Abstract
Intravenous immunoglobulin has been shown to decrease the risk of post-transplant infections in heart recipients with IgG hypogammaglobulinemia, however the use of subcutaneous immunoglobulin has not been reported. We report on immune reconstitution, clinical efficacy and tolerability of subcutaneous immunoglobulin replacement therapy in a heart transplant recipient with secondary antibody deficiency. Maintenance of IgG levels, specific antibodies and control of infections were observed after change from intravenous immunoglobulin to subcutaneous immunoglobulin due to poor intravenous access. Recurrences of severe infections were observed when subcutaneous immunoglobulin infusions were stopped. Our observations suggest that subcutaneous immunoglobulin replacement therapy might be effective and well tolerated in selected heart recipients.
Keywords: heart transplantation, hypogammaglobulinemia, infection, subcutaneous immunoglobulin
Introduction
Intravenous immunoglobulin (IVIG) replacement therapy is safe and useful to reconstitute IgG levels in heart recipients with severe infections and IgG hypogammaglobulinemia after transplantation [1].
The potential role of subcutaneous immunoglobulin (SCIG) replacement therapy in this setting has not been described in heart transplantation [2].
We describe our experience in the use of SCIG in a heart recipient with combined secondary post-transplant antibody and functional cellular deficiency and recurrent severe infections.
IVIG and SCIG were used in a compassionate use basis. Ethical committee approval was obtained. Bacterial infections were diagnosed by culture, cytomegalovirus (CMV) infection by CMV antigenemia and aspergillosis by Aspergillus fumigatus isolation. The patient gave written informed consent.
Case Report
A 61-year-old man received a heart transplantation. The patient was CMV seronegative and the donor CMV seropositive. In the pre-transplant period he did not have infections.
Induction therapy included daclizumab, methylprednisolone and mofetil mycophenolate.
There was no evidence of primary allograft failure. Maintenance immunosuppressive therapy included tacrolimus (from transplantation to month 26), mofetil mycophenolate (from transplantation to month 9), azathioprine (from month 9), everolimus (from month 26) and prednisone. Prophylaxis included IV gancyclovir followed by oral valgancyclovir during 12 weeks. Infectious episodes were as follows: at day 14, Pseudomonas aeruginosa bacteremia, Haemophilus influenzae and methicillin resistant staphylococcal respiratory infection; at month 5, late CMV disease and at month 9, invasive Aspergillus fumigatus infection (renal and prostatic).
Antibody deficiency was documented by a decrease of distinct antibodies as follows: on day 7 and month 1 post-transplantation total IgG (nephelometry) and specific antibody levels (ELISA) were 776 and 454 mg/dL, respectively; anti-HBs, 37.7 and 16 mU/mL; anti-pneumococcal polysaccharide, 7.6 and 2.5 mg/dL; anti-tetanus toxoid, 0.7 and 0.2 IU/dL and anti-CMV titer, 3958 and 597.
The evaluation of cellular immunity disclosed a progressive decrease in the percentage of interferon-producing CD8 T cells against intermediate-1 CMV antigen from baseline (pre-transplantation 0.64%) to 3 months after transplantation (0%). In the evaluation of innate immunity the patient was found to have very low mannose binding levels before heart transplantation, at one week and one month after transplantation (25 ng/mL). IgA and complement C3 levels were within normal ranges during follow-up.
The patient received replacement IVIG therapy in hospital from months 2 to 8 (6 months) and from month 10 to 20 (10 months) after transplantation because of recurrent severe infections with post heart transplant hypogammaglobulinemia (defined as serum IgG < 600 mg/dL) and decreased specific antibody levels. At month 16 disappearance of aspergillus lesions was demonstrated after combined use of voriconazole and IVIG.
At month 20, bronchoalveolar lung carcinoma was diagnosed.
Due to poor intravenous access, the patient was changed from IVIG to SCIG infusions (Vivaglobin 16%, CSL Behring), at 100 mg/kg/week. SCIG infusions were administered 3 months at the hospital and then at home, when infusions proved to be well tolerated.
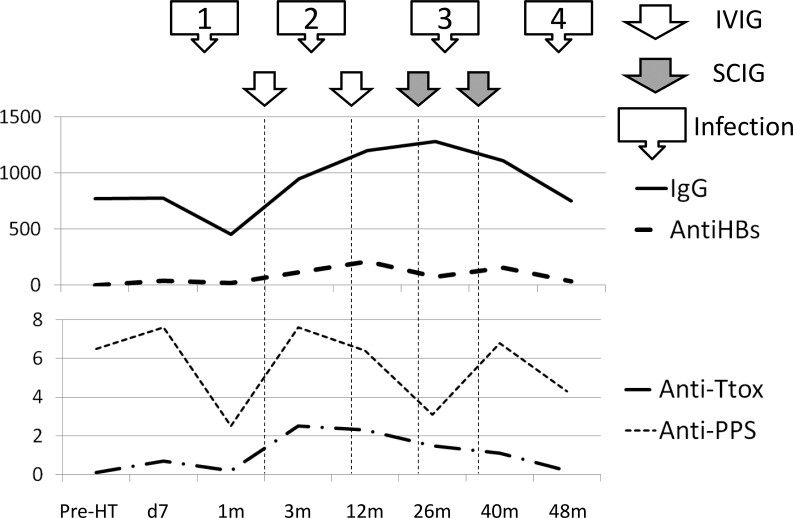
During the 6-month clinical follow-up with SCIG from month 22 to 28 (6 months), IgG levels were maintained at over 1000 mg/dL, the patient tolerated the infusions well and no infectious complications were observed (Figure 1).
Figure 1.
IVIG was started at months 2 and 10. SCIG was started at month 22 and 36. 48m: Latest study time during follow-up, 2 months after SCIG was stopped. Anti-PPS: anti-pneumococcal polysaccharyde 23 serotypes (mg/dL); anti-HBS: anti-hepatitis B surface antigen (mU/mL); anti-Ttox: anti-tetanus toxoid (mg/dL); IgG: serum IgG levels (mg/dL); pre-HT: pre heart transplantation. Infection 1-4: infectious episodes.
IVIG = intravenous immunoglobulin; SCIG = subcutaneous immunoglobulin.
At month 28 SCIG infusions were stopped.
Recurrent bacterial pneumonia, Clostridium-difficile-associated diarrhea and respiratory syncytial virus pneumonia occurred between months 29 to 36 beginning 1 month after SCIG infusions were stopped.
The patient restarted SCIG replacement therapy from month 36 to 46 (10 months) without further recurrence of infections during this period. At month 46 SCIG were stopped again.
Extrahospitalary pneumonia followed by Enterococcus faecalis septic shock presented at month 48, 2 months after SCIG infusions were stopped.
No systemic or local reactions were observed during SCIG infusions. There was no evidence of renal failure during IVIG or SCIG administration periods. During follow-up there were no episodes of acute cellular rejection or cardiac vascular allograft disease.
Discussion
SCIG was well tolerated and associated with control of infections in a heart recipient with post-transplant antibody and cellular deficiency.
The potential protective mechanisms included maintenance of IgG and specific antibodies against microbial polysaccharides and proteins.
A previous study performed in 10 lung recipients with hypogammaglobulinemia demonstrated increase in IgG levels at three months that was sustained at 6-12 months with SCIG replacement therapy [2]. Long-term IgG replacement with SCIG may be necessary for selected patients with sustained antibody deficiency and recurrent infections. Potential advantages of SCIG in these patients include independence from hospital-based infusion settings and an alternative for patients with poor venous access [3].
Higher infusion frequency is the main disadvantage of SCIG [4].
We describe an innovative approach to IgG replacement in a heart recipient with antibody deficiency and severe infections, but the effectiveness of this intervention cannot be inferred based on a single case report.
The safety and efficacy of SCIG in this setting warrant evaluation in a clinical trial [5].
Footnotes
Source of Support Nil.
Disclosures None declared.
Cite as: Carbone J, Palomo J, Fernandez-Yañez J, Sarmiento E. Subcutaneous immunoglobulin replacement therapy in a heart transplant recipient with severe recurrent infections. Heart, Lung and Vessels. 2015; 7(3): 256-259.
References
- Carbone J, Sarmiento E, Del Pozo N, Rodriguez-Molina J J, Navarro J, Fernandez-Yañez J. et al. Restoration of humoral immunity after intravenous immunoglobulin replacement therapy in heart recipients with post-transplant antibody deficiency and severe infections. Clin Transplant. 2012;26:277–283. doi: 10.1111/j.1399-0012.2012.01653.x. [DOI] [PubMed] [Google Scholar]
- Shankar T, Gribowicz J, Crespo M, Silveira F P, Pilewski J, Petrov A A. Subcutaneous IgG replacement therapy is safe and well tolerated in lung transplant recipients. Int Immunopharmacol. 2013;15:752–755. doi: 10.1016/j.intimp.2013.02.021. [DOI] [PubMed] [Google Scholar]
- Shapiro R S. Why I use subcutaneous immunoglobulin (SCIG). J Clin Immunol. 2013;33:95–98. doi: 10.1007/s10875-012-9853-2. [DOI] [PubMed] [Google Scholar]
- Bonilla F A. Pharmacokinetics of immunoglobulin administered via intravenous or subcutaneous routes. Immunol Allergy Clin North Am. 2008;28:803–819. doi: 10.1016/j.iac.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Yahyazadeh A, Beckebaum S, Cicinnati V, Klein C, Paul A, Pascher A. et al. Efficacy and safety of subcutaneous human HBV-immunoglobulin (Zutectra) in liver transplantation: an open, prospective, single-arm phase III study. Transpl Int. 2011;24:441–450. doi: 10.1111/j.1432-2277.2011.01222.x. [DOI] [PubMed] [Google Scholar]