Abstract
With dengue fever emerging as a global health problem and more Australians travelling to endemic areas, imported dengue infection is on the rise and clinicians need to remain vigilant. Primary cardiac and neurologic involvement in dengue infection has been rarely described in the medical literature and the pathophysiology is poorly understood. A rare and fatal case of primary dengue infection in a 34-yearold woman who returned from Papua New Guinea is reported; the unusual features of this case include severe primary dengue infection, myocarditis, and acute cerebral oedema resulting in death. This case demonstrates that severe atypical manifestations and fatality can occur with primary dengue infection.
Keywords: Dengue, myocarditis, cerebral oedema, infection, Australia
Implications for Practice:
-
What is known about this subject?
In Australia, North Queensland is at risk but fatal dengue cases are unusual. Severe primary infection with myocarditis and cerebral oedema are extremely rare.
-
What new information is offered in this case study?
Severe atypical manifestations can occur in dengue without haemorrhage or plasma leakage. Rarely, severe primary infection with myocarditis and acute cerebral oedema can be fatal.
-
What are the implications for research, policy, or practice?
Dengue should be suspected in travellers returning from endemic areas. Rapid deterioration can occur in these patients who are ideally managed in high dependency units.
Background
Dengue is an arthropod-borne viral infection that is transmitted in humans by mosquitoes and is rapidly emerging as a global health problem.1 According to the World Health Organization (WHO), dengue is endemic in more than 100 countries, with 2.5 billion people at risk.1 In Australia, North Queensland is a recognised area at risk with the predominance of dengue virus serotype 2.2 Dramatically increased international travel to endemic countries is considered to be a contributing factor; O’Brien et al. found dengue infection in eight per cent of travellers returning to Australia with a febrile illness.3 In general, fatal dengue cases are extremely rare in Australia.4
The clinical presentation of dengue ranges from selflimiting, mild febrile illness to severe dengue haemorrhagic fever and dengue shock syndrome. A dengue co-infection in a blood stream infection caused by Stenotrophomonas maltophila, causing a diagnostic dilemma has been reported.5 Shock due to myocarditis and acute cerebral oedema resulting in fatality are rarely described.6,7 The incidence is variable and the pathogenesis is not well understood. Life-threatening primary dengue infection has been previously described,8 and secondary infection by two different serotypes is considered an important epidemiologic risk factor for severe disease through a mechanism termed as “antibody dependent enhancement”.9
We report on a fatal case of dengue in a young woman returning from Papua New Guinea with severe primary dengue infection. The case is noteworthy for unusual features that are rarely described in the literature, including myocarditis and acute cerebral oedema.
Case details
A 34-year-old woman presented to the emergency department in January 2013 with a two-day history of fever, headache, and photophobia following a holiday in Port Moresby, Papua New Guinea. She was haemodynamically stable, demonstrated neck stiffness with no hepatosplenomegaly, mucosal bleeding, or rash. Mosquito bite marks were evident on her lower extremities.
Initial laboratory investigations (Table 1), chest X-ray, electrocardiogram, and computed tomography (CT) scan of the brain were unremarkable. Serial blood cultures, blood smear for malarial parasites, Rickettsial serology were negative. Cerebrospinal fluid (CSF) was normal except mildly elevated white blood cell count (9x106 cells/L, RI <5x106 cells/L, inadequate cells on smear for differential count). She subsequently deteriorated with worsening fever (41.3°C), shock, and agitation. She was intubated and transferred to the intensive care unit (ICU). Neurological examination revealed fixed and dilated pupils.
Table 1: Laboratory investigations in the emergency department and subsequently in the ICU.
| Laboratory Parameter | Emergency Department (on admission) | Intensive Care Unit (worst value) | Reference Interval |
|---|---|---|---|
| Haemoglobin | 144 G/L | 147 G/L | 135–180 G/L |
| Haematocrit | 0.42 | 0.44 | 0.39–0.52 |
| White Cell count | 12 X 109/L | 2 X 109/L | 4-11 X 109/L |
| Neutrophil count | 8.7 X 109/L | 1.5 X 109/L | 2-8 X 109/L |
| Platelet Count | 245 X 109/L | 76 X 109/L | 140-400 X 109/L |
| International normalised ratio | 1.2 | 1.5 | 0.9–1.2 |
| Prothrombin Time | 13s | 16s | 10–13s |
| Activated Partial Thromboplastin Time | N/A* | 53s | 26–41s |
| Fibrinogen | N/A* | 2.7 G/L | 1.7–4.5 G/L |
| Bilirubin | 17 umol/L | 20 umol/L | <20 umol/L |
| Aspartate Transaminase | 22 U/L | 3500 U/L | <35 U/L |
| Alanine Transaminase | 15 U/L | 1080 U/L | <45 U/L |
| Troponin I | N/A* | 27 ug/L | <0.04 ug/L |
| Creatine Kinase | N/A* | 1530 U/L | 46–71 U/L |
| Lactate Dehydrogenase | N/A* | 4010 U/L | 150–280 U/L |
A repeat CT scan of the brain revealed cerebral oedema. A transthoracic echocardiogram revealed severe systolic dysfunction and ejection fraction of approximately 25–30 per cent. The pattern of regional wall motion abnormality (RWMA) was suggestive of “inverted” Takotsubo cardiomyopathy demonstrated by a preserved function of the apex with hypokinesis of the base. There was no evidence of haemorrhage, pleural effusion, ascites, or plasma leakage. She received broad-spectrum antibiotics, inotropes and 8L of crystalloids in the first 24 hours.
Primary dengue infection was diagnosed by detecting dengue IgM (Negative IgG), NS1 (Non-structural protein 1) antigen in serum and positive dengue polymerase chain reaction (PCR) in the serum and CSF for virus serotype 3. There was no neurologic improvement after four days of treatment in the ICU and life support was discontinued after discussion with the family.
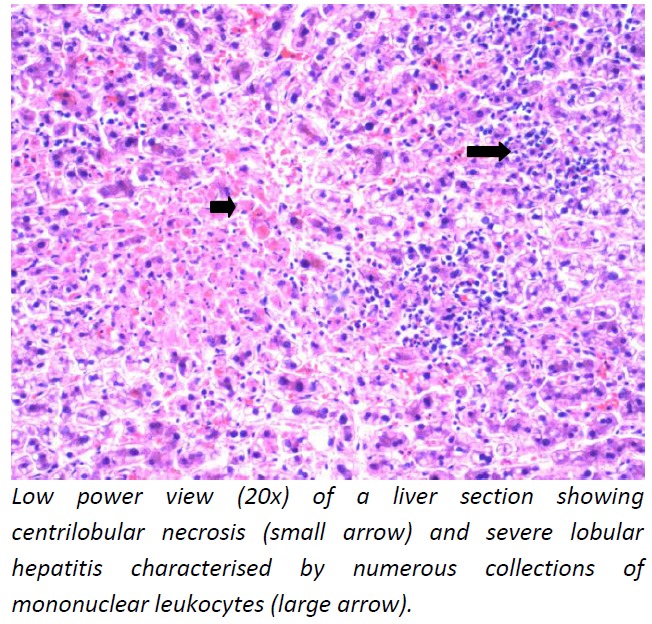
Post-mortem diagnosis of dengue was confirmed by detecting dengue RNA in the blood and myocardium. The brain showed features suggestive of acute global hypoxic ischemic encephalopathy. The myocardium showed widespread interstitial oedema and increased mononuclear leukocytes suggestive of myocarditis (Figure 1). There was evidence of pulmonary oedema with foci of intra-alveolar haemorrhage (Figure 2) and lobular hepatitis with centrilobular necrosis (Figure 3).
Figure 1: Heart–high power view (40x).
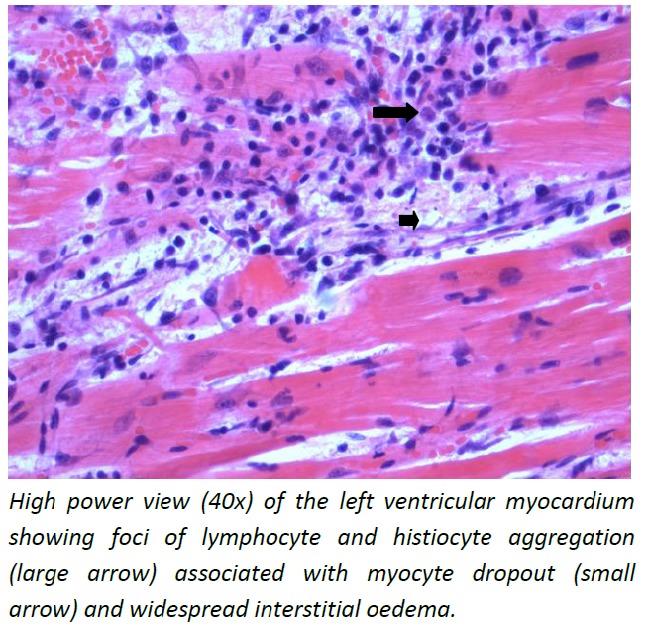
Figure 2: Lung–microphotography (20x).
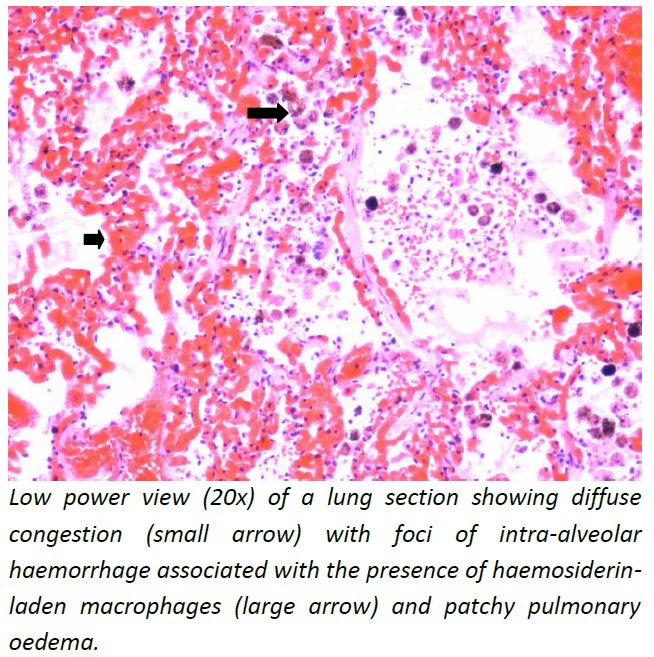
Figure 3: Liver–microphotography (20x).
Discussion
We report a fatal case of dengue fever that included severe primary dengue infection with cardiac and neurologic involvement, a case that is unusual for the concomitant myocarditis and cerebral oedema.
Cardiac complications in dengue
Cardiac complications in dengue range from asymptomatic atrio-ventricular dysrrhythmias and mild elevation of biomarkers to myocarditis and severe left ventricular failure.6, 10Wali et al. found global hypokinesis in 70 per cent of patients (12/17) with dengue haemorrhagic fever/dengue shock syndrome, which reverted back to normal in three weeks.11 Similarly, 17 per cent of children (9/54) with varying grades of disease had a reduced ejection fraction (<50 per cent).12
The pathophysiology of cardiac involvement is poorly understood. Either direct dengue invasion of cardiac muscle or cytokine mediated immunological response or both are incriminated. Myocarditis was a major contributor to shock in our case as there was no evidence of bleeding or plasma leakage. Although the echocardiogram in our patient suggested a RWMA pattern of “inverted” Takotsubo cardiomyopathy, it could not be classified as such; the Mayo Clinic criteria for Takotsubo cardiomyopathy excludes myocarditis.13
Neurologic complications in dengue
The most common neurologic manifestation in dengue is encephalitis, followed by meningitis, seizures, acute flaccid paralysis, and Guillain-Barré syndrome.14 Reported mortality rates are variable (3.7 per cent to 47 per cent).14,15 Our patient rapidly developed cerebral oedema and brain death similar to a case described by Janssen et al.7
Acute cerebral oedema is extremely unusual in patients with dengue. It is usually mild and occurs in later stages of the disease as a part of multisystem involvement. Three pathogenic mechanisms of neurologic involvement have been proposed; direct neuronal infiltration, encephalopathy due to systemic complications and post-infectious immunemediated complications. A direct involvement was demonstrated in our patient with dengue PCR in the CSF; however, the autopsy did not reveal meningoencephalitis.
All four dengue virus serotypes are reported in relation to neurological manifestations; however, dengue virus serotype 3 is more commonly related to neurovirulence16 and was identified in our case.
Fatal primary dengue infection
Although life-threatening primary dengue infection has been described,8 secondary infection with two sequential infections by different serotypes is considered an important epidemiologic risk factor for severe disease. The mechanism is thought to be “antibody dependent enhancement”, which leads to raised in-vivo concentration of virus and a large infected cell mass resulting in activation of complement cascade and accelerated release of vasoactive substances leading to capillary leakage.9
Conclusion
This case demonstrates that severe atypical cardiovascular and neurologic manifestations can occur in dengue, and that even a primary infection can result in fatality. Clinicians therefore should remain vigilant to diagnose dengue early and provide supportive therapy. With increasing numbers of Australians travelling to dengue endemic areas, dengue viral infection could become a significant issue for the Australian health system and clinicians should remain aware of the potential consequences of infection.
ACKNOWLEDGEMENTS
None
Footnotes
PEER REVIEW
Not commissioned. Externally peer reviewed.
CONFLICTS OF INTEREST
The authors declare that they have no competing interests.
FUNDING
None
ETHICS COMMITTEE APPROVAL
An exemption from requiring a formal ethical review was granted by the local “Metro South Human Research Ethics Committee” based in Princess Alexandra Hospital, Brisbane, Queensland. HREC Reference number: HREC/15/QPAH/509. A copy of the exemption letter is available from the corresponding author.
PATIENT CONSENT
The authors, Sane S, Saulova A, McLaren R, White H, declare that:
- They have obtained an informed telephonic consent for the publication of the details relating to the patient in this report from the parents of the patient as her next of kin. A written entry has been made to that effect into the medical records kept at Logan Hospital, Queensland.
- All possible steps have been taken to safeguard the identity of the patient.
- This submission is compliant with the requirements of local research ethics committees.
Please cite this paper as: Sane S, Saulova A, McLaren R, White H. A fatal case of primary dengue infection with myocarditis and cerebral oedema. AMJ 2015;8(9): 299–303. http//dx.doi.org/10.4066/AMJ.2015.2489
References
- 1.World Health Organization. Dengue: guidelines for diagnosis, treatment, prevention and control. [Accessed 2015 Aug 1]. Available from: http://www.who.int/tdr/publications/documents/deng ue-diagnosis.pdf. [PubMed] [Google Scholar]
- 2.Leggat PA. Dengue in Northern Queensland, Australia: Risk from travellers or risk to travellers? Travel Med Infect Dis. 2009 Jul;7(4):212–4. doi: 10.1016/j.tmaid.2009.03.007. doi: 10.1016/j.tmaid.2009.03.007. Epub 2009 Apr 19. [DOI] [PubMed] [Google Scholar]
- 3.O’Brien D, Tobin S, Brown GV. et al. Fever in returned travellers: Review of hospital admissions for a 3-year period. Clin Inf Dis. 2001;33:603–9. doi: 10.1086/322602. [DOI] [PubMed] [Google Scholar]
- 4.McBride WJH. Deaths associated with dengue haemorrhagic fever: the first in Australia and over a century. Med J Aust. 2005;183(1):35–7. doi: 10.5694/j.1326-5377.2005.tb06889.x. [DOI] [PubMed] [Google Scholar]
- 5.Srirangaraj S, Kali A, Vijayan S. Dengue co-infection in a blood stream infection caused by Stenotrophomonas maltophila: A case report. Australas Med J. 2014 Nov 30;7(11):441–4. doi: 10.4066/AMJ.2014.2205. doi: 10.4066/AMJ.2014.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee IK, Lee WH, Liu JW. et al. Acute myocarditis in dengue haemorrhagic fever: a case report and review of cardiac complications in dengue-affected patients. Int J Infect Dis. 2010 Oct;14(10):e919–22. doi: 10.1016/j.ijid.2010.06.011. doi: 10.1016/j.ijid.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 7.Janssen HLA, Bienfait HP, Jansen CL. et al. Fatal cerebral oedema associated with primary dengue infection. J Infect. 1998;36(3):344–6. doi: 10.1016/s0163-4453(98)94783-1. [DOI] [PubMed] [Google Scholar]
- 8.Barnes WJ, Rosen L. Fatal haemorrhagic disease and shock associated with primary dengue infection on a Pacific island. Am J Trop Med Hyg. 1974;23:495–506. doi: 10.4269/ajtmh.1974.23.495. [DOI] [PubMed] [Google Scholar]
- 9.Simmons CP, Farrar JJ, Chau NV. et al. Current concepts-dengue. N Engl J Med. 2012;366:1423–32. doi: 10.1056/NEJMra1110265. [DOI] [PubMed] [Google Scholar]
- 10.Miranda CH, Borges M, Matsuno AK. et al. Evaluation of cardiac involvement during dengue viral infection. Clin Infect Dis. 2013 Sep;57(6):812–9. doi: 10.1093/cid/cit403. doi: 10.1093/cid/cit403. [DOI] [PubMed] [Google Scholar]
- 11.Wali JP, Biswas A, Chandra S. et al. Cardiac involvement in dengue haemorrhagic fever. Int J Cardiol. 1998;64:31–6. doi: 10.1016/s0167-5273(98)00008-4. [DOI] [PubMed] [Google Scholar]
- 12.Kabra Sk, Juneja R, Madhulika. et al. Myocardial dysfunction in children with dengue haemorrhagic fever. Natl Med J India. 1998;11:59–61. [PubMed] [Google Scholar]
- 13.Akashi YJ, Goldstein DS, Barbaro G. et al. Takotsubo Cardiomyopathy: A New Form of Acute, reversible Heart Failure. Circulation. 2008;118:2751–62. doi: 10.1161/CIRCULATIONAHA.108.767012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jackson ST, Mullings A, Bennett F. et al. Dengue infection in patients presenting with neurological manifestations in a dengue endemic population. West Indian Med J. 2008;57(4):373–6. [PubMed] [Google Scholar]
- 15.Malavige GN, Ranatunga PK, Jayaratne SD. et al. Dengue viral infection as a cause of encephalopathy. Indian J Med Microbiol. 2007;25(2):143–5. doi: 10.4103/0255-0857.32722. [DOI] [PubMed] [Google Scholar]
- 16.Solomon T, Dung NM, Vaughn DW. et al. Neurological manifestations of dengue infection. Lancet. 2000;355:1053–9. doi: 10.1016/S0140-6736(00)02036-5. [DOI] [PubMed] [Google Scholar]


