Abstract
LinA is the first enzyme of the microbial degradation pathway of a chlorinated insecticide, hexachlorocyclohexane (HCH), and mediates the dehydrochlorination of α-, γ-, and δ-HCH. Its two variants, LinA type 1 and LinA type 2, which differ at 10 out of 156 amino acid residues, have been described. Their activities for the metabolism of different HCH isomers differ considerably but overall are high for γ-HCH, moderate for α-HCH, low for δ-HCH, and lacking for β-HCH. Here, we describe the characterization of a new variant of this enzyme, LinA type 3, whose gene was identified from the metagenome of an HCH-contaminated soil sample. Its deduced primary structure in the region spanning amino acid residues 1 to 147 of the protein exhibits 17 and 12 differences from LinA type 1 and LinA type 2, respectively. In addition, the residues GIHFAPS, present at the region spanning residues 148 to 154 in both LinA type 1 and LinA type 2, are deleted in LinA type 3.The activity of LinA type 3 for the metabolism of δ-HCH is several orders of magnitude higher than that of LinA type 1 or LinA type 2 and can be useful for improvement of the metabolism of δ-HCH.
INTRODUCTION
Technical hexachlorocyclohexane (t-HCH) is a chlorinated insecticide consisting of α (60 to 70%)-, β (5 to 12%)-, γ (10 to 12%)-, δ (6 to 10%)-, and ε (3 to 4%)-isomers (1, 2). These isomers differ from each other in the spatial orientation of chlorine atoms on the cyclohexane ring. Their solubility, reactivity, and toxicity also differ considerably (1, 2). Several microorganisms that mediate the biodegradation of one or more of these isomers under aerobic conditions have been characterized (3, 4). The available information suggests that the degradation of γ-HCH is initiated by the activity of the enzyme HCH dehydrochlorinase (LinA), which mediates its dehydrochlorination in two steps, i.e., the formation of pentachlorocyclohexene (PCCH) in the first step, which is converted into 1,3,4,6-tetrachloro-1,4-cyclohexadiene (1,4-TCDN) in the second step (3, 4). The TCDN formed is further metabolized by the serial activity of the enzymes LinB, LinC, LinD, LinE, LinF, and LinGHJ to form products that enter the tricarboxylic acid (TCA) cycle and support cell growth (3, 4). The biodegradation of α-HCH, although not studied in detail, is expected to follow a similar pathway (4). The degradation pathway for β-HCH, however, is distinct from these pathways and is initiated by the enzyme LinB. Briefly, it is first metabolized to 2,3,4,5,6-pentachlorocyclohexanol, which is converted into 2,3,5,6-tetrachlorocyclohexanediol in the second step (5, 6). These metabolites have also been referred to as B1 and B2, respectively (7).
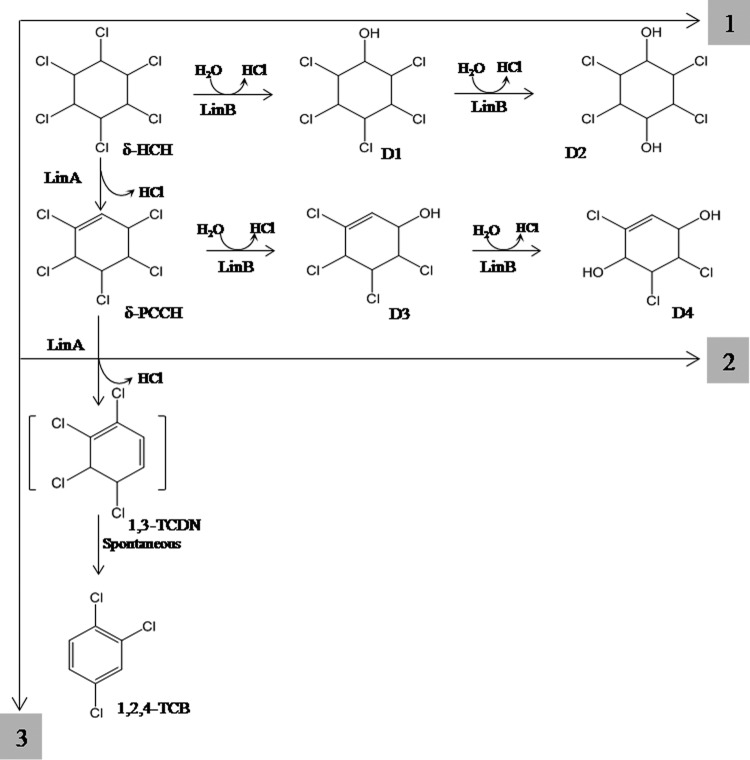
Degradation of δ-HCH, which carries two adjacent trans-diaxial HCl pairs (8), however, can proceed by three different pathways (Fig. 1). One pathway is similar to that for γ-HCH, where LinA has been proposed to cause anti-1,2-elimination of HCl by abstraction of H+ at C-2 or C-6, leading to the formation of either δ-PCCH-1 or δ-PCCH-2, which undergoes a syn-1,4-elimination (H+ abstraction at C-6) by the activity of LinA to form 1,2,5,6-tetrachlorocyclohexa-1,3-diene (8). A syn-1,2-elimination reaction leads to the formation of a mixture of 1,2,4- or 1,2,3-trichlorobenzene (TCB) (8). The second pathway is similar to the β-HCH pathway, wherein its two-step dehalogenation by LinB leads to the formation of the corresponding pentachlorocyclohexanol and tetrachlorocyclohexanediol, also referred to as D1 and D2, respectively (7). In the third pathway, δ-HCH undergoes dehydrochlorination by LinA to δ-PCCH, which is metabolized further by LinB in two steps to the metabolites 2,3,4,5-tetrachloro-5-cyclohexene-1-ol and 2,3,5-trichloro-5-cyclohexene-1,4-diol, also referred to as D3 and D4, respectively (7, 8).
FIG 1.
Three possible pathways for metabolism of δ-HCH, including its conversion by LinB into the metabolites D1 (δ-2,3,4,5,6-pentachlorocyclohexanol) and D2 (δ-2,3,5,6-tetrachlorocyclohexane-1,4-diol) (1); its conversion by LinA into δ-PCCH, followed by conversion by LinB into D3 (δ-3,4,5,6-tetrachloro-2-cyclohexenol) and D4 (δ-3,5,6-trichloro-2-cyclohexene-1,4-diol) (2); and its conversion by LinA into 1,3,4,6-tetrachlorocyclohexa-1,4-diene (1,4-TCDN), followed by spontaneous metabolism to 1,2,4-trichlorobenzene (3).
Two variants of LinA, LinA type 1 and LinA type 2, have been characterized (9). While LinA type 1, also referred to as LinA-UT26 or LinA2 in some studies (8, 10), was first described for Sphingobium japonicum UT26 (11) and subsequently described for several other organisms (3, 4), the gene for LinA type 2 was discovered in a soil metagenome (9). Both proteins consist of 156 amino acid residues, but they differ from each other at 10 positions (Fig. 2). LinA type 2 is distinct from another variant, LinA1, which was characterized in Sphingobium indicum B90 and consists of 154 amino acids (4, 8, 10). LinA1 is 99% identical to LinA type 2 in the region from amino acids 1 to 148 but differs completely from it in the C-terminal region (residues 149 to 156) due to a replacement of the amino acids IHFAPSGA by ALLQKS (9). LinA type 1 and LinA type 2 exhibit substantial differences in enantioselectivity and thermostability (9). Thus, while LinA type 1 exhibits enantioselectivity for the transformation of (−)-α-HCH, LinA type 2 shows a preference for (+)-α-HCH (9). Also, while LinA type 1 undergoes rapid denaturation after it is preincubated at 45°C, LinA type 2 remains stable for up to 8 h (9, 12). The activities of both variants are high with γ-HCH, moderate with α-HCH, and low with δ-HCH, and no activity was observed with β-HCH (12). In this report, we describe the identification and characterization of a new variant, LinA type 3, whose gene was obtained from the metagenome of an HCH-contaminated soil sample.
FIG 2.
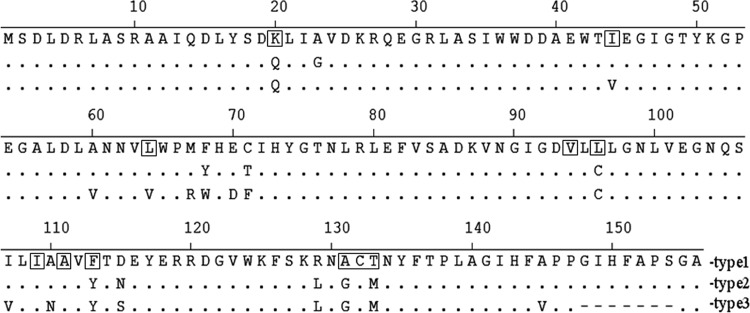
Primary structures of the various LinA enzymes described in this study. The amino acid residues that are identical to LinA type 1 are shown as dots, and those absent in other LinA enzymes are shown as dashes. The structure of LinA type 3 was deduced from the nucleotide sequence of its gene, identified in this study (GenBank accession number KF534798), and those of type 1 (accession number D90355) and type 2 (accession number EU863871) were reported previously. The boxed residues represent those identified previously (18) for their role in degradation and their enantiomeric preference for various HCH isomers.
MATERIALS AND METHODS
Soil samples.
Several soil samples were collected from the vicinity of an HCH-manufacturing unit, India Pesticides Ltd., located near Lucknow, India. Levels of HCH residues in these soils exhibited large variations in terms of both their total concentrations and the relative levels of the different isomers. Six samples, S1 to S6, whose ∑-HCH levels, i.e., the sum of α-, β-, γ-, and δ-HCH levels, were 159.6, 33.3, 16.4, 0.2, 0.3, and 0.9 mg g−1 soil, respectively, were selected for this study.
PCR amplification of linA genes and nucleotide sequencing.
Metagenomic DNA was extracted from the soil samples, and PCR amplification of linA genes from this DNA was done as described previously (12), except that the Platinum Taq thermostable high-fidelity DNA polymerase enzyme (Invitrogen, Carlsbad, CA, USA) was used. The forward primer (5′-CATATGAGTGATCTAGACAGACTTGCAA-3′) consisted of 1 to 25 nucleotides from the 5′ end of linA type 1 with an NdeI site at its 5′ end (underlined). The reverse primer (5′-CTCGAGCGATTTTTGCAACAGAGC-3′) consisted of nucleotides 1 to 21 from the 5′ end of IS6100, which was shown previously to be present downstream of the termination codon of several linA genes (3), and an overhang of the XhoI site at its 5′ end (underlined). The selected amplified DNA product was eluted from the agarose gel and ligated with the TA cloning vector pGEM-T Easy (Promega, Madison, WI, USA), and Escherichia coli DH5α cells were transformed as described previously (12). Inserts from the clones were sequenced on an ABI Prism-3100 genetic analyzer (Applied Biosystems, Foster City, CA, USA) by using universal forward and reverse M13 primers. Alignment of the sequences was done by using the Clustal W algorithm (DNAStar Inc., WI, USA).
Expression of selected LinA enzymes.
Inserts in the clones that were carrying the desired linA genes, i.e., identical to linA type 1 (GenBank accession number D90355), linA type 2 (accession number EU863871), and the newly identified linA type 3, were reamplified by using primers that contained NdeI and XhoI sites in the forward primer described above and a reverse primer that consisted of nucleotides 448 to 471 of linA type 1, respectively. These inserts were ligated with the pET-28a(+) vector (Novagen, Darmstadt, Germany) and cloned into E. coli BL21(DE3) cells, as described previously (9). After induction with 0.1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) and cell lysis, the expressed proteins present in the clear supernatant were purified by using a Ni-nitrilotriacetic acid (NTA) Superflow column (Qiagen, Hilden, Germany) at 4°C (9). Aliquots (0.5 ml) containing 0.5 mg protein in 50 mM sodium phosphate buffer (pH 8.0) and 15% glycerol were stored at −20°C for further studies.
Chemical synthesis of β-, γ-, and δ-PCCH.
γ-PCCH and δ-PCCH were synthesized according to a previously reported method (13). Briefly, 100 mg γ-HCH or δ-HCH, dissolved in 10 ml acetonitrile, was mixed with 5 ml 0.1 M NaOH and incubated at 40°C. After 20 min, the content was extracted in n-hexane. The formed PCCHs were purified by column chromatography using silica gel 60 (63 to 100 μm; Merck, Darmstadt, Germany) as the stationary phase. The solvents n-hexane–acetone (98:2) and cyclohexane-acetone (98:2) were used as the mobile phases for γ-PCCH and δ-PCCH, respectively. For the preparation of β-PCCH, however, a method described previously by Buser and Müller (14) was used. Briefly, 100 mg α-HCH, dissolved in 5 ml pyridine, was incubated at 65°C for 14 h. The contents were then extracted with n-hexane and washed serially with 1 N HCl, 5% NaHCO3, and water. The formed β-PCCH was purified by column chromatography, as described above, using n-hexane–ethyl acetate (95:5) as the mobile phase.
Enzymatic synthesis of γ-PCCH enantiomers.
For the preparation of γ-PCCH1, 10 mg of chemically synthesized γ-PCCH–racemate was incubated with the LinΑ type 1 enzyme (5 μg ml−1) for 30 min in a 100-ml reaction mixture containing 50 mM Tris-Cl (pH 8.0), 2% dimethyl sulfoxide (DMSO), and 10% glycerol. The remaining γ-PCCH-1, along with the formed trichlorobenzenes (TCBs), was extracted with n-hexane. γ-PCCH-2 was prepared in the same manner, except that the enzyme used was LinA type 2 (40 μg ml−1), and the reaction time was 60 min. The γ-PCCH enantiomers were purified from the TCBs by column chromatography, as described above.
Analytical methods. (i) Kinetics of α-HCH, γ-HCH, and δ-HCH conversion by LinA type 1, LinA type 2, and LinA type 3 enzymes.
The conversion of selected HCH isomers by LinA variants was analyzed by monitoring the consumption of a particular substrate at 30°C in a solution containing 50 mM Tris-HCl buffer (pH 8.0), 10% glycerol, 10% DMSO, and the HCH substrate at two initial concentrations (250 and 500 μM). The reaction was monitored by withdrawing 0.5-ml samples from the reaction mixture and stopped by mixing the samples with 0.5 ml of n-hexane–acetone (in a ratio of 8/1) containing 1,2,3-trichloropropane as an internal standard. After extraction, samples were analyzed by using gas chromatography-mass spectrometry (GC-MS). For measurement of the levels of LinA type 2 with δ-HCH and LinA type 3 with α-HCH and γ-HCH, the reaction mixture volume was reduced to 1 ml, and the progress of the reaction was monitored by withdrawing 1-μl samples, followed by direct injection into a GC-MS instrument without extraction. For experiments where the activity of the mixture of LinA and LinB was to be evaluated, reactions were performed by using 340 μM δ-HCH, 6 nM LinA, and/or 3 nM LinB (same as LinBMI [6], cloned and purified in the laboratory), as described above. The thermostability of different LinA enzymes was evaluated after preincubation at 60°C for different time periods, as described previously (9).
(ii) Analysis of residual HCH.
Residual HCH isomers were quantified by gas chromatography (9). Briefly, suitable aliquots were run on a capillary gas chromatograph (Clarus-500; PerkinElmer, Waltham, MA, USA) equipped with a low-bleed column (TraceGOLD TG-1MS, 30 m by 0.25 mm by 0.25 μm; Thermo Scientific) and an electron capture detector. Run conditions were as follows: the initial oven temperature was held at 150°C for 1 min, increased to 200°C at a rate of 10°C min−1, and further increased to 250°C at 5°C min−1, where it was held for another 10 min. Injector and detector temperatures were fixed at 250°C and 350°C, respectively. Nitrogen gas was used as the carrier at a flow rate of 1.0 ml min−1.
For enantiomer analysis, an Agilent 7890A gas chromatograph that was equipped with a chiral column (CP-Chirasil-Dex-CB, 25 m by 0.32 mm by 0.25 μm) and an electron capture detector was used (15). The initial oven temperature was 80°C (2-min hold), which was increased to 180°C at a rate of 2°C min−1 and then held for 10 min. The injector and detector were fixed at 250°C.
(iii) GC-MS analysis.
Residual HCH isomers were quantified by using a gas chromatograph (catalog number 7890; Agilent, USA) equipped with a ZB-5 capillary column (30 m by 0.25 mm by 0.25 μm; Phenomenex) and connected to a mass spectrometer (catalog number 5975C; Agilent, USA). One microliter of the sample was injected into a split-splitless inlet at 250°C, at a split ratio of 1:50. The temperature program was an isothermal step at 40°C for 1 min, followed by an increase to 250°C at a rate of 20°C/min and a hold for 2 min. The flow rate of the carrier gas (He) was 1.2 ml min−1. The mass spectrometer was operated in scan mode (30 to 320 atomic mass units [amu]). The temperatures of the ion source and the GC-MS interface were 230°C and 250°C, respectively.
(iv) Data analysis and statistics.
For each reaction, full time courses measured for two different starting concentrations of the substrate were simultaneously fitted to a three-step model (see the equation below) by using the dynamic kinetic simulation program KinTek Global Kinetic Explorer version 4.0 (KinTek, USA). During data fitting based on numerical integration of rate equations from an input model, the program sought parameters that resulted in minimum χ2 value using nonlinear regression based on the Levenberg-Marquardt method. The standard error (SE) was calculated from the covariance matrix during nonlinear regression for global fitting of the data for both experiments for each reaction. In addition to SE values, more rigorous analysis of the variation of these kinetic parameters was accomplished by confidence contour analysis using FitSpace Explorer (KinTek, USA). For this analysis, the lower and upper limits for each parameter were derived from the threshold in the confidence contours at 1.2 times the minimum χ2 value (16). The steady-state kinetic constants were derived as follows, where the turnover number is calculated as kcat = k2, the specificity constant is calculated as kcat/Km = k2/(k−1/k1), and the product dissociation constant is calculated as Ki = k−3/k3:
where E is enzyme, S is substrate, P is product, E.S is enzyme-substrate complex, and E.P is enzyme-product complex.
Nucleotide sequence accession number.
The nucleotide sequence of linA type 3 has been submitted to GenBank under accession number KF534798.
RESULTS AND DISCUSSION
Several LinA variants are present in soil metagenomes.
Southern hybridization by the linA probe revealed the presence of several cross-reactive products that were formed from the different soil samples after PCR amplification. The major ones included a 2.0-kb product from S1; 1.8- and 1.7-kb products from S2; 1.8- and 1.7-kb products from S3; 1.8- and 1.5-kb products from S4; and 1.8-, 1.7-, 1.0-, and 0.5-kb products from both S5 and S6 (data not shown). Nucleotide sequencing of several randomly selected clones, obtained from each of the amplified fragments that were cross-reactive with the linA probe, revealed that their size heterogeneity was due to the presence of IS6100 0, 444, 1,084, 1,251, 1,352, or 1,567 nucleotides downstream of the linA gene. The sequence of the region between linA and IS6100 in all the clones was >99% identical to that of the corresponding region of the archetypal strain UT26 (data not shown). Sequencing of the linA regions in these amplified products revealed the presence of three major types of linA variants, i.e., either >95% identical to previously described linA type 1 or linA type 2 (9) or belonging to a new group, linA type 3. The linA type 3 gene differed from linA type 1 in the region spanning nucleotides 1 to 440 at 21 positions, which led to changes in 17 amino acid residues, i.e., K20Q, I44V, A60V, L64V, M67R, F68W, E70D, C71F, L96C, I107V, A110N, F113Y, D115S, R129L, A131G, T133M, and A145V, in the deduced primary structure of the LinA type 3 protein (Fig. 2). Similarly, linA type 3 differed from linA type 2 at 14 nucleotide positions and caused differences in 12 amino acid residues, i.e., K20Q, I44V A60V, L64V, M67R, Y68W, E70D, T71F, I107V, A110N, N115S, and A145V, in the LinA type 3 protein (Fig. 2). In addition to these differences, the nucleotides present at positions 445 to 465 in linA type 1 and linA type 2 are deleted in linA type 3 (Fig. 2) and correspondingly lead to deletions of the encoded 7 amino acid residues GIHFAPS present at positions 148 to 154 in the LinA type 1 and LinA type 2 proteins. However, amino acid residues D25 and H73, previously described to form a catalytic dyad in LinA, were found to be conserved in LinA type 3.
Activity of the LinA variants with various HCH isomers.
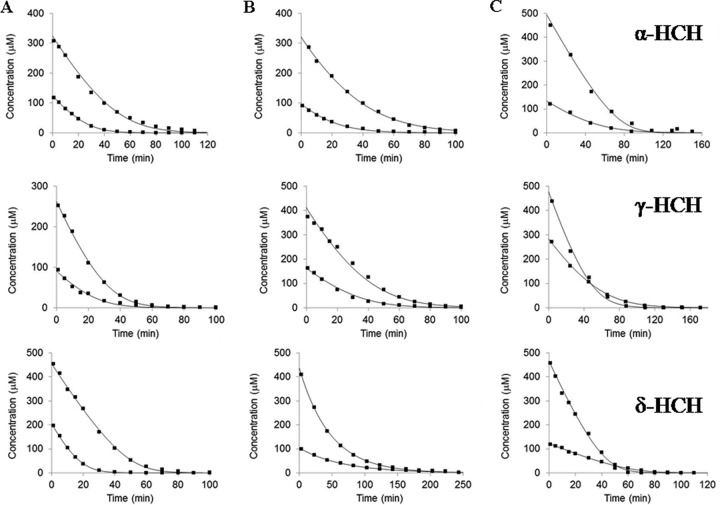
All three variants, i.e., LinA type 1, LinA type 2, and LinA type 3, were able to mediate the transformation of α-HCH, γ-HCH, and δ-HCH (Fig. 3). Their catalytic efficiencies (kcat/Km) for dehydrochlorination of α-HCH were comparable (Table 1). The reactions were accompanied by the formation of β-PCCH and 1,2,4-trichlorobenzene (see Fig. S1 in the supplemental material). Similarly to α-HCH, all three variants were also able to mediate the dechlorination of γ-HCH. The catalytic efficiency (kcat/Km) was highest for LinA type 1 (86.2 ± 10.1 min−1 μM−1), followed by LinA type 2 (59.3 ± 6.7 min−1 μM−1) and LinA type 3 (59.3 ± 6.7 min−1 μM−1). The activity of LinA type 1 or LinA type 2 with γ-HCH led to the formation of γ-PCCH and a mixture of 1,2,4-TCB and 1,2,3-TCB. With LinA type 3, however, formation of 1,3,5-TCB was also observed after the reaction, in addition to the formation of γ-PCCH, 1,2,4-TCB, and 1,2,3-TCB (see Fig. S1 in the supplemental material). Similarly to α-HCH and γ-HCH, all the LinA proteins mediated the metabolism of δ-HCH to δ-PCCH and 1,2,4-TCB, along with trace amounts of 1,2,3-TCB (see Fig. S1 in the supplemental material). The catalytic efficiency (kcat/Km) of LinA type 3 for δ-HCH was 50 times higher than that of LinA type 1 and several orders of magnitude higher than that of LinA type 2 (Table 1).
FIG 3.
Metabolism of α-HCH, γ-HCH, and δ-HCH by LinA type 1 (A), type 2 (B), and type 3 (C). In each case, two concentrations of the substrate and enzyme were used. Thus, 125 and 325 μM α-HCH were incubated with 0.74 and 1.01 μM LinA type 1, respectively; 90 and 260 μM γ-HCH were incubated with 0.201 and 0.324 μM LinA type 1, respectively; and both 205 and 455 μM δ-HCH were incubated with 5.07 μM LinA type 1. Similarly, 95 and 320 μM α-HCH were incubated with 0.53 and 0.84 μM LinA type 2, respectively; 170 and 410 μM γ-HCH were incubated with 1.38 and 2.97 μM LinA type 2, respectively; and 105 and 435 μM δ-HCH were incubated with 36 and 91 μM LinA type 2, respectively. Likewise, 130 and 495 μM α-HCH were incubated with 0.21 and 0.42 μM LinA type 3, respectively; 280 and 480 μM γ-HCH were incubated with 4.51 and 11.27 μM LinA type 3, respectively; and 120 and 465 μM δ-HCH were incubated with 0.04 and 0.21 μM LinA type 3, respectively.
TABLE 1.
Kinetic constants for LinA enzymes with HCH isomers
| LinA variant type | Mean kcat/Km (min−1 μM−1) ± SE |
Mean kcat (min−1) ± SE |
||||
|---|---|---|---|---|---|---|
| α-HCH | γ-HCH | δ-HCH | α-HCH | γ-HCH | δ-HCH | |
| 1 | 0.52 ± 0.02 | 86.2 ± 10.1 | 7.18 ± 0.35 | 7.0 ± 0.5 | 21.1 ± 2.6 | 2.1 ± 0.1 |
| 2 | 0.35 ± 0.02 | 59.3 ± 6.7 | 0.0006 ± 0.0001 | 10.1 ± 0.8 | 3.6 ± 0.5 | 0.18 ± 0.02 |
| 3 | 0.21 ± 0.02 | 18.4 ± 1.5 | 343.7 ± 15.8 | 20.6 ± 2.9 | 1.1 ± 0.1 | 57.4 ± 2.7 |
The observed metabolism of δ-HCH is in agreement with data from previous studies (8, 17) where this isomer was found to be a substrate for the LinA enzymes, due to the presence of a 1,2-biaxial HCl pair(s) that was necessary for the dehydrochlorination activity of LinA (13). The high activity of LinA type 3 for δ-HCH, compared to that of LinA type 1 or LinA type 2, could be due to any one or more of the 17 amino acid residues in LinA type 1 or the 12 residues in LinA type 2 that are different in LinA type 3 (Fig. 2). Incidentally, these different amino acid residues include 7 residues, i.e., K20Q, I44V, L64V, L96C, F113Y, A131G, and T133M, that are among the 11 residues that were identified previously for their role in the metabolism of various isomers (18). The high activity of LinA type 3 for δ-HCH occurs at the cost of its activity for γ-HCH, which correspondingly is considerably lower than those of LinA type 1 and LinA type 2 (Table 1). The low activity of LinA type 3 for γ-HCH could be the reason for the nonidentification of this enzyme in previous studies, where the majority of the LinA enzymes were obtained from organisms that were isolated by selective enrichment on γ-HCH (3, 4).
Enantioselectivity analysis revealed that LinA type 1 and LinA type 2 exhibited a preference for the metabolism of the (−) and (+) enantiomers, respectively (see Fig. S1 in the supplemental material), as reported previously (9). However, both α-HCH enantiomers were metabolized by LinA type 3 at comparable rates. The reaction results in the formation of β-PCCH-1 and β-PCCH-2 enantiomers, but β-PCCH-2 was observed as the major product (65%) (see Fig. S1 in the supplemental material). Similarly, after the reaction of γ-HCH with LinA type 1 and LinA type 3, γ-PCCH-2 was the major product formed, i.e., 95% and 60%, respectively. The reaction of LinA type 2 with γ-HCH, however, led to the formation of a racemic mixture of γ-PCCH-1 and γ-PCCH-2 (see Fig. S1 in the supplemental material). Enantioselectivity was also observed for the metabolism of δ-HCH by LinA type 1 and LinA type 3, and δ-PCCH-2 was the major product (>95%) formed. Reactions with LinA type 2, however, led to the formation of a racemic mixture of δ-PCCH-1 and δ-PCCH-2 (see Fig. S1 in the supplemental material).
The observed enantioselectivity of LinA type 1 and LinA type 2 is in agreement with previously reported findings for α-HCH (9, 10), γ-HCH (13), and δ-HCH (8), but new information about the selectivity of LinA type 3 with all these substrates has become available in this study. It has been proposed that LinA type 1 enantiotopically differentiates between two 1,2-biaxial HCl pairs present on γ-HCH and gives rise to a single PCCH enantiomer, 1,3(R),4(S),5(S),6(R)-PCCH, which is then converted to 1,2,4-TCB via the presumed but instable intermediate (3R,6R)-1,3,4,6-tetrachlorocyclohexa-1,4-diene. However, the formation of a mixture of γ-PCCH-1 and γ-PCCH-2 by the enzymes LinA type 2 and LinA type 3 suggests that these variants can recognize both biaxial pairs of γ-HCH and lead to the formation of the two γ-PCCH enantiomers, which can be explained by different binding orientations in the enzyme active site.
Enantioselective metabolism of δ-HCH by the variant LinA type 3 to δ-PCCH-2, whose further metabolism to TCB is extremely slow, can be useful for the enzymatic preparation of δ-PCCH-2. Likewise, the LinA type 1 and LinA type 2 enzymes can be used for obtaining nearly pure preparations of γ-PCCH-1 and γ-PCCH-2 enantiomers, respectively, as was done for this study.
Activity of the LinA variants with different PCCHs.
Enantioselectivity analysis revealed that chemically synthesized β-PCCH, γ-PCCH, and δ-PCCH consisted of racemic mixtures of two enantiomers in each case. The activities of all three LinA enzymes with chemically synthesized β-PCCH revealed that the reactions in either case were accompanied by the formation of 1,2,4-TCB (see Fig. S2 in the supplemental material). During this activity, while LinA type 1 exhibited a preference for the metabolism of β-PCCH-1, LinA type 2 and LinA type 3 used β-PCCH-2 as the preferred substrate (see Fig. S2 in the supplemental material). The results for LinA type 1 and LinA type 2 are in agreement with data from previous studies (9, 13).
Unlike with β-PCCH, 1,2,4-TCB and 1,2,3-TCB were formed by the activities of LinA type 1 and LinA type 2 on γ-PCCH. However, additional formation of 1,3,5-TCB was observed after the activity of LinA type 3, as was also seen when γ-HCH was used as the substrate, as described above. Enantioselectivity analysis revealed that while LinA type 1 exhibited a preference for the metabolism of γ-PCCH-2, LinA type 2 and type 3 used γ-PCCH-1 as the preferred substrate (see Fig. S2 in the supplemental material). Studies with purified γ-PCCH-1 or γ-PCCH-2 revealed that the formation of 1,3,5-TCB, in addition to 1,2,4-TCB and 1,2,3-TCB, after the activity of LinA type 3 was due to the metabolism of γ-PCCH-2 and not γ-PCCH-1 (data not shown).
Similarly to β-PCCH and γ-PCCH, the three LinA variants also mediate the transformation of δ-PCCH, and 1,2,4-TCB was formed as the major metabolite, along with trace amounts of 1,2,3-TCB. Enantioselectivity analysis revealed that while LinA type 1 exhibited a preference for the metabolism of δ-PCCH-2, LinA type 2 and LinA type 3 used δ-PCCH-1 preferentially (see Fig. S2 in the supplemental material).
While the observations for LinA type 1 and LinA type 2 are in agreement with previously reported findings (8), information on LinA type 3 was obtained in this study.
Thermostability of the different LinA enzymes.
The three LinA variants exhibited considerable differences in their thermostability (see Fig. S3 in the supplemental material). Thus, after preincubation at 60°C for 2 h, while the residual activity of LinA type 1 was only ∼15% of its initial activity, nearly 100% of the initial activity of LinA type 2 and 50% of the initial activity of LinA type 3 were observed. The thermostability of an enzyme has important implications for its potential role in biocatalytic applications (19), and features responsible for conferring this property to different enzymes have been studied extensively (20). The observed moderate thermostability of LinA type 3 could be due to the presence of Q20 in its structure, which is among the two amino acid residues, i.e., Q20 and G23, that were identified previously (9) as conferring stability to LinA proteins. However, a role for other different amino acid residues cannot be ruled out at this stage.
Metabolism of δ-HCH by LinA and LinB.
While the metabolism of δ-HCH to δ-PCCH and TCBs occurs with different LinA enzymes, as described above and also as described in previous studies (8, 17), δ-HCH can also be metabolized by LinB to the metabolites D1 (δ-2,3,4,5,6-pentachlorocyclohexanol) and D2 (δ-2,3,5,6-tetrachlorocyclohexane-1,4-diol) (Fig. 1). In the present study, when δ-HCH was incubated with a mixture of LinA type 1 and LinB, formation of the metabolite D2 was observed (see Fig. S4 in the supplemental material), suggesting that the activity of LinB dominated over that of LinA in this reaction. However, when the same reaction was done by using LinA type 3, a shift in the product profile was observed, and the metabolite D4 was formed, which results from the activity of LinB on δ-PCCH (Fig. 1). The formation of different metabolites, D2 during the metabolism of δ-HCH with a combination of the enzymes LinA type 1 and LinB but D4 with a combination of the enzymes LinA type 3 and LinB, suggests that different pathways for the metabolism of δ-HCH might operate in contaminated soils, depending on the nature of the LinA proteins present.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a grant from the Department of Biotechnology, Government of India. N.S. thanks the Council of Scientific and Industrial Research, India, for the fellowship support. The RECETOX research infrastructure was supported by the projects of the Czech Ministry of Education (LO1214 and LM2011028).
We are grateful to Hana Moskalíková of Masaryk University, Brno, Czech Republic, for her expert technical assistance in doing the kinetic experiments with the enzymes. We also thank Yuji Nagata of Tohoku University, Japan, for his valuable comments on the manuscript.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.01683-15.
REFERENCES
- 1.Willett KL, Ulrich EM, Hites RA. 1998. Differential toxicity and environmental fates of hexachlorocyclohexane isomers. Environ Sci Technol 32:2197–2207. doi: 10.1021/es9708530. [DOI] [Google Scholar]
- 2.Agency for Toxic Substances and Disease Registry, US Department of Health and Human Services. 2005. Toxicological profile for alpha-, beta-, gamma- and delta-hexachlorocyclohexane. Centers for Disease Control and Prevention, Atlanta, GA: http://www.atsdr.cdc.gov/toxprofiles/tp43.pdf. [Google Scholar]
- 3.Nagata Y, Endo R, Ito M, Ohtsubo Y, Tsuda M. 2007. Aerobic degradation of lindane (γ-hexachlorocyclohexane) in bacteria and its biochemical and molecular basis. Appl Microbiol Biotechnol 76:741–752. doi: 10.1007/s00253-007-1066-x. [DOI] [PubMed] [Google Scholar]
- 4.Lal R, Pandey G, Sharma P, Kumari K, Malhotra S, Pandey R, Raina V, Kohler HPE, Holliger C, Jackson C, Oakeshott JG. 2010. Biochemistry of microbial degradation of hexachlorocyclohexane and prospects for bioremediation. Microbiol Mol Biol Rev 74:58–80. doi: 10.1128/MMBR.00029-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma P, Raina V, Kumari R, Malhotra S, Dogra C, Kumari H, Kohler HPE, Buser HR, Holliger C, Lal R. 2006. Haloalkane dehalogenase LinB is responsible for β- and δ-hexachlorocyclohexane transformation in Sphingobium indicum B90A. Appl Environ Microbiol 72:5720–5727. doi: 10.1128/AEM.00192-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ito M, Prokop Z, Klvana M, Otsubo Y, Tsuda M, Damborsky J, Nagata Y. 2007. Degradation of beta-hexachlorocyclohexane by haloalkane dehalogenase LinB from gamma-hexachlorocyclohexane-utilizing bacterium Sphingobium sp. MI1205. Arch Microbiol 188:313–325. doi: 10.1007/s00203-007-0251-8. [DOI] [PubMed] [Google Scholar]
- 7.Raina V, Hauser A, Buser RH, Rentsch D, Sharma P, Lal R, Holliger C, Poiger JT, Kohler HPE. 2007. Hydroxylated metabolites of β- and δ-hexachlorocyclohexane: bacterial formation, stereochemical configuration, and occurrence in groundwater at a former production site. Environ Sci Technol 41:4292–4298. doi: 10.1021/es062908g. [DOI] [PubMed] [Google Scholar]
- 8.Geueke B, Miska ME, Poiger T, Rentsch D, Lal R, Holliger C, Kohler HPE. 2013. Enantioselective dehydrochlorination of δ-hexachlorocyclohexane and δ-pentachlorocyclohexene by LinA1 and LinA2 from Sphingobium indicum B90A. Appl Environ Microbiol 79:6180–6183. doi: 10.1128/AEM.01770-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Macwan AS, Kukshal V, Srivastava N, Javed S, Kumar A, Ramachandran R. 2012. Crystal structure of the hexachlorocyclohexane dehydrochlorinase (LinA-type 2): mutational analysis, thermostability and enantioselectivity. PLoS One 7:e50373. doi: 10.1371/journal.pone.0050373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suar M, Hauser A, Poiger T, Buser HR, Müller MD, Dogra C, Raina V, Holliger C, Vander Meer JR, Lal R, Kohler HPE. 2005. Enantioselective transformation of alpha-hexachlorocyclohexane by the dehydrochlorinases LinA1 and LinA2 from the soil bacterium Sphingomonas paucimobilis B90A. Appl Environ Microbiol 71:8514–8518. doi: 10.1128/AEM.71.12.8514-8518.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imai R, Nagata Y, Fukuda M, Takagi M, Yano K. 1991. Molecular cloning of a Pseudomonas paucimobilis gene encoding a 17-kilodalton polypeptide that eliminates HCl molecules from γ-hexachlorocyclohexane. J Bacteriol 173:6811–6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Macwan AS, Javed S, Kumar A. 2011. Isolation of a novel thermostable dehydrochlorinase (LinA) from a soil metagenome. 3 Biotech 1:193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trantírek L, Hynková K, Nagata Y, Murzin A, Ansorgová A, Sklenář V, Damborský J. 2001. Reaction mechanism and stereochemistry of γ-hexachlorocyclohexane dehydrochlorinase LinA. J Biol Chem 276:7734–7740. doi: 10.1074/jbc.M007452200. [DOI] [PubMed] [Google Scholar]
- 14.Buser HR, Müller MD. 1995. Isomer and enantioselective degradation of hexachlorocyclohexane isomers in sewage sludge under anaerobic conditions. Environ Sci Technol 29:664–672. doi: 10.1021/es00003a013. [DOI] [PubMed] [Google Scholar]
- 15.Zhao R, Chu S, Zhao R, Xu X, Liu X. 2005. Ultrasonic extraction followed by sulfuric acid silica gel cleanup for the determination of α-hexachlorocyclohexane enantiomers in biota samples. Anal Bioanal Chem 381:1248–1252. doi: 10.1007/s00216-004-3041-z. [DOI] [PubMed] [Google Scholar]
- 16.Johnson KA, Simpson ZB, Blom T. 2009. FitSpace explorer: an algorithm to evaluate multidimensional parameter space in fitting kinetic data. Anal Biochem 387:30–41. doi: 10.1016/j.ab.2008.12.025. [DOI] [PubMed] [Google Scholar]
- 17.Wu J, Hong Q, Sun Y, Hong Y, Yan Q, Li S. 2007. Analysis of the role of LinA and LinB in biodegradation of δ-hexachlorocyclohexane. Environ Microbiol 9:2331–2340. doi: 10.1111/j.1462-2920.2007.01350.x. [DOI] [PubMed] [Google Scholar]
- 18.Okai M, Kubota K, Fukuda M, Nagata Y, Nagata K, Tanokura M. 2010. Crystal structure of γ-hexachlorocyclohexane dehydrochlorinase LinA from Sphingobium japonicum UT26. J Mol Biol 403:260–269. doi: 10.1016/j.jmb.2010.08.043. [DOI] [PubMed] [Google Scholar]
- 19.Turner P, Mamo G, Karlsson EN. 2007. Potential and utilization of thermophiles and thermostable enzymes in biorefining. Microb Cell Fact 6:9. doi: 10.1186/1475-2859-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar S, Tsai CJ, Nussinov R. 2000. Factors enhancing protein thermostability. Protein Eng 13:179–191. doi: 10.1093/protein/13.3.179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.