Abstract
Wolfram syndrome (WS) is a rare, progressive, neurodegenerative disorder that has an autosomal recessive pattern of inheritance. The gene for WS, wolfram syndrome 1 gene (WFS1), is located on human chromosome 4p16.1 and encodes a transmembrane protein. To date, approximately 230 mutations in WFS1 have been confirmed, in which nonsynonymous single nucleotide polymorphisms (nsSNPs) are the most common forms of genetic variation. Nonetheless, there is poor knowledge on the relationship between SNP genotype and phenotype in other nsSNPs of the WFS1 gene. Here, we analysed 395 nsSNPs associated with the WFS1 gene using different computational methods and identified 20 nsSNPs to be potentially pathogenic. Furthermore, to identify the amino acid distributions and significances of pathogenic nsSNPs in the protein of WFS1, its transmembrane domain was constructed by the TMHMM server, which suggested that mutations outside of the TMhelix could have more effects on protein function. The predicted pathogenic mutations for the nsSNPs of the WFS1 gene provide an excellent guide for screening pathogenic mutations.
Wolfram syndrome (WS) (MIM 222300), also known as DIDMOAD (diabetes insipidus, insulin-deficient diabetes mellitus, optic atrophy and deafness), is a rare neurodegenerative disorder of autosomal recessive inheritance, characterised by diabetes insipidus, insulin-deficient diabetes mellitus, optic atrophy and deafness. Of these symptoms, diabetes mellitus is the most common manifestation of WS with a median onset age of 6 years1 and always presents before the age of 162. The prevalence of WS is approximately 1/700,000 individuals in the UK, and 1/100,000 individuals in North America3. Since the first report for WS by Wolfram and Wagener in 19384, progressively more cases have been observed. Many studies have been performed to investigate the genetic basis of this hereditary disease and have identified that loss-of-function mutations in the WFS1 gene are the main cause of the syndrome5.
WFS1, located on human chromosome 4p16.1, is composed of eight exons, of which only the first exon is a noncoding exon, and most mutations in WFS1 have been identified in exon 8 but also in exons 3, 4, 5 and 66,7,8. WFS1 encodes the protein wolframin, which is abundantly expressed in pancreas, brain, heart, and muscle and is thought to be a novel endoplasmic reticulum (ER) calcium channel or a regulator of channel activity9,10. Additionally, wolframin appears to be involved in membrane trafficking, protein processing11, regulation of intracellular Ca2+ homeostasis12 and β-cell dysfunction13,14. Mutations in the WFS1 gene may result in instability and a significantly reduced half-life of wolframin in the endoplasmic reticulum and then may cause disease15.
To date, approximately 230 mutations in WFS1 have been reported (https://lovd.euro-wabb.org/home.php?select_db=WFS1). Although nsSNPs are the most common form of genetic variation in these mutations, the relationship between the genotype and phenotype of other nsSNPs in the WFS1 gene is unclear. Given the large number of nsSNPs in the WFS1 gene, it is expensive and time-consuming to experimentally explore the functional effects of these SNPs. The prediction of the phenotypic effects of nsSNPs based on different computational methods has become a well-known methodology16,17, and several research articles have cited its effectiveness in identifying deleterious, disease-related mutations18,19. In those methods, predicting pathogenic nsSNPs is based on identifying structural and functional damaging properties. This study will facilitate the investigation of the role of nsSNPs in WFS1 and identify pathogenic nsSNPs associated with the WFS1 gene based on different computational methods. Among these methods, the prediction of deleterious and damaging nsSNPs was performed by SIFT and PolyPhen-2. A support vector machine (SVM) along with the SIFT algorithm, PhD-SNP and MutPred were used to detect disease-associated nsSNPs. In addition, to identify the amino acid distributions and significances of pathogenic nsSNPs in the protein of WFS1, we constructed the transmembrane domain by the TMHMM server v2.0.
Results
SNP dataset from databases
The nsSNPs were collected from the NCBI dbSNP, HGMD, Deafness Variation Databases and the Locus Specific Database, in which the NCBI dbSNP database was the primary source, containing approximately 1,500 SNPs, and the other three were as supplemental. After filtering, a total of 395 nsSNPs were identified.
NsSNP prediction results of WFS1
To identify deleterious mutations from the nsSNPs in the WFS1 gene, the SIFT and PolyPhen-2 server were used to predict whether the mutations were deleterious/damaging. The SIFT server was used to calculate the tolerance index of all 395 collected nsSNPs with evolutionary conservation analysis, and a SIFT score value of <0.05 was considered to be deleterious. Meanwhile, we subjected all 395 nsSNPs to the PolyPhen-2 structure-based analysis server to further analyze the effects of amino acid substitutions (AAS) on the structures and functions. Of the 395 nsSNPs in the WFS1 gene, 174 nsSNPs were predicted to be deleterious by SIFT and the remaining nsSNPs were tolerated except for nonsense mutations for which SIFT provided no score. Among these deleterious nsSNPs, 32 mutations (P7L, G154A, W314R, P346L, Y351C, S353C, R375C, E394V, E394K, S430L, S430W, Y528D, P533S, A684V, A684T, A684G, C690R, C690G, G695V, Y699H, Y699C, Y699S, G702S, G702D, R708C, N714T, G736R, G736D, G736S, G834S, L842F and P885L) were reported to be highly deleterious with SIFT scores of 0.000. Obviously, in these highly deleterious nsSNPs, the mutation frequencies in the amino acid loci 394, 430, 684, 690, 699, 702 and 736 were higher than other loci. In PolyPhen-2, 235 nsSNPs were predicted to be damaging to protein structure and function, of which 89 mutations were predicted to be highly deleterious with PolyPhen-2 scores of 1.000. A total of 156 nsSNPs were predicted to be deleterious and damaging by both SIFT and PolyPhen-2 (Table 1) after excluding all nonsense mutations. Additionally, of these 156 nsSNPs, 28 nsSNPs (P346L, Y351C, S353C, R375C, E394V, E394K, S430L, S430W, Y528D, P533S, Y669H, Y669C, Y669S, A684T, A684G , A684V, C690R, C690G, G695V, G702D, G702S, R708C, G736D, G736R, G736S, G834S, L842F and P885L) were predicted to be highly deleterious and damaging by both algorithms with SIFT scores of 0.000 and PolyPhen-2 scores of 1 (Table 1).
Table 1. Deleterious and damaging nsSNPs of WFS1 prioritised using SIFT and PolyPhen-2 scores.
| Amino Acid Change | Nucleotide Variation | SIFT Score | PolyPhen-2 Score | SNP ID* |
|---|---|---|---|---|
| R24H | G/A | 0.011 | 0.999 | rs71524364 |
| T104I | C/T | 0.021 | 0.992 | |
| G107E | G/A | 0.004 | 1 | rs71530914 |
| G107R | G/A | 0.003 | 1 | WFS1_00227 |
| Y110N | T/A | 0.023 | 0.999 | CM050353 |
| D118A | A/C | 0.004 | 0.999 | rs71524349 |
| A126T | G/A | 0.007 | 1 | rs145639028 |
| G154A | G/C | 0 | 0.996 | rs71530927 |
| T156M | C/T | 0.002 | 1 | |
| D171N | G/A | 0.049 | 0.953 | |
| R177P | G/C | 0.010 | 1 | CM083208 |
| A198V | C/T | 0.047 | 0.875 | rs142687752 |
| E202G | A/G | 0.043 | 0.998 | WFS1_00230 |
| D211N | G/A | 0.017 | 0.813 | rs138682654 |
| R228H | G/A | 0.037 | 1 | rs150771247 |
| E273K | G/A | 0.018 | 0.904 | rs142428158 |
| P292S | C/T | 0.008 | 1 | CM992981 |
| I296S | T/G | 0.003 | 0.688 | CM992982 |
| W314R | T/A | 0 | 0.999 | WFS1_00229 |
| L327I | C/A | 0.013 | 1 | rs71537678 |
| F329I | T/A | 0.031 | 0.99 | rs188848517 |
| P346L | C/T | 0 | 1 | CM073420 |
| F350V | T/G | 0.045 | 0.999 | |
| Y351C | A/G | 0 | 1 | rs181988441 |
| S353C | C/G | 0 | 1 | rs143547567 |
| C360Y | G/A | 0.001 | 0.999 | rs147157374 |
| T361I | C/T | 0.002 | 1 | WFS1_00075 |
| R375C | C/T | 0 | 1 | rs200095753 |
| R375H | G/A | 0.003 | 1 | rs142671083 |
| T378N | C/A | 0.007 | 0.999 | WFS1_00097 |
| D389E | T/G | 0.007 | 0.978 | rs201282601 |
| E394K | G/A | 0 | 1 | rs373146435 |
| E394V | A/T | 0 | 1 | rs146563951 |
| L402P | T/C | 0.001 | 1 | CM112216 |
| H407R | A/G | 0.010 | 0.684 | rs140407862 |
| V412A | T/C | 0.021 | 0.981 | rs144951440 |
| F417S | T/C | 0.002 | 0.95 | rs111570388 |
| I427S | T/G | 0.005 | 0.903 | CM073419 |
| S430L | C/T | 0 | 1 | WFS1_00218 |
| S430W | C/G | 0 | 1 | WFS1_00194 |
| L432V | C/G | 0.027 | 1 | rs35031397 |
| F439C | T/G | 0.002 | 0.913 | rs141585847 |
| S443I | G/T | 0.002 | 0.997 | CM015195 |
| T455M | C/T | 0.027 | 1 | rs139361521 |
| R456C | C/T | 0.010 | 0.689 | rs144452795 |
| E462G | A/G | 0.016 | 0.99 | rs398123066 |
| E462G | A/G | 0.016 | 0.99 | |
| C505Y | G/A | 0.001 | 0.998 | CM031397 |
| L506R | T/G | 0.003 | 0.95 | CM043878 |
| L511P | T/C | 0.001 | 0.949 | |
| Y513S | A/C | 0.036 | 0.98 | |
| R517H | G/A | 0.024 | 0.986 | rs150394063 |
| R517P | G/C | 0.022 | 0.904 | |
| M518I | G/A | 0.013 | 0.978 | rs138232538 |
| A519V | C/T | 0.047 | 1 | rs201557396 |
| Y528D | T/G | 0 | 1 | CM087003 |
| P533S | C/T | 0 | 1 | rs146132083 |
| C537Y | G/A | 0.003 | 0.999 | rs199910987 |
| L543R | T/G | 0.003 | 1 | CM031400 |
| V545M | G/A | 0.038 | 0.992 | rs201993978 |
| V546D | T/A | 0.004 | 0.999 | CM031401 |
| R558C | C/T | 0.001 | 1 | rs199946797 |
| R558H | G/A | 0.002 | 1 | CM031402 |
| A575G | C/G | 0.018 | 0.528 | rs71524360 |
| G576S | G/A | 0.031 | 0.882 | rs1805069 |
| V582M | G/A | 0.009 | 0.916 | rs377677092 |
| R587W | C/T | 0.005 | 0.999 | rs138968466 |
| L594R | T/G | 0.001 | 0.999 | rs200288171 |
| A602E | C/A | 0.011 | 0.74 | rs2230720 |
| A602G | C/G | 0.001 | 0.74 | |
| P607L | C/T | 0.040 | 0.999 | rs373862003 |
| P607R | C/G | 0.010 | 1 | CM033825 |
| R611C | C/T | 0.008 | 0.999 | rs144993516 |
| L637P | T/C | 0.002 | 1 | WFS1_00215 |
| T641M | C/T | 0.018 | 0.985 | rs376626985 |
| R653C | C/T | 0.007 | 1 | rs201064551 |
| E655G | A/G | 0.006 | 0.999 | CM024439 |
| E655K | G/A | 0.015 | 0.995 | CM108408 |
| S662P | T/C | 0.004 | 1 | rs376341411 |
| L664R | T/G | 0.001 | 1 | CM090453 |
| T665I | C/T | 0.002 | 0.976 | |
| T665N | C/A | 0.005 | 0.544 | rs138258392 |
| T665P | A/C | 0.004 | 0.544 | rs369656458 |
| Y669C | A/G | 0 | 1 | CM983479 |
| Y669H | T/C | 0 | 1 | CM072120 |
| Y669S | A/C | 0 | 1 | CM090454 |
| L672P | T/C | 0.026 | 0.998 | CM056420 |
| G674E | G/A | 0.029 | 1 | CM020990 |
| G674R | G/A | 0.024 | 1 | rs200672755 |
| G674V | G/T | 0.013 | 1 | CM020991 |
| R676C | C/T | 0.030 | 1 | rs201623184 |
| W678L | G/T | 0.008 | 0.999 | CM073425 |
| A684G | C/G | 0 | 1 | |
| A684T | G/A | 0 | 1 | |
| A684V | C/T | 0 | 1 | rs387906930 |
| R685C | C/T | 0.003 | 1 | rs112967046 |
| R685P | G/C | 0.023 | 0.999 | CM081852 |
| R685P | G/C | 0.023 | 0.999 | |
| I688T | T/C | 0.002 | 0.999 | |
| C690G | T/G | 0 | 1 | CM087004 |
| C690R | T/C | 0 | 1 | CM992988 |
| G695V | G/T | 0 | 1 | rs28937891 |
| T699M | C/T | 0.001 | 1 | rs28937894 |
| W700C | G/T | 0.001 | 1 | CM992989 |
| G702D | G/A | 0 | 1 | CM090455 |
| G702S | G/A | 0 | 1 | rs71532862 |
| R703C | C/T | 0.024 | 1 | rs201888856 |
| K705N | G/C | 0.032 | 0.997 | CM032680 |
| R708C | C/T | 0 | 1 | rs200099217 |
| R708H | G/A | 0.003 | 1 | rs369062548 |
| D713G | A/G | 0.012 | 0.999 | rs143280847 |
| N714T | A/C | 0 | 0.998 | rs397517196 |
| L723P | T/C | 0.001 | 1 | |
| P724L | C/T | 0.002 | 1 | rs28937890 |
| P724S | C/T | 0.043 | 1 | |
| R732C | C/T | 0.007 | 1 | rs71526458 |
| R732H | G/A | 0.018 | 1 | rs149013740 |
| G736D | G/A | 0 | 1 | rs71530912 |
| G736R | G/C | 0 | 1 | |
| G736S | G/A | 0 | 1 | rs71532864 |
| Y739D | T/G | 0.006 | 1 | rs367737581 |
| C742R | T/C | 0.010 | 1 | rs71532865 |
| C742W | C/G | 0.002 | 1 | rs71532866 |
| R756C | C/T | 0.002 | 1 | rs138127684 |
| A761V | C/T | 0.031 | 0.818 | rs71526459 |
| H763P | A/C | 0.014 | 0.995 | |
| D771G | A/G | 0.011 | 1 | CM015267 |
| D771H | G/C | 0.003 | 1 | CM052942 |
| R772C | C/T | 0.005 | 1 | rs149540655 |
| E776V | A/T | 0.001 | 1 | rs56002719 |
| G780R | G/C | 0.046 | 0.989 | CM012813 |
| G780S | G/A | 0.049 | 0.896 | rs387906931 |
| R791C | C/T | 0.019 | 0.982 | rs200528166 |
| K800E | A/G | 0.038 | 0.958 | rs55674815 |
| L804P | T/C | 0.001 | 1 | WFS1_00226 |
| S807R | A/C | 0.012 | 0.973 | CM020992 |
| E809K | G/A | 0.042 | 0.999 | rs71539673 |
| R818C | C/T | 0.014 | 1 | rs35932623 |
| L829P | T/C | 0.001 | 1 | rs104893883 |
| G831D | G/A | 0.012 | 1 | rs28937895 |
| R832C | C/T | 0.010 | 1 | rs148089728 |
| G834S | G/A | 0 | 1 | rs398124214 |
| L842F | C/T | 0 | 1 | rs71530915 |
| A844T | G/A | 0.047 | 0.973 | CM053436 |
| A844V | C/T | 0.036 | 0.999 | rs200192011 |
| R859P | G/C | 0.004 | 1 | CM052943 |
| R859W | C/T | 0.001 | 1 | rs372298367 |
| H860D | C/G | 0.007 | 0.96 | CM043881 |
| I863M | C/G | 0.003 | 0.977 | rs71524393 |
| E864K | G/A | 0.045 | 1 | rs74315205 |
| R868C | C/T | 0.008 | 1 | rs148611943 |
| R868H | G/A | 0.031 | 1 | rs56393026 |
| A874T | G/A | 0.006 | 1 | rs200775335 |
| K876T | A/C | 0.006 | 0.98 | rs144900514 |
| P885L | C/T | 0 | 1 | rs372855769 |
| A889V | C/T | 0.024 | 0.855 | rs147934586 |
*In the SNP ID column, the nsSNPs with the prefix “rs” are from dbSNP, and those with the prefix “CM” and “WFS1_” are from HGMD and Locus Specific Database, respectively, and the remaining with no SNP ID are in the Deafness Variation Database. The nsSNPs highlighted in bold are predicted to be highly deleterious and damaging, with a SIFT score of 0, and PolyPhen-2 score of 1.
For further study, we used PhD-SNP and MutPred to investigate whether these 156 filtered deleterious and damaging nsSNPs were associated with disease. PhD-SNP is optimised to classify disease-causing point mutations from the given datasets, and MutPred is also a web application tool developed to classify an AAS as either disease-associated or neutral in humans but also predicts the molecular cause of disease/deleterious AASs. Of the 156 nsSNPs, 97 diseased-associated nsSNPs were predicted by PhD-SNP and 91 nsSNPs were predicted to be disease-associated by MutPred tools. But it is worth noting that some of the 28 mutations with scores of 0.000 for SIFT and 1.000 for Polyphen-2 in Table 1 like P346L, Y351C, G834S or L842F were not predicted as diseased-associated by both PhD-SNP and MutPred, this might be because the loci of these amino acid were conserved, but the mutants on these loci could not cause the molecular changes or affect the whole protein structure. Finally, 70 nsSNPs were predicted to be diseased-associated using both PhD-SNP and MutPred, in which the numbers of mutations predicted as very confident hypotheses, confident hypotheses and actionable hypotheses were 16, 33 and 21, respectively. The most common changes in the molecular mechanisms in the mutants predicted by MutPred were gains or losses of helixes and sheets. Representative diseased-associated nsSNPs and the corresponding AAS of nsSNPs in the WFS1 gene are provided in Table 2. After inspecting these mutations in their reference sources, most of the nsSNPs predicted have also been reported, demonstrating that the nsSNPs predicted were credible from multiple computational methods. Finally, we predicted 20 mutations (F329I, S353C, R375H, R375C, E394K, F439C, R517P, L594R, P607L, S662P, T665I, R732C, R732H, G736D, Y739D, C742R, R832C, R859W, R868C and A874T) to be potentially pathogenic mutations, and 50 other mutations had been previously published or cited (Table 2).
Table 2. Diseased-associated nsSNPs of WFS1 predicted using the PhD-SNP and MutPred servers.
| Amino Acid Change | g Value | p Value | Molecular Change | Prediction Reliability | SNP ID* | Reported or not |
|---|---|---|---|---|---|---|
| Y110N | 0.849 | 0.0133 | Gain of disorder | Confident Hypotheses | CM050353 | Y41 |
| R177P | 0.817 | 0.0021 | Loss of MoRF binding | Very Confident Hypotheses | CM083208 | Y42 |
| P292S | 0.942 | 0.0093 | Gain of helix | Very Confident Hypotheses | CM992981 | Y20 |
| I296S | 0.867 | 0.0051 | Gain of loop | Very Confident Hypotheses | CM992982 | Y20 |
| W314R | 0.884 | 0.0162 | Gain of methylation at W314 | Confident Hypotheses | WFS1_00229 | Y43 |
| F329I | 0.774 | 0.0344 | Gain of sheet | Actionable Hypotheses | rs188848517 | N |
| S353C | 0.502 | 0.0266 | Gain of sheet | Actionable Hypotheses | rs143547567 | N |
| R375H | 0.670 | 0.0444 | Loss of helix | Actionable Hypotheses | rs142671083 | N |
| R375C | 0.669 | 0.0444 | Loss of helix | Actionable Hypotheses | rs200095753 | N |
| E394V | 0.811 | 0.0425 | Gain of helix | Confident Hypotheses | rs146563951 | Y44 |
| E394K | 0.826 | 0.0176 | Gain of methylation at E394 | Confident Hypotheses | rs373146435 | N |
| L402P | 0.679 | 0.0215 | Gain of relative solvent accessibility | Actionable Hypotheses | CM112216 | Y23 |
| I427S | 0.828 | 0.0082 | Gain of disorder | Very Confident Hypotheses | CM073419 | Y45 |
| S430L | 0.793 | 0.0203 | Loss of loop | Confident Hypotheses | WFS1_00218 | Y22 |
| S430W | 0.790 | 0.0266 | Gain of sheet | Confident Hypotheses | WFS1_00194 | Y23 |
| F439C | 0.835 | 0.0357 | Loss of sheet | Confident Hypotheses | rs141585847 | N |
| S443I | 0.836 | 0.0221 | Gain of sheet | Confident Hypotheses | CM015195 | Y21 |
| C505Y | 0.975 | 0.0062 | Loss of catalytic residue at P504 | Very Confident Hypotheses | CM031397 | Y46 |
| L506R | 0.858 | 0.0196 | Loss of helix | Confident Hypotheses | CM043878 | Y47 |
| L511P | 0.748 | 0.0016 | Gain of sheet | Actionable Hypotheses | Y25 | |
| R517P | 0.534 | 0.0072 | Loss of helix | Actionable Hypotheses | N | |
| Y528D | 0.939 | 0.0037 | Loss of sheet | Very Confident Hypotheses | CM087003 | Y48 |
| P533S | 0.886 | 0.0228 | Loss of sheet | Confident Hypotheses | rs146132083 | Y44 |
| L543R | 0.768 | 0.0228 | Loss of sheet | Actionable Hypotheses | CM031400 | Y46 |
| V546D | 0.828 | 0.0037 | Loss of sheet | Very Confident Hypotheses | CM031401 | Y46 |
| R558C | 0.890 | 0.0296 | Loss of methylation at R558 | Confident Hypotheses | rs199946797 | Y49 |
| R558H | 0.950 | 0.0296 | Loss of methylation at R558 | Confident Hypotheses | CM031402 | Y46 |
| L594R | 0.688 | 0.0344 | Gain of sheet | Actionable Hypotheses | rs200288171 | N |
| P607L | 0.748 | 0.0022 | Gain of helix | Actionable Hypotheses | rs373862003 | N |
| P607R | 0.954 | 0.0005 | Gain of MoRF binding | Very Confident Hypotheses | CM033825 | Y50 |
| L637P | 0.683 | 0.0072 | Loss of helix | Actionable Hypotheses | WFS1_00215 | Y51 |
| E655G | 0.756 | 0.0187 | Loss of solvent accessibility | Actionable Hypotheses | CM024439 | Y44 |
| E655K | 0.811 | 0.0049 | Gain of MoRF binding | Very Confident Hypotheses | CM108408 | Y52 |
| S662P | 0.816 | 0.0312 | Gain of loop | Confident Hypotheses | rs376341411 | N |
| L664R | 0.926 | 0.0090 | Gain of MoRF binding | Very Confident Hypotheses | CM090453 | Y53 |
| T665I | 0.821 | 0.0117 | Gain of helix | Confident Hypotheses | N | |
| L672P | 0.874 | 0.0076 | Loss of helix | Very Confident Hypotheses | CM056420 | Y54 |
| G674R | 0.964 | 0.0328 | Gain of MoRF binding | Confident Hypotheses | rs200672755 | Y55 |
| G674V | 0.958 | 0.0325 | Gain of helix | Confident Hypotheses | CM020991 | Y56 |
| W678L | 0.933 | 0.0132 | Loss of catalytic residue at A677 | Confident Hypotheses | CM073425 | Y57 |
| A684V | 0.755 | 0.0104 | Loss of helix | Actionable Hypotheses | rs387906930 | Y21 |
| R685P | 0.859 | 0.0033 | Loss of helix | Very Confident Hypotheses | Y58 | |
| C690R | 0.945 | 0.0008 | Gain of MoRF binding | Very Confident Hypotheses | CM992988 | Y20 |
| C690G | 0.955 | 0.0115 | Gain of disorder | Confident Hypotheses | CM087004 | Y48 |
| G695V | 0.911 | 0.0036 | Gain of sheet | Very Confident Hypotheses | rs28937891 | Y6 |
| H696Y | 0.764 | 0.0390 | Gain of sheet | Actionable Hypotheses | WFS1_00098 | Y59 |
| W700C | 0.942 | 0.0157 | Loss of MoRF binding | Confident Hypotheses | CM992989 | Y20 |
| G702S | 0.887 | 0.0315 | Loss of sheet | Confident Hypotheses | rs71532862 | Y23 |
| G702D | 0.96 | 0.0315 | Loss of sheet | Confident Hypotheses | CM090455 | Y53 |
| R708C | 0.921 | 0.0182 | Loss of MoRF binding | Confident Hypotheses | rs200099217 | Y21 |
| L723P | 0.731 | 0.0045 | Gain of loop | Actionable Hypotheses | Y23 | |
| P724L | 0.926 | 0.0336 | Loss of catalyticresi due at P724 | Confident Hypotheses | rs28937890 | Y6 |
| R732H | 0.855 | 0.0444 | Loss of helix | Confident Hypotheses | rs149013740 | N |
| R732C | 0.848 | 0.0376 | Loss of helix | Confident Hypotheses | rs71526458 | N |
| G736D | 0.934 | 0.0425 | Gain of helix | Confident Hypotheses | rs71530912 | N |
| G736R | 0.965 | 0.0117 | Gain of helix | Confident Hypotheses | Y60 | |
| Y739D | 0.736 | 0.0332 | Gain of disorder | Actionable Hypotheses | rs367737581 | N |
| C742R | 0.814 | 0.013 | Gain of disorder | Confident Hypotheses | rs71532865 | N |
| E776V | 0.939 | 0.050 | Gain of MoRF binding | Confident Hypotheses | rs56002719 | Y47 |
| L804P | 0.768 | 0.0063 | Loss of sheet | Actionable Hypotheses | WFS1_00226 | Y26 |
| L829P | 0.928 | 0.0079 | Gain of loop | Very Confident Hypotheses | rs104893883 | Y61 |
| G831D | 0.923 | 0.0143 | Gain of helix | Confident Hypotheses | rs28937895 | Y61 |
| R832C | 0.505 | 0.0228 | Loss of sheet | Actionable Hypotheses | rs148089728 | N |
| R859W | 0.596 | 0.0152 | Loss of disorder | Actionable Hypotheses | rs372298367 | N |
| R859P | 0.853 | 0.0315 | Loss of sheet | Confident Hypotheses | CM052943 | Y27 |
| H860D | 0.769 | 0.0104 | Loss of sheet | Actionable Hypotheses | CM043881 | Y47 |
| E864K | 0.901 | 0.0016 | Gain of MoRF binding | Very Confident Hypotheses | rs74315205 | Y62 |
| R868C | 0.843 | 0.0179 | Loss of disorder | Confident Hypotheses | rs148611943 | N |
| A874T | 0.769 | 0.0061 | Gain of sheet | Actionable Hypotheses | rs200775335 | N |
| P885L | 0.953 | 0.0117 | Gain of helix | Confident Hypotheses | rs372855769 | Y20 |
*In the SNP ID column, the nsSNPs with the prefix “rs” are from dbSNP, and those with the prefix “CM” and “WFS1_” are from HGMD and Locus Specific Database, respectively, and the remaining with no SNP ID are in the Deafness Variation Database.The nsSNPs highlighted in bold are potential pathogenic nsSNPs which have not been reported.
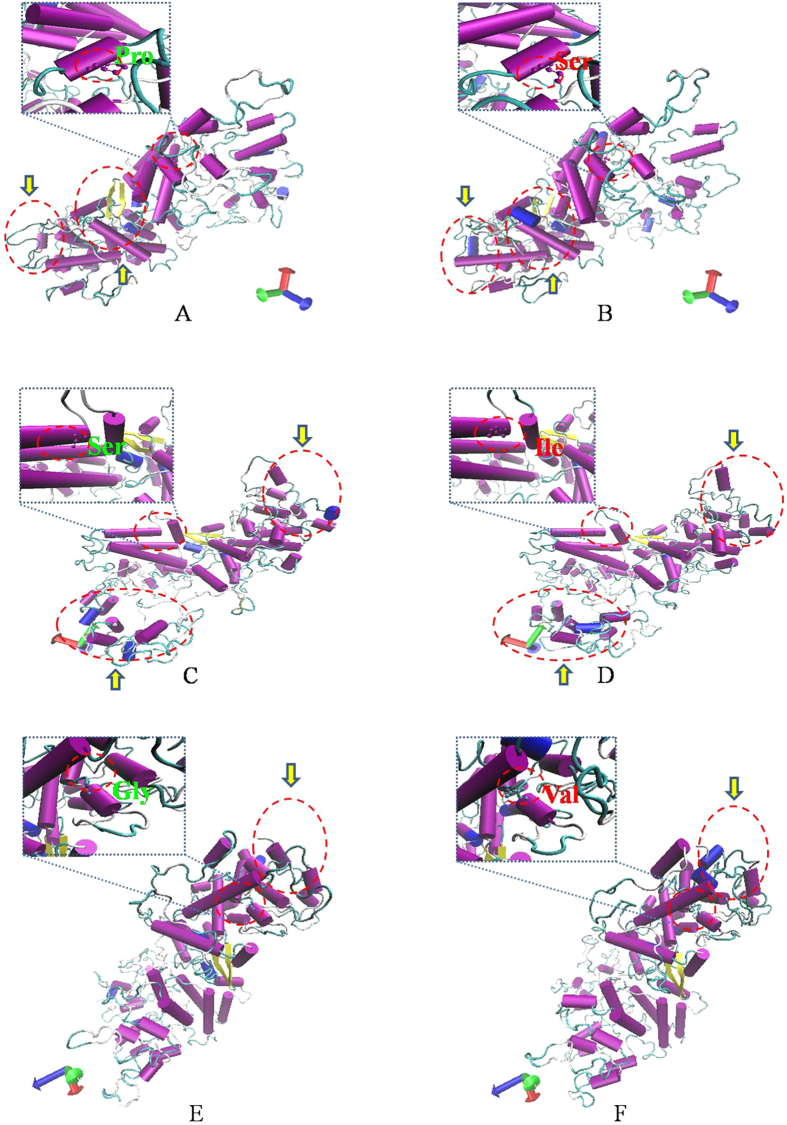
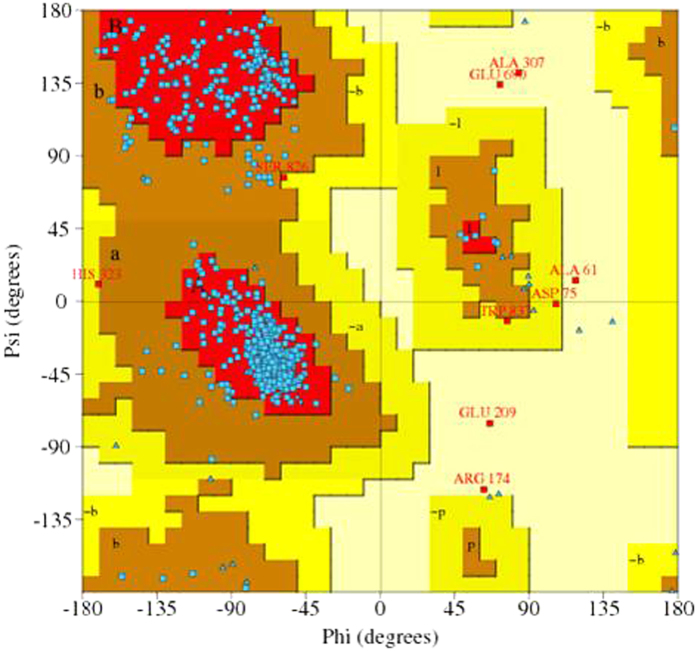
Additionally, to better understand how the pathogenic nsSNPs affect protein conformation and result in disease states, we constructed wild type and mutant proteins via the Robetta and SWISS-MODEL tools (Fig. 1, Supplementary file 1–4). And the geometric evaluations of the modeled 3D structure were performed using PROCHECK by calculating the Ramachandran plot (Fig. 2). The wild type protein showed 99.4% of residues in most favoured and allowed region and the overall average of G factors was 0.27 which showed the structure was usual. In this step, we randomly selected three predicted nsSNPs (P292S, S443I and G695V) that have been reported to be pathogenic6,20,21 and compared the structures between the wild type and mutant proteins. We observed that after mutation, not only did the amino acid change, but it also affected the entire protein structure. All of the three protein structures (P292S, S443I and G695V) representing different mutations gained or lost some α-helixes, suggesting a potential molecular mechanism resulting in WS.
Figure 1. Protein structure predicted by the SWISS-MODEL server.
(A,B) indicate the changes between wild type and mutant wolframin with the amino acid change P292S, (C,D) depict the structural changes between wild type and mutant S443I, and E and F illustrate the effects of G695V. (A,C,E) are protein structures of the wild type wolframin, and (B,D,F) are structures of the mutant proteins (created by SWISS-MODEL and illustrated with VMD). The arrows in yellow and the circles in red indicate the differences between the wild type and the mutant.
Figure 2. Ramachandran Plot of the wild type wolframin protein structure evaluated by PROCHECK.
Amino acid distribution in the transmembrane domain
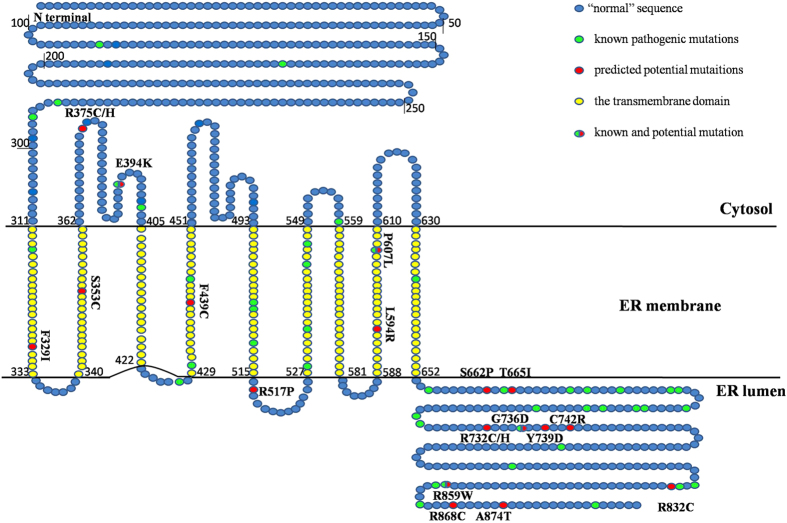
To elucidate the amino acid distributions and significances of predicted pathogenic nsSNPs in wolframin, we constructed its transmembrane domain using the TMHMM server v2.0 (Fig. 3). In this analysis, the transmembrane domain of wolframin was divided into 9 TMhelixes, with each TMhelix being approximately 23-amino acids long. Except for the third and seventh TMhelix, 18 pathogenic mutations were distributed across the other seven TMhelixes, accounting for 25.71% of all 70 pathogenic mutations, of which 13 were previously known. Notably, most pathogenic mutations in our study were not located in the transmembrane domain but in the C-terminal domain of wolframin (Table 3). In all 70 pathogenic mutations, approximately 52 were not located in the TMhelix (74.29%), 39 of which were located in the C-terminal domain. Thirty-seven pathogenic mutations have been previously reported in the 52 mutations not located in the TMhelix, and only 15 mutations were predicted to be potentially pathogenic.
Figure 3. Transmembrane domain structure of wolframin and its distribution of mutations22.
The 70 predicted pathogenic mutations are highlighted with green/red coloured circles compared to “normal” sequence with blue circles  . The 50 known pathogenic mutations are depicted in green
. The 50 known pathogenic mutations are depicted in green  and the 20 predicted potentially pathogenic mutations are in red
and the 20 predicted potentially pathogenic mutations are in red  . The transmembrane domain is depicted in yellow
. The transmembrane domain is depicted in yellow  . The circle with green and red
. The circle with green and red  denotes that the locus has a known and predicted mutation.
denotes that the locus has a known and predicted mutation.
Table 3. NsSNP distributions of the transmembrane domain of wolframin from the TMHMM server.
| Distribution of Transmembrane Domain | Range of Amino Acid | Number of Reported Pathogenic nsSNPs | Number of Predicted Pathogenic nsSNPs | Total Number of nsSNPs in Each Domain | Ratio of Each Domain (%) |
|---|---|---|---|---|---|
| Outside | 1–310 | 4 | 0 | 4 | 5.714 |
| TMhelix 1 | 311–333 | 1 | 1 | 2 | 2.857 |
| Inside | 334–339 | 0 | 0 | 0 | 0 |
| TMhelix 2 | 340–362 | 0 | 1 | 1 | 1.429 |
| Outside | 363–404 | 2 | 3 | 5 | 7.142 |
| TMhelix 3 | 405–422 | 0 | 0 | 0 | 0 |
| Inside | 423–428 | 1 | 0 | 1 | 1.429 |
| TMhelix 4 | 429–451 | 3 | 1 | 4 | 5.714 |
| Outside | 452–492 | 0 | 0 | 0 | 0 |
| TMhelix 5 | 493–515 | 3 | 0 | 3 | 4.286 |
| Inside | 516–526 | 0 | 1 | 1 | 1.429 |
| TMhelix 6 | 527–549 | 4 | 0 | 4 | 5.714 |
| Outside | 550–558 | 2 | 0 | 2 | 2.857 |
| TMhelix 7 | 559–581 | 0 | 0 | 0 | 0 |
| Inside | 582–587 | 0 | 0 | 0 | 0 |
| TMhelix 8 | 588–610 | 1 | 2 | 3 | 4.286 |
| Outside | 611–629 | 0 | 0 | 0 | 0 |
| TMhelix 9 | 630–652 | 1 | 0 | 1 | 1.429 |
| Inside* | 653–890 | 28 | 11 | 39 | 55.714 |
| Total | 890-amino acids | 50 | 20 | 70 | 100 |
*The domain highlighted in bold is the distribution of the C terminal domain.
Discussion
WS is a rare autosomal recessive disorder with a number of loss-of-function mutations of the WFS1, both within and between most affected patients/families. Wide tissue distribution of wolframin and many mutations in WFS1 resulting in WS may contribute to different phenotypes. Growing evidences have presented many clinical signs and possible correlations between the genotype and the development of the neurologic manifestations, the age at onset of diabetes mellitus, hearing defects, and diabetes insipidus in WS on the cohort of WS patients22,23. So far, although a large number of variants of the WFS1 gene have been identified, novel mutations are continuously found in this gene. Furthermore, the pathogenic role of different mutations, polymorphisms and sequencing variants of the gene remains largely unknown. Phenotypic prediction of the effects of nsSNPs might identify meaningful changes in genes that alter protein function to induce phenotypic consequences. The sheer number of SNPs in online databases provides an abundant resource to predict the phenotypic effects of nsSNPs, and known pathogenic mutations from the literature provide us an opportunity to inspect prediction accuracy, which indicates whether the relationships between nsSNP prediction results and known pathogenic mutations are confirmed by in vivo and in vitro experiments.
In the present study, we predicted 20 potentially pathogenic mutations and 50 known pathogenic mutations using in silico methods, and combined the results of the most common changes by MutPred and the predictions of the three protein structures by the SWISS-MODEL to determine that the most probable mutational effects causing WS might be the gains or losses of α-helixes. It is worth to consider that some predicted pathogenic nsSNPs have been confirmed by in vitro functional studies and genetic analysis for WS families, which could indirectly verify the accuracy of our methods. For example, p.P724L(c.2171C>T) and p.G695V(c.2084G>T) of WFS1 have been reported to lead to WS and which cause the formation of detergent-insoluble aggregates of wolframin when was expressed in COS-7 cells24; the p.A684V(c.2051C>T) and p.L511P (c.1532T>C) were ectopically expressed in HEK293 cells which showed reduced protein levels compared to wild type wolframin, strongly indicating that the mutation is disease-causing21,25. Meanwhile, by direct DNA sequencing and linkage analysis, p.L804P (c.2411T>C) and p.R859P (c.2576G>C) were identified after screening the entire coding region of the WFS1 gene in a Chinese WS family and in a US family with the nonsyndromic hearing loss, respectively26,27.
WFS1 spanning approximately 33.4 kb of genomic DNA, consists of eight exons and produces a peptide product which is 890-amino acid long (wolframin). The amino acid distribution results of wolframin suggest that wolframin contains 9 transmembrane domains. These results are consistent with the previous research which provides experimental evidence that wolframin contains 9 transmembrane segments and is embedded in the membrane in an Ncyt/Clum topology15. However, the prediction for wolframin available at UniProt database gives 11 transmembrane domains (http://www.uniprot.org/uniprot/O76024) (Table 4), and the difference between the two predicted results was mainly in the TMhelix 5, TMhelix 6 and TMhelix 11. In our result, the 493–515 amino acids are located in TMhelix5; while in UniProt, this region has been divided into TMhelix 5 and TMhelix 6 domains, respectively; the 653–890 amino acids have also been predicted as two TMhelixes in the same way in the UniProt. With reference to most researches, the wolframin were considered as 9 transmembrane domains with some evidences, and this is due to the differences in the execution of algorithm. Additionally, our results also indicate that the mutations outside of the TMhelix could have more pronounced functional effects, especially in the C-terminal with 39 predicted mutations. Many of the reported missense mutations are located in the C-terminal hydrophilic part of the protein15, and the experiments also support these predictions. Just as de Heredia et al. found that besides the transmembrane domains, the mutations identified in WS patients also concentrate in the last 100 amino acids in the C-terminal1. Using yeast two-hybrid analysis, Zatyka et al. identified that the C-terminal domain of wolframin, which is positioned in the ER lumen, bound the C-terminal domain (amino acids 652–890) of the ER-localized Na+/K+ ATPase beta-1 subunit (ATP1B1)28. And the Na+/K+ ATPase deficiency has a crucial role in apoptosis and in neural degenerative disease which can be induced by mutations in WFS1, leading to the development of WS29.
Table 4. The prediction results to the transmembrane domain of wolframin from the TMHMM server and UniProt database.
| TMHMM server |
UniProt database |
||
|---|---|---|---|
| Distribution of Transmembrane Domain | Range of Amino Acid | Distribution of Transmembrane Domain | Range of Amino Acid |
| Outside | 1–310 | Outside | 1–313 |
| TMhelix-1 | 311–333 | TMhelix-1 | 314–334 |
| Inside | 334–339 | Inside | 335–339 |
| TMhelix-2 | 340–362 | TMhelix-2 | 340–360 |
| Outside | 363–404 | Outside | 361–401 |
| TMhelix-3 | 405–422 | TMhelix-3 | 402–422 |
| Inside | 423–428 | Inside | 423–426 |
| TMhelix-4 | 429–451 | TMhelix-4 | 427–447 |
| Outside | 452–492 | Outside | 448–464 |
| TMhelix-5 | 493–515 | TMhelix-5 | 465–485 |
| Inside | 486–495 | ||
| TMhelix-6 | 496–516 | ||
| Inside | 516–526 | Outside | 517–528 |
| TMhelix-6 | 527–549 | TMhelix-7 | 529–549 |
| Outside | 550–558 | Inside | 550–562 |
| TMhelix-7 | 559–581 | TMhelix-8 | 563–583 |
| Inside | 582–587 | Outside | 584–588 |
| TMhelix-8 | 588–610 | TMhelix-9 | 589–609 |
| Outside | 611–629 | Inside | 610–631 |
| TMhelix-9 | 630–652 | TMhelix-10 | 632–652 |
| Inside* | 653–890 | Topological domain | 653–869 |
| TMhelix-11 | 870–890 | ||
| Total | 890-amino acids | Total | 890-amino acids |
*The domains highlighted in bold are the distributions of the C terminal domain.
In summary, we used extensive functional and structural level analyses to predict potentially pathogenic mutations for nsSNPs in the WFS1 gene and analysed the amino acid distributions of wolframin to provide a guide for screening pathogenic mutations and investigating the function of wolframin. Furthermore, we provide information for predicting the effects of nsSNPs in genes encoding transmembrane proteins and for further research in variant effect prediction.
Materials and Methods
Dataset collection
NsSNP datasets of the WFS1 gene were obtained from the NCBI dbSNP database (http://www.ncbi.nlm.nih.gov/projects/SNP/)30, HGMD (http://www.hgmd.cf.ac.uk/ac)31, Deafness Variation Database (http://deafnessvariationdatabase.org) and the Locus Specific Database (https://lovd.euro-wabb.org/home.php?select_db=WFS1). The amino acid sequence of wolframin was retrieved from the UniProt database (http://www.uniprot.org/). Data for the WFS1 gene were collected from Entrez Gene on the NCBI web site (http://www.ncbi.nlm.nih.gov/genbank/), and the literature search was performed using PubMed, Science Direct, and Web of Science.
Filtering and mining of nsSNPs
Because SNPs from the databases were not initially nsSNPs, we needed to perform some manual filtering. In this process, we eliminated SNPs in 3′ or 5′UTRs and synonymous SNPs. For prediction and analysis, SNP ID, gene name, protein accession, amino acid residue 1 (wild type), amino acid position, and amino acid residue 2 (missense) for all nsSNPs were collected from the NCBI dbSNP database, HGMD, and Deafness Variation Databases.
Predicting the phenotype of nsSNPs with the SIFT and PolyPhen-2 tools
After filtering the nsSNPs, we predicted their functional effects with the SIFT (http://sift-dna.org) and PolyPhen-2 (http://genetics.bwh.harvard.edu/pph2/) tools. In SIFT server, a highly conserved position is more likely to be deleterious with a SIFT score <0.05, whereas a tolerant mutation will have a SIFT score >0.0532,33. PolyPhen-2 extracts various sequence- and structure-based features of the substitution site and inputs them into a probabilistic classifier based on a given AAS and protein accession. The mutation is appraised qualitatively, as benign, possibly damaging, or most likely damaging34.
Identifying disease-associated nsSNPs using the PhD-SNP and MutPred tools
PhD-SNP (http://snps.biofold.org/phd-snp) and MutPred (http://mutpred.mutdb.org/) were based on a support vector machine (SVM) and the SIFT algorithm. To PhD-SNP, in briefly, after inputting the protein sequence, position and new residue, the substitution from the wild type residue to the mutant is encoded in a 20-element vector that is −1 in position relative to the wild type residue, 1 in the position relative to the mutant residues and 0 in the remaining 18 positions. Next, a second 20-element vector encoding the sequence environment is constructed to report the occurrence of residues in a window of 19 residues around the mutated residue. With this supervised learning approach, a given mutation is classified as disease or neutral35,36.
MutPred is based on SIFT scores, the gain or loss of 14 different structural and functional properties. Two important scores are contained in the output of MutPred: a general score (g), and top 5 property score (p). The general score (g) indicates the probability that the AAS is deleterious/disease-associated, whereas the top 5 property score (p) is the P-value that indicates whether certain structural and functional properties are affected. The combinations of high general scores and low property scores are referred to as actionable hypotheses, confident hypotheses, and very confident hypotheses37.
Protein structure prediction of pathogenic nsSNPs via Robetta and SWISS-MODEL tools
As the structure of wolframin is not available and there is not suitable template for modelling, so we choose the Robetta server (http://robetta.bakerlab.org/) to construct the protein structure. The Robetta server is a full chain protein structure prediction server for ab initio and comparative modeling, and the SWISS-MODEL (http://swissmodel.expasy.org/) is a fully automated, dedicated protein structure homology-modelling server38,39. The amino acid sequence of wolframin was retrieved from NCBI (accession number: NP_005996.2). 3D-structure of wolframin was performed using Robetta server. And the mutant proteins were constructed by SWISS-MODEL with the template performed using Robetta server (Sup.file S). The quality of the modelled structure of native and mutant protein was evaluated by the PROCHECK (http://services.mbi.ucla.edu/SAVES/).
Analysis of the transmembrane domain by the TMHMM server v2.0
TMHMM server v2.0 (http://www.cbs.dtu.dk/services/TMHMM/), based on a hidden Markov model (HMM) with an architecture that corresponds closely to the biological system, is a membrane protein topology prediction method. Compared with other servers, TMHMM server v2.0, which is thought to be currently the best performing transmembrane prediction program, can model and predict the location and orientation of alpha helices in membrane-spanning proteins with high accuracy40.
Additional Information
How to cite this article: Qian, X. et al. Phenotype Prediction of Pathogenic Nonsynonymous Single Nucleotide Polymorphisms in WFS1. Sci. Rep. 5, 14731; doi: 10.1038/srep14731 (2015).
Supplementary Material
Acknowledgments
This research was supported in part by the National Natural Science Foundation of China (No. 31171217) to XC, the Grant from Jiangsu Health Administration of China (LJ201120) and the Research Special Fund for Public Welfare Industry of Health, Ministry of Health of China (No. 201202005) to GX.
Footnotes
Author Contributions Conceived and designed the experiments: X.C. Analyzed the data: X.Q. and G.X. Wrote the first draft of the manuscript: X.Q. L.Q. partially modified the manuscript in the later phases of revised versions. Reviewed, edited and approved the manuscript: X.C.
References
- de Heredia M. L., Clèries R. & Nunes V. Genotypic classification of patients with Wolfram syndrome: insights into the natural history of the disease and correlation with phenotype. Genetics in Medicine 15, 497–506 (2013). [DOI] [PubMed] [Google Scholar]
- Ayme S. et al. Diagnosis and clinical features of Wolfram Syndrome. Management of Wolfram Syndrome: A Clinical Guideline. (2014) (http://euro-wabb.org/images/euro-wabb/guidelines/Wolfram_guideline_V14_%2028_04_2014.pdf). EURO-WABB Project. Accessed: 28th April 2014.
- Barrett T. G., Bundey S. E. & Macleod A. F. Neurodegeneration and diabetes: UK nationwide study of wolfram (didmoad) syndrome. Lancet 346, 1458–1463 (1995). [DOI] [PubMed] [Google Scholar]
- Wolfram D. J. & Wagener H. P. Diabetes mellitus and simple optic atrophy among siblings: report of four cases. Mayo Clinic Proceedings 13, 715–718 (1938). [Google Scholar]
- Matsunaga K. et al. Wolfram syndrome in the Japanese population: molecular analysis of WFS1 gene and characterization of clinical features. PLoS One 9, e106906 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H. et al. A gene encoding a transmembrane protein is mutated in patients with diabetes mellitus and optic atrophy (wolfram syndrome). Nature Genetics 20, 143–148 (1998). [DOI] [PubMed] [Google Scholar]
- Strom T. M. et al. Diabetes insipidus, diabetes mellitus, optic atrophy and deafness (didmoad) caused by mutations in a novel gene (wolframin) coding for a predicted transmembrane protein. Human Molecular Genetics 7, 2021–2028 (1998). [DOI] [PubMed] [Google Scholar]
- Rigoli L., Lombardo F. & i Bella C. D. Wolfram syndrome and WFS1 gene. Clinical Genetic 79, 103–117 (2011). [DOI] [PubMed] [Google Scholar]
- Gasparin M. R. et al. Identification of novel mutations of the WFS1 gene in brazilian patients with wolfram syndrome. European Journal of Endocrinology 160, 309–316 (2009). [DOI] [PubMed] [Google Scholar]
- Sütt S. et al. Wfs1-deficient animals have brain-region-specific changes of Na+, K+ -ATPase activity and mRNA expression of α1 and β1 subunits. Journal of Neuroscience Research 93, 530–537 (2015). [DOI] [PubMed] [Google Scholar]
- Takeda K. et al. WFS1 (wolfram syndrome 1) gene product: Predominant subcellular localization to endoplasmic reticulum in cultured cells and neuronal expression in rat brain. Human Molecular Genetics 10, 5477–5484 (2001). [DOI] [PubMed] [Google Scholar]
- Osman A. A. et al. Wolframin expression induces novel ion channel activity in endoplasmic reticulum membranes and increases intracellular calcium. Journal of Biological Chemistry 278, 52755–52762 (2003). [DOI] [PubMed] [Google Scholar]
- McBain S. C & Morgan N. G. Functional effects of expression of wolframin-antisense transcripts in brin-bd11 beta-cells. Biochemical and Biophysical Research Communications 307, 684–688 (2003). [DOI] [PubMed] [Google Scholar]
- Shang L. et al. β-cell dysfunction due to increased ER stress in a stem cell model of Wolfram syndrome. Diabete 63, 923–933 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann S. et al. Wolfram syndrome: structural and functional analyses of mutant and wild-type wolframin, the WFS1 gene product. Human Molecular Genetics 12, 16 (2003). [DOI] [PubMed] [Google Scholar]
- Kumar A. & Purohit R. Computational investigation of pathogenic nsSNPs in CEP63 protein. Gene 503, 75–82 (2012). [DOI] [PubMed] [Google Scholar]
- Naresh K. et al. Computational screening of disease associated mutations on NPC1 gene and its structural consequence in Niemann-Pick type-C1. Frontiers in Biology 9, 410–421 (2014). [Google Scholar]
- Banerjee S. et al. In silico analysis of all point mutations on the 2B domain of K5/K14 causing epidermolysis bullosa simplex: a genotype-phenotype correlation. Molecular Biosystems 10, 2567–2577 (2014). [DOI] [PubMed] [Google Scholar]
- Carvalho M. A. et al. Determination of cancer risk associated with germ line brca1 missense variants by functional analysis. Cancer Research 67, 1494–1501 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy C. et al. Clinical and molecular genetic analysis of 19 wolfram syndrome kindreds demonstrating a wide spectrum of mutations in WFS1. American Journal of Human Genetics 65, 1279–1290 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessa A. et al. Identification of novel WFS1 mutations in Italian children with Wolfram syndrome. Human Mutation 17, 348–349 (2001). [DOI] [PubMed] [Google Scholar]
- Rohayem J. et al. Diabetes and neurodegeneration in Wolfram syndrome: a multicenter study of phenotype and genotype. Diabetes Care 34, 1503–1510 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaussenot A. et al. Neurologic features and genotype-phenotype correlation in Wolfram syndrome. Ann Neurol 69, 501–508 (2011). [DOI] [PubMed] [Google Scholar]
- Fonseca S. G. et al. WFS1 is a novel component of the unfolded response and protein maintains homeostasis of the endoplasmic reticulum in pancreatic beta-cells. Journal of Biological Chemistry 280, 39609–39615 (2005). [DOI] [PubMed] [Google Scholar]
- Yuca S. A. et al. Rapidly progressive renal disease as part of Wolfram syndrome in a large inbred Turkish family due to a novel WFS1 mutation (p.Leu511Pro). European Journal of Medical Genetics 55, 37–42 (2012). [DOI] [PubMed] [Google Scholar]
- Xu Q., Qu H. & Wei S. Clinical and molecular genetic analysis of a new mutation in children with Wolfram syndrome: a case report. Molecular Medicine Reports 7, 965–968 (2013). [DOI] [PubMed] [Google Scholar]
- Gurtler N. et al. Two families with nonsyndromic low-frequency hearing loss harbor novel mutations in wolfram syndrome gene 1. Journal of Molecular Medicine 83, 553–560 (2005). [DOI] [PubMed] [Google Scholar]
- Zatyka M. et al. Sodium-potassium ATPase 1 subunit is a molecular partner of Wolframin, an endoplasmic reticulum protein involved in ER stress. Human Molecular Genetics 17, 190–200 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanna D. et al. Identification of p.A684V missense mutation in the WFS1 gene as a frequent cause of autosomal dominant optic atrophy and hearing impairment. American Journal of Medical Genetics Part A 155, 1298–1313 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry S. T. et al. dbsnp: the NCBI database of genetic variation. Nucleic Acids Research 29, 308–311 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenson P. D. et al. The human gene mutation database: Building a comprehensive mutation repository for clinical and molecular genetics, diagnostic testing and personalized genomic medicine. Human Genetics 133, 1–9 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim N. L. et al. Sift web server: Predicting effects of amino acid substitutions on proteins. Nucleic Acids Research 40, 452–457 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng P. C. & Henikoff S. Sift: Predicting amino acid changes that affect protein function. Nucleic Acids Research 31, 3812–3814 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adzhubei I. A. et al. A method and server for predicting damaging missense mutations. Nature Methods 7, 248–249 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capriotti E., Fariselli P., Calabrese R. & Casadio R.. Predicting protein stability changes from sequences using support vector machines. Bioinformatics 21 Suppl 2, 54–58 (2005). [DOI] [PubMed] [Google Scholar]
- Capriotti E., Calabrese R. & Casadio R. Predicting the insurgence of human genetic diseases associated to single point protein mutations with support vector machines and evolutionary information. Bioinformatics 22, 2729–2734 (2006). [DOI] [PubMed] [Google Scholar]
- Li B. et al. Automated inference of molecular mechanisms of disease from amino acid substitutions. Bioinformatics 25, 2744–2750 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold K., Bordoli L., Kopp J. & Schwede T. The SWISS-MODEL workspace: a web-based environment for protein structure homology modeling. Bioinformatics 22, 195–201 (2006). [DOI] [PubMed] [Google Scholar]
- Schwede T., Kopp J. Guex N. & Peitsch. SWISS-MODEL: An automated protein homology-modeling server. Nucleic Acids Research 31, 3381–3385 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller S., Croning M. D. R. & Apweiler R. Evaluation of methods for the prediction of membrane spanning regions. Bioinformatics 17, 646–653 (2001). [DOI] [PubMed] [Google Scholar]
- Giuliano F. et al. Wolfram syndrome in French population: Characterization of novel mutations and polymorphisms in the WFS1 gene. Human Mutation 25, 99–100 (2005). [DOI] [PubMed] [Google Scholar]
- Zenteno J. C., Ruiz G., Perez-Cano H. J. & Camargo M.. Familial wolfram syndrome due to compound heterozygosity for two novel WFS1 mutations. Molecular Vision 14, 1353–1357 (2008). [PMC free article] [PubMed] [Google Scholar]
- Bonnycastle L. et al. Autosomal dominant diabetes arising from a Wolfram syndrome 1 mutation. Diabetes 62, 3943–3950 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford J. et al. Is there a relationship between wolfram syndrome carrier status and suicide. American Journal of Medical Genetics 114, 343–346 (2002). [DOI] [PubMed] [Google Scholar]
- Cano A. et al. Identification of novel mutations in WFS1 and genotype-phenotype correlation in wolfram syndrome. American Journal of Medical Genetics 143, 1605–1612 (2007). [DOI] [PubMed] [Google Scholar]
- Colosimo A. et al. Molecular detection of novel WFS1 mutations in patients with wolfram syndrome by a dhplc-based assay. Human Mutation 21, 622–629 (2003). [DOI] [PubMed] [Google Scholar]
- Smith C. J. et al. Phenotype-genotype correlations in a series of wolfram syndrome families. Diabetes Care 27, 2003–2009 (2004). [DOI] [PubMed] [Google Scholar]
- Zalloua P. A. et al. WFS1 mutations are frequent monogenic causes of Juvenile-onset diabetes mellitus in Lebanon. Human Molecular Genetics 17, 4012–4021 (2008). [DOI] [PubMed] [Google Scholar]
- Torres R. et al. Mutation screening of the Wolfram syndrome gene in psychiatric patients. Molecular Psychiatry 6, 39–43 (2001). [DOI] [PubMed] [Google Scholar]
- van ven Ouweland J. M. et al. Molecular characterization of WFS1 in patients with wolfram syndrome. Journal of Molecular Diagnostics 5, 88–95 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganie M. A. et al. Presentation and clinical course of Wolfram (DIDMOAD) syndrome from North India. Diabetic Medicine 28, 1337–1342 (2011). [DOI] [PubMed] [Google Scholar]
- Yu G. et al. WS1 gene mutation analysis of wolfram syndrome in a Chinese patient and a systematic review of literatures. Endocrine 38, 147–152 (2010). [DOI] [PubMed] [Google Scholar]
- Gasparin M. R. et al. Identification of novel mutations of the WFS1 gene in brazilian patients with wolfram syndrome. European Journal of Endocrinology 160, 309–316 (2009). [DOI] [PubMed] [Google Scholar]
- Swift M. & Swift R. G. Wolframin mutations and hospitalization for psychiatric illness. Molecular Psychiatry 10, 799–803 (2005). [DOI] [PubMed] [Google Scholar]
- Khanim F., Kirk, Latif J. F. & Barrett T. G. WFS1/wolframin mutations, wolfram syndrome, and associated diseases. Human Mutation 17, 357–367 (2001). [DOI] [PubMed] [Google Scholar]
- Cryns K. et al. Mutations in the WFS1 gene that cause low-frequency sensorineural hearing loss are small non-inactivating mutations. Human Genetics 110, 389–394 (2002). [DOI] [PubMed] [Google Scholar]
- Fukuoka H. Mutations in the WFS1 gene are a frequent cause of autosomal dominant nonsyndromic low-frequency hearing loss in Japanese. Journal of Human Genetics 52, 510–515 (2007). [DOI] [PubMed] [Google Scholar]
- Bramhall N. F. et al. A novel WFS1 mutation in a family with dominant low frequency sensorineural hearing loss with normal VEMP and EcochG findings. BMC Medical Genetics 2, 48 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y. et al. Identification of two novel missense WFS1 mutations, H696Y and R703H, in patients with non-syndromic low-frequency sensorineural hearing loss. Journal of Genetics and Genomics 38, 71–76 (2011). [DOI] [PubMed] [Google Scholar]
- Domenech E., Gomez-Zaera M. & Nunes V. Study of the WFS1 gene and mitochondrial DNA in Spanish wolfram syndrome families. Clinical Genetics 65, 463–469 (2004). [DOI] [PubMed] [Google Scholar]
- Bespalova I. N. et al. Mutations in the wolfram syndrome 1 gene (WFS1) are a common cause of low frequency sensorineural hearing loss. Human Molecular Genetics 10, 2501–2508 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiberg H. et al. Autosomal dominant optic atrophy associated with hearing impairment and impaired glucose regulation caused by a missense mutation in the WFS1 gene. Journal of Medical Genetic 43, 435–440 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.