Abstract
Vibrio parahaemolyticus, Vibrio vulnificus, and Vibrio cholerae of the non-O1/non-O139 serotype are present in coastal lagoons of southern France. In these Mediterranean regions, the rivers have long low-flow periods followed by short-duration or flash floods during and after heavy intense rainstorms, particularly at the end of the summer and in autumn. These floods bring large volumes of freshwater into the lagoons, reducing their salinity. Water temperatures recorded during sampling (15 to 24°C) were favorable for the presence and multiplication of vibrios. In autumn 2011, before heavy rainfalls and flash floods, salinities ranged from 31.4 to 36.1‰ and concentrations of V. parahaemolyticus, V. vulnificus, and V. cholerae varied from 0 to 1.5 × 103 most probable number (MPN)/liter, 0.7 to 2.1 × 103 MPN/liter, and 0 to 93 MPN/liter, respectively. Following heavy rainstorms that generated severe flash flooding and heavy discharge of freshwater, salinity decreased, reaching 2.2 to 16.4‰ within 15 days, depending on the site, with a concomitant increase in Vibrio concentration to ca. 104 MPN/liter. The highest concentrations were reached with salinities between 10 and 20‰ for V. parahaemolyticus, 10 and 15‰ for V. vulnificus, and 5 and 12‰ for V. cholerae. Thus, an abrupt decrease in salinity caused by heavy rainfall and major flooding favored growth of human-pathogenic Vibrio spp. and their proliferation in the Languedocian lagoons. Based on these results, it is recommended that temperature and salinity monitoring be done to predict the presence of these Vibrio spp. in shellfish-harvesting areas of the lagoons.
INTRODUCTION
Vibrio parahaemolyticus, Vibrio vulnificus, and Vibrio cholerae are Gram-negative halophilic bacteria autochthonous to marine and estuarine environments and components of those ecosystems (1). These vibrios are recognized throughout the world as agents of gastroenteritis resulting from consumption of raw or undercooked seafood and serious infections caused by exposure of skin wounds to seawater (2).
Subpopulations of V. parahaemolyticus and V. vulnificus are potential agents of disease outbreaks. For example, enteropathogenic strains of V. parahaemolyticus produce a thermostable direct hemolysin (TDH) and/or a TDH-related hemolysin (TRH), and the genes tdh and trh code for TDH and TRH, respectively (3). Other factors are associated with virulence of V. vulnificus, including the vvhA gene encoding hemolytic cytolysin (4, 5). It is important to note that V. parahaemolyticus is the leading cause of bacterial human gastroenteritis associated with seafood consumption, especially in the United States and Japan (6, 7, 8). In the United States, V. vulnificus is responsible for 95% of all seafood-related deaths, with a mortality rate of around 50% (5). V. cholerae, the etiologic agent of cholera, has been detected in natural freshwater and brackish water worldwide, even in areas where no clinical cases of cholera have been reported (1). Most environmental isolates of V. cholerae are of the non-O1/non-O139 serotype but are capable of causing diarrheal outbreaks (7, 9). The presence and isolation of these three Vibrio spp. have also been documented to occur in coastal waters and shellfish-rearing areas in Europe, i.e., Spain (10), Italy (11, 12), Denmark (13), and Norway (14). These vibrios have been isolated in French coastal waters and shellfish at different locations along the English Channel, the Atlantic Ocean, and the Mediterranean Sea (15, 16, 17, 18).
Vibrios are less frequently associated with outbreaks of disease in Europe than in the United States and Asia, and specifically, the risk of V. parahaemolyticus infection is considered to be low (8, 19). In France, 100 cases of V. parahaemolyticus infection were reported in 2001, following consumption of mussels imported from Ireland (20). Since then, only sporadic cases of Vibrio infections have been described (21, 22).
From the perspective of vibrio ecology, the spatiotemporal distribution of vibrios has been linked to environmental factors, with temperature being one of the most important, because it is related to seasonal distribution in temperate coastal areas, with maximal abundance during summer through early fall (23, 24, 25, 26, 27, 28). Many studies have shown that these bacteria enter a viable-but-nonculturable state when water temperatures average less than 15°C and temperatures above 20°C favor their growth (23, 24, 25, 26, 28). Salinity is also an important parameter influencing the dynamics of human-pathogenic vibrios in aquatic systems. Lower salinity favors Vibrio growth and proliferation, particularly in brackish waters (29, 30, 31). Other biotic factors, such as phytoplankton and zooplankton populations, have also been found to be important in the ecology and dynamics of vibrios (32, 33, 34).
In a previous investigation, we studied the occurrence of V. parahaemolyticus, V. vulnificus, and non-O1/non-O139 V. cholerae in the coastal lagoons of southern France, showing three human-pathogenic Vibrio spp. to be present in water, shellfish, and sediment of those lagoons (15). The lagoons receive watershed input that is exchanged with seawater through small channels, the “grau.” Mediterranean rainfall is short in duration (a few days) but intense, resulting in flash floods, particularly in the autumn, bringing large volumes of freshwater into the lagoons and reducing their salinity (35, 36). Fishing and other recreational activities occur in some of the lagoons, and mollusk farming as well. The objective of the study reported here was to investigate the effect of Mediterranean autumnal flash floods on the occurrence and distribution of three pathogenic Vibrio species in these lagoons and channels.
MATERIALS AND METHODS
Area of study and sample collection.
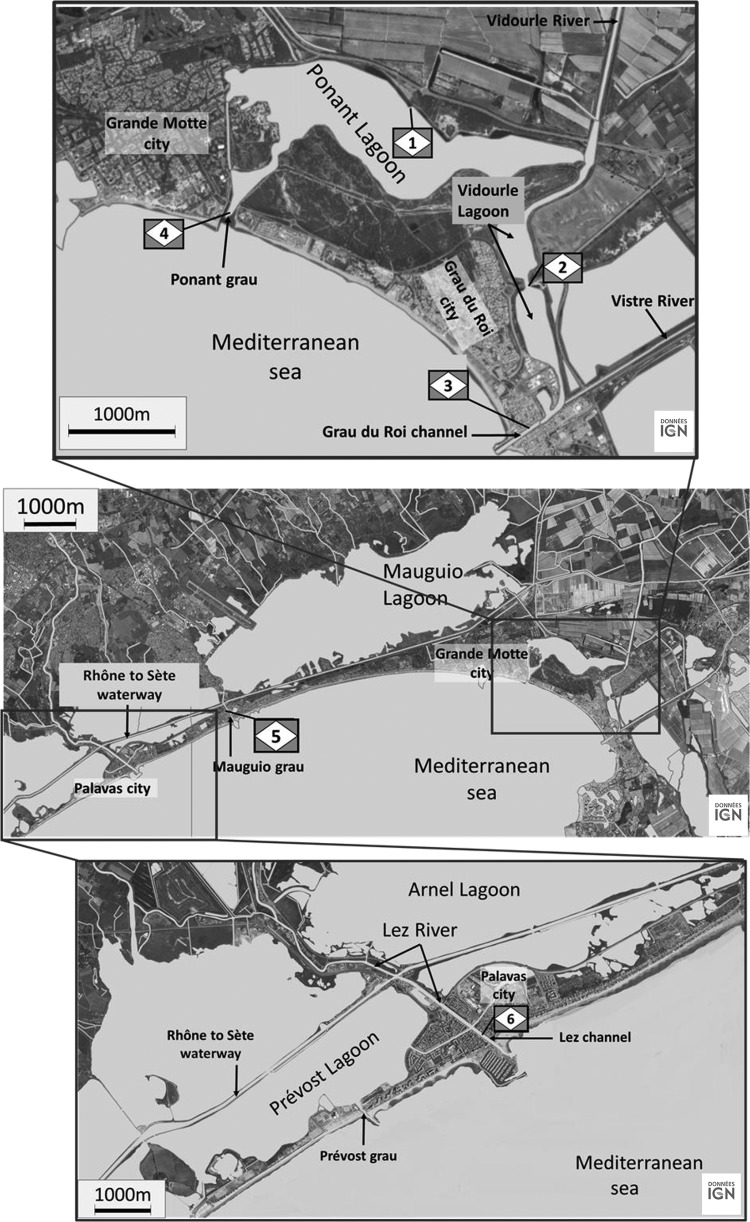
Figure 1 shows the location of sampling sites along the French Mediterranean coast (Languedoc area) characterized by a succession of lagoons (saline marshes). These lagoons are of different sizes and volumes and are connected to the sea by short channels, or “grau” (an Occitan term). All are connected by a navigable waterway linking the Rhône River to Sète (the Rhône-to-Sète waterway). In addition, coastal rivers flow into the lagoons (e.g., the Vidourle River), and others run through the lagoons by a channel to the sea and connect with the lagoons (e.g., the Lez River). Thus, these lagoons form a complex hydrosystem that receives input of seawater or freshwater, depending on rainfall, drought, and whether winds are from the north or south.
FIG 1.
Location of sampling sites along the French Mediterranean coast (Languedoc area). Station 1, Ponant Lagoon; station 2, Vidourle Lagoon; station 3, Grau du Roi channel; station 4, Ponant grau; station 5, Mauguio grau; station 6, Lez channel. Maps © IGN—2015.
Sampling stations were selected based on the hydrological complexity of the lagoons and their representation of various hydrological conditions. Station 1 is positioned in the Ponant Lagoon (2.0 km2 wide with a 2.7-m mean depth but 4 m deep in the center), and it is the site of some recreational activity. During floods, part of the Vidourle River is diverted into the Ponant Lagoon to protect the Grau du Roi city from flooding. Station 2 is situated in the Vidourle Lagoon (0.405-km2 area and 2-m mean depth), which receives the main flow of the Vidourle River. The Grau du Roi channel (station 3) is where the Vidourle and Vistre rivers flow to the sea. Station 4, Ponant grau, is where the Ponant Lagoon connects with the sea. The Mauguio Lagoon (31.7-km2 area and 0.8-m mean depth, with a depth of 1.3 m deep in the center) has controlled seawater entry and significantly lower salinity than the other lagoons. Station 5 is located in the Mauguio grau that communicates with the Mauguio Lagoon and the sea but also with the Rhône-to-Sète waterway. The Lez River crosses the Arnel and Prévost lagoons and exchanges water with these lagoons through small channels and the Rhône-to-Sète waterway. Station 6 is situated in the channel of Lez that leads to the sea.
Surface water samples (one sample of 5 liters) were collected at each station during the rainy season, in September, October, and November 2011 and in September 2012. Samples were transported in coolers (15 to 18°C) to the laboratory and processed within 4 h after collection.
Enumeration of V. parahaemolyticus, V. vulnificus, and V. cholerae by MPN employing real-time PCR.
Enumeration of the three Vibrio spp. in water samples was accomplished by enrichment in alkaline peptone water (APW) and using a combined most-probable-number (MPN)–real-time PCR method (15, 37). Water samples (1 liter, 100 ml, and 10 ml) were filtered in triplicate through 0.45-μm-pore-size nitrocellulose membranes (Whatman, GE Healthcare, Versailles, France), and the filters were incubated in APW at 41.5°C for 18 h. Smaller volumes (i.e., 1 ml of undiluted sample and 1 ml of 10-fold and 100-fold dilutions) were inoculated, in triplicate, directly into APW broth and incubated at 41.5°C for 18 h. After enrichment, bacterial DNA was extracted from 1 ml of the APW by boiling and used as the template (10-fold-dilution) for PCR.
Real-time PCR amplification of toxR (total V. parahaemolyticus) and tdh and trh2 (enteropathogenic V. parahaemolyticus), when the APW was positive for toxR, was run in a 10-μl volume containing 0.1 mM forward primer and 0.3 mM reverse primer, 0.3 mM TaqMan probe, 2 mM MgCl2, 5 μl of 2× Platinum quantitative PCR SuperMix-UDG (Invitrogen, Life Technologies, Carlsbad, CA), 1.05 μl of RNase-, DNase-, and protease-free water (5 Prime, Hamburg, Germany), and 2 μl of a DNA extract diluted 10-fold or 2 μl of RNase-, DNase-, and protease-free water for a negative control (15, 38). The PCR positive control was a plasmid with the amplicon of the target gene (2 μl of a 106 copies/μl). The presence of potential PCR inhibitors was detected simultaneously by real-time PCR amplification using the TaqMan kit exogenous internal positive control reagents (Invitrogen; Life Technologies, Carlsbad, CA). Real-time PCR thermal cycling was run using the LightCycler 480 (Roche Diagnostics, Mannheim, Germany). The thermal program for V. parahaemolyticus consisted of a 10-min denaturation step at 95°C, followed by 45 cycles of amplification at 95°C for 10 s and 60°C for 1 min.
Real-time PCR amplification for V. vulnificus and V. cholerae was performed using primers for the dnaJ gene and 16S-23S rRNA intergenic spacer region, respectively (38, 39, 40). The real-time PCR mixture of 10 μl was composed of 0.4 mM each primer for V. cholerae and 0.5 mM forward primer and 0.3 mM reverse primer for V. vulnificus plus 5 μl of 2× mixture of Sybr 250 buffer (Roche Diagnostics, Mannheim, Germany), 2.2 μl of RNase-, DNase-, and protease-free water (5 Prime, Hamburg, Germany), and 2 μl of a DNA extract diluted 10-fold or 2 μl of RNase-, DNase-, and protease-free water serving as a negative control. The PCR positive control was a plasmid with the amplicon of the target gene (2 μl at 106 copies/μl). The real-time PCR program was run in a LightCycler 480 (Roche), consisting of one cycle at 95°C for 10 min and 45 cycles at 95°C for 15 s, 63°C for 15 s, and 72°C for 15 s. The program terminated with a final dissociation curve analysis consisting of one step at 63°C for 30 s and gradual heating to 95°C.
Environmental parameters.
Temperature and salinity were recorded in situ using a WTW LF 196 conductimeter at the time of sampling. For chlorophyll a (Chl a) analysis, ca. 25 to 200 ml water was filtered under vacuum on GF/F filters (Whatman, GE Healthcare, Versailles, France) and stored at −20°C in glass tubes. Upon thawing, filters were sonicated in 5 ml of 90% acetone and incubated for 24 h in the dark at 4°C. After centrifugation, Chl a concentrations were measured in the supernatant by spectrofluorimetry (41). For analysis of particulate organic carbon and nitrogen (POC and PON, respectively), filtration (25 to 500 ml of water) was done using precombusted GF/F filters (450°C for 4 h), and samples were stored frozen at −80°C until analyzed using the Dumas combustion method (42) and the FlashEA 1112 organic elemental analyzer. To quantify suspended particulate matter (SPM), 25 to 500 ml of each water sample was filtered under vacuum on a GF/F membrane dried at 105°C for 2 h and weighed. The filters were washed with the same quantity of distilled water, dried overnight at 105°C, and weighed again. Data for river flow were provided by Banque Hydro (Eau-France) at three gauging stations situated upstream of the lagoon area on the Vidourle, Vistre, and Lez rivers (station Y3464010 at Marsillargues, station Y3534010 at Cailar, and station Y3204030 at Montpellier, respectively). Rainfall data were provided by Météo-France at three rain gauges representative of coastal rainfall located on catchments of the Vidourle, Vistre, and Lez rivers at Villevielle, Nîmes Courbessac, and Prades le Lez, respectively. River flow was recorded every 5 min during flooding and every hour outside the flood periods. Rainfall was also recorded hourly and daily, respectively. Mean daily river discharge and daily rainfall are calculated using these data.
Sampling strategy.
Sampling was organized according to when the weather forecast was for heavy rain. The objective was to sample the different stations before any freshwater input to the lagoons and until, during, and after rain in order to observe the effect on salinity. As the Vibrio quantification method is time-consuming, sampling and monitoring at the six stations were done only weekly. Sampling was done on the first day of the rain and depended on the duration and intensity of the rain.
The sites were sampled first on only on 1 day, 5 September, 2011, when the rain was brief. The second sampling began on 24 October 2011, when there was a forecast for a very strong atmospheric depression. Sampling occurred during significant rain and flooding on 27 October, 2 November, and 9 November. On 3 and 24 September 2012, sampling was done following a short rain.
Statistical analyses.
Data were analyzed using R software available at http://www.R-project.org and R package FactoMineR (43). The distribution of data was determined by Shapiro-Wilk test. Principal component analysis (PCA) was used to visualize relationships between Vibrio abundance and environmental variables, and Spearman's rank analysis was used to define statistically significant relationships.
RESULTS
Distribution of V. parahaemolyticus, V. vulnificus, and V. cholerae.
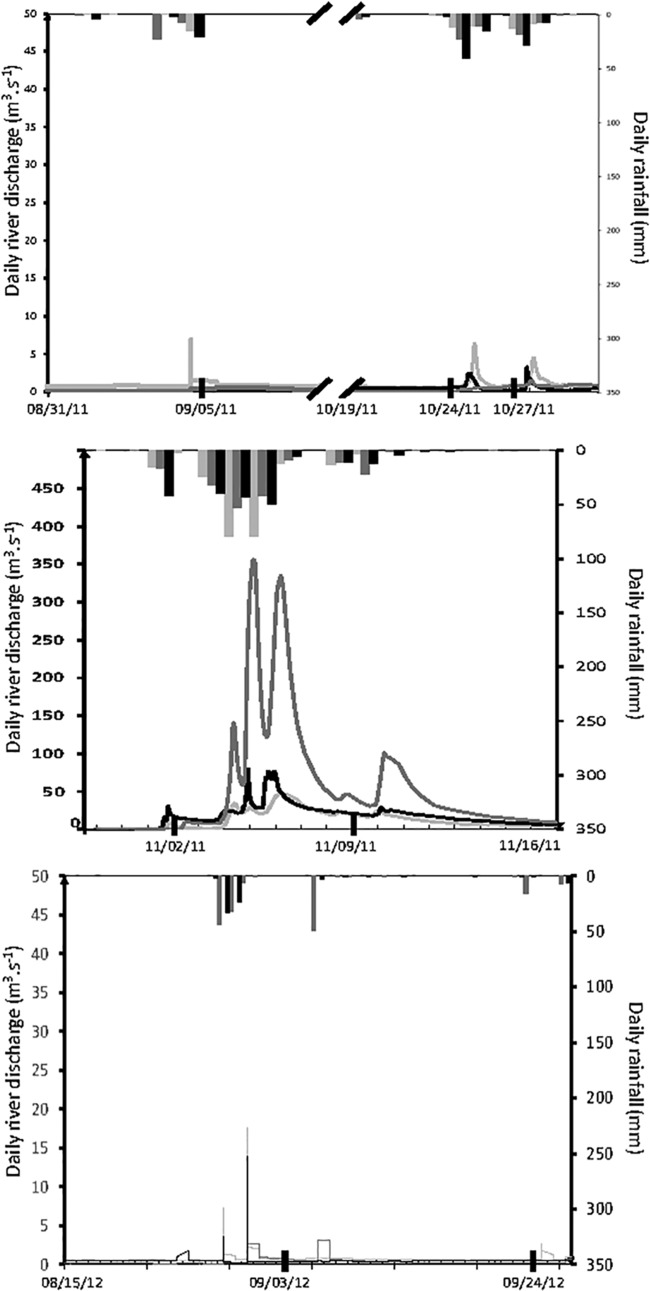
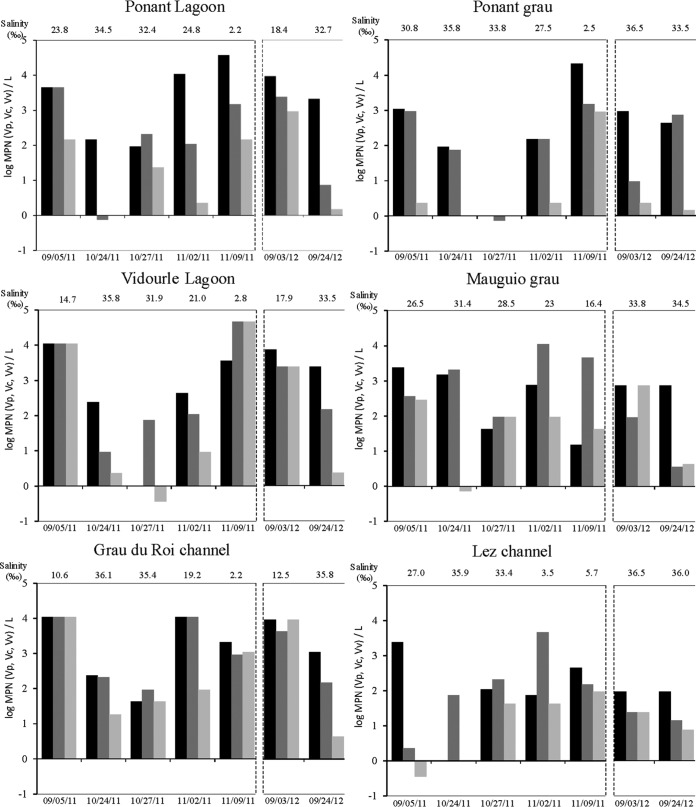
On 5 September 2011, sampling was performed after a short rainfall that lasted from 3 to 4 September (Fig. 2). Rainfall was 32 mm at Villevielle (Vidourle watershed), 16 mm at Nîmes Courbessac (Vistre watershed), and 24 mm at Montpellier (Lez watershed). During this period of time, the flows of the Vidourle, Vistre, and Lez rivers increased slightly from 0.1 to 0.56, 0.8 to 1.3, and 0.45 to 7.8 m3/s, respectively. The three sampling stations were more directly influenced by coastal river flow (Ponant Lagoon, Vidourle Lagoon, and Grau du Roi channel), with lower salinities (23.8, 14.7, and 10.6‰, respectively), than the three stations by seawater (Ponant grau, Mauguio grau, and Lez channel) (30.8, 26.5, and 27‰, respectively) (Fig. 3). Vibrio concentrations were higher in the Ponant Lagoon, Vidourle Lagoon, and Grau du Roi channel (4.6 × 103 to 1.1 × 104 MPN/liter V. parahaemolyticus and V. vulnificus and 1.5 × 102 to 1.1 × 104 MPN/liter V. cholerae) than in the Ponant grau, Mauguio grau, and Lez channel (1.1 × 103 to 2.4 × 103 MPN/liter V. parahaemolyticus, 2.3 to 9.3 × 102 MPN/liter V. vulnificus, and 0.36 to 2.9 × 102 MPN/liter V. cholerae) (Fig. 3). Water temperatures ranged from 23 to 23.9°C at all sampling sites.
FIG 2.
Daily rainfall and river discharge versus time: The hydrograph (lines) was combined with the hyetograph (vertical bars): dark gray lines/bars represent Vidourle stations, light gray lines/bars represent Vistre stations, and black lines/bars represent Lez stations. The heavy black bars on the date axis indicate the dates of sampling.
FIG 3.
Concentrations of Vibrio parahaemolyticus (Vp [black bars]), V. vulnificus (Vv [dark gray bars]), and V. cholerae (Vc [light gray bars]) in water samples collected from the Ponant Lagoon, Ponant grau, Vidourle Lagoon, Mauguio grau, Grau du Roi channel, and Lez channel in September, October, and November 2011 and in September 2012. Salinity is indicated on the graphs. The temperature was stable (see the text) and hence is not shown.
The sampling for October to November 2011 was done during a flood period (Fig. 2). Water temperatures ranged from 15 to 17°C during this time. Three intense rain showers (24 to 25 and 27 to 28 October and 1 November) with 15 to 56 mm accumulated rainfall measured at each rain gauge were responsible for three successive increases in river flow: up 10, 9, and 24 m3/s for the Vidourle, Vistre, and Lez rivers, respectively. At the time of sampling (24 and 27 October), water salinity ranged from 31.4 to 36.1‰ at all sampling sites, except Mauguio grau, on 27 October (28.5‰) (Fig. 3). These salinities were high and close to that measured on the seacoast (36.5‰). On these dates, V. parahaemolyticus, V. vulnificus, and V. cholerae concentrations varied from 0 to 1.5 × 103 MPN/liter, 0.7 to 2.1 × 103 MPN/liter, and 0 to 93 MPN/liter, respectively (Fig. 3).
Heavy rainstorms (3 to 6 November) (between 135 and 195 mm accumulated rainfall measured at each rain gauge) generated a major flood. The flows of the Vidourle, Vistre, and Lez rivers corresponded to discharges of up to 350, 47, and 80 m3/s, respectively (Fig. 2). The last rain showers on 8 to 9 November resulted in accumulated rainfalls of 16 and 32 mm, as measured at each rain gauge, and high river discharge lasted until 16 November. This was followed by a rapid decrease in salinity, reaching between 19 and 27‰ at all sampling sites on 2 November, except at the Lez channel (3.5‰), and values of between 2.2 and 5.7‰ on 9 November, except at the Mauguio grau (16.4‰) (Fig. 3). V. parahaemolyticus, V. vulnificus, and V. cholerae concentrations increased abruptly during the 6 days after the first intense rains, reaching between 74 and 1.1 × 104 MPN/liter, 1.1 × 102 and 1.1 × 104 MPN/liter, and 2.3 and 93 MPN/liter, respectively, on 2 November (Fig. 3). On 9 November, the Vibrio concentrations were up to 3.8 × 104 MPN/liter for V. parahaemolyticus in the Ponant Lagoon and 2.1 × 104 MPN/liter in the Ponant grau and up to 4.6 × 104 MPN/liter for V. vulnificus and V. cholerae in the Vidourle Lagoon.
The dynamics of Vibrio spp. in September 2012 showed similar patterns to those observed during the autumn of 2011. Short rain storms from 27 to 30 August 2012 (between 58 and 82 mm of accumulated rainfall at each rain gauge) generated brief increases in river discharge from 0.9 to 17 m3/s, from 0.2 to 10 m3/s, and from 0.5 to 27 m3/s for the Vidourle, Vistre, and Lez rivers, respectively (Fig. 2). Water temperatures ranged from 17 to 20°C. Concentrations of vibrios ranged from 9.3 × 102 to 9.3 × 103 MPN/liter at sites where the salinity was lower (Ponant Lagoon, 18.4‰; Vidourle Lagoon, 17.9‰; and the Grau du Roi channel, 12.5‰) and from 2.3 to 9.3 × 102 MPN/liter at sites where the salinity was high (Ponant grau, 36.5‰; Mauguio grau, 33.8‰; and Lez channel, 36.5‰) (Fig. 3).
There was very little rain before the 24 September 2012 sampling (Fig. 2). Water salinity ranged from 32.7 to 36‰ and water temperature from 17 to 20°C at all sampling sites. V. parahaemolyticus, V. vulnificus, and V. cholerae concentrations varied from 93 to 2.4 × 103 MPN/liter, 3.6 to 7.4 × 102 MPN/liter, and 1.4 to 7.5 MPN/liter, respectively (Fig. 3).
Enteropathogenic tdh+ V. parahaemolyticus was not detected in any of the water samples collected at the stations included in this study. Enteropathogenic trh2+ V. parahaemolyticus was detected in 36 of 42 water samples collected at all sites, at concentrations ranging from 0.9 to 4.6 × 103 MPN/liter (Table 1). These data did not show any detectible pattern with respect to site, date, or salinity.
TABLE 1.
Concentration of enteropathogenic trh2+ V. parahaemolyticus in water samples collected in September to November 2011 and September 2012 from the Ponant Lagoon, Ponant grau, Vidourle Lagoon, Mauguio grau, Grau du Roi channel, and Lez channel
| Sampling date (mo/day/yr) |
trh2+
V. parahaemolyticus concn (MPN/liter) in samples from: |
|||||
|---|---|---|---|---|---|---|
| Ponant Lagoon | Vidourle Lagoon | Grau du Roi channel | Ponant grau | Mauguio grau | Lez channel | |
| 09/05/11 | 240 | 1,100 | 240 | 240 | 93 | 240 |
| 10/24/11 | 110 | 46 | 0 | 1.1 | 240 | 0 |
| 10/27/11 | 24 | 0 | 0.36 | 0 | 0.36 | 0 |
| 11/02/11 | 2,400 | 430 | 4,600 | 0 | 7.5 | 0.92 |
| 11/09/11 | 0.92 | 0.92 | 2.3 | 1.4 | 4.3 | 9.3 |
| 09/03/12 | 43 | 93 | 430 | 23 | 24 | 15 |
| 09/24/12 | 38 | 240 | 230 | 240 | 240 | 46 |
Relationship between V. parahaemolyticus, V. vulnificus, and V. cholerae and environmental parameters.
Concentrations of chlorophyll a (Chl a), suspended particulate matter (SPM), particulate organic carbon (POC), and particulate organic nitrogen (PON) were relatively high and varied regardless of site or date, from 1 to 48 μg/liter, 2.5 to 83 g/liter, 2 to 64 mg/liter, and 0.25 to 8.7 mg/liter, respectively. The pH was stable at ca. 8 (7.64 to 8.17).
Principal component analysis (PCA) of environmental parameters and Vibrio abundance, except SPM which was highly autocorrelated with POC and PON, explained 46.13% of the total variance (Fig. 4). Abundance of V. vulnificus, salinity, and pH were principal factors contributing to axis 1. POC, temperature, and abundance of trh2+ V. parahaemolyticus contributed to axis 2. A highly significant negative correlation was observed between abundance of V. parahaemolyticus (r = −0.55, P < 0.001), V. cholerae (r = −0.71, P < 0.001), and V. vulnificus (r = −0.51, P < 0.001) and salinity, whereas a highly significant positive correlation was observed between abundance of enteropathogenic trh2+ V. parahaemolyticus and V. parahaemolyticus (r = 0.53, P < 0.001) and temperature (r = 0.57, P < 0.001).
FIG 4.
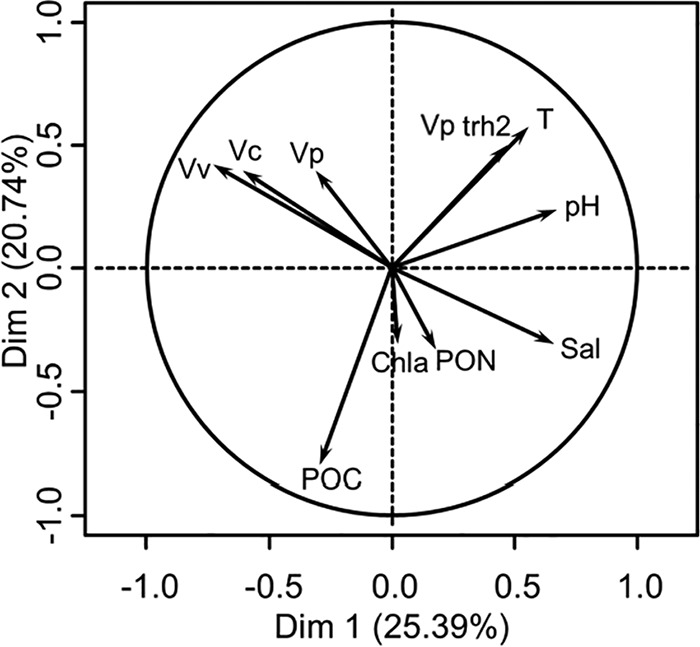
Principal component analysis of data. Vp, V. parahaemolyticus; Vv, V. vulnificus; Vc, V. cholerae; Vp trh2, trh2+ V. parahaemolyticus; T, temperature; Sal, salinity; Chla, chlorophyll a; POC, particulate organic carbon; PON, particulate organic nitrogen; Dim, dimension.
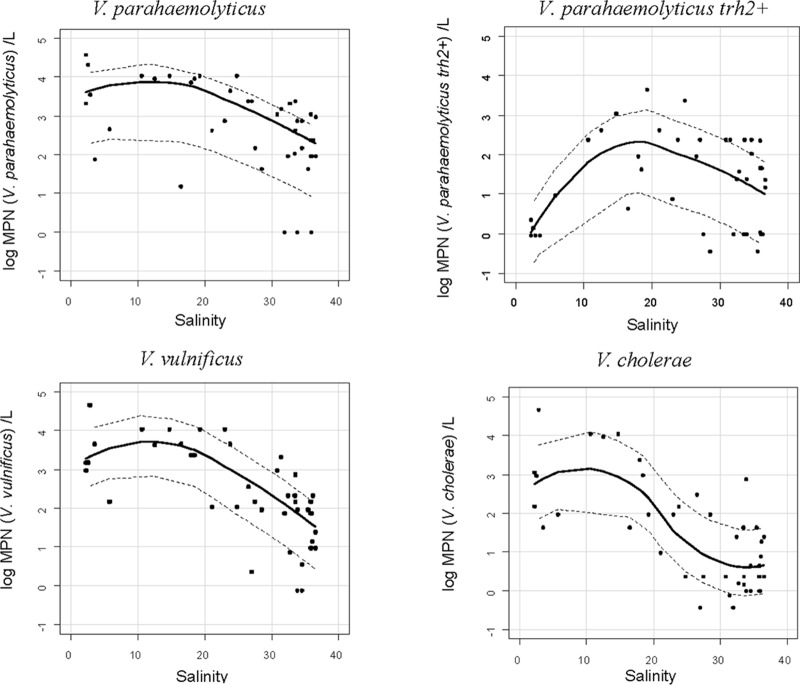
The results of the statistical analysis showed that salinity was a determining factor explaining the dynamics of the three Vibrio spp. Furthermore, when the log concentration of the vibrios was plotted against salinity, an adverse effect of salinity for all vibrios was detected: namely, their concentration decreased when salinity increased (Fig. 5). The relationships were not linear, and smoothed curves showed the most favorable salinity for these Vibrio spp. varied according to Vibrio species between 10 and 20‰ for total V. parahaemolyticus, ∼18‰ for enteropathogenic trh2+ V. parahaemolyticus, 10 to 15‰ for V. vulnificus, and 5 to 12‰ for V. cholerae. A salinity higher than 20‰ caused a drop in concentration of all three of the vibrios but most dramatically for V. cholerae: i.e., at a salinity higher than 30‰, the concentration of V. cholerae was less than 10 MPN/liter, and for V. vulnificus and V. parahaemolyticus, the concentrations were between 10 and 100 MPN/liter and 100 and 1,000 MPN/liter, respectively.
FIG 5.
Log concentrations of V. parahaemolyticus, V. vulnificus, V. cholerae, and trh2+ V. parahaemolyticus versus salinity measured in situ at the time of sampling.
DISCUSSION
Temperature has been shown by many investigators to be a major factor explaining the population dynamics of vibrios in coastal marine ecosystems (15, 16, 23, 27, 32, 33, 44). Water temperatures recorded during sampling done in this study (15 to 24°C) were favorable for both the presence and multiplication of V. parahaemolyticus, V. vulnificus, and V. cholerae, as has been reported by other investigators (23, 24, 25, 26, 28). The concentrations of the three Vibrio spp. ranged from less than 10 MPN/liter to more than 104 MPN/liter.
The highest concentrations were detected when the salinity was between 10 and 20‰ for V. parahaemolyticus, 10 and 15‰ for V. vulnificus, and 5 and 12‰ for V. cholerae. V. vulnificus and V. cholerae showed less tolerance to high salinity than V. parahaemolyticus (45). It had been shown previously that salinity has a significant and highly negative effect on the dynamics of these three Vibrio spp. in aquatic systems (30). The results of this study are consistent with those of the meta-analysis of Takemura et al. (46) and the observations of many other environmental studies showing a strong correlation between the presence of these Vibrio spp. and temperature and salinity (1, 25, 26, 47, 58). Interestingly, salinity was not found to be significantly correlated with the concentration of enteropathogenic trh2+ V. parahaemolyticus, with the highest numbers at a salinity of ca. 18‰. Temperature was positively correlated with this pathogenic Vibrio and consistent with observations along the French Atlantic coast reported by Deter et al. (16).
Other environmental parameters measured in this study (Chl a, SPM, POC, and PON) did not show significant correlation with the concentration of any of the three vibrios, even though these parameters have been shown by other investigators to influence the dynamics of these bacteria (32, 46). It may be possible that, in this study, temperature and salinity have a stronger measurable effect than the other parameters.
In the lagoons, temperature alone did not modulate the concentration of these vibrios. It is concluded that in the absence of a significant change in temperature, salinity becomes the determining factor controlling the number of vibrios, with low salinity related to increased numbers of V. parahaemolyticus, V. vulnificus, and V. cholerae in the lagoons during the summer and autumn months. This effect of salinity on Vibrio concentrations has been demonstrated for V. parahaemolyticus in the rias of Galicia, Spain, by Martinez-Urtiza et al. (10), for V. vulnificus in Barnegat Bay, NJ, by Randa et al. (31), and for V. cholerae in the Mississippi Sound by Griffitt and Grimes (29).
Climate anomalies, such as El Niño, and increase in sea surface temperature (SST)—two consequences of climate change—have been linked to cholera epidemics (48) and to the spread of V. parahaemolyticus and V. vulnificus in coastal marine systems, increasing the risk of Vibrio illnesses in the latter case (49). SST anomalies were concluded to explain V. parahaemolyticus outbreaks in Alaska in 2004, Galicia, Spain, in 1999, and Peru in 1997 and the lengthening of the summer season to V. vulnificus illnesses in the United States (50, 51). Exceptional weather events, such Hurricane Irene in the Chesapeake Bay in 2011 (52) or the storm Xynthia in the Pertuis Breton (Atlantic coast, France) in 2010 (53), have been related to ecosystem disruption and, thereby, to changes in the concentration of V. parahaemolyticus and V. vulnificus or the emergence of enteropathogenic tdh+ V. parahaemolyticus, respectively.
Our results show that an abrupt decrease in salinity caused by heavy rainfall and major flooding notably favored growth of V. parahaemolyticus, V. vulnificus, and V. cholerae and was linked to their rapid proliferation in the brackish waters of the Languedocian lagoons. These results clearly indicate that flood events can strongly affect the abundance of these vibrios, as already shown for components of the microbial food web in the Thau Lagoon (35). To our knowledge, this is the first time that such rapid proliferation of vibrios has been shown in situ following coastal flooding and sudden decrease in salinity.
Prediction of a changing climate portends an increase in the frequency and/or intensity of heavy rainstorms (54). Our results suggest that such storm events will result in increased presence and abundance of these three Vibrio spp. in the lagoons of southern France. Some of these lagoons are sites of significant shellfish production, and previous research has shown the presence of V. parahaemolyticus and V. vulnificus in mussels and clams (15). It is clear that shellfish harvesting from the lagoons where the salinity has significantly decreased may contain dangerously high numbers of human-pathogenic Vibrio concentrations posing a significant risk to public health.
Monitoring temperature and salinity in lagoons would allow mapping of the two parameters using spatial interpolation. It would then be possible to build a prediction system for the risk posed by V. parahaemolyticus, V. vulnificus, and V. cholerae of the non-O1/non-O139 serotype in shellfish-breeding zones, independent of bacteriological analysis, as has been done for the Chesapeake Bay (55, 56, 57). Future work that includes monitoring these parameters and modeling the presence of pathogenic vibrios surely will provide preventive measures to be developed for management of shellfish safety.
ACKNOWLEDGMENTS
This work was supported by funding provided by the GIS “Climat Environnement Société”,“ by the Observatoire Hommes-Milieux (OHM)” “Littoral Méditerrannéen,” and by the Programme National EC2CO “Ecosphère Continentale et Côtière” and by a doctoral fellowship (K.E.) from CNRS and Université Montpellier 2. National Institutes of Health grant no. R01AI039129 is also acknowledged.
REFERENCES
- 1.Colwell RR, Kaper J, Joseph SW. 1977. Vibrio cholerae, Vibrio parahaemolyticus and other vibrios: occurrence and distribution in Chesapeake Bay. Science 198:394–396. doi: 10.1126/science.198.4315.394-a. [DOI] [PubMed] [Google Scholar]
- 2.Pruzzo C, Huq A, Colwell RR, Donelli G. 2005. Pathogenic Vibrio species in the marine and estuarine environment, p 217–252. In Belkin S, Colwell RR (ed), Oceans and health: pathogens in the marine environment. Springer, New York, NY. [Google Scholar]
- 3.Iida T, Park KS, Honda T. 2006. Vibrio parahaemolyticus, p 340–348. In Thompson F, Austin B, Swings J (ed), The biology of vibrios. ASM Press, Washington, DC. [Google Scholar]
- 4.Jones MK, Oliver JD. 2009. Vibrio vulnificus: disease and pathogenesis. Infect Immun 77:1723–1733. doi: 10.1128/IAI.01046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oliver JD. 2006. Vibrio vulnificus, p 349–366. In Thompson F, Austin B, Swings J (ed), The biology of vibrios. ASM Press, Washington, DC. [Google Scholar]
- 6.Daniels NA, MacKinnon L, Bishop R, Altekruse S, Ray B, Hammond RM, Thompson S, Wilson S, Bean NH, Griffin PM, Slutsker L. 2000. Vibrio parahaemolyticus infections in the United States, 1973-1998. J Infect Dis 181:1661–1666. doi: 10.1086/315459. [DOI] [PubMed] [Google Scholar]
- 7.Rippey SR. 1994. Infectious diseases associated with molluscan shellfish consumption. Clin Microbiol Rev 7:419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su YC, Liu C. 2007. Vibrio parahaemolyticus: a concern of seafood safety. Food Microbiol 24:549–558. doi: 10.1016/j.fm.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Dutta D, Chowdhury G, Pazhani GP, Guin S, Dutta S, Ghosh S, Rajendran K, Nandy RK, Mukhopadhyay AK, Bhattacharya MK, Mitra U, Takeda Y, Nair GB, Ramamurthy T. 2013. Vibrio cholerae non-O1, non-O139 serogroups and cholera-like diarrhea, Kolkata, India. Emerg Infect Dis 19:464–467. doi: 10.3201/eid1903.121156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez-Urtaza J, Lozano-Leon A, Varela-Pet J, Trinanes J, Pazos Y, Garcia-Martin O. 2008. Environmental determinants of the occurrence and distribution of Vibrio parahaemolyticus in the rias of Galicia, Spain. Appl Environ Microbiol 74:265–274. doi: 10.1128/AEM.01307-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barbieri E, Falzano L, Fiorentini C, Pianetti A, Baffone W, Fabbri A, Matarrese P, Casiere A, Katouli M, Kuhn I, Mollby R, Bruscolini F, Donelli G. 1999. Occurrence, diversity, and pathogenicity of halophilic Vibrio spp. and non-O1 Vibrio cholerae from estuarine waters along the Italian Adriatic coast. Appl Environ Microbiol 65:2748–2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vezzulli L, Pezzati E, Moreno M, Fabiano M, Pane L, Pruzzo C, VibrioSea Consortium. 2009. Benthic ecology of Vibrio spp. and pathogenic Vibrio species in a coastal Mediterranean environment (La Spezia Gulf, Italy). Microb Ecol 58:808–818. doi: 10.1007/s00248-009-9542-8. [DOI] [PubMed] [Google Scholar]
- 13.Hoi L, Larsen JL, Dalsgaard I, Dalsgaard A. 1998. Occurrence of Vibrio vulnificus biotypes in Danish marine environments. Appl Environ Microbiol 64:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bauer A, Ostensvik Y, Florvag M, Ormen O, Rorvik LM. 2006. Occurrence of Vibrio parahaemolyticus, V. cholerae, and V. vulnificus in Norwegian Blue mussels (Mytilus edulis). Appl Environ Microbiol 72:3058–3061. doi: 10.1128/AEM.72.4.3058-3061.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cantet F, Hervio-Heath D, Caro A, Le Mennec C, Monteil C, Quemere C, Jolivet-Gougeon A, Colwell RR, Monfort P. 2013. Quantification of Vibrio parahaemolyticus, Vibrio vulnificus and Vibrio cholerae in French Mediterranean coastal lagoons. Res Microbiol 164:867–874. doi: 10.1016/j.resmic.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deter J, Lozach S, Veron A, Chollet J, Derrien A, Hervio-Heath D. 2010. Ecology of pathogenic and non-pathogenic Vibrio parahaemolyticus on the French Atlantic coast. Effects of temperature, salinity, turbidity and chlorophyll a. Environ Microbiol 12:929–937. doi: 10.1111/j.1462-2920.2009.02136.x. [DOI] [PubMed] [Google Scholar]
- 17.Hervio-Heath D, Colwell RR, Derrien A, Robert-Pillot A, Fournier JM, Pommepuy M. 2002. Occurrence of pathogenic vibrios in coastal areas of France. J Appl Microbiol 92:1123–1135. doi: 10.1046/j.1365-2672.2002.01663.x. [DOI] [PubMed] [Google Scholar]
- 18.Robert-Pillot A, Guénolé A, Lesne J, Delesmont R, Fournier JM, Quilici ML. 2004. Occurrence of the tdh and trh genes in Vibrio parahaemolyticus isolates from waters and raw shellfish collected in two French coastal areas and from seafood imported into France. Int J Food Microbiol 91:319–325. doi: 10.1016/j.ijfoodmicro.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Geneste C, Dab W, Cabanes PA, Vaillant V, Quilici ML, Fournier JM. 2000. Les vibrioses noncholériques en France: cas identifiés de 1995 à 1998 par le Centre National de Référence. Bull Epidemiol Hebdo 9:38–40. [Google Scholar]
- 20.Hervio Heath D, Zidane M, Le Saux J-C, Lozach S, Vaillant V, Le Guyader S, Pommepuy M. 2005. Toxi-infections alimentaires collectives liées à la consommation de moules contaminées par Vibrio parahaemolyticus: enquête environnementale. Bull Epidemiol AFSSA 17:1–2. [Google Scholar]
- 21.Quilici ML, Robert-Pillot A. 2011. Infections à vibrions non cholériques. Med Maladies Infect 8:1–12. [Google Scholar]
- 22.Quilici ML, Robert-Pillot A, Picart J, Fournier JM. 2005. Pandernic Vibrio parahaemolyticus O3:K6 spread, France. Emerg Infect Dis 11:1148–1149. doi: 10.3201/eid1107.041008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blackwell KD, Oliver JD. 2008. The ecology of Vibrio vulnificus, Vibrio cholerae, and Vibrio parahaemolyticus in North Carolina estuaries. J Microbiol 46:146–153. doi: 10.1007/s12275-007-0216-2. [DOI] [PubMed] [Google Scholar]
- 24.Colwell RR, Grimes DJ. 2000. Nonculturable microorganisms in the environment. ASM Press, Washington, DC. [Google Scholar]
- 25.DePaola A, Nordstrom JL, Bowers JC, Wells JG, Cook DW. 2003. Seasonal abundance of total and pathogenic Vibrio parahaemolyticus in Alabama oysters. Appl Environ Microbiol 69:1521–1526. doi: 10.1128/AEM.69.3.1521-1526.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Motes ML, DePaola A, Cook DW, Veazey JE, Hunsucker JC, Garthright WE, Blodgett RJ, Chirtel SJ. 1998. Influence of water temperature and salinity on Vibrio vulnificus in Northern Gulf and Atlantic Coast oysters (Crassostrea virginica). Appl Environ Microbiol 64:1459–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pfeffer CS, Hite MF, Oliver JD. 2003. Ecology of Vibrio vulnificus in estuarine waters of eastern North Carolina. Appl Environ Microbiol 69:3526–3531. doi: 10.1128/AEM.69.6.3526-3531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roszak DB, Colwell RR. 1987. Survival strategies of bacteria in the natural environment. Microbiol Rev 51:365–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Griffitt KJ, Grimes DJ. 2013. Abundance and distribution of Vibrio cholerae, V. parahaemolyticus, and V. vulnificus following a major freshwater intrusion into the Mississippi Sound. Microb Ecol 65:578–583. doi: 10.1007/s00248-013-0203-6. [DOI] [PubMed] [Google Scholar]
- 30.Hsieh JL, Fries JS, Noble RT. 2008. Dynamics and predictive modelling of Vibrio spp. in the Neuse River Estuary, North Carolina, USA. Environ Microbiol 10:57–64. doi: 10.1111/j.1462-2920.2007.01429.x. [DOI] [PubMed] [Google Scholar]
- 31.Randa MA, Polz MF, Lim E. 2004. Effects of temperature and salinity on Vibrio vulnificus population dynamics as assessed by quantitative PCR. Appl Environ Microbiol 70:5469–5476. doi: 10.1128/AEM.70.9.5469-5476.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson CN, Bowers JC, Griffitt KJ, Molina V, Clostio RW, Pei S, Laws E, Paranjpye RN, Strom MS, Chen A, Hasan NA, Huq A, Noriea I, Nicholas F, Grimes DJ, Colwell RR. 2012. Ecology of Vibrio parahaemolyticus and Vibrio vulnificus in the coastal and estuarine waters of Louisiana, Maryland, Mississippi, and Washington (United States). Appl Environ Microbiol 78:7249–7257. doi: 10.1128/AEM.01296-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson CN, Flowers AR, Noriea NF III, Zimmerman AM, Bowers JC, DePaola A, Grimes DJ. 2010. Relationships between environmental factors and pathogenic vibrios in the northern Gulf of Mexico. Appl Environ Microbiol 76:7076–7084. doi: 10.1128/AEM.00697-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martinez-Urtaza J, Blanco-Abad V, Rodriguez-Castro A, Ansede-Bermejo J, Miranda A, Rodriguez-Alvarez MX. 2012. Ecological determinants of the occurrence and dynamics of Vibrio parahaemolyticus in offshore areas. ISME J 6:994–1006. doi: 10.1038/ismej.2011.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pecqueur D, Vidussi F, Fouilland E, Floc'h EL, Mas S, Roques C, Salles C, Tournoud M-G, Mostajir B. 2011. Dynamics of microbial planktonic food web components during a river flash flood in a Mediterranean coastal lagoon. Hydrobiologia 673:13–27. doi: 10.1007/s10750-011-0745-x. [DOI] [Google Scholar]
- 36.Perrin JL, Tournoud MG. 2009. Hydrological processes controlling flow generation in a small Mediterranean catchment under karstic influence. Hydrol Sci J 54:1125–1140. doi: 10.1623/hysj.54.6.1125. [DOI] [Google Scholar]
- 37.Luan X, Chen J, Liu Y, Li Y, Jia J, Liu R, Zhang X-H. 2008. Rapid quantitative detection of Vibrio parahaemolyticus in seafood by MPN-PCR. Curr Microbiol 57:218–221. doi: 10.1007/s00284-008-9177-x. [DOI] [PubMed] [Google Scholar]
- 38.Tall A, Teillon A, Boisset C, Delesmont R, Touron-Bodilis A, Hervio-Heath D. 2012. Real-time PCR optimization to identify environmental Vibrio spp. strains. J Appl Microbiol 113:361–372. doi: 10.1111/j.1365-2672.2012.05350.x. [DOI] [PubMed] [Google Scholar]
- 39.Chun J, Huq A, Colwell RR. 1999. Analysis of 16S-23S rRNA intergenic spacer regions of Vibrio cholerae and Vibrio mimicus. Appl Environ Microbiol 65:2202–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nhung PH, Ohkusu K, Miyasaka J, Sun XS, Ezaki T. 2007. Rapid and specific identification of 5 human pathogenic Vibrio species by multiplex polymerase chain reaction targeted to dnaJ gene. Diagn Microbiol Infect Dis 59:271–275. doi: 10.1016/j.diagmicrobio.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 41.Neveux J, Lantoine F. 1993. Spectrofluorometric assay of chlorophylls and phaeopigments using the least squares approximation technique. Deep Sea Res 40:1747–1765. doi: 10.1016/0967-0637(93)90030-7. [DOI] [Google Scholar]
- 42.Dumas JBA. 1831. Procédés de l'analyse organique. Ann Chim Phys 47:198–205. [Google Scholar]
- 43.Lê S, Josse J, Husson F. 2008. FactoMineR: an R package for multivariate analysis. J Stat Softw 25:1–18. [Google Scholar]
- 44.Parveen S, Hettiarachchi KA, Bowers JC, Jones JL, Tamplin ML, Mckay R, Beatty W, Brohawn K, DaSilva LV, DePaola A. 2008. Seasonal distribution of total and pathogenic Vibrio parahaemolyticus in Chesapeake Bay oysters and waters. Int J Food Microbiol 128:354–361. doi: 10.1016/j.ijfoodmicro.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 45.Naughton LM, Blumerman SL, Carlberg M, Boyd EF. 2009. Osmoadaptation among Vibrio species and unique genomic features and physiological responses of Vibrio parahaemolyticus. Appl Environ Microbiol 75:2802–2810. doi: 10.1128/AEM.01698-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takemura AE, Chien DM, Polz ME. 2014. Associations and dynamics of Vibrionaceae in the environment, from the genus to the population level. Front Microbiol 5:38. doi: 10.3389/fmicb.2014.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wright AC, Hill RT, Johnson JA, Roghman MC, Colwell RR, Morris JG. 1996. Distribution of Vibrio vulnificus in the Chesapeake Bay. Appl Environ Microbiol 62:717–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Constantin de Magny G, Murtugudde R, Sapiano MRP, Nizam A, Brown CW, Busalacchi AJ, Yunus M, Nair GB, Gil AI, Lanata CF, Calkins J, Manna B, Rajendran K, Bhattacharya MK, Huq A, Sack RB, Colwell RR. 2008. Environmental signatures associated with cholera epidemics. Proc Natl Acad Sci U S A 105:17676–17681. doi: 10.1073/pnas.0809654105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vezzulli L, Colwell RR, Pruzzo C. 2013. Ocean warming and spread of pathogenic vibrios in the aquatic environment. Microb Ecol 65:817–825. doi: 10.1007/s00248-012-0163-2. [DOI] [PubMed] [Google Scholar]
- 50.Baker-Austin C, Stockley L, Rangdale R, Martinez-Urtaza J. 2010. Environmental occurrence and clinical impact of Vibrio vulnificus and Vibrio parahaemolyticus: a European perspective. Environ Microbiol Rep 2:7–18. doi: 10.1111/j.1758-2229.2009.00096.x. [DOI] [PubMed] [Google Scholar]
- 51.Martinez-Urtaza J, Bowers JC, Trinanes J, DePaola A. 2010. Climate anomalies and the increasing risk of Vibrio parahaemolyticus and Vibrio vulnificus illnesses. Food Res Int 43:1780–1790. doi: 10.1016/j.foodres.2010.04.001. [DOI] [Google Scholar]
- 52.Shaw KS, Jacobs JM, Crump BC. 2014. Impact of Hurricane Irene on Vibrio vulnificus and Vibrio parahaemolyticus concentrations in surface water, sediment, and cultured oysters in the Chesapeake Bay, MD, USA. Front Microbiol 5:204. doi: 10.3389/fmicb.2014.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hervio-Heath D, Constantin de Magny G, Deter J, Teillon A, Lozach S, Derrien A. 2012. A survey of Vibrio parahaemolyticus in French Atlantic coastal waters, p 11–16. In Pathogenic Vibrio spp. in Northern European Waters: International Symposium. Bundesanstalt für Gewässerkunde, Koblenz, Germany. [Google Scholar]
- 54.Milly PCD, Wetherald RT, Dunne KA, Delworth TL. 2002. Increasing risk of great floods in a changing climate. Nature 415:514–517. doi: 10.1038/415514a. [DOI] [PubMed] [Google Scholar]
- 55.Constantin de Magny G, Long W, Brown CW, Hood RR, Huq A, Murtugudde R, Colwell RR. 2009. Predicting the distribution of Vibrio spp. in the Chesapeake Bay: a Vibrio cholerae case study. Ecohealth 6:378–389. doi: 10.1007/s10393-009-0273-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Urquhart EA, Hoffman MJ, Murphy RR, Zaitchik BF. 2013. Geospatial interpolation of MODIS-derived salinity and temperature in the Chesapeake Bay. Remote Sens Environ 135:167–177. doi: 10.1016/j.rse.2013.03.034. [DOI] [Google Scholar]
- 57.Urquhart EA, Zaitchik BF, Waugh DW, Guikema SD, Del Castillo CE. 2014. Uncertainty in model predictions of Vibrio vulnificus response to climate variability and change: a Chesapeake Bay case study. PLoS One 9:e98256. doi: 10.1371/journal.pone.0098256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Froelich BA, Williams TC, Noble RT, Oliver JD. 2012. Apparent loss of Vibrio vulnificus from North Carolina oysters coincides with a drought-induced increase in salinity. Appl Environ Microbiol 78:3885–3889. doi: 10.1128/AEM.07855-11. [DOI] [PMC free article] [PubMed] [Google Scholar]