Abstract
In North America, Lyme disease (LD) is a tick-borne zoonosis caused by the spirochete bacterium Borrelia burgdorferi sensu stricto, which is maintained by wildlife. Tick vectors and bacteria are currently spreading into Canada and causing increasing numbers of cases of LD in humans and raising a pressing need for public health responses. There is no vaccine, and LD prevention depends on knowing who is at risk and informing them how to protect themselves from infection. Recently, it was found in the United States that some strains of B. burgdorferi sensu stricto cause severe disease, whereas others cause mild, self-limiting disease. While many strains occurring in the United States also occur in Canada, strains in some parts of Canada are different from those in the United States. We therefore recognize a need to identify which strains specific to Canada can cause severe disease and to characterize their geographic distribution to determine which Canadians are particularly at risk. In this review, we summarize the history of emergence of LD in North America, our current knowledge of B. burgdorferi sensu stricto diversity, its intriguing origins in the ecology and evolution of the bacterium, and its importance for the epidemiology and clinical and laboratory diagnosis of LD. We propose methods for investigating associations between B. burgdorferi sensu stricto diversity, ecology, and pathogenicity and for developing predictive tools to guide public health interventions. We also highlight the emergence of B. burgdorferi sensu stricto in Canada as a unique opportunity for exploring the evolutionary aspects of tick-borne pathogen emergence.
INTRODUCTION
Lyme disease (LD; also called Lyme borreliosis) occurs throughout the temperate region of the Northern Hemisphere and is caused by species of the Borrelia burgdorferi sensu lato species complex. In Canada and the United States, B. burgdorferi sensu stricto (referred to here as B. burgdorferi) is the sole species known to be associated with human disease (1). These bacteria are maintained in nature by wild-animal reservoir hosts (rodents and other mammals as well as birds) and are transmitted by hard-bodied (ixodid) ticks which feed on these animals. LD was first recognized and began emerging in the United States in the 1970s (2). Since then, the incidence of the disease has increased in the United States to reach an estimated 300,000 cases a year at the time of writing (3). In the United States, 90% of LD cases occur in two foci, the Northeast and the upper Midwest, while in the Pacific and southern regions of the United States, human cases are less frequent (4). The geographic pattern of high-risk areas is associated with the occurrence of the tick Ixodes scapularis and, in part, with the distribution of certain Borrelia genotypes (5, 6). The emergence (or, probably more accurately, reemergence) of LD in the United States is thought to be due to reforestation following land use changes during the 20th century which led to expansions of populations of the wild-animal hosts of I. scapularis (7). Possibly driven by a warming climate (8), I. scapularis is now expanding its range into Canada from Manitoba through Ontario and Quebec to New Brunswick and Nova Scotia (9), with LD spirochetes invading newly established tick populations (10), resulting in increases in annual reported human case numbers (from <50 in 2004 to 682 in 2013 [11]). Risk modeling suggests that case numbers will increase rapidly in the coming years in Canada as I. scapularis invades the most heavily populated southern parts of Canada (9, 12).
The emergence of LD in North America in general, and in Canada in particular, involves the spread of a wildlife-borne zoonosis of considerable significance for human health, driven by environmental change, and, as such, is an issue of “One Health” (the interconnected and mutually dependent nature of human, animal, and environmental health [13, 14]). Understanding the mechanisms of emergence of LD, useful for prediction, therefore requires simultaneous consideration of the three components of One Health (13).
As in the United States, LD cases in Canada have three main stages: (i) early LD, (ii) early disseminated LD, and (iii) late LD (15). Erythema migrans (EM), a spreading painless erythematous skin lesion of ≥5 cm in diameter at the site of the infective tick bite, is the typical first distinct symptom of early LD (16–18). EM disappears within 1 to 2 weeks, when the spirochetes disseminate hematogenously from the skin, marking the onset of early disseminated LD. The main manifestations of early disseminated LD are neurological (radiculopathy, cranial neuropathy, and mononeuropathy multiplex), cardiac (atrioventricular heart block that can cause sudden death [19]), and cutaneous (multiple EM lesions). Neurological disease mainly affects the peripheral nervous system, while late LD comprises arthritis and neurological manifestations (17). Reinfection following treatment and recovery is common, particularly if individuals are reinfected with a different strain (20, 21). In up to 20% of patients, symptoms that can be debilitating persist following treatment, a condition known as posttreatment Lyme disease syndrome (PLDS) (17, 22).
Detection of antibodies to B. burgdorferi is the mainstay of LD diagnosis but is complicated by specificity and sensitivity issues (23). Consequently, the gold standard method of serodiagnosis is a two-tier approach comprising a screening immunofluorescence assay (IFA) or enzyme immunoassays (EIA) followed by Western blotting using standardized criteria to interpret the bands to obtain the best balance of sensitivity and specificity (24). Test sensitivity is low in early LD but increases as the disease progresses (25).
Whether or not untreated infections go on to produce disseminated LD may depend (among other factors) on the infecting strain of B. burgdorferi (see below). Identification of which strains of B. burgdorferi may cause disseminated LD and where they occur is of importance in Canada for the following reasons. First, numbers of cases of LD are currently increasing almost exponentially in Canada (11), consistent with earlier assessments of increasing risk due to tick range expansion (12). Second, there is currently no vaccine for humans, and effective public health actions resulting in tick avoidance/infection prevention, targeted at those who are at risk, are paramount for reducing the impact of emerging LD in Canada (26). As the clinical impacts of LD and postinfection immunity are strain specific (21, 27), the strain structure of B. burgdorferi in a particular locality will determine the extent to which a local population is at risk from disseminated LD and can suffer multiple reinfections year after year. The ability to identify which strains cause disseminated LD, and to be able to predict their occurrence in Canada by understanding any associations with environmental factors or animal hosts, would permit implementation of preventive public health actions that are “smarter” by targeting the greatest effort at protecting the populations at the greatest risk from severe LD. We also need to identify pathogenic strains for future studies that can elucidate the interactions of the human, animal, and bacterial genomes that result in infection and disease (28, 29, 30, 31).
DIVERSITY OF BORRELIA BURGDORFERI
The etiological agent of LD was identified in 1981 and named Borrelia burgdorferi (32, 33). The LD group of spirochetes consisted of >20 named or proposed species at the time of writing, with 8 in North America: B. burgdorferi, B. andersoni, B. bissettii, B. californiensis, B. carolinensis, B. americana, B. kurtenbachii, and B. garinii (although this species is found only in cliff-nesting seabird colonies in Newfoundland) (34). B. burgdorferi is itself a diverse species. The genome of B. burgdorferi comprises a linear chromosome carrying genes needed for cell maintenance and replication (the occurrence of genes encoding proteins for metabolic purposes is very limited, likely as an adaptation to a strictly parasitic life style) and a large number of circular and linear plasmids which carry the genes encoding most of the outer surface proteins (Osp), which are involved in interactions with host and vector (35). Borrelia species have been delineated using DNA-DNA association, 23S-5S intergenic spacer (IGS), and 16S sequences and multilocus sequence analysis (MLSA) (34, 36, 37). A number of methods of strain typing and genotyping B. burgdorferi have been used (38). These include determination of the chromosomal 16S–23S (rrs-rrlA) IGS by restriction fragment length polymorphism (RFLP) analysis, which classifies isolates into one of three groups of ribosomal sequence types (RSTs) (39), and by typing of IGS sequences (40) and analysis of sequences of the plasmid-encoded OspA and OspC (41). More recently, multilocus sequence typing (MLST) using sequences of housekeeping genes has become available (42). There is considerable correlation among the strain typing classifications obtained by analysis of IGS sequences and ospC alleles due to extensive linkage disequilibrium in the genome of B. burgdorferi. This is thought to be due to the low rates of multiplication in this organism and, relative to other bacteria, the more limited opportunities for horizontal transfer of genomic DNA and plasmids due to its vector-borne ecology (40). For this reason, it has been suggested that ospC is a lineage-defining gene (43), but this linkage has proved not to be absolute, and the use of sequences of multiple housekeeping genes, as used in MLST, is currently considered to be the method of choice for phylogenetic and phylogeographic analyses (34). Next-generation sequencing and analysis of single nucleotide polymorphisms (SNP) shows promise for the future (44, 45, 46, 47) but has yet to be readily applicable (mostly in terms of bioinformatics management) to larger, epidemiologically useful sample sizes for the study of B. burgdorferi in North America to the degree that these techniques are beginning to be applied to enterobacteriaciae (48).
At the time of writing, 111 sequence types (STs) of B. burgdorferi have been identified by MLST in North America (49) and their occurrence shows clear geographic patterns—for the most part, different STs are found in the northeastern United States, in the midwestern United States, and in California (Fig. 1), and geographic and landscape barriers currently limit gene flow between these groups (50). However, the main phylogenetic pattern of clades is not geographically defined, as illustrated in Fig. 2: clonal complexes (which mostly equate with clades in the phylogenetic tree [49]) comprise STs from multiple geographic locations, as illustrated in Fig. 2 by the color coding of STs according to the geographic location of origin described in the Fig. 1 legend. The initial population expansion of B. burgdorferi in North America has been estimated to have occurred thousands to a million years ago (51). The origin of the clades is unclear, and hypotheses for their occurrence include introductions from Europe and glacial-interglacial cycles altering ecological suitability for population expansion. Either way, because the main clades are not associated with geographical isolation, we speculate that clades may relate broadly to some form of ecological “isolation,” which for B. burgdorferi may be host species association. In this scenario, successful expansions involved strains particularly adapted to hosts that were abundant at that time and whose descendants may persist today (49). There is evidence that some B. burgdorferi strains may be more efficiently transmitted by, or more frequently associated with, some host species than others (52, 53, 54, 55). This evidence comprises statistical associations from field and laboratory observations (including traits such as longer periods of postinfection transmissibility [52, 54]) rather than “smoking-gun,” experimentally demonstrated mechanisms such as sensitivity to an alternative pathway complement (see reference 56 and below); here we use the term “host species association” to mean that certain strains are more efficiently transmitted by, or persist in, infected individuals of certain host species. However, the niche breadth of B. burgdorferi strains is wider than that of other Borrelia species: there is certainly not strong host specialization such as there is for B. burgdorferi sensu lato species in Europe (57), and, to our knowledge, B. burgdorferi strains in North America remain host generalists (i.e., can infect and be transmitted from a wide range of wild-animal host species [58]).
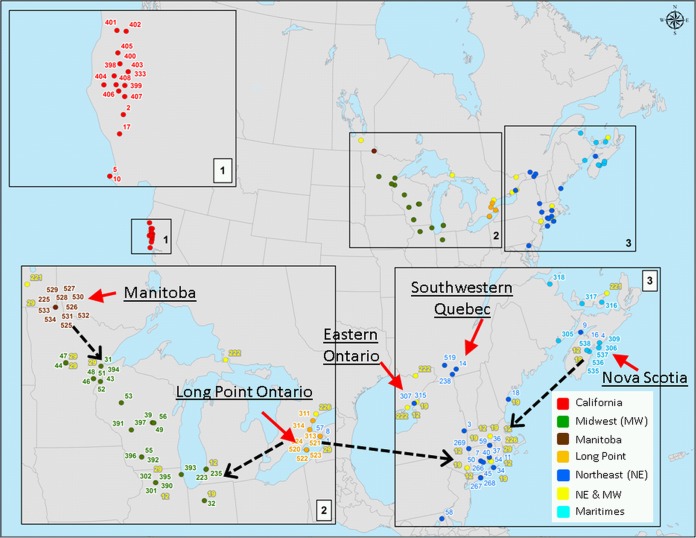
FIG 1.
Locations where B. burgdorferi samples that have been subjected to MLST analysis have been collected in North America. The colored points indicate locations where all samples analyzed were collected, while red arrows indicate the locations of field sites where new samples in a recent study in Canada were obtained (49). The different colored points correspond to sequence types (STs) found in different geographic regions. Cyan, STs found only in the Maritimes, Canada; orange, STs found only at Long Point, Ontario, Canada; brown, STs found only in Manitoba, Canada; blue, STs found across the northeastern United States and southern Quebec, southeastern Ontario, and the Maritimes in Canada; green, STs found in the midwestern United States; yellow, STs found in both northeastern and midwestern locations of the United States and longitudinally corresponding regions of Canada; red, STs found only in California. The dashed black arrows indicate the likely locations of immediate ancestors of novel STs found in Canada. Maps were created in ArcGIS. (Adapted from reference 49.)
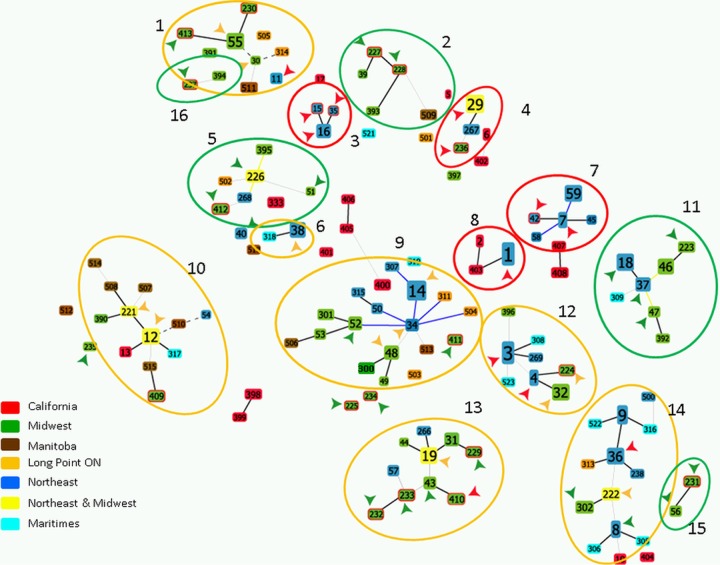
FIG 2.
A population snapshot created in goeBurst of B. burgdorferi sequence types (STs; i.e., strains which are individually numbered) identified by MLST in samples from the United States and Canada (49). Many STs are linked (being single- or double-locus variants) into clonal complexes in which the deduced relationships are identified by the color of the lines connecting STs as follows: gray and blue lines indicate (respectively) double- and single-locus variants, while yellow and black lines indicate relationships inferred (respectively) without tiebreak rules and with tiebreak rules. STs are identified by their individual number and color coded by location of origin as follows: blue, STs common to the northeastern United States, Quebec, eastern Ontario, and the Maritimes; green, STs common to the midwestern United States, Manitoba, and western Ontario; yellow, STs occurring in both the northeastern and midwestern United States and Canada; red, STs from California; cyan STs occurring only in the Maritimes; orange, STs occurring only at Long Point Ontario; brown, STs occurring only in Manitoba. Arrows indicate pathogenicity as follows: red, STs always (to date) associated with disseminated LD; orange, STs sometimes associated with disseminated LD; green, STs not associated with disseminated LD. Red circles surround clonal complexes of STs that mostly cause disseminated LD, orange circles surround clonal complexes of STs that sometimes cause disseminated LD, and green circles surround clonal complexes of STs that do not (to our current knowledge) cause disseminated LD (27).
It would be expected that the strains of B. burgdorferi now emerging in Canada would be the same as those occurring in the United States in locations directly to the south, assuming that migratory birds and other hosts are carrying ticks and B. burgdorferi from south to north each spring (59). Initial MLST-based studies of the diversity of B. burgdorferi in ticks collected in passive surveillance in Canada have tended to support this hypothesis (55). However, there is uncertainty as to the origin of ticks collected in passive surveillance, particularly when this involves ticks collected by veterinarians, because dogs very readily pick up ticks, including “adventitious” ticks, which may be dispersed from their location of origin (sometimes long distances of >500 km) by migratory birds or other hosts (60). More recently, we studied the diversity of B. burgdorferi in ticks collected from the environment or from hosts in locations in southeastern and south central Canada, where reproducing and self-sustaining populations of I. scapularis ticks and B. burgdorferi are known to be endemic and emerging (the locations of the sites of the populations are shown in Fig. 1). In that study, we found that strains in some Canadian populations (those in Manitoba and western Ontario and the Maritimes) are frequently different from those in the United States (albeit with recent ancestors immediately to the south in the United States), while in one region (extending from southeastern Ontario to southwestern Quebec), the strains are almost all the same as those in the northeastern United States (49). Details of the geographic occurrence of these strains are shown in Fig. 1. We have proposed that the occurrence of the novel strains is due to immigration of B. burgdorferi strains from multiple refugial populations in northern parts of the United States adjoining Canada in which studies of B. burgdorferi strain structure have not been conducted to date. This conclusion was reached because the novel strains in each site were mostly scattered about the phylogenetic tree and among clonal complexes (illustrated in Fig. 2) rather than comprising the distinct clades that would be expected if the new strains had arisen simply from recent founders or strains surviving in Canadian refuges (49). The increasing recognition that certain reservoir host species of B. burgdorferi such as the deer mouse (Peromyscus maniculatus) and the eastern chipmunk (Tamias striatus) may have survived glacial periods in northern refugia in North America may support the idea of the occurrence of multiple small refugia in the northern United States (61, 62). Even so, it is likely that strains occurring in these regions are uncommon in the United States, and they have not been detected in human cases to date (25), which could in part be due to low human population densities in the northern parts of states bordering Canada. Importantly, for Canada, where (in contrast to the United States) human population densities are highest close to the Canada-United States border, the clinical and diagnostic consequences of infection with these strains are currently unknown.
ECOLOGICAL ORIGINS AND CLINICAL AND DIAGNOSTIC IMPORTANCE OF B. BURGDORFERI DIVERSITY
In Europe, genospecies of the B. burgdorferi sensu lato species complex have almost absolute specialization for different reservoir host species and often produce a different range of LD symptoms when they infect humans (56, 63). A key molecular mechanism of this quasi-host species specialism (sensitivity to alternative pathway complement) is well established (56), although the precise mechanisms whereby different genospecies cause different types of disease in humans are not fully understood. Possible mechanisms include genospecies-specific tissue tropism, proliferation, and elicitation of pathology-causing immune responses (64, 65, 66). In North America, there is evidence that different strains of B. burgdorferi vary in their capacity to elicit an immune response in humans that is detectable, in the early stages of infection, by current methods of serodiagnosis (i.e., the two-tiered test comprising EIA followed by Western blot analysis interpreted by CDC-recommended criteria [1, 24]). Studies in mouse models suggest the possibility that some strains cause at least some pathology associated with disseminated infection, despite a failure to elicit a serological response detectable by two-tier method; one RST-3 strain of B. burgdorferi (determined by RFLP analysis of 16S-23S rRNA gene spacer sequences) produced some evidence of carditis in C3H mice but did not elicit a detectable immune response (67). While the two-tiered serological test has good sensitivity in later LD, sensitivity is low in very early disease (i.e., at the EM stage), and up to 20% of patients may test negative in very early disseminated LD (25). To date, there have been no discoveries of specific determinants of pathogenicity or virulence factors of B. burgdorferi such as those represented by the toxin secretion and invasion-encoding pathogenicity islands of many other pathogenic bacteria (68, 69, 70), and it is thought that most of the pathogenic effects are due to local inflammation induced by the bacterium and effects of autoantibodies (71, 72). The capacity of B. burgdorferi strains to systemically disseminate in humans has been considered equivalent to pathogenicity for this species complex (1, 73). The molecular determinants of “pathogenicity” of B. burgdorferi are therefore those that permit generalized dissemination in humans and subsequent persistence, implying that there are likely multiple determinants with complex functions in pathogenicity (74, 75). Studies have demonstrated that the pathogenicity of North American B. burgdorferi in humans, defined by whether disease manifestations are limited to the initial EM stage or instead progress to hematogenous dissemination and subsequent development of disseminated disease symptoms, can vary with infecting strain. The plasmid-borne, highly polymorphic ospC gene and the 16S-23S (rrs-rrlA) IGS have been the most commonly used genetic markers for identifying U.S. strains of B. burgdorferi that cause disseminated or localized LD (1, 65, 73, 76, 77, 78). Strains exhibiting restriction fragment length polymorphism in the 16S-23S rRNA intergenic spacer that are designated RST-1 and RST-2 types or that belong to major ospC allele groups A, B, H, I, and K are more frequently associated with hematogenous dissemination early in the course of LD, while RST-3 types and strains carrying ospC major groups other than A, B, H, I, and K (particularly T and U) are more likely to cause EM lesions alone and nondisseminated infection (1, 65, 73, 76, 77, 78). Different RST groups are also associated with different rates of antibiotic-refractory Lyme arthritis (79), indicating that knowledge of the infecting strain genotype may be important for predicting disease outcome and treatment planning.
Both RST and ospC typing methods have proved to be useful tools for classifying B. burgdorferi strains by their tendency to disseminate in humans. However, RST typing has limited discriminatory power for this purpose (6, 80), and the suitability of ospC typing may also be restricted, since the highly variable ospC gene is subject to strong selection by the host immune system (38, 41, 42). While associations of genotypes with dissemination are clear, specific genetic determinants facilitating dissemination remain unknown, although it could be argued that ospC could be a direct determinant of dissemination as it is mechanistically involved in B. burgdorferi-host interactions during the early phases of infection (81, 82, 83). A recent study showed that pathogenicity is often (genetically) predictable by examination of the housekeeping gene sequences used in MLST for phylogenetic analyses and that these sequences predicted pathogenicity better than ospC sequences (27). In Fig. 2, a population snapshot created in goeBurst of B. burgdorferi MLST sequence types (STs) from the United States and Canada is shown in which many STs are linked into clonal complexes. Some of these clonal complexes (which mostly correspond to clades in the MLST phylogenetic tree [49]) comprise a mix of STs that have been shown to date to be pathogenic (i.e., produce disseminated LD) with STs that are not pathogenic (i.e., do not produce disseminated LD). However, half of the clonal complexes comprise STs that are either all pathogenic or all nonpathogenic (Fig. 2 [27]). Associations of pathogenicity (as a phenotype) and MLST strain types are unlikely to be mechanistic as the housekeeping genes amplified and sequenced in MLST are not surface expressed and are not involved in interactions with the host. However, the fact that some MLST strain types are particularly associated with pathogenicity is highly significant because it suggests that pathogenicity in humans, who are not involved in the ecology of B. burgdorferi, is a phenotype associated with different lineages of the bacterium. In turn, this implies that the bacterial genetic determinants of pathogenicity in humans have origins in the evolution of the bacterium, driven by the ecological factors that have shaped its phylogeny. Because geographic isolation has not been a factor involved in the divergence of the phylogenetic groups (and clonal complexes; Fig. 2) that also appears to describe B. burgdorferi pathogenicity (50), alternative ecological factors, including those relating to associations with reservoir host species, may have been influential.
Horizontal gene transfer contributes to genetic diversity in B. burgdorferi sensu lato, although, apart from some specific loci, rates of horizontal gene transfer are generally low (84). However, recombination within B. burgdorferi sensu lato species has been shown to be 50 times higher than recombination between species (46). Furthermore, in B. burgdorferi sensu stricto, most recombination occurs within clades (49). The clades are not geographically defined or isolated, so this may support a hypothesis of host species association of clades, because both the donor strain and the recipient strain must be capable of infecting the same host for recombination to occur in that host or in a tick that subsequently feeds on that host. Current evidence suggests that host species association is mechanistically possible even if that does not mean that strains of B. burgdorferi are specialists but may be evolving toward specialism and may have done so in the past (52, 53, 54, 55). Together, these findings support the use of MLST as a strain typing tool useful for exploring the host species associations and pathogenicity of B. burgdorferi.
There are currently recognized ecological drivers of diversification of tick-borne pathogens such as B. burgdorferi, including drivers of host specialism. To persist in nature, tick-borne pathogens must be transmissible from ticks to hosts and the pathogen must disseminate in the host to locations where uninfected ticks can then acquire the pathogen and, following successful feeding and molting, subsequently transmit the pathogen to another host. In so doing, the pathogen must mechanistically negotiate multiple host environments, evade the innate and acquired immune response, and not kill or debilitate the host, which would prevent onward transmission to ticks (56). There are four key phases to host infection and onward transmission from the host (reviewed in reference 85): (i) initial infection in the skin of the host at the site of inoculation by an infected tick, which requires evasion of the host innate immune response, potentially via exploitation or binding of vector and/or host molecules (56); (ii) dissemination from the site of infection, which requires motility, binding of host receptors on vascular surfaces, immune evasion, and systemic dissemination during a short period of spirochetemia (86, 87, 88); (iii) persistence of infection to maximize the number of uninfected ticks the infected host can infect, which requires evasion of the host innate and acquired immune response (89, 90, 91, 92); and (iv) transmission from the host to a feeding uninfected tick, which likely requires either persistence in the dermis or some form of redissemination back to the dermis, where the spirochetes can be picked up by ticks (93). Although all of these processes are important determinants of whether or not, and how efficiently, a wild-animal species acts as a reservoir host for a particular strain, processes i to iii are important for infection in humans, and processes ii and iii are likely important in determining the capacity of different strains to cause disseminated LD.
Observed differences in transmission of different strains from reservoir host species in North America (which may underpin field-observed associations of strains with host species) in experimental settings have involved differences in the lengths of the periods postinfection during which the strain is efficiently transmitted from the infected hosts to ticks. Some strains are transmitted almost lifelong by Peromyscus leucopus (the white-footed mouse) following infection, while others are transmitted for only a short period postinfection and infection may be eliminated, presumably by the host immune response (51, 54). Those demonstrated to be transmitted almost lifelong by P. leucopus were of the RST-1 type of strains that are also known to often cause disseminated LD in humans, while those strains that were transmitted only for short periods were of the RST-3 type of strains that are less associated with disease manifestations involving dissemination of the bacteria (51, 54). Therefore, in some circumstances, RST-1 type strains may predominate in locations where P. leucopus mice are dominant hosts for immature ticks. However, short-lived infections were not a general characteristic of the RST-3 strain used because when mouse species other than P. leucopus were infected with this strain, efficient transmission to ticks persisted for long period postinfection (54). This could mean that hosts other than mice may be preferred hosts for the RST-3 strain used in these experiments. Therefore, different strains, which show different degrees of pathogenicity in humans, may have adapted to some extent for longer-term persistence in, and transmission from, different host species. This provides experimental support for the hypothesis that the phenotypic characteristics of strains of B. burgdorferi that determine their pathogenicity in humans may have their origins in adaptations to host species that advantage their transmissibility and thus persistence in nature.
Geographic variations in tick seasonality likely interplay with host species-strain associations driven by immune or other mechanisms: the more separated are the seasons of activity of infecting nymphal ticks and infection-acquiring larval ticks, the longer the host must remain alive and infective (56). Greater seasonal synchrony of nymphal and larval I. scapularis ticks in the upper Midwest of the United States (94) is associated with greater B. burgdorferi diversity (27). A recent key finding is that many of the B. burgdorferi strains occurring in the more western parts of Ontario and Manitoba and in the Maritimes are different from those found to date in the United States (49) (Fig. 1 and 2). Strains occurring in southern Quebec and eastern Ontario are nearly all the same as those occurring in the northeastern United States, so understanding the pathogenicity of strains in the northeastern United States is more relevant to this region of Canada. However, while the strains here may be the same, we are seeing skewing of strain frequencies compared to those seen in the northeastern United States due to founder events and other processes associated with recent invasion of the bacterium (59). Therefore, we need a greater understanding of which strains in Canada are pathogenic and where they occur. The possible existence of host species association of B. burgdorferi strains would mean that the occurrence of different strains may be predictable by the occurrence of particular host species and perhaps also of lineages of key Peromyscus sp. rodent reservoir hosts. These lineages have a geographic pattern in North America that is much more complex (95, 96) than that currently considered for B. burgdorferi phylogeography (49, 50, 51). This pattern has already proven to be compellingly associated with the phylogeographic pattern of zoonoses other than B. burgdorferi (e.g., Sin Nombre virus [97]) and could be similarly associated with B. burgdorferi, given recent evidence indicating that exposure of rodent hosts to B. burgdorferi infections may reciprocally shape their populations (30). The emergence of B. burgdorferi in North America, and particularly in Canada, driven by climate and habitat change, provides an intriguing opportunity to explore the evolutionary aspect of a key emerging vector-borne zoonosis, and evolutionary aspects are recognized as a neglected but critical factor in understanding how interactions among environment, pathogens, and animal hosts determine risks for human health (98, 99).
HOW TO STUDY AND PREDICT THE ECOLOGICAL ORIGINS AND CLINICAL AND DIAGNOSTIC IMPORTANCE OF B. BURGDORFERI DIVERSITY
Prospective studies are clearly needed to study the associations of novel B. burgdorferi strains in Canada with disseminated LD and their capacity to be detected in diagnostic tests and are also needed to elucidate their ecological origins to allow spatiotemporal prediction of their occurrence. The tools for this are at our fingertips. Apart from the currently available molecular phylogenetic methods of MLST, other methods such as genomic SNP analyses for cultured Borrelia strains may help to develop sufficiently sensitive typing tools to obtain information about relevant samples from infected patients and assess strain associations with clinical observations (27). A downside to this is that samples for such study are not usually collected in the course of routine diagnosis, and this will mean designing prospective studies. Systematic studies of wild animals and ticks collected from the field will be essential for a number of reasons. First, using clinical samples from infected people diagnosed using current diagnostic tests to search for novel strains may be equivalent to confining our search to what is visible under the lamppost that currently illuminates human LD cases. Second, in order to develop predictive methods that will help us to understand risk in the environments to which humans are exposed, samples from these environments must be collected and the pathogenic potential of strains occurring there must be characterized. Collection and analysis of relevant samples from ticks and animal hosts is routinely conducted in Canada using well-established field and laboratory protocols (100), and culture of pure strains from mixed infections is very feasible (101) when next-generation approaches (see, e.g., reference 47) for analysis of mixed infections are not available. Differences in disease severity and dissemination properties among B. burgdorferi genotypes have been experimentally tested and corroborated for selected strains in C3H/HeJ mice (67, 102), indicating that mouse pathogenesis studies are very useful for prediction of disease outcomes in humans. Therefore, methods for exploring associations between strains occurring in the field and pathogenicity in humans already exist. Wild-rodent host lineages can be identified using analysis of standard mitochondrial gene targets (96) and host community structure, which may be to some extent estimated by analysis of remote-sensed habitat or environmental data or ground-level landscape analysis (103, 104, 105, 106) and may indicate occurrences of different strains. With current geomatics technology, this information can be readily synthesized into risk maps that permit identification of the populations at risk from different strains by public health end users (12).
CONCLUSIONS
LD continues to emerge in the United States and is now emerging in Canada. Novel strains of B. burgdorferi occur in Canada, and these may originate from as-yet-unexplored refugia in the very northernmost parts of the United States bordering Canada. Increasingly, it is recognized that strains of B. burgdorferi differ in their capacity to disseminate and cause disease of different severities in humans. Humans do not take part in the natural transmission cycle of B. burgdorferi, and pathogenicity in humans is most likely to have evolved as a consequence of interactions with natural reservoir hosts. The potential association of pathogenicity of B. burgdorferi strains with different lineages, each in turn associated with different reservoir host species, raises the intriguing possibility that the occurrence of pathogenic B. burgdorferi strains may be predictable in ways that are practicable for public health end users. The tools to explore this hypothesis already exist, so such an endeavor would be practical and feasible. The emergence of LD in Canada due to the spread of the tick vector, and B. burgdorferi, represents a public health challenge requiring exploration of the ecological and evolutionary origins of genetic diversity of B. burgdorferi and its epidemiological and pathological consequences for practical public health purposes. But it also represents an interesting and unique opportunity to explore the evolutionary aspects of the emergence by invasion of a tick-borne pathogen.
REFERENCES
- 1.Wormser GP, Liveris D, Hanincová K, Brisson D, Ludin S, Stracuzzi VJ, Embers ME, Philipp MT, Levin A, Aguero-Rosenfeld M, Schwartz I. 2008. Effect of Borrelia burgdorferi genotype on the sensitivity of C6 and 2-tier testing in North American patients with culture-confirmed Lyme disease. Clin Infect Dis 47:910–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steere AC, Malawista SE, Snydman DR, Shope RE, Andiman WA, Ross MR, Steele FM. 1977. An epidemic of oligoarticular arthritis in children and adults in three Connecticut communities. Arthritis Rheum 20:7–17. [DOI] [PubMed] [Google Scholar]
- 3.CDC. 2013. How many people get Lyme disease? CDC, Atlanta, GA. http://www.cdc.gov/lyme/stats/humanCases.html. [Google Scholar]
- 4.Bacon RM, Kugeler KJ, Mead PS, Centers for Disease Control and Prevention (CDC). 2008. Surveillance for Lyme disease-United States, 1992–2006. MMWR Surveill Summ 57:1–9. [PubMed] [Google Scholar]
- 5.Pepin KM, Eisen RJ, Mead PS, Piesman J, Fish D, Hoen AG, Barbour AG, Hamer S, Diuk-Wasser MA. 2012. Geographic variation in the relationship between human Lyme disease incidence and density of infected host-seeking Ixodes scapularis nymphs in the Eastern United States. Am J Trop Med Hyg 86:1062–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brisson D, Vandermause MF, Meece JK, Reed KD, Dykhuizen DE. 2010. Evolution of northeastern and midwestern Borrelia burgdorferi, United States. Emerg Infect Dis 16:911–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wood CL, Lafferty KD. 2013. Biodiversity and disease: a synthesis of ecological perspectives on Lyme disease transmission. Trends Ecol Evol 28:239–247. [DOI] [PubMed] [Google Scholar]
- 8.Ogden NH, Radojevic M, Wu X, Duvvuri VR, Leighton PA, Wu J. 2014. Estimated effects of projected climate change on the basic reproductive number of the tick vector of Lyme disease Ixodes scapularis. Environ Health Perspect 122:631–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leighton P, Koffi J, Pelcat Y, Lindsay LR, Ogden NH. 2012. Predicting the speed of tick invasion: an empirical model of range expansion for the Lyme disease vector Ixodes scapularis in Canada. J Appl Ecol 49:457–464. doi: 10.1111/j.1365-2664.2012.02112.x. [DOI] [Google Scholar]
- 10.Ogden NH, Lindsay LR, Leighton PA. 2013. Predicting the rate of invasion of the agent of Lyme disease Borrelia burgdorferi. J Appl Ecol 50:510–518. doi: 10.1111/1365-2664.12050. [DOI] [Google Scholar]
- 11.Public Health Agency of Canada. 2015. Surveillance of Lyme disease. Public Health Agency of Canada, Ottawa, Ontario, Canada: http://www.healthycanadians.gc.ca/diseases-conditions-maladies-affections/disease-maladie/lyme/surveillance-eng.php. [Google Scholar]
- 12.Ogden NH, St-Onge L, Barker IK, Brazeau S, Bigras-Poulin M, Charron DF, Francis CM, Heagy A, Lindsay LR, Maarouf A, Michel P, Milord F, O'Callaghan CJ, Trudel L, Thompson RA. 2008. Risk maps for range expansion of the Lyme disease vector, Ixodes scapularis, in Canada now and with climate change. Int J Health Geogr 7:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zinsstag J, Schelling E, Waltner-Toews D, Tanner M. 2011. From “one medicine” to “one health” and systemic approaches to health and well-being. Prev Vet Med 101:148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bidaisee S, Macpherson CN. 2014. Zoonoses and one health: a review of the literature. J Parasitol Res 2014:874345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ogden NH, Koffi JK, Lindsay LR, Fleming S, Mombourquette DC, Sanford C, Badcock J, Gad RR, Jain-Sheehan N, Moore S, Russell C, Hobbs L, Baydack R, Graham-Derham S, Lachance L, Simmonds K, Scott AN. 2015. Surveillance for Lyme disease in Canada, 2009–2012. Can Commun Dis Rep 41:132–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steere AC, Sikand VK, Meurice F, Parenti DL, Fikrig E, Schoen RT, Nowakowski J, Schmid CH, Laukamp S, Buscarino C, Krause DS. 1998. Vaccination against Lyme disease with recombinant Borrelia burgdorferi outer-surface lipoprotein A with adjuvant. Lyme Disease Vaccine Study Group. N Engl J Med 339:209–215. [DOI] [PubMed] [Google Scholar]
- 17.Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS, Krause PJ, Bakken JS, Strle F, Stanek G, Bockenstedt L, Fish D, Dumler JS, Nadelman RB. 2006. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 43:1089–1134. [DOI] [PubMed] [Google Scholar]
- 18.Nadelman RB, Nowakowski J, Forseter G, Goldberg NS, Bittker S, Cooper D, Aguero-Rosenfeld M, Wormser GP. 1996. The clinical spectrum of early Lyme borreliosis in patients with culture-confirmed erythema migrans. Am J Med 100:502–508. [DOI] [PubMed] [Google Scholar]
- 19.Forrester JD, Meiman J, Mullins J, Nelson R, Ertel SH, Cartter M, Brown CM, Lijewski V, Schiffman E, Neitzel D, Daly ER, Mathewson AA, Howe W, Lowe LA, Kratz NR, Semple S, Backenson PB, White JL, Kurpiel PM, Rockwell R, Waller K, Johnson DH, Steward C, Batten B, Blau D, DeLeon-Carnes M, Drew C, Muehlenbachs A, Ritter J, Sanders J, Zaki SR, Molins C, Schriefer M, Perea A, Kugeler K, Nelson C, Hinckley A, Mead P, Centers for Disease Control and Prevention (CDC). 2014. Notes from the field: update on Lyme carditis, groups at high risk, and frequency of associated sudden cardiac death—United States. MMWR Morb Mortal Wkly Rep 63:982–983. [PMC free article] [PubMed] [Google Scholar]
- 20.Nadelman RB, Hanincová K, Mukherjee P, Liveris D, Nowakowski J, McKenna D, Brisson D, Cooper D, Bittker S, Madison G, Holmgren D, Schwartz I, Wormser GP. 2012. Differentiation of reinfection from relapse in recurrent Lyme disease. N Engl J Med 367:1883–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khatchikian CE, Nadelman RB, Nowakowski J, Schwartz I, Wormser GP, Brisson D. 2014. Evidence for strain-specific immunity in patients treated for early Lyme disease. Infect Immun 82:1408–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feder HM Jr, Johnson BJ, O'Connell S, Shapiro ED, Steere AC, Wormser GP, Ad Hoc International Lyme Disease Group. 2007. A critical appraisal of “chronic Lyme disease”. N Engl J Med 357:1422–1430. [DOI] [PubMed] [Google Scholar]
- 23.Bruckbauer HR, Preac-Mursic V, Fuchs R, Wilske B. 1992. Crossreactive proteins of Borrelia burgdorferi. Eur J Clin Microbiol Infect Dis 11:224–232. [DOI] [PubMed] [Google Scholar]
- 24.CDC. 1995. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. MMWR Morb Mortal Wkly Rep 44:590–591. [PubMed] [Google Scholar]
- 25.Aguero-Rosenfeld ME, Wang G, Schwartz I, Wormser GP. 2005. Diagnosis of Lyme borreliosis. Clin Microbiol Rev 18:484–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogden NH, Lindsay LR, Schofield SW. Methods to prevent tick bites and Lyme disease. Clin Lab Med, in press. [DOI] [PubMed] [Google Scholar]
- 27.Hanincova K, Mukherjee P, Ogden NH, Margos G, Wormser GP, Reed KD, Meece JK, Vandermause MF, Schwartz I. 2013. Multilocus sequence typing of Borrelia burgdorferi suggests existence of lineages with differential pathogenic properties in humans. PLoS One 8:e73066. doi: 10.1371/journal.pone.0073066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iliopoulou BP, Guerau-de-Arellano M, Huber BT. 2009. HLA-DR alleles determine responsiveness to Borrelia burgdorferi antigens in a mouse model of self-perpetuating arthritis. Arthritis Rheum 60:3831–3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strle K, Shin JJ, Glickstein LJ, Steere AC. 2012. Association of a Toll-like receptor 1 polymorphism with heightened Th1 inflammatory responses and antibiotic-refractory Lyme arthritis. Arthritis Rheum 64:1497–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tschirren B, Andersson M, Scherman K, Westerdahl H, Mittl PR, Råberg L. 2013. Polymorphisms at the innate immune receptor TLR2 are associated with Borrelia infection in a wild rodent population. Proc Biol Sci 280:20130364. doi: 10.1098/rspb.2013.0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jungnick S, Margos G, Rieger M, Dzaferovic E, Bent S, Overzier E, Silaghi C, Walder G, Wex F, Koloczec J, Sing A, Fingerle V. Borrelia burgdorferi sensu stricto and Borrelia afzelii: population structure and differential pathogenicity. Ticks Tick Borne Dis, in press. [DOI] [PubMed] [Google Scholar]
- 32.Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, Davis JP. 1982. Lyme disease—a tick-borne spirochetosis? Science 216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- 33.Johnson SE, Klein GC, Schmid GP, Bowen GS, Feeley JC, Schulze T. 1984. Lyme disease: a selective medium for isolation of the suspected etiological agent, a spirochete. J Clin Microbiol 19:81–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Margos G, Vollmer SA, Ogden NH, Fish D. 2011. Population genetics, taxonomy, phylogeny and evolution of Borrelia burgdorferi sensu lato. Infect Genet Evol 11:1545–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Casjens SR, Mongodin EF, Qiu WG, Luft BJ, Schutzer SE, Gilcrease EB, Huang WM, Vujadinovic M, Aron JK, Vargas LC, Freeman S, Radune D, Weidman JF, Dimitrov GI, Khouri HM, Sosa JE, Halpin RA, Dunn JJ, Fraser CM. 2012. Genome stability of Lyme disease spirochetes: comparative genomics of Borrelia burgdorferi plasmids. PLoS One 7:e33280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olsen I, Paster BJ, Dewhirst FE. 2000. Taxonomy of spirochetes. Anaerobe 6:39–57. doi: 10.1006/anae.1999.0319. [DOI] [Google Scholar]
- 37.Masuzawa T, Takada N, Kudeken M, Fukui T, Yano Y, Ishiguro F, Kawamura Y, Imai Y, Ezaki T. 2001. Borrelia sinica sp. nov., a Lyme disease-related Borrelia species isolated in China. Int J Syst Evol Microbiol 51:1817–1824. doi: 10.1099/00207713-51-5-1817. [DOI] [PubMed] [Google Scholar]
- 38.Wang G, Liveris D, Mukherjee P, Jungnick S, Margos G, Schwartz I. 2014. Molecular typing of Borrelia burgdorferi. Curr Protoc Microbiol 34:12C.5.1–12C.5.31. doi: 10.1002/9780471729259.mc12c05s34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liveris D, Gazumyan A, Schwartz I. 1995. Molecular typing of Borrelia burgdorferi sensu lato by PCR-restriction fragment length polymorphism analysis. J Clin Microbiol 33:589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bunikis J, Garpmo U, Tsao J, Berglund J, Fish D, Barbour AG. 2004. Sequence typing reveals extensive strain diversity of the Lyme borreliosis agents Borrelia burgdorferi in North America and Borrelia afzelii in Europe. Microbiology 150:1741–1755. [DOI] [PubMed] [Google Scholar]
- 41.Qiu WG, Dykhuizen DE, Acosta MS, Luft BJ. 2002. Geographic uniformity of the Lyme disease spirochete (Borrelia burgdorferi) and its shared history with tick vector (Ixodes scapularis) in the Northeastern United States. Genetics 160:833–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Margos G, Gatewood AG, Aanensen DM, Hanincová K, Terekhova D, Vollmer SA, Cornet M, Piesman J, Donaghy M, Bormane A, Hurn MA, Feil EJ, Fish D, Casjens S, Wormser GP, Schwartz I, Kurtenbach K. 2008. MLST of housekeeping genes captures geographic population structure and suggests a European origin of Borrelia burgdorferi. Proc Natl Acad Sci U S A 105:8730–8735. doi: 10.1073/pnas.0800323105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barbour AG, Travinsky B. 2010. Evolution and distribution of the ospC gene, a transferable serotype determinant of Borrelia burgdorferi. mBio 1:e00153-10. doi: 10.1128/mBio.00153-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leichty AR, Brisson D. 2014. Selective whole genome amplification for resequencing target microbial species from complex natural samples. Genetics 198:473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gatzmann F, Metzler D, Krebs S, Blum H, Sing A, Takano A, Kawabata H, Fingerle V, Margos G, Becker NS. 2015. NGS population genetics analyses reveal divergent evolution of a Lyme borreliosis agent in Europe and Asia. Ticks Tick Borne Dis 6:344–351. [DOI] [PubMed] [Google Scholar]
- 46.Jacquot M, Gonnet M, Ferquel E, Abrial D, Claude A, Gasqui P, Choumet V, Charras-Garrido M, Garnier M, Faure B, Sertour N, Dorr N, De Goër J, Vourc'h G, Bailly X. 2014. Comparative population genomics of the Borrelia burgdorferi species complex reveals high degree of genetic isolation among species and underscores benefits and constraints to studying intra-specific epidemiological processes. PLoS One 9:e94384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carpi G, Walter KS, Bent SJ, Hoen AG, Diuk-Wasser M, Caccone A. 2015. Whole genome capture of vector-borne pathogens from mixed DNA samples: a case study of Borrelia burgdorferi. BMC Genomics 16:434. doi: 10.1186/s12864-015-1634-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Franz E, Delaquis P, Morabito S, Beutin L, Gobius K, Rasko DA, Bono J, French N, Osek J, Lindstedt BA, Muniesa M, Manning S, LeJeune J, Callaway T, Beatson S, Eppinger M, Dallman T, Forbes KJ, Aarts H, Pearl DL, Gannon VP, Laing CR, Strachan NJ. 2014. Exploiting the explosion of information associated with whole genome sequencing to tackle Shiga toxin-producing Escherichia coli (STEC) in global food production systems. Int J Food Microbiol 187:57–72. [DOI] [PubMed] [Google Scholar]
- 49.Mechai S, Margos G, Feil EJ, Lindsay LR, Ogden NH. 2015. Phylogeographic analysis reveals a complex population structure of Borrelia burgdorferi in southern Canada. Appl Environ Microbiol 81:1309–1318. doi: 10.1128/AEM.03730-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Margos G, Tsao JI, Castillo-Ramírez S, Girard YA, Hamer SA, Hoen AG, Lane RS, Raper SL, Ogden NH. 2012. Two boundaries separate Borrelia burgdorferi populations in North America. Appl Environ Microbiol 78:6059–6067. doi: 10.1128/AEM.00231-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoen AG, Margos G, Bent SJ, Diuk-Wasser MA, Barbour A, Kurtenbach K, Fish D. 2009. Phylogeography of Borrelia burgdorferi in the eastern United States reflects multiple independent Lyme disease emergence events. Proc Natl Acad Sci U S A 106:15013–15038. doi: 10.1073/pnas.0903810106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Derdáková M, Dudiòák V, Brei B, Brownstein JS, Schwartz I, Fish D. 2004. Interaction and transmission of two Borrelia burgdorferi sensu stricto strains in a tick-rodent maintenance system. Appl Environ Microbiol 70:6783–6788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brisson D, Dykhuizen DE. 2004. ospC diversity in Borrelia burgdorferi: different hosts are different niches. Genetics 168:713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hanincová K, Ogden NH, Diuk-Wasser M, Pappas CJ, Lyer R, Fish D, Schwartz I, Kurtenbach K. 2008. Fitness variation of Borrelia burgdorferi sensu stricto strains in mice. Appl Environ Microbiol 74:153–157. doi: 10.1128/AEM.01567-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ogden NH, Margos G, Aanensen DM, Drebot MA, Feil EJ, Hanincová K, Schwartz I, Tyler S, Lindsay LR. 2011. Investigation of genotypes of Borrelia burgdorferi in Ixodes scapularis ticks collected during surveillance in Canada. Appl Environ Microbiol 77:3244–3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kurtenbach K, Hanincová K, Tsao J, Margos G, Fish D, Ogden NH. 2006. Key processes in the evolutionary ecology of Lyme borreliosis. Nat Rev Microbiol 4:660–669. [DOI] [PubMed] [Google Scholar]
- 57.Piesman J, Gern L. 2004. Lyme borreliosis in Europe and North America. Parasitology 129:S191–S220. [DOI] [PubMed] [Google Scholar]
- 58.Hanincová K, Kurtenbach K, Diuk-Wasser M, Brei B, Fish D. 2006. Epidemic spread of Lyme borreliosis, northeastern United States. Emerg Infect Dis 12:604–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ogden NH, Mechai S, Margos G. 2013. Changing geographic ranges of ticks and tick-borne pathogens: drivers, mechanisms and consequences for pathogen diversity. Front Cell Infect Microbiol 3:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ogden NH, Trudel L, Artsob H, Barker IK, Beauchamp G, Charron DF, Drebot MA, Galloway TD, O'Handley R, Thompson RA, Lindsay LR. 2006. Ixodes scapularis ticks collected by passive surveillance in Canada: analysis of geographic distribution and infection with the Lyme borreliosis agent Borrelia burgdorferi. J Med Entomol 43:600–609. [DOI] [PubMed] [Google Scholar]
- 61.Rowe KC, Heske EJ, Brown PW, Paige KN. 2004. Surviving the ice: Northern refugia and postglacial colonization. Proc Natl Acad Sci U S A 101:10355–10359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang D-S, Kenagy GJ. 2009. Nuclear and mitochondrial DNA reveal contrasting evolutionary processes in populations of deer mice (Peromyscus maniculatus). Mol Ecol 18:5115–5125. doi: 10.1111/j.1365-294X.2009.04399.x. [DOI] [PubMed] [Google Scholar]
- 63.Strle F, Ruzic-Sabljic E, Cimperman J, Lotric-Furlan S, Maraspin V. 2006. Comparison of findings for patients with Borrelia garinii and Borrelia afzelii isolated from cerebrospinal fluid. Clin Infect Dis 43:704–710. [DOI] [PubMed] [Google Scholar]
- 64.Brisson D, Baxamusa N, Schwartz I, Wormser GP. 2011. Biodiversity of Borrelia burgdorferi strains in tissues of Lyme disease patients. PLoS One 6:e22926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Strle K, Jones KL, Drouin EE, Li X, Steere AC. 2011. Borrelia burgdorferi RST1 (OspC type A) genotype is associated with greater inflammation and more severe Lyme disease. Am J Pathol 178:2726–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu Q, Liu Z, Wang J, Li Y, Guan G, Yang J, Chen Z, Luo J, Yin H. 2013. Pathogenic analysis of Borrelia garinii strain SZ isolated from Northeastern China. Parasit Vectors 6:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang G, Ojaimi C, Wu H, Saksenberg V, Lyer R, Liveris D, McClain SA, Wormser GP, Schwartz I. 2002. Disease severity in a murine model of Lyme borreliosis is associated with the genotype of the infecting Borrelia burgdorferi sensu stricto strain. J Infect Dis 186:782–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hacker J, Kaper JB. 2000. Pathogenicity islands and the evolution of microbes. Annu Rev Microbiol 54:641–679. [DOI] [PubMed] [Google Scholar]
- 69.Pearson JS, Zhang Y, Newton HJ, Hartland EL. 2015. Post-modern pathogens: surprising activities of translocated effectors from E. coli and Legionella. Curr Opin Microbiol 23:73–79. doi: 10.1016/j.mib.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 70.Krüger A, Lucchesi PM. 2015. Shiga toxins and stx phages: highly diverse entities. Microbiology 161:451–462. doi: 10.1099/mic.0.000003. [DOI] [PubMed] [Google Scholar]
- 71.Stanek G, Strle F. 2008. Lyme disease: European perspective. Infect Dis Clin North Am 22:327–339. doi: 10.1016/j.idc.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 72.Weis JJ, Bockenstedt LK. 2010. Host response, p 413–442. In Samuels DS, Radolph JD (ed), Borrelia—molecular biology, host interaction and pathogenesis. Caister Academic Press, Norfolk, United Kingdom. [Google Scholar]
- 73.Wormser GP, Liveris D, Nowakowski J, Nadelman RB, Cavaliere LF, McKenna D, Holmgren D, Schwartz I. 1999. Association of specific subtypes of Borrelia burgdorferi with hematogenous dissemination in early Lyme disease. J Infect Dis 180:720–725. [DOI] [PubMed] [Google Scholar]
- 74.Brissette CA, Gaultney RA. 2014. That's my story, and I'm sticking to it—an update on B. burgdorferi adhesins. Front Cell Infect Microbiol 4:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu Q, Guan G, Liu Z, Li Y, Luo J, Yin H. 2015. RNA-Seq-based analysis of changes in Borrelia burgdorferi gene expression linked to pathogenicity. Parasit Vectors 8:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Seinost G, Dykhuizen DE, Dattwyler RJ, Golde WT, Dunn JJ, Wang IN, Wormser GP, Schriefer ME, Luft BJ. 1999. Four clones of Borrelia burgdorferi sensu stricto cause invasive infection in humans. Infect Immun 67:3518–3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jones KL, Glickstein LJ, Damle N, Sikand VK, McHugh G, Steere AC. 2006. Borrelia burgdorferi genetic markers and disseminated disease in patients with early Lyme disease. J Clin Microbiol 44:4407–4413. doi: 10.1128/JCM.01077-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dykhuizen DE, Brisson D, Sandigursky S, Wormser GP, Nowakowski J, Nadelman RB, Schwartz I. 2008. The propensity of different Borrelia burgdorferi sensu stricto genotypes to cause disseminated infections in humans. Am J Trop Med Hyg 78:806–810. [PMC free article] [PubMed] [Google Scholar]
- 79.Jones KL, McHugh GA, Glickstein LJ, Steere AC. 2009. Analysis of Borrelia burgdorferi genotypes in patients with Lyme arthritis: high frequency of ribosomal RNA intergenic spacer type 1 strains in antibiotic-refractory arthritis. Arthritis Rheum 60:2174–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hanincová K, Liveris D, Sandigursky S, Wormser GP, Schwartz I. 2008. Borrelia burgdorferi sensu stricto is clonal in patients with early Lyme borreliosis. Appl Environ Microbiol 74:5008–5014. doi: 10.1128/AEM.00479-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tilly K, Krum JG, Bestor A, Jewett MW, Grimm D, Bueschel D, Byram R, Dorward D, Vanraden MJ, Stewart P, Rosa P. 2006. Borrelia burgdorferi OspC protein required exclusively in a crucial early stage of mammalian infection. Infect Immun 74:3554–3564. doi: 10.1128/IAI.01950-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tilly K, Bestor A, Rosa PA. 2013. Lipoprotein succession in Borrelia burgdorferi: similar but distinct roles for OspC and VlsE at different stages of mammalian infection. Mol Microbiol 89:216–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Radolf JD, Caimano MJ. 2008. The long strange trip of Borrelia burgdorferi outer-surface protein C. Mol Microbiol 69:1–4. doi: 10.1111/j.1365-2958.2008.06226.x. [DOI] [PubMed] [Google Scholar]
- 84.Seifert SN, Khatchikian CE, Zhou W, Brisson D. 2015. Evolution and population genomics of the Lyme borreliosis pathogen, Borrelia burgdorferi. Trends Genet 31:201–207. doi: 10.1016/j.tig.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ogden NH, Margos G, Artsob H, Tsao JI. 2014. Tick-borne bacteria: Lyme disease, relapsing fever and tularaemia, p 278–312. In Sonenshine D, Rowe M (ed), The biology of ticks, vol 2 Oxford University Press, Oxford, United Kingdom. [Google Scholar]
- 86.Coleman JL, Sellati TJ, Testa JE, Kew RR, Furie MB, Benach JL. 1995. Borrelia burgdorferi binds plasminogen, resulting in enhanced penetration of endothelial monolayers. Infect Immun 63:2478–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liveris D, Wang G, Girao G, Byrne DW, Nowakowski J, McKenna D, Nadelman R, Wormser GP, Schwartz I. 2002. Quantitative detection of Borrelia burgdorferi in 2-millimeter skin samples of erythema migrans lesions: correlation of results with clinical and laboratory findings. J Clin Microbiol 40:1249–1253. doi: 10.1128/JCM.40.4.1249-1253.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Moriarty TJ, Shi M, Lin YP, Ebady R, Zhou H, Odisho T, Hardy PO, Salman-Dilgimen A, Wu J, Weening EH, Skare JT, Kubes P, Leong J, Chaconas G. 2012. Vascular binding of a pathogen under shear force through mechanistically distinct sequential interactions with host macromolecules. Mol Microbiol 86:1116–1131. doi: 10.1111/mmi.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Benach JL, Habicht GS, Gocinski BL, Coleman JL. 1984. Phagocytic cell responses to in vivo and in vitro exposure to the Lyme disease spirochaete. Yale J Biol Med 57:599–605. [PMC free article] [PubMed] [Google Scholar]
- 90.Barthold S, Feng S, Bockenstedt L, Fikrig E, Feen K. 1997. Protective and arthritis-resolving activity in sera of mice infected with Borrelia burgdorferi. Clin Infect Dis 25:S9–S17. [DOI] [PubMed] [Google Scholar]
- 91.Liang FT, Yan J, Mbow ML, Sviat SL, Gilmore RD, Mamula M, Fikrig E. 2004. Borrelia burgdorferi changes its surface antigenic expression in response to host immune responses. Infect Immun 72:5759–5767. doi: 10.1128/IAI.72.10.5759-5767.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Coutte L, Botkin DJ, Gao L, Norris SJ. 2009. Detailed analysis of sequence changes occurring during vlsE antigenic variation in the mouse model of Borrelia burgdorferi infection. PLoS Pathog 5:e1000293. doi: 10.1371/journal.ppat.1000293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shih CM, Chao LL, Yu CP. 2002. Chemotactic migration of the Lyme disease spirochaete (Borrelia burgdorferi) to salivary gland extracts of vector ticks. Am J Trop Med Hyg 66:616–621. [DOI] [PubMed] [Google Scholar]
- 94.Gatewood AG, Liebman KA, Vourc'h G, Bunikis J, Hamer SA, Cortinas R, Melton F, Cislo P, Kitron U, Tsao J, Barbour AG, Fish D, Diuk-Wasser MA. 2009. Climate and tick seasonality are predictors of Borrelia burgdorferi genotype distribution. Appl Environ Microbiol 75:2476–2483. doi: 10.1128/AEM.02633-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Waters JH. 1963. Biochemical relationships of the mouse Peromyscus in New England. Syst Zool 12:122–133. doi: 10.2307/2411532. [DOI] [Google Scholar]
- 96.Dragoo JW, Lackey JA, Moore KE, Lessa EP, Cook JA, Yates TL. 2006. Phylogeography of the deer mouse (Peromyscus maniculatus) provides a predictive framework for research on hantaviruses. J Gen Virol 87:1997–2003. [DOI] [PubMed] [Google Scholar]
- 97.Drebot MA, Gavrilovskaya I, Mackow ER, Chen Z, Lindsay R, Sanchez AJ, Nichol ST, Artsob H. 2001. Genetic and serotypic characterization of Sin Nombre-like viruses in Canadian Peromyscus maniculatus mice. Virus Res 75:75–86. doi: 10.1016/S0168-1702(01)00227-1. [DOI] [PubMed] [Google Scholar]
- 98.Travis DA, Sriramarao P, Cardona C, Steer CJ, Kennedy S, Sreevatsan S, Murtaugh MP. 2014. One Medicine One Science: a framework for exploring challenges at the intersection of animals, humans, and the environment. Ann N Y Acad Sci 1334:26–44. doi: 10.1111/nyas.12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Suzán G, García-Peña GE, Castro-Arellano I, Rico O, Rubio AV, Tolsá MJ, Roche B, Hosseini PR, Rizzoli A, Murray KA, Zambrana-Torrelio C, Vittecoq M, Bailly X, Aguirre AA, Daszak P, Prieur-Richard AH, Mills JN, Guégan JF. 2015. Metacommunity and phylogenetic structure determine wildlife and zoonotic infectious disease patterns in time and space. Ecol Evol 5:865–873. doi: 10.1002/ece3.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dibernardo A, Cote T, Ogden NH, Lindsay LR. 2014. The prevalence of Borrelia miyamotoi infection, and coinfections with other Borrelia spp. in Ixodes scapularis ticks collected in Canada. Parasit Vectors 7:183. doi: 10.1186/1756-3305-7-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hinnebusch J, Barbour AG. 1992. Linear- and circular-plasmid copy numbers in Borrelia burgdorferi. J Bacteriol 174:5251–5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang G, Ojaimi C, Iyer R, Saksenberg V, McClain SA, Wormser GP, Schwartz I. 2001. Impact of genotypic variation of Borrelia burgdorferi sensu stricto on kinetics of dissemination and severity of disease in C3H/HeJ mice. Infect Immun 69:4303–4312. doi: 10.1128/IAI.69.7.4303-4312.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ogden NH, Barker IK, Beauchamp G, Brazeau S, Charron DF, Maarouf A, Morshed MG, O'Callaghan CJ, Thompson RA, Waltner-Toews D, Waltner-Toews M, Lindsay LR. 2006. Investigation of ground level and remote-sensed data for habitat classification and prediction of survival of Ixodes scapularis ticks in habitats of southeastern Canada. J Med Entomol 43:403–414. [DOI] [PubMed] [Google Scholar]
- 104.Bouchard C, Beauchamp G, Leighton PA, Lindsay R, Bélanger D, Ogden NH. 2013. Does biodiversity prevent Lyme disease invasion? Parasit Vectors 6:195. doi: 10.1186/1756-3305-6-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gabriele-Rivet V, Arsenault J, Badcock J, Cheng A, Edsall J, Goltz J, Kennedy J, Lindsay LR, Pelcat Y, Ogden NH. 2015. Different ecological niches for ticks of public health significance in Canada. PLoS One 10:e0131282. doi: 10.1371/journal.pone.0131282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Leyequien E, Verrelst J, Slot M, Schaepman-Strub G, Heitkönig IMA, Skidmore A. 2007. Capturing the fugitive: applying remote sensing to terrestrial animal distribution and diversity. Int J Appl Earth Obs Geoinf 9:1–20. doi: 10.1016/j.jag.2006.08.002. [DOI] [Google Scholar]