Abstract
We recently reported that glycated albumin (GA) is increased in subjects with longer duration of diabetes and with decreased insulin secretory function. Based on this, we investigated whether GA increases with time relative to glycated hemoglobin (HbA1c) and the association between GA and beta-cell function. We analyzed 340 type 2 diabetes patients whose serum GA and HbA1c levels had been repeatedly measured over 4 years. We assessed the pattern of changes with time in glycemic indices (GA, HbA1c, and GA/HbA1c ratio) and their relationship with beta-cell function. In all patients, glycemic indices decreased and maintained low levels around 15 and 27 months. However, from 39 months to 51 months, GA significantly increased but HbA1c tended to increase without statistical significance. We defined ΔGA/HbA1c as the difference between the nadir point (at 15 to 27 months) and the end point (at 39 to 51 months) and found that ΔGA/HbA1c was positively correlated with diabetes duration and negatively related to beta-cell function. In multivariable linear regression analyses, ΔGA/HbA1c was independently associated with diabetes duration. In conclusion, this study demonstrated that serum GA levels increase relative to HbA1c levels with time.
1. Introduction
Glucose monitoring is essential for the appropriate care and treatment of patients with diabetes in order to avoid diabetic complications and hypoglycemia. An accurate measure of glucose level allows physicians and patients to make optimal decisions about food, physical activity, and medications [1]. Of the glycemic indices, the American Diabetes Association recommends glycated hemoglobin (HbA1c) testing in all diabetic patients as an initial assessment and then as a part of continuing care [2]. This recommendation is derived from clinical data that shows that HbA1c reflects average glycemic status over 2-3 months and predicts diabetic complications [3, 4]. Although HbA1c provides useful information, it might be inadequate in clinical situations such as anemia, renal insufficiency, and gestational diabetes. Glycated albumin (GA) has been gaining popularity as an indicator in several physiologic and pathologic conditions [5] because it provides more information than the gold standard HbA1c. In line with this trend, we have demonstrated the clinical relevance of GA in type 2 diabetes mellitus (T2D) with insulin secretory dysfunction rather than insulin resistance [6], fluctuating or poorly controlled glycemic excursions [7], and progressing atherosclerosis [8].
In the natural course of T2D, however, beta-cell function decreases as duration of diabetes increases [9]. Moreover, glycemic excursions worsen due to decreased beta-cell function [10]. In a recent cross-sectional study, we reported that the levels of GA/HbA1c were significantly elevated in subjects with long diabetic duration, largely attributed to the inverse relationships between GA and pancreatic beta-cell secretory indices [11], and suggested that clinicians should be careful in interpreting GA as only an indicator of glycemic control in T2D cases of longer duration. However, no longitudinal studies investigating the change in GA and HbA1c over time in patients with T2D have been published.
In this longitudinal observational study, we investigated the changing pattern of glycemic indices such as GA, HbA1c, and GA/HbA1c over 4 years in order to determine whether GA increases more with time relative to HbA1c in subjects with T2D. We also investigated which clinical and biochemical parameters are associated with changes in the GA/HbA1c ratio.
2. Research Design and Methods
2.1. Subjects and Data Collection
In this longitudinal observational study, we recruited patients with T2D who had enrolled in previous studies [6, 7] between May 2009 and June 2011 and who were followed up in June 2014. Using electronic medical records, we reviewed and rechecked demographic and clinical data for age, gender, metabolic parameters, and duration of diabetes. The diabetic duration was defined from the date the patients were first diagnosed with diabetes by blood tests or by patient recall from interviews.
To investigate the changes in glycemic indices with time, we tried to include patients whose duration of diabetes was less than 5 years. Patients were included if they were (1) aged ≥20 years, (2) had repeated laboratory data for both HbA1c and GA up to the final follow-up point, and (3) had undergone a baseline standardized liquid meal test (Ensure, Meiji Dairies Corporation, Tokyo, Japan; 500 kcal, 17.5 g fat (31.5%), 68.5 g carbohydrate (54.5%), and 17.5 g protein (14.0%)) after an overnight fast. Patients were excluded if they had any medical conditions that could alter HbA1c or GA levels such as liver cirrhosis or chronic kidney diseases (estimated glomerular filtration rate (GFR) by chronic kidney disease epidemiology collaboration formula <60 mL/min/1.73 m2), pregnancy, or hematologic disorders or if they were being treated with steroids.
The protocol of this study was approved by the Institutional Review Board at Severance Hospital (IRB numbers 4-2009-0656, 4-2012-0398, and 4-2014-0507). Written informed consent for this study was not required by the Institutional Review Board because researchers only accessed the database for analysis purposes, and personal information was not used.
2.2. Laboratory Measurements
The baseline glycemic indices (GA, HbA1c, and GA/HbA1c) were defined as the values measured at enrollment. Subsequently, serum GA and HbA1c were measured every 3 or 6 months. The end point glycemic indices of each subject were measured between 39 and 51 months. For glucose and C-peptide analyses, blood samples were collected at 0 and 90 min (basal and stimulated values) as part of the standardized liquid meal test. Pancreatic beta-cell functions in the context of ambient insulin secretory function were assessed using the following indices: (1) PCGR (stimulated C-peptide level/stimulated glucose level × 100), (2) C-peptide increment (ΔC-peptide = stimulated C-peptide − basal C-peptide), and (3) C-peptide-genic index [CGI = (stimulated C-peptide − basal C-peptide)/(stimulated glucose − basal glucose)]. Measurement techniques included the hexokinase method for glucose and high-performance liquid chromatography using Variant II Turbo (Bio-Rad Laboratories, Hercules, CA) for HbA1c. Serum GA was analyzed by an enzymatic method using an albumin-specific proteinase, ketoamine oxidase, an albumin assay reagent (LUCICA GA-L; Asahi Kasei Pharma Co., Tokyo, Japan), and a Hitachi 7699 P module autoanalyzer (Hitachi Instruments Service, Tokyo, Japan). GA values were calculated from the ratio of GA to total serum albumin and expressed as a percentage. Serum C-peptide levels were measured in duplicate using an immunoradiometric assay method (Beckman Coulter, Fullerton, CA).
2.3. Statistical Analysis
All continuous variables were presented as mean ± standard deviation (SD) or median (quartiles) or as mean ± standard error (SE) for variables on the graphs. Categorical variables were described as N (%). Differences were analyzed using Student's t-test for the continuous variables and the chi-square test for categorical variables.
Repeated measured analysis of variance (ANOVA) with Bonferroni correction and paired t-test were used to determine the significance of differences in glycemic indices according to duration of diabetes. We compared GA, HbA1c, and GA/HbA1c levels at baseline and 3, 15, 27, and 39 to 51 months after enrollment in all patients who had glycemic values available at that time point. Because all glycemic indices reached their lowest level between 15 and 27 months (arbitrarily defined nadir point), we defined ΔGA/HbA1c as the difference in GA/HbA1c between the end point (39 to 51 months) and nadir point (15 to 27 months). One-way ANOVA with Tukey correction was used to compare the differences of duration of diabetes and PCGR according to the tertiles of ΔGA/HbA1c ratio. Multivariable linear regression analysis was performed to determine the independent relationship of the studied variables including duration of diabetes associated with ΔGA/HbA1c increase. Statistical analyses were performed using PASW Statistics version 18.0 for Windows (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Study Population Characteristics
A total of 340 subjects (71% men, mean age 61.3 ± 11.6 years) were enrolled in this study. The patient characteristics of the cohort are shown in Table 1. The mean body mass index (BMI) was 25.4 ± 3.6 kg/m2 and the prevalence of hypertension was 57% (n = 195). Median duration of diabetes and levels of mean HbA1c were 1 (range: 0–5.0) year and 7.0% + 0.9%, respectively. We also measured baseline insulin secretory beta-cell function indices such as PCGR (3.24 ± 2.1), ΔC-peptide level (4.13 ± 2.6), and CGI (0.08 ± 0.4). All glycemic indices, GA (19.3 ± 6.6 versus 16.5 ± 4.9), HbA1c (7.7 ± 1.6 versus 7.0 ± 1.2), and GA/HbA1c (2.47 ± 0.5 versus 2.33 ± 0.4), were decreased at final follow-up compared to those at baseline. At the time of enrollment, the patients were being treated with metformin (221 patients; 65% of the study population), sulfonylurea (88; 26%), DPP-IV inhibitors (59; 17%), or insulin (63; 19%).
Table 1.
Baseline characteristics of the study population.
| Variables | All (N = 340) |
|---|---|
| Demographics | |
| Age (years) | 61.3 ± 11.6 |
| Male, N (%) | 204 (71) |
| BMI (kg/m2) | 25.4 ± 3.6 |
| Waist circumference (cm) | 88.1 ± 9.0 |
| Hypertension, N (%) | 195 (57) |
| Duration of diabetes (years) | 1.0 (0–5.0) |
| Biochemistry profiles | |
| Creatinine (mg/dL) | 0.93 ± 0.2 |
| Estimated GFR (mL/min/1.73 m2) | 81.5 ± 17.7 |
| Albumin (g/dL) | 4.6 ± 0.4 |
| Total cholesterol (mg/dL) | 177.2 ± 48.5 |
| Triglyceride (mg/dL) | 152.5 ± 110.7 |
| HDL-cholesterol (mg/dL) | 47.7 ± 14.3 |
| LDL-cholesterol (mg/dL) | 99.7 ± 38.7 |
| Beta-cell function indices at baseline | |
| Basal glucose (mg/dL) | 138.0 ± 50.9 |
| Stimulated glucose (mg/dL) | 231.8 ± 87.3 |
| Basal C-peptide (ng/mL) | 2.35 ± 1.2 |
| Stimulated C-peptide (ng/mL) | 6.50 ± 3.3 |
| ΔC-peptide (ng/ml) | 4.13 ± 2.6 |
| PCGR | 3.24 ± 2.1 |
| CGI | 0.08 ± 0.4 |
| Glycemic indices | |
| GA at baseline (%) | 19.3 ± 6.6 |
| HbA1c at baseline (%) | 7.7 ± 1.6 |
| HbA1c at baseline (mmol/mol) | 60.8 ± 16.9 |
| GA/HbA1c ratio at baseline | 2.47 ± 0.5 |
| GA at end point (%) | 16.5 ± 4.9 |
| HbA1c at end point (%) | 7.0 ± 1.2 |
| HbA1c at end point (mmol/mol) | 53.2 ± 13.1 |
| GA/HbA1c ratio at end point | 2.33 ± 0.4 |
| Mean GA (%) | 16.5 ± 4.0 |
| Mean HbA1c (%) | 7.0 ± 0.9 |
| Medications at baseline | |
| Insulin, N (%) | 63 (19) |
| Metformin, N (%) | 221 (65) |
| DPP-IV inhibitor, N (%) | 59 (17) |
| Thiazolidinediones, N (%) | 40 (12) |
| Sulfonylurea, N (%) | 88 (26) |
| Medications at 27 months | |
| Insulin, N (%) | 52 (15) |
| Metformin, N (%) | 254 (75) |
| DPP-IV inhibitor, N (%) | 98 (29) |
| Thiazolidinediones, N (%) | 65 (19) |
| Sulfonylurea, N (%) | 99 (29) |
Continuous variables were described as mean ± SD or median (quartiles), N (%) for categorical variables.
BMI, body mass index; GFR, glomerular filtration rate; GA, glycated albumin; CGI, C-peptide-genic index; PCGR, postprandial C-peptide to glucose ratio.
3.2. Glycated Albumin and GA/HbA1c Ratio Levels Increased Relative to HbA1c Levels over Time
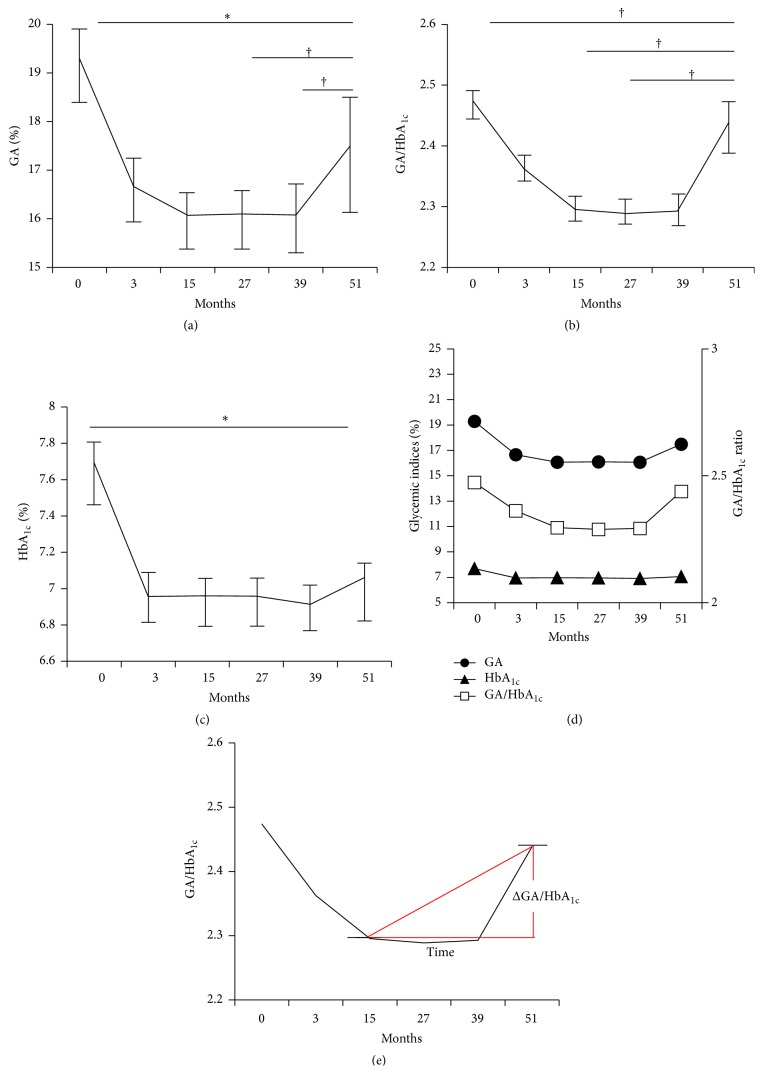
In all patients, both levels of GA (16.1% ± 4.0%) and GA/HbA1c ratio (2.30 ± 0.4) improved and reached the nadir points on glucose control at 15 months (Figure 1). From 15 months to 39 months, the nadir glycemic indices were stably maintained. From 39 months to 51 months, GA significantly increased (16.1% ± 4.8% to 17.5% ± 4.9%, p = 0.028), but HbA1c tended to increase without statistical significance (6.9% ± 1.0% to 7.1% ± 1.2%, p = 0.389). The levels of GA at 27 and 39 months (p = 0.029 and 0.028, resp.) as well as GA/HbA1c ratio at 15 and 27 months (p = 0.038, p = 0.039) were significantly lower than at 51 months (Figures 1(a) and 1(b)). However, statistical differences in HbA1c between each time point (3, 15, 27, and 39 months) and the last time point (51 months) were not significant except for baseline (Figure 1(c)). In sum, GA levels and the GA/HbA1c ratio, but not HbA1c levels, were significantly increased at the final follow-up compared with those at the nadir time point (Figure 1(d)).
Figure 1.
Changing patterns of glycemic indices over 4 years. (a) GA, (b) GA/HbA1c ratio, (c) HbA1c, (d) changing patterns of glycemic indices, (e) ΔGA/HbA1c, calculated by end point GA/HbA1c – nadir point GA/HbA1c. Data are presented as mean with SE. ∗ p < 0.001, † p < 0.05 for the comparison with 51 months.
3.3. Associations between ΔGA/HbA1c and Clinical and Biochemical Parameters
Since the GA/HbA1c ratio significantly increased from the nadir point to the final follow-up point, which was designated as ΔGA/HbA1c (Figure 1(e)), we tried to determine the clinical and biochemical parameters that are associated with ΔGA/HbA1c (Table 2). In the univariate linear regression analysis, duration of diabetes (standardized β coefficient (STD β) = 0.187, p = 0.001), mean GA (STD β = 0.345, p < 0.001), and mean HbA1c (STD β = 0.128, p = 0.018) were positively associated with ΔGA/HbA1c. On the other hand, beta-cell function indices were negatively related to ΔGA/HbA1c. In particular, PCGR (STD β = −0.145, p = 0.007) was more strongly associated with ΔGA/HbA1c than ΔC-peptide (STD β = −0.139, p = 0.011).
Table 2.
Univariate linear regression analysis to determine the variables associated with ΔGA/HbA1c.
| Variables | STD β | p |
|---|---|---|
| Age (year) | 0.063 | 0.246 |
| BMI (kg/m2) | −0.063 | 0.251 |
| Waist circumference (cm) | 0.004 | 0.940 |
| Estimated GFR (mL/min/1.73 m2) | −0.032 | 0.552 |
| Albumin (g/dL) | 0.008 | 0.886 |
| Total cholesterol (mg/dL) | −0.029 | 0.599 |
| Triglyceride (mg/dL) | −0.080 | 0.141 |
| HDL-cholesterol (mg/dL) | 0.023 | 0.674 |
| LDL-cholesterol (mg/dL) | −0.007 | 0.903 |
| GA at baseline (%) | 0.166 | 0.002 |
| HbA1c at baseline (%) | 0.017 | 0.753 |
| Mean GA (%) | 0.345 | <0.001 |
| Mean HbA1c (%) | 0.128 | 0.018 |
| Duration of diabetes (year) | 0.187 | 0.001 |
| ΔC-peptide (ng/mL) | −0.139 | 0.011 |
| PCGR | −0.145 | 0.007 |
| CGI | −0.059 | 0.284 |
BMI, body mass index; GFR, glomerular filtration rate; GA, glycated albumin; PCGR, postprandial C-peptide to glucose ratio; CGI, C-peptide-genic index. Values with statistical significance are printed in bold.
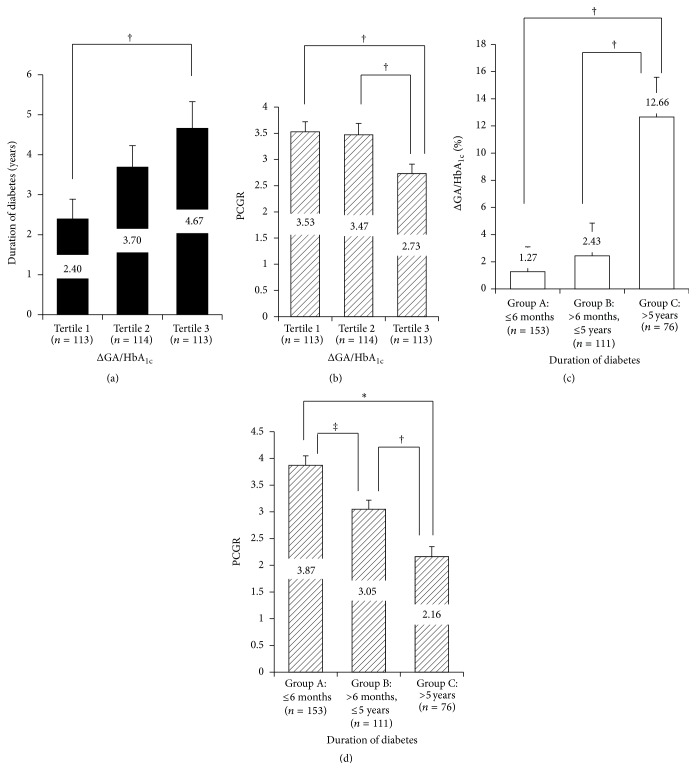
We classified study subjects according to tertiles of ΔGA/HbA1c. Individuals in higher tertiles for ΔGA/HbA1c had longer duration of diabetes (2.4 versus 3.7 versus 4.7 years; tertile 1 versus tertile 3, p = 0.013) and lower levels of PCGR (3.5 versus 3.5 versus 2.7; tertile 1 versus tertile 3, p = 0.011; tertile 2 versus tertile 3, p = 0.021) (Figures 2(a) and 2(b)). Moreover, study subjects were categorized into three groups based on duration of diabetes (Group A: ≤6 months, n = 153; Group B: >6 months and ≤5 years, n = 111; Group C: >5 years, n = 76) to investigate the impact of diabetes duration on ΔGA/HbA1c ratio and PCGR. The ΔGA/HbA1c ratios (expressed as percentages) were significantly elevated in patients with diabetes of duration >5 years compared to other groups (Figure 2(c)), whereas PCGR was decreased in patients with longer duration of diabetes (Figure 2(d)).
Figure 2.
Correlations between ΔGA/HbA1c and duration of diabetes, beta-cell function. (a, b) Differences of duration of diabetes (a) and PCGR (b) in subjects according to the tertiles of ΔGA/HbA1c. (c, d) Differences of ΔGA/HbA1c (c) and PCGR (d) in subjects according to duration of diabetes. † p < 0.05, ‡ p < 0.01, ∗ p < 0.001; ΔGA/HbA1c (%) = ΔGA/HbA1c/nadir point GA/HbA1c ∗100.
3.4. ΔGA/HbA1c Was Independently Associated with Duration of Diabetes
Multivariable linear regression models were applied to determine the clinical and laboratory variables associated with ΔGA/HbA1c (Table 3). We focused on certain parameters that can directly or indirectly reflect the insulin secretory function, such as PCGR, duration of diabetes, and medication history of DPP-IV inhibitor which can effectively reduce postprandial glucose. After adjustment for clinically important variables such as age, sex, BMI, waist circumference, and estimated GFR in model 1, history of DPP-IV inhibitor use was negatively associated with ΔGA/HbA1c (STD β = −0.111, p = 0.049). After additional inclusion of PCGR in model 2, PCGR showed significant correlation with ΔGA/HbA1c (STD β = −0.161, p = 0.009), but history of DPP-IV inhibitor use lost its significance. In model 3, duration of diabetes was further adjusted and the significant correlation of PCGR with ΔGA/HbA1c disappeared (STD β = −0.111, p = 0.080). However, duration of diabetes was still independently associated with ΔGA/HbA1c (STD β = 0.172, p = 0.005). Moreover, this association remained significant even after adjustment for glycemic status of subjects (inclusion of mean GA in model 4 and mean HbA1c in model 5, resp.).
Table 3.
Multivariable linear regression analyses to determine the variables associated with ΔGA/HbA1c.
| Models | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Conventional confounders |
Model 1 + PCGR |
Model 2 + duration of diabetes |
Model 3 + mean GA |
Model 3 + mean HbA1c |
|||||
| STD β | p | STD β | p | STD β | p | STD β | p | STD β | p | |
| DPP-IV inhibitor use | −0.111 | 0.049 | −0.109 | 0.053 | −0.089 | 0.111 | −0.084 | 0.133 | −0.088 | 0.116 |
| PCGR | — | — | −0.161 | 0.009 | −0.111 | 0.080 | −0.059 | 0.396 | −0.106 | 0.113 |
| Duration of diabetes | — | — | 0.172 | 0.005 | 0.166 | 0.007 | 0.170 | 0.007 | ||
Conventional confounders: age (years), sex (0 = female, 1 = male), body mass index (kg/m2), waist circumference (cm), and estimated glomerular filtration rate (mL/min/1.73 m2).
PCGR, postprandial C-peptide to glucose ratio; STD β, standardized β coefficient. Values with statistical significance are printed in bold.
Additionally, we conducted multiple linear regression analyses to determine variables associated with PCGR at baseline (Supplementary Table 1 in Supplementary Material available online at http://dx.doi.org/10.1155/2015/576306). PCGR showed the strongest relationship with mean GA (STD β = −0.336, p < 0.001). It also had significant correlation with duration of diabetes (STD β = −0.133, p = 0.010) and insulin use (STD β = −0.119, p = 0.029) (model 1). To evaluate the association between PCGR and ΔGA/HbA1c, model 2 was developed, which showed a significant negative relationship (STD β = −0.107, p = 0.032).
4. Discussion
Evidence has accumulated on the clinical relevance of GA as a glycemic index. However, the optimal use of GA as a glucose monitoring tool has not been fully investigated. Based on a previous cross-sectional study that showed that GA values are significantly influenced by the duration of T2D in cases where beta-cell function gradually decreases with time, we hypothesized that the ratio of GA to HbA1c might not be constant over time. In this study of more than 4 years, we assessed glycemic excursion by measuring HbA1c and GA and investigated discrepancy between two glycemic indices according to multiple time points. This study has three main findings: first, we found an initial sharp decrease in these glycemic indices, followed by maintenance at a low level, and then a gradual increase. Unlike for GA, the HbA1c increase was statistically insignificant. Second, the change in GA/HbA1c ratios, defined as the difference between the nadir point and the end point, was independently associated with baseline duration of diabetes. Third, impaired beta-cell function accounted for the association between longer duration of diabetes and increase in GA relative to HbA1c, as well as the increase in the GA/HbA1c ratio.
Because HbA1c is formed via a nonenzymatic glycation process of hemoglobin in erythrocytes [12], medical conditions such as pregnancy, hemolytic anemia, chronic kidney disease, or end stage renal disease with dialysis could alter HbA1c levels. In those cases, GA may be a more reliable marker than HbA1c [5]. In contrast to HbA1c formation, which requires intracellular glucose and protein metabolism, GA is formed directly via an extracellular nonenzymatic glycation process in plasma. However, medical conditions associated with albumin metabolism such as obesity, hyperthyroidism, and nephrotic syndrome, as well as glucocorticoid treatment [5], are known to affect GA levels. To avoid complications, we did not include patients with liver cirrhosis, chronic kidney diseases, pregnancy, and hematologic disorders or those who were being treated with steroid therapy.
With respect to the clinical relevance of the GA/HbA1c ratio, it is known that the ratio is significantly correlated with insulin secretory beta-cell function but not with insulin resistance [6]. Recent study also showed that lower insulin secretory capacity predicted increased levels of GA/HbA1c ratio in subjects with T2D [13]. Moreover, the GA/HbA1c ratio in patients with T1D and T2D more accurately reflected glucose excursion [7, 14–16] and diabetic vasculopathy [8, 17] than HbA1c alone. The GA/HbA1c ratio was significantly higher in T2D patients treated with insulin than in those treated with either diet or oral hypoglycemic agents [7, 18]. This observation might explain why history of insulin use is associated with either significant hyperglycemia or decreased beta-cell function. Our study also showed that ΔGA/HbA1c between end point and nadir point is significantly associated with decreased insulin secretory function-related clinical and laboratory variables such as baseline and mean GA, mean HbA1c PCGR, ΔC-peptide, and diabetic duration (Table 2). Of the assessed glycemic indices, baseline HbA1c did not predict the changes in the GA/HbA1c ratio. With respect to the effect of insulin secretory factors on GA values, a recent cross-sectional study reported that GA levels significantly increased more in patients with longer duration of T2D and impaired beta-cell function measured by ΔC-peptide regardless of HbA1c levels [11]. Consistent with this finding, our longitudinal study also showed that patients with higher levels of ΔGA/HbA1c had longer duration of diabetes and lower levels of PCGR (Figure 2). Furthermore, PCGR representing beta-cell function was associated with diabetic duration and insulin use at baseline and mean GA but not with mean HbA1c. Based on these findings, we could infer that patients with T2D of longer duration and with higher GA/HbA1c are more likely to have impaired beta-cell function and need insulin.
Our study had several strengths. First, this study is a longitudinal study with a long follow-up period of more than 4 years, which allowed us to investigate the changes in GA and HbA1c levels over time. Second, about 80% of participants had a relatively short duration of diabetes (≤5 years) at enrollment. Lastly, we conducted mixed meal tests to obtain basal and stimulated C-peptide levels, which were then used to calculate PCGR as a measure of beta-cell function. That allowed for standardization of the stimulation calories and glucose content. Because it can be easily calculated and is a reliable indicator of beta-cell function, the PCGR is being used more frequently to help determine the optimal antidiabetic drug treatment [19, 20]. In our study, PCGR levels were strongly associated with ΔC-peptide (r = 0.808, p < 0.001) which strongly predicted beta-cell function (Supplementary Figure 1). In multivariable linear regression analyses, PCGR was also associated with ΔGA/HbA1c. However, because the duration of diabetes strongly affects ΔGA/HbA1c, after adjusting for duration of diabetes, the association between ΔGA/HbA1c and PCGR disappeared (Table 3).
This study has the following limitations. First, we did not measure beta-cell function or glucose levels during follow-up period or at the end point. Thus, we did not prove that the difference between GA and HbA1c is caused by a decline in beta-cell function during the follow-up period. Second, since this is a retrospective study, the follow-up period varied among the participants. Third, because we did not assess changes in medication, we could not adjust for its effects.
5. Conclusions
We conclude that both impaired beta-cell function and longer duration of diabetes are associated with an increase in GA relative to HbA1c and an increase in the GA/HbA1c ratio. The GA/HbA1c ratio was significantly correlated with insulin secretory beta-cell function and increased as duration of diabetes increased. In this regard, clinicians should be extra careful when interpreting GA and GA/HbA1c ratio values in subjects with longer duration of diabetes. Further well-designed prospective studies enrolling larger populations are warranted.
Supplementary Material
Supplementary Table 1 Analyses to determine the variables associated with PCGR *Model 1 was adjusted for age (years), sex (0=female, 1=male), body mass index (kg/m2), waist circumference (cm), hypertension (0=no, 1=yes), and estimated glomerular filtration rate (ml/min/1.73m2). * *Model 2 was additionally adjusted for ΔGA/HbA1c (end-point, baseline). Supplementary Figure 1 Correlation between ΔC-peptide and PCGR
Conflict of Interests
The authors declare that there is no competing financial interest associated with this paper.
Authors' Contribution
Byung-Wan Lee, Yong-ho Lee, and Hye-jin Yoon carried out the concept and design of the study. Hye-jin Yoon, Yong-ho Lee, So Ra Kim, Byung-Wan Lee, and Hyun Chul Lee carried out data analysis and interpretation. Hye-jin Yoon, Yong-ho Lee, and Byung-Wan Lee were responsible for the drafting of the paper. Kwang Joon Kim, Eun Seok Kang, Bong Soo Cha, and Hyun Chul Lee were responsible for the critical revision of the paper. Hye-jin Yoon, Yong-ho Lee, and Kwang Joon Kim were responsible for the statistics. Hye-jin Yoon and Yong-ho Lee were responsible for the data collection. Hye-jin Yoon and Yong-ho Lee contributed equally to this study.
References
- 1.Lee Y. K., Song S. O., Kim K. J., et al. Glycemic effectiveness of metformin-based dual-combination therapies with sulphonylurea, pioglitazone, or DPP4-inhibitor in drug-naïve Korean type 2 diabetic patients. Diabetes & Metabolism Journal. 2013;37(6):465–474. doi: 10.4093/dmj.2013.37.6.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care. 2014;37(supplement 1):S14–S80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 3.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) The Lancet. 1998;352(9131):837–853. doi: 10.1016/s0140-6736(98)07019-6. [DOI] [PubMed] [Google Scholar]
- 4.Lee E. J., Kim Y. J., Kim T. N., et al. A1c variability can predict coronary artery disease in patients with type 2 diabetes with mean A1c levels greater than 7. Endocrinology and Metabolism. 2013;28(2):125–132. doi: 10.3803/EnM.2013.28.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim K. J., Lee B.-W. The roles of glycated albumin as intermediate glycation index and pathogenic protein. Diabetes & Metabolism Journal. 2012;36(2):98–107. doi: 10.4093/dmj.2012.36.2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim D., Kim K. J., Huh J. H., et al. The ratio of glycated albumin to glycated haemoglobin correlates with insulin secretory function. Clinical Endocrinology. 2012;77(5):679–683. doi: 10.1111/j.1365-2265.2011.04312.x. [DOI] [PubMed] [Google Scholar]
- 7.Lee E. Y., Lee B.-W., Kim D., et al. Glycated albumin is a useful glycation index for monitoring fluctuating and poorly controlled type 2 diabetic patients. Acta Diabetologica. 2011;48(2):167–172. doi: 10.1007/s00592-010-0242-0. [DOI] [PubMed] [Google Scholar]
- 8.Song S. O., Kim K. J., Lee B.-W., Kang E. S., Cha B. S., Lee H. C. Serum glycated albumin predicts the progression of carotid arterial atherosclerosis. Atherosclerosis. 2012;225(2):450–455. doi: 10.1016/j.atherosclerosis.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Madsbad S., Faber O. K., Binder C., McNair P., Christiansen C., Transbøl I. Prevalence of residual beta-cell function in insulin-dependent diabetics in relation to age at onset and duration of diabetes. Diabetes. 1978;27(supplement 1):262–264. doi: 10.2337/diab.27.1.s262. [DOI] [PubMed] [Google Scholar]
- 10.Jensen C. C., Cnop M., Hull R. L., Fujimoto W. Y., Kahn S. E. β-cell function is a major contributor to oral glucose tolerance in high-risk relatives of four ethnic groups in the U.S. Diabetes. 2002;51(7):2170–2178. doi: 10.2337/diabetes.51.7.2170. [DOI] [PubMed] [Google Scholar]
- 11.Lee Y. H., Kown M. H., Kim K. J., et al. Inverse association between glycated albumin and insulin secretory function may explain higher levels of glycated albumin in subjects with longer duration of diabetes. PLoS ONE. 2014;9(9) doi: 10.1371/journal.pone.0108772.e108772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hare M. J. L., Shaw J. E., Zimmet P. Z. Current controversies in the use of haemoglobin A1c . Journal of Internal Medicine. 2012;271(3):227–236. doi: 10.1111/j.1365-2796.2012.02513.x. [DOI] [PubMed] [Google Scholar]
- 13.Saisho Y., Tanaka K., Abe T., Kawai T., Itoh H. Lower beta cell function relates to sustained higher glycated albumin to glycated hemoglobin ratio in Japanese patients with type 2 diabetes. Endocrine Journal. 2014;61(2):149–157. doi: 10.1507/endocrj.ej13-0376. [DOI] [PubMed] [Google Scholar]
- 14.Suwa T., Ohta A., Matsui T., et al. Relationship between clinical markers of glycemia and glucose excursion evaluated by continuous glucose monitoring (CGM) Endocrine Journal. 2010;57(2):135–140. doi: 10.1507/endocrj.k09e-234. [DOI] [PubMed] [Google Scholar]
- 15.Yoshiuchi K., Matsuhisa M., Katakami N., et al. Glycated albumin is a better indicator for glucose excursion than glycated hemoglobin in type 1 and type 2 diabetes. Endocrine Journal. 2008;55(3):503–507. doi: 10.1507/endocrj.k07e-089. [DOI] [PubMed] [Google Scholar]
- 16.Saisho Y., Tanaka K., Abe T., Shimada A., Kawai T., Itoh H. Glycated albumin to glycated hemoglobin ratio reflects postprandial glucose excursion and relates to beta cell function in both type 1 and type 2 diabetes. Diabetology International. 2011;2(3):146–153. doi: 10.1007/s13340-011-0035-x. [DOI] [Google Scholar]
- 17.Kim W., Kim K. J., Lee B.-W., Kang E. S., Cha B. S., Lee H. C. The glycated albumin to glycated hemoglobin ratio might not be associated with carotid atherosclerosis in patients with type 1 diabetes. Diabetes & Metabolism Journal. 2014;38(6):456–463. doi: 10.4093/dmj.2014.38.6.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koga M., Murai J., Saito H., Kasayama S. Glycated albumin and glycated hemoglobin are influenced differently by endogenous insulin secretion in patients with type 2 diabetes. Diabetes Care. 2010;33(2):270–272. doi: 10.2337/dc09-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okuno Y., Komada H., Sakaguchi K., et al. Postprandial serum C-peptide to plasma glucose concentration ratio correlates with oral glucose tolerance test- and glucose clamp-based disposition indexes. Metabolism: Clinical and Experimental. 2013;62(10):1470–1476. doi: 10.1016/j.metabol.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 20.Lee E. Y., Hwang S., Lee S. H., et al. Postprandial C-peptide to glucose ratio as a predictor of beta-cell function and its usefulness for staged management of type 2 diabetes. Journal of Diabetes Investigation. 2014;5(5):517–524. doi: 10.1111/jdi.12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1 Analyses to determine the variables associated with PCGR *Model 1 was adjusted for age (years), sex (0=female, 1=male), body mass index (kg/m2), waist circumference (cm), hypertension (0=no, 1=yes), and estimated glomerular filtration rate (ml/min/1.73m2). * *Model 2 was additionally adjusted for ΔGA/HbA1c (end-point, baseline). Supplementary Figure 1 Correlation between ΔC-peptide and PCGR