Abstract
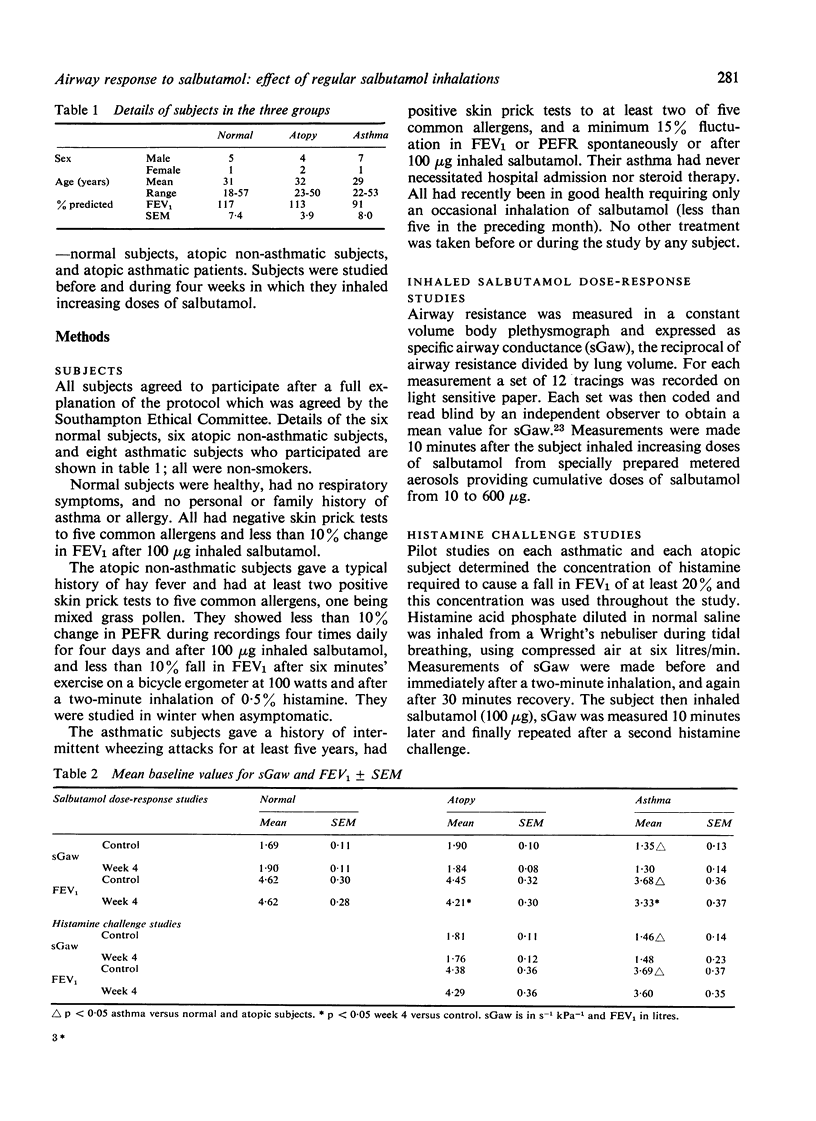
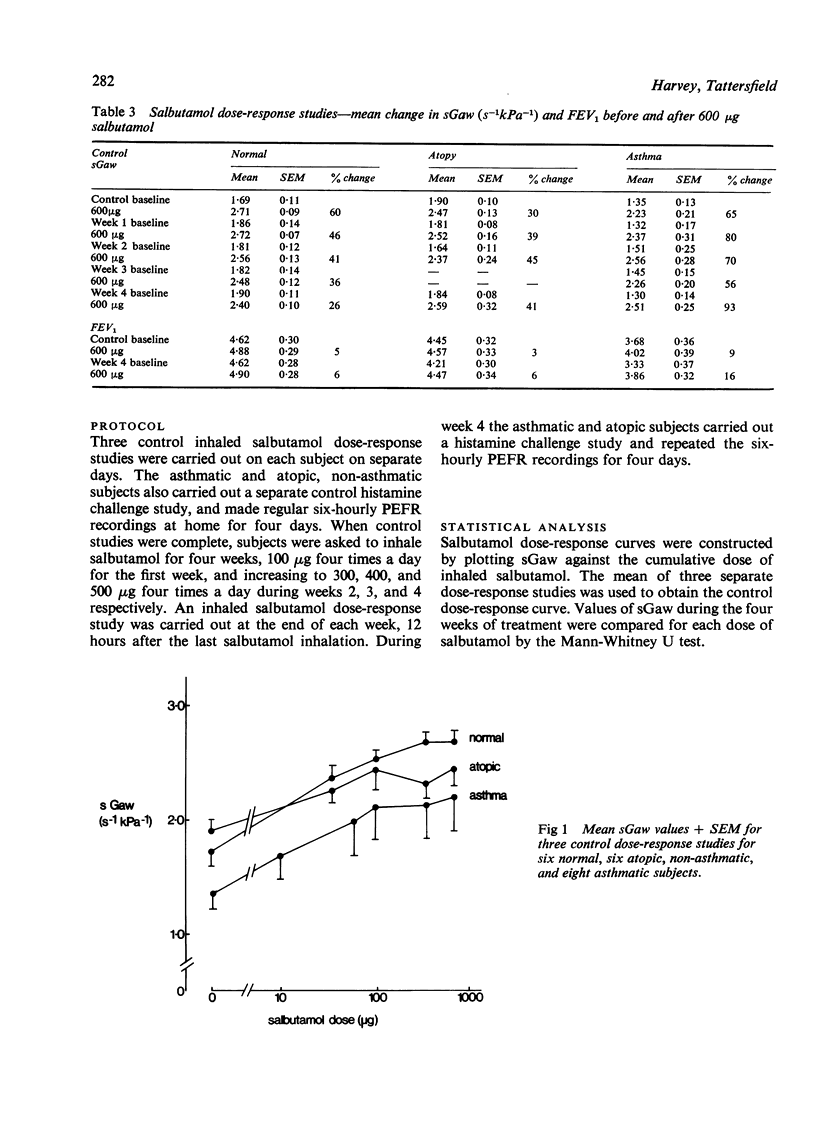
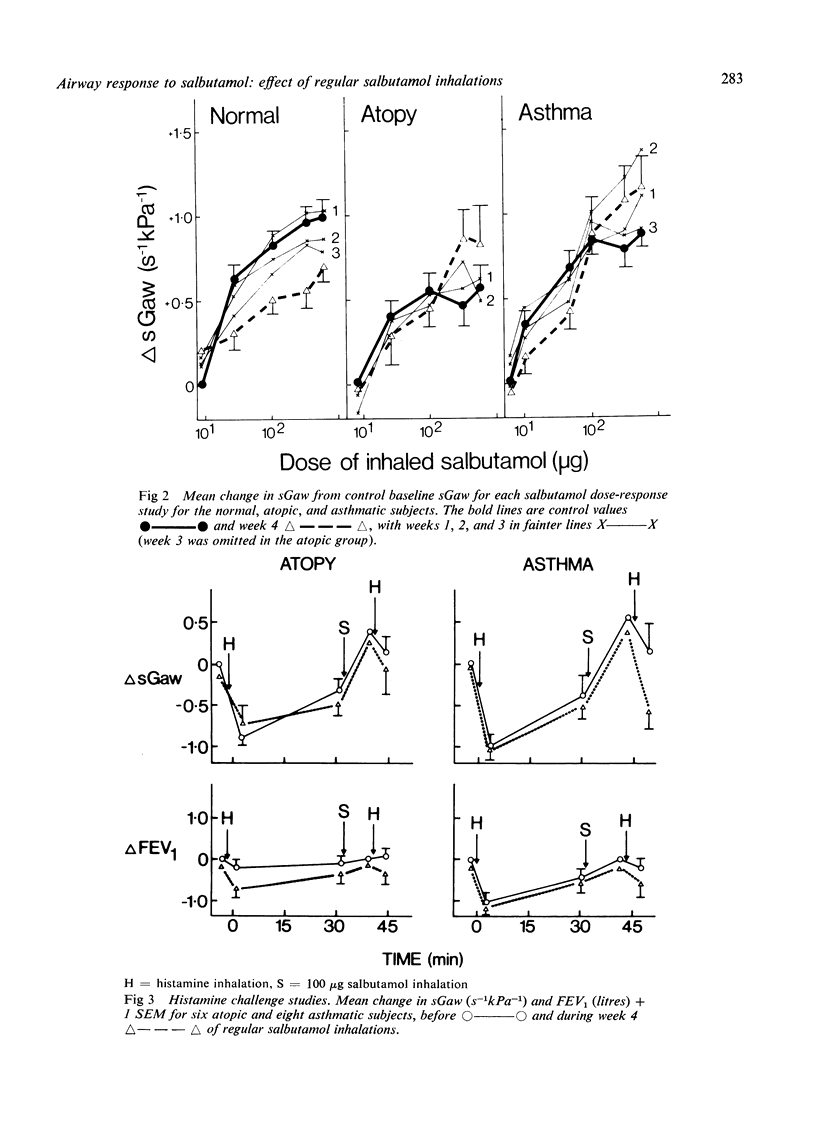
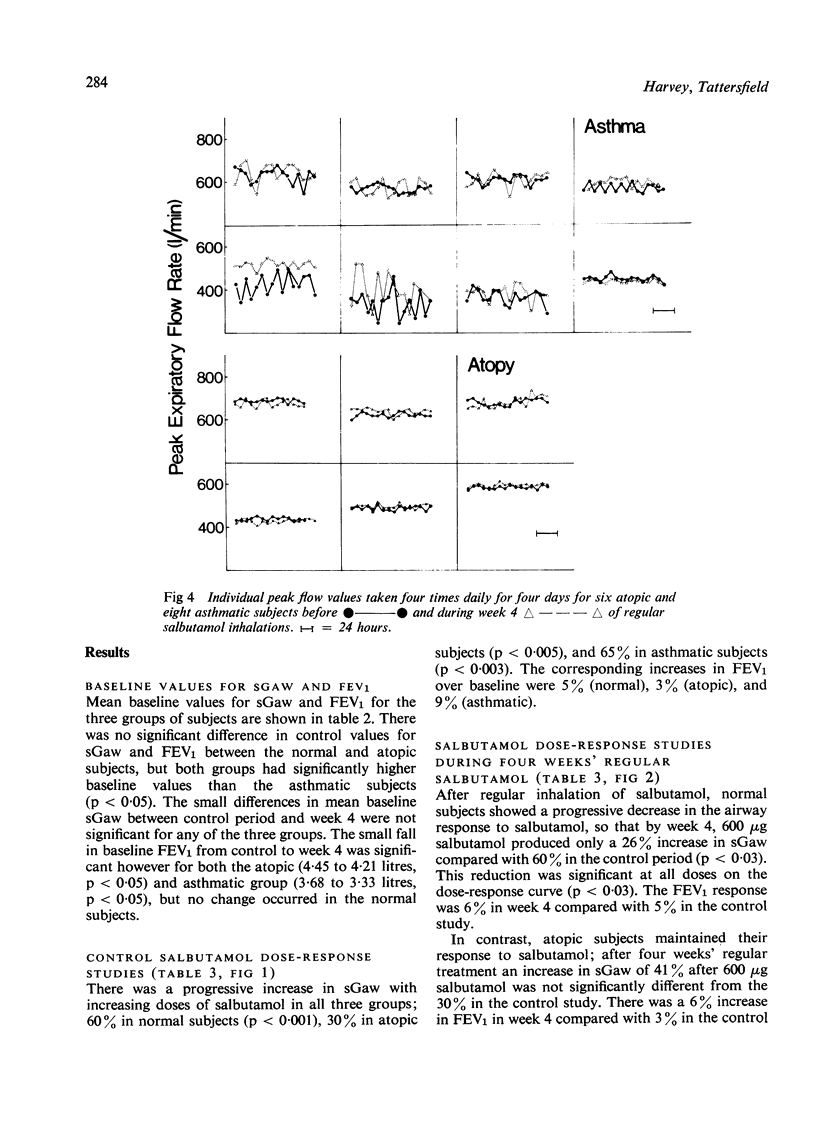
This study was designed to determine whether resistance to the airway effects of the beta-agonist, salbutamol, would develop in three groups of subjects while taking large doses of inhaled salbutamol. Six normal non-atopic, six atopic non-asthmatic, and eight atopic asthmatic subjects were studied by an identical technique. The development of resistance was assessed from salbutamol dose-response studies in which the airway response was measured as specific airway conductance (sGaw). Further evidence was sought in the atopic and asthmatic subjects by measuring the airway response to a standard histamine inhalation challenge and the protective effect of 100 micrograms salbutamol on this challenge, and by six-hourly peak flow recordings. Subjects were assessed before and during four weeks in which they took inhaled salbutamol regularly in doses increasing to 500 microgram quid in week 4. Normal subjects showed a progressive reduction in the bronchodilator (sGaw) response to salbutamol during the four weeks, indicating the progressive development of resistance. The atopic subjects, both asthmatic and non-asthmatic, showed no reduction in the response to salbutamol during the four weeks, nor any change in the response to histamine challenge or in regular peak flow readings. These results demonstrate that asthmatic patients do not develop bronchial beta-adrenoceptor resistance easily and suggests that they and atopic non-asthmatic subjects are less susceptible to its development than normal subjects.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes P. J., Dollery C. T., MacDermot J. Increased pulmonary alpha-adrenergic and reduced beta-adrenergic receptors in experimental asthma. Nature. 1980 Jun 19;285(5766):569–571. doi: 10.1038/285569a0. [DOI] [PubMed] [Google Scholar]
- Brooks S. M., McGowan K., Bernstein I. L., Altenau P., Peagler J. Relationship between numbers of beta adrenergic receptors in lymphocytes and disease severity in asthma. J Allergy Clin Immunol. 1979 Jun;63(6):401–406. doi: 10.1016/0091-6749(79)90213-6. [DOI] [PubMed] [Google Scholar]
- Campbell A. H. Mortality from asthma and bronchodilator aerosols. Med J Aust. 1976 Mar 20;1(12):386–391. [PubMed] [Google Scholar]
- Conolly M. E., Davies D. S., Dollery C. T., George C. F. Resistance to -adrenoceptor stimulants (a possible explanation for the rise in ashtma deaths). Br J Pharmacol. 1971 Oct;43(2):389–402. [PMC free article] [PubMed] [Google Scholar]
- Formgren H. The therapeutic value of oral long-term treatment with terbutaline (Bricanyl) in asthma. A follow-up study of its efficacy and side effects. Scand J Respir Dis. 1975;56(6):321–328. [PubMed] [Google Scholar]
- Fuller D. J., Byus C. V., Russell D. H. Specific regulation by steroid hormones of the amount of type I cyclic AMP-dependent protein kinase holoenzyme. Proc Natl Acad Sci U S A. 1978 Jan;75(1):223–227. doi: 10.1073/pnas.75.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galant S. P., Duriseti L., Underwood S., Allred S., Insel P. A. Beta adrenergic receptors of polymorphonuclear particulates in bronchial asthma. J Clin Invest. 1980 Mar;65(3):577–585. doi: 10.1172/JCI109702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galant S. P., Duriseti L., Underwood S., Insel P. A. Decreased beta-adrenergic receptors on polymorphonuclear leukocytes after adrenergic therapy. N Engl J Med. 1978 Oct 26;299(17):933–936. doi: 10.1056/NEJM197810262991707. [DOI] [PubMed] [Google Scholar]
- Gallagher J. T., Kent P. W., Passatore M., Phipps R. J., Richardson P. S. The composition of tracheal mucus and the nervous control of its secretion in the cat. Proc R Soc Lond B Biol Sci. 1975 Dec 31;192(1106):49–76. doi: 10.1098/rspb.1975.0151. [DOI] [PubMed] [Google Scholar]
- Gibson G. J., Greenacre J. K., König P., Conolly M. E., Pride N. B. Use of exercise challenge to investigate possible tolerance to beta-adrenoceptor stimulation in asthma. Br J Dis Chest. 1978 Jul;72(3):199–206. doi: 10.1016/0007-0971(78)90042-6. [DOI] [PubMed] [Google Scholar]
- Greenacre J. K., Schofield P., Conolly M. E. Desensitization of the beta-adrenoceptor of lymphocytes from normal subjects and asthmatic patients in vitro. Br J Clin Pharmacol. 1978 Mar;5(3):199–206. doi: 10.1111/j.1365-2125.1978.tb01624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey J. E., Baldwin C. J., Wood P. J., Alberti K. G., Tattersfield A. E. Airway and metabolic responsiveness to intravenous salbutamol in asthma: effect of regular inhaled salbutamol. Clin Sci (Lond) 1981 May;60(5):579–585. doi: 10.1042/cs0600579. [DOI] [PubMed] [Google Scholar]
- Heaf P. J. Deaths in asthma: a therapeutic misadventure? Br Med Bull. 1970 Sep;26(3):245–247. doi: 10.1093/oxfordjournals.bmb.a070792. [DOI] [PubMed] [Google Scholar]
- Holgate S. T., Baldwin C. J., Tattersfield A. E. beta-adrenergic agonist resistance in normal human airways. Lancet. 1977 Aug 20;2(8034):375–377. doi: 10.1016/s0140-6736(77)90304-x. [DOI] [PubMed] [Google Scholar]
- Holgate S. T., Stubbs W. A., Wood P. J., McCaughey E. S., Alberti K. G., Tattersfield A. E. Airway and metabolic resistance to intravenous salbutamol: a study in normal man. Clin Sci (Lond) 1980 Sep;59(3):155–161. doi: 10.1042/cs0590155. [DOI] [PubMed] [Google Scholar]
- Inman W. H., Adelstein A. M. Rise and fall of asthma mortality in England and Wales in relation to use of pressurised aerosols. Lancet. 1969 Aug 9;2(7615):279–285. doi: 10.1016/s0140-6736(69)90051-8. [DOI] [PubMed] [Google Scholar]
- Jenne J. W., Chick T. W., Strickland R. D., Wall F. J. Subsensitivity of beta responses during therapy with a long-acting beta-2 preparation. J Allergy Clin Immunol. 1977 May;59(5):383–390. doi: 10.1016/0091-6749(77)90023-9. [DOI] [PubMed] [Google Scholar]
- Larsson S., Svedmyr N., Thiringer G. Lack of bronchial beta adrenoceptor resistance in asthmatics during long-term treatment with terbutaline. J Allergy Clin Immunol. 1977 Feb;59(2):93–100. doi: 10.1016/0091-6749(77)90209-3. [DOI] [PubMed] [Google Scholar]
- Miller J., Wallace D., Grieco M. H., Frenkel R., Larsen K. Double-blind trial of oral carbuterol in bronchial asthma. Ann Allergy. 1977 Jul;39(1):12–17. [PubMed] [Google Scholar]
- Morris H. G., Rusnak S. A., Selner J. C., Barzens K., Barnes J. Comparative effects of ephedrine on adrenergic responsiveness in normal and asthmatic subjects. J Allergy Clin Immunol. 1978 May;61(5):294–302. doi: 10.1016/0091-6749(78)90050-7. [DOI] [PubMed] [Google Scholar]
- Mukherjee C., Caron M. G., Lefkowitz R. J. Regulation of adenylate cyclase coupled beta-adrenergic receptors by beta-adrenergic catecholamines. Endocrinology. 1976 Aug;99(2):347–357. doi: 10.1210/endo-99-2-347. [DOI] [PubMed] [Google Scholar]
- Nelson H. S., Black J. W., Branch L. B., Pfuetze B., Spaulding H., Summers R., Wood D. Subsensitivity to epinephrine following the administration of epinephrine and ephedrine to normal individuals. J Allergy Clin Immunol. 1975 May;55(5):299–309. doi: 10.1016/0091-6749(75)90002-0. [DOI] [PubMed] [Google Scholar]
- Parker C. W., Smith J. W. Alterations in cyclic adenosine monophosphate metabolism in human bronchial asthma. I. Leukocyte responsiveness to -adrenergic agents. J Clin Invest. 1973 Jan;52(1):48–59. doi: 10.1172/JCI107173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson J. W., Conolly M. E., Davies D. S., Dollery C. T. Isoprenaline resistance and the use of pressurised aerosols in asthma. Lancet. 1968 Aug 24;2(7565):426–429. doi: 10.1016/s0140-6736(68)90467-4. [DOI] [PubMed] [Google Scholar]
- Peel E. T., Gibson G. J. Effects of long-term inhaled salbutamol therapy on the provocation of asthma by histamine. Am Rev Respir Dis. 1980 Jun;121(6):973–978. doi: 10.1164/arrd.1980.121.6.973. [DOI] [PubMed] [Google Scholar]
- Plummer A. L. The development of drug tolerance to beta2 adrenergic agents. Chest. 1978 Jun;73(6 Suppl):949–957. doi: 10.1378/chest.73.6.949. [DOI] [PubMed] [Google Scholar]
- Reisman R. E. Asthma induced by adrenergic aerosols. J Allergy. 1970 Sep;46(3):162–177. doi: 10.1016/0021-8707(70)90095-x. [DOI] [PubMed] [Google Scholar]
- Szentivanyi A. The radioligand binding approach in the study of lymphocytic adrenoceptors and the constitutional basis of atopy. J Allergy Clin Immunol. 1980 Jan;65(1):5–11. doi: 10.1016/0091-6749(80)90170-0. [DOI] [PubMed] [Google Scholar]
- Tattersfield A. E., Keeping I. M. Assessing change in airway calibre--measurement of airway resistance. Br J Clin Pharmacol. 1979 Oct;8(4):307–319. doi: 10.1111/j.1365-2125.1979.tb04711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Metre T. E., Jr Adverse effects of inhalation of excessive amounts of nebulized isoproterenol in status asthmaticus. J Allergy. 1969 Feb;43(2):101–113. doi: 10.1016/0021-8707(69)90130-0. [DOI] [PubMed] [Google Scholar]
- VanArsdel P. P., Jr, Schaffrin R. M., Rosenblatt J., Sprenkle A. C., Altman L. C. Evaluation of oral fenoterol in chronic asthmatic patients. Chest. 1978 Jun;73(6 Suppl):997–998. doi: 10.1378/chest.73.6_supplement.997. [DOI] [PubMed] [Google Scholar]
- Wilson A. F., Novey H. S., Cloninger P., Davis J., White D. Cardiopulmonary effects of long-term bronchodilator administration. J Allergy Clin Immunol. 1976 Jul;58(1 Pt 2):204–212. doi: 10.1016/0091-6749(76)90156-1. [DOI] [PubMed] [Google Scholar]



