Chem A 116:7474–7490. doi: 10.1021/jp300522h. [DOI] [PubMed] [Google Scholar]
- 6.Laser N, Feitelson J. 1977. Protonation and deprotonation equilibria of lumichrome. Photochem Photobiol 25:451–456. doi: 10.1111/j.1751-1097.1977.tb09170.x. [DOI] [Google Scholar]
- 7.Sikorska E, Koziołowa A. 1996. Excited state proton transfer of methyl- and cyano-substituted alloxazines in the presence of acetic acid. J Photochem Photobiol A 95:215–221. doi: 10.1016/1010-6030(95)04258-X. [DOI] [Google Scholar]
- 8.Koziołowa A, Visser VN, Kozioł J, Szafran MM. 1996. Phototautomerism of 5-deazalumichrome (in the presence of acetic acid). J Photochem Photobiol A 93:157–163. doi: 10.1016/1010-6030(95)04198-2. [DOI] [Google Scholar]
- 9.Koziołowa A. 1979. Solvent and methyl substituent effect on phototautomerism and ionization of alloxazines. Photochem Photobiol 29:459–471. doi: 10.1111/j.1751-1097.1979.tb07076.x. [DOI] [Google Scholar]
- 10.Miskolczy Z, Bizcók L. 2005. Anion-induced changes in the absorption and fluorescence properties of lumichrome: a new off-the-shelf fluorescent probe. Chem Phys Lett 411:238–242. doi: 10.1016/j.cplett.2005.06.049. [DOI] [Google Scholar]
- 11.Bairi P, Chakraborty P, Roy B, Nandi KA. 2014. Sensing of Hg+2 and Ag+ through a pH dependent FRET system: fabrication of molecular logic gates. Sens Actuators B 193:349–355. doi: 10.1016/j.snb.2013.11.119. [DOI] [Google Scholar]
- 12.Platz SM, Goodrich PR Jr. December 2004. Isoalloxazine derivatives to neutralize biological contaminants. US patent 6,828,323.
- 13.Goodrich PR Jr, Hlavinka D. August 2006. Method and apparatus for inactivation of biological contaminants using photosensitizers. US patent 7,094,378.
- 14.Yukawa M. May 2011. Memory device and a semiconductor device. US patent 7,935,957.
- 15.Suzuki TA, Ohishi N, Yagi K. 1979. Simple procedure for the separation of lumiflavine and lumichrome from photolysates of riboflavine. J Chromatogr A 169:459–461. doi: 10.1016/0021-9673(75)85082-5. [DOI] [Google Scholar]
- 16.Kino K, Kobayashi T, Arima E, Komori R, Kobayashi T, Miyazawa H. 2009. Photoirradiation products of flavin derivatives, and the effects of photooxidation on guanine. Bioorg Med Chem Lett 19:2070–2074. doi: 10.1016/j.bmcl.2009.01.112. [DOI] [PubMed] [Google Scholar]
- 17.Seng F, Ley K. 1972. A new synthesis of lumichrome. Angew Chem Int Ed Engl 11:1010–1011. doi: 10.1002/anie.197210101. [DOI] [Google Scholar]
- 18.Foster WJ. 1944. Microbiological aspects of riboflavin. I. Introduction. II. Bacterial oxidation of riboflavin to lumichrome. J Bacteriol 47:27–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamada K, Sakai M, Yoshimura K. 1956. Two new types of riboflavin-decomposing bacteria isolated from human feces. J Vitaminol 2:307–315. doi: 10.5925/jnsv1954.2.307. [DOI] [PubMed] [Google Scholar]
- 20.Schumann P, Rainey AF, Burghardt J, Stackebrandt E, Weiss N. 1999. Reclassification of Brevibacterium oxydans (Chatelain and Second 1966) as Microbacterium oxydans comb. nov. Int J Syst Bacteriol 49:175–177. doi: 10.1099/00207713-49-1-175. [DOI] [PubMed] [Google Scholar]
- 21.Takeuchi M, Hatano K. 1998. Proposal of six new species in the genus Microbacterium and transfer of Flavobacterium marinotypicum ZoBell and Upham to the genus Microbacterium as Microbacterium maritypicum comb. nov. Int J Syst Bacteriol 48:973–982. doi: 10.1099/00207713-48-3-973. [DOI] [PubMed] [Google Scholar]
- 22.Coffman ER, Kildsig OD. 1996. Effect of nicotinamide and urea on the solubility of riboflavin in various solvents. J Pharm Sci 85:951–954. doi: 10.1021/js960012b. [DOI] [PubMed] [Google Scholar]
- 23.Rajamani S, Bauer DW, Robinson BJ, Farrow MJ III, Pesci CE, Teplitski M, Gao M, Sayre TR, Phillips AD. 2008. The vitamin riboflavin and its derivative lumichrome activate the LasR bacterial quorum-sensing receptor. Mol Plant Microbe Interact 21:1184–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Massey V. 2000. The chemical and biological versatility of riboflavin. Biochem Soc Trans 28:283–296. doi: 10.1042/bst0280283. [DOI] [PubMed] [Google Scholar]
- 25.Foster WE, Gyure CD, Heefner LD, Weaver AC, Yarus JM, Burdzinski AL. June 1992. Riboflavin producing strains of microorganisms, method for selecting, and method for fermentation. US patent 5,120,655.
- 26.Yanagita T, Foster WJ. 1956. A bacterial riboflavin hydrolase. J Biol Chem 221:593–607. [PubMed] [Google Scholar]
- 27.Yang SC, McCormic BD. 1967. Substrate specificity of riboflavin hydrolase from Pseudomonas riboflavina. Biochim Biophys Acta 132:511–513. doi: 10.1016/0005-2744(67)90171-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.