Abstract
Introduction
Insufficient mesenteric perfusion is a dramatic complication in critically ill patients. Hydrogen sulfide, a newly recognized endogenous gaseous mediator, acts as an intestinal vasoactive agent and seems to protect against mesenteric ischemic damage. We investigated whether sodium hydrogen sulfide, a hydrogen sulfide donor, can improve mesenteric perfusion in an experimental model of pigs, both in physiological and ischemic conditions.
Methods
The study was conducted at Careggi University Hospital (Florence, IT). Fourteen male domestic pigs (≈10 Kg) were anesthetized and mechanically ventilated. Animals were randomized in control and ischemia groups. Mesenteric ischemia was induced with a positive end-expiratory pressure of 15 cmH2O. After mini-laparotomy, each animal received incremental doses of sodium hydrogen sulfide every 20 minutes. Perfusion of both the jejunal mucosa and sternal skin were measured by laser Doppler flowmeter, and systemic hemodynamic parameters were monitored.
Results
In the control group, sodium hydrogen sulfide was able to significantly improve the mesenteric perfusion, showing a 50% increase from the baseline blood flow. In the ischemia group, NaHS-induced a two-fold increase of the mesenteric post-ischemic perfusion with a recovery up to 70% of pre- positive end-expiratory pressure mesenteric blood flow. Sodium hydrogen sulfide did not directly or indirectly (by blood flow redistribution) affect the sternal skin microcirculation, heart rates, or mean arterial pressure, suggesting a tissue-specific micro-vascular action.
Conclusions
In a porcine model, we observed a mesenteric perfusion recovery mediated by administration of hydrogen sulfide donor without affecting general hemodynamic.
Keywords: hydrogen sulfide, mesenteric perfusion, intestinal ischemia
Introduction
Hydrogen sulfide (H2S), recognized as a new gaseous mediator, plays numerous roles both in normal physiological and pathophysiological conditions. Three enzymes endogenously synthesize H2S: cystathionine-β-synthase (CBS), cystathionine-β-lyase (or cystathionase, CSE) and 3-mercapto-sulphurtransferase [2, 3]. H2S plays numerous roles both in normal physiological and pathophysiological conditions [1]. As nitric oxide (NO), H2S is involved in the regulation of vascular tone. Experimental evidence has shown that H2S evokes concentration-dependent relaxation of many vascular districts, such as the aorta, through ATP-sensitive potassium (K+ATP)-channels opening [4], the portal vein [5], and the mesenteric artery bed [6]. In particular, according to the Authors, the sensitivity of mesenteric artery bed to H2S was about fivefold higher than in the aorta in rats. The supposed H2S-selectivity for the mesenteric vascular system has recently been confirmed. Yang et al. demonstrated that CSE knock-out mice displayed hypertension and diminished endothelium-dependent vascular relaxation, which were more pronounced in the mesenteric district [7].
Decreased mesenteric perfusion is a severe complication occurring in critically ill patients [8, 9]. The mesenteric hypoperfusion can be linked to an increase in intra-abdominal pressure (e.g. traumatic injury, retroperitoneal hematoma) or shock. The switch from aerobic to anaerobic metabolism in the mesenteric bed promotes the secretion of multiple vasoactive substances (e.g. nitric oxide) to improve tissue perfusion through local vasodilation. Eventually, persistent vasodilation may adversely affect both macro- and microcirculation, leading to tissue hypoperfusion [10]. In mechanically ventilated patients affected by acute respiratory distress syndrome, high respiratory pressures, including positive end-expiratory pressure (PEEP), may exacerbate mesenteric hypoperfusion [10]. High PEEP may lead to both regional and systemic adverse effects due to increased intra-thoracic pressures and decreased venous return to right atrium [11]. The consequent reduction in cardiac output redistributes blood flow away from the splanchnic circulation with an elevated risk for mesenteric ischemia, as already confirmed in rodents and pigs [11].
The aim of the present study was to examine the effect of incremental intravenous doses of sodium hydrogen sulfide (NaHS), a H2S donor, within a physiological range on pig mesenteric microcirculatory blood flow in both physiological and PEEP-induced ischemic conditions.
Methods
Animals and compounds. Fourteen male pigs, weight 10 Kg±1 (SEM), were used with the approval of the Local Animal Experiment Ethics Committee. All procedures were carried out according to the guidelines of the National Institute of Health for the care and use of laboratory animals.
As a source of H2S in sodium-chloride solution (0.9%, vehicle), we used sodium hydrogen sulfide (NaHS, Sigma-Aldrich Srl, Milano, Italy), which reacts with water (1 ml/kg), producing H2S [5].
Anesthesia. After overnight fast, pigs were anesthetized with ketamine (10 mg/Kg i.m.) and maintained under anesthesia with nitrous oxide (N2O) and sevoflurane (Sevorane® Baxter) at 1.0 MAC. Neuromuscular block was achieved with a bolus of vecuronium bromide (0.1 mg/Kg) and maintained with additional boluses of 0.015 mg/kg every 30 min. Animals were intubated in a supine position with a 4.5-5.0 mm inner diameter cuffed endotracheal tube and mechanically ventilated with tidal volume of 8 ml/Kg and FiO2 set at 50%.
The following physiological parameters were monitored:
electrocardiogram (lead DII);
inspired and expired oxygen and carbon dioxide concentrations (%)
SpO2 (ear lobe);
invasive femoral arterial pressure (20 G).
Surgical preparation. A mini-laparotomy was performed to expose the Treitz muscle. The Treitz muscle was sectioned to access the jejunum. In order to evaluate the mesenteric perfusion, a laser Doppler flowmeter probe was positioned in the first jejunal loop [12]. For better evaluation, the probe was placed near a micro-vessel on the same course of mesenteric artery root and kept in place with a 3/0 Dexon suture. A second Doppler flowmeter probe was placed on the sternal skin to measure cutaneous perfusion. At the end of each experimental surgery, the mini-laparotomy was sutured and the animal was sacrificed with an intravenous bolus of KCl.
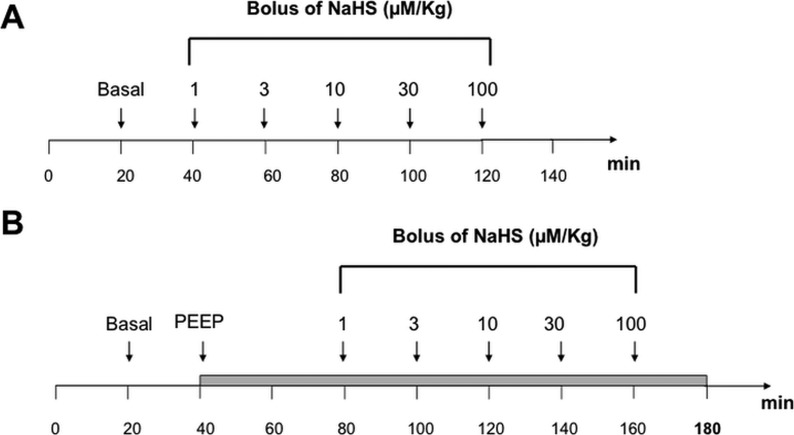
Experimental protocol. The study protocol is summarized in Figure 1.
Figure 1.
Study protocol. Each point time represent the timing of data collection.
Panel A: control animals.
Panel B: ischemia-induced animals. PEEP ventilation has been fixed at 15 cm H2O and 40 minutes have been waited for the stabilization of hemodynamic parameters of the animal.
PEEP = positive end-expiratory pressure.
Panel A and Panel B illustrate respectively the experimental protocol for both control and ischemia-induced groups. In the ischemia groups, a PEEP of 15 cmH2O was applied to achieve mesenteric hypo-perfusion, as according to many other similar study protocols [13, 14]. The administration of the drug was shifted 40 minutes later than the control group in order to achieve hemodynamic stabilization. An intravenous bolus of NaHS (10 ml) was administrated at a constant rate of 60 seconds every 20 minutes in the following order: 1 µM/Kg, 3 µM/Kg, 10 µM/Kg, 30 µM/Kg, 100 µM/Kg, 300 µM/Kg, 1000 µM/Kg. No artifact signal due to the injection procedure has been noticed. In both groups, the baseline measurements were performed twenty minutes after the induction of anesthesia (stabilization period), and subsequently repeated 10 minutes after each NaHS administration. In both groups, mesenteric perfusion was recorded from the end of the stabilization period until the death of the animal (Figure 1 panel A and B).
Laser Doppler Flow Measurements. In order to measure the tissue perfusion, a Laser Doppler Flowmeter (Periflux System 5000, Perimed, Milan, Italy) was used, with a small straight probe (probe 407-1) on the sternal skin and a needle probe (probe 411) in the jejunal mucosa (see above). Basal blood flow was defined as the mean of blood flow (expressed as Perfusion Unit, PU) within a 10-minute interval immediately after the stabilization period. The response to each NaHS dose was expressed as the mean of blood flow (expressed as Perfusion Unit, PU). Changes in blood flow were expressed as percentage increases relative to the basal level. For the ischemia group, we considered as basal level the mean blood flow within a 10 minutes interval after the PEEP stabilization period.
Statistics. GraphPad Prism 5 (GraphPad Software Inc., San Diego, CA) was used for statistical analysis. All results were expressed as mean±standard error of mean (SEM). Statistical analysis was made by t-test or one-way analysis of variance (ANOVA) for repeated measures followed by Bonferroni post hoc test. P value was considered significant if greater than 0.05.
Results
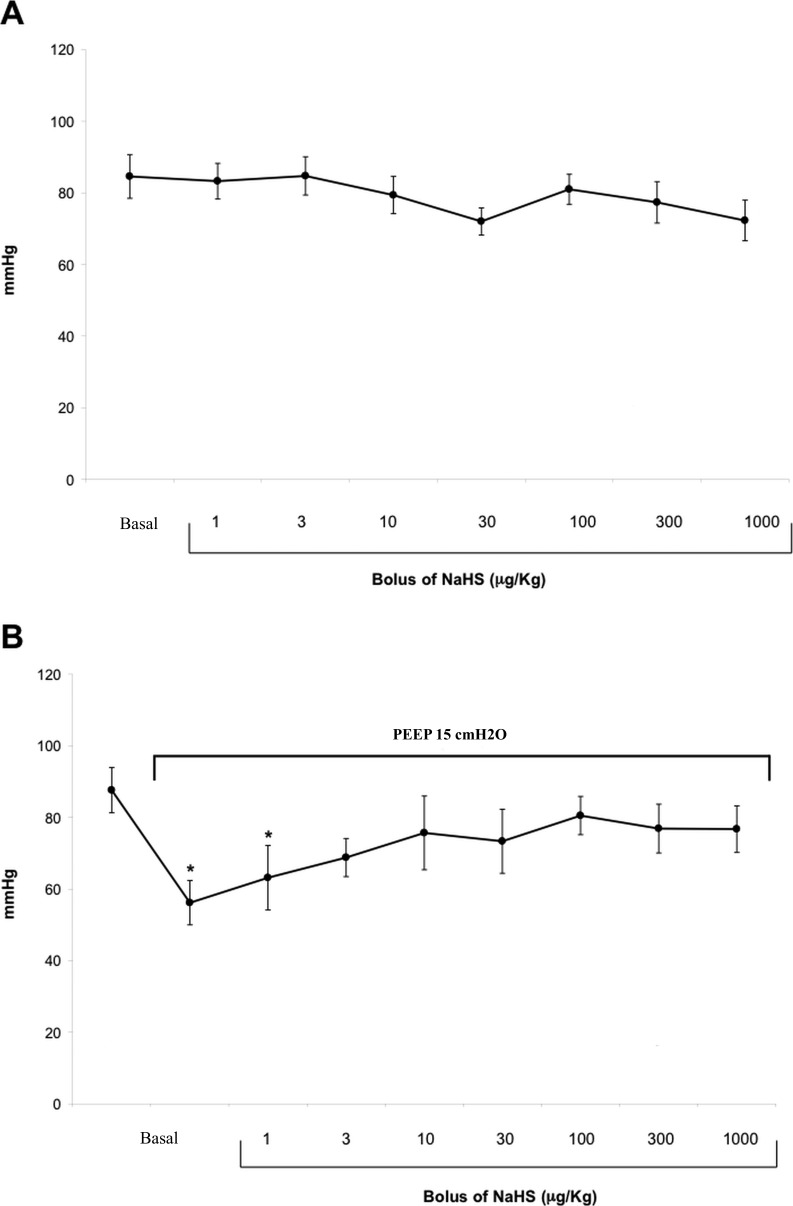
Effect of NaHS on hemodynamic parameters. In the control group, the serial intravenous doses of NaHS did not significantly modify the mean arterial pressure(MAP) (Figure 2, panel A), and the HR. In the ischemia-induced group, the increase in intra-thoracic pressures caused a significant reduction in MAP (Figure 2, panel B) associated with not-significant changes in HR.
Figure 2.
Effect of serial intravenous bolus of NaHS on mean arterial pressure in control (Panel A) and ischemia groups (Panel B). Data are expressed as mean ± SEM. Statistical analysis: ANOVA; *p < 0.05.
NaHS = sodium hydrogen sulfide; SEM = standard error of mean; ANOVA = analysis of variance.
After incremental bolus of NaHS, MAP values progressively increased, recovering values comparable with the pre-ischemic measures after the 3 µg/kg bolus (p=0.03; Figure 2, panel B). Gas exchange (pO2, pCO2) and metabolic status (pH, lactate) did not change significantly during H2S infusion in both groups. In particular, mean (±SEM) PaCO2 was 38.6±1.1 mmHg in control group and 37.9±0.9 in the experimental group.
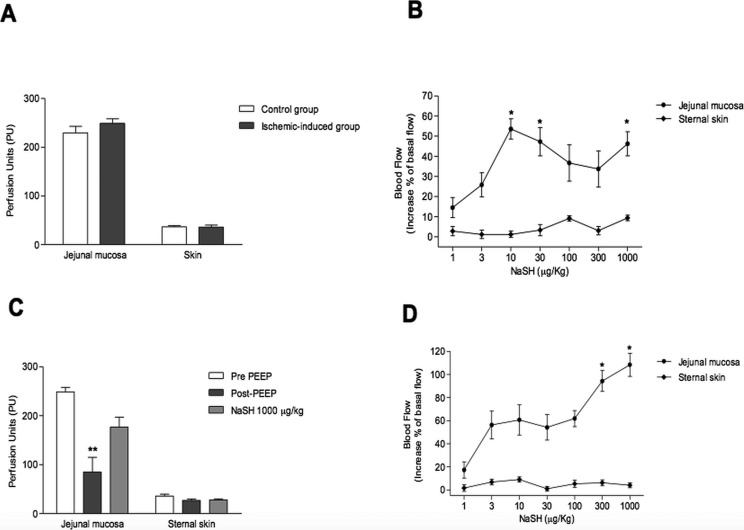
Effect of NaHS on perfusion of jejunal mucosa and sternal skin. The baseline flow did not differ between the two studied groups, neither in the mesenteric nor cutaneous districts (248 vs. 229 PU, p=0.8; 35.7 vs. 36.3 PU, p=0.8, respectively; Figure 3, panel A).
In the control group, jejunal mucosa perfusion increased with a peak at 10 µM/Kg (Figure 3, panel B).
Conversely, a not-significant effect was observed on the sternal skin, suggesting that NaHS did not affect the cutaneous microcirculation (Figure 3, panel B) (p < 0.05).
In the PEEP-induced ischemia group, the jejunal mucosa perfusion significantly decreased 66% from the baseline immediately after application of the PEEP (p=0.009, Figure 3, panel C), whereas skin perfusion was not significantly affected, suggesting that PEEP application selectively impaired the mesenteric perfusion (Figure 3, panel C). As observed in the control group, in the ischemia group the administration of NaHS significantly increased perfusion of jejunal mucosa, with a recovery of 70% of pre-ischemic mesenteric flow (Figure 3, panel D) (p < 0.05). Finally, ischemic animals showed higher increases in jejunal mucosa blood flow than animals in the control group (p=0.02, Figure 3).
Figure 3.
Panel A: Baseline mesenteric and cutaneous blood flow (expressed in Perfusion Units) in both control and PEEP-induced ischemia groups.
Panel B: Effect of serial intravenous doses of NaHS on jejunal mucosa perfusion and sternal skin in control group. Doses were administrated each 20 minutes. Changes in blood flow are expressed in percentage of the basal level.
Panel C: Effect of PEEP (15 cmH2O) on pig mesenteric and cutaneous blood flow (expressed as perfusion units, PU).
Panel D: Effect of serial intravenous doses of NaHS on jejunal mucosa perfusion and sternal skin in ischemia-induced group. Doses were administrated each 20 minutes. Changes in blood flow are expressed in percentage of the basal level.
Data are expressed as mean ± SEM. Statistical analysis: t-test (panel A); ANOVA (panels B, C and D); *p < 0.05.
PEEP = positive end-expiratory pressure; NaHS = sodium hydrogen sulfide; ANOVA = analysis of variance; SEM = standard error of mean.
Discussion
Our study shows that NaHS, an H2S donor, can restore PEEP-mediated splanchnic hypoperfusion to 70% of pre-PEEP values. These results are important if considering that with the aim to improve splanchnic perfusion, the most widely utilized drugs are dopamine receptor agonists (dopamine or fenoldopam), since they are supposedly free from side effects [15, 16] but without definitive evidence of their beneficial role in intestinal mucosa perfusion [17].
We also observed that, in the control group, low concentrations of H2S were able to increase the intestinal mucosal perfusion up to 50% of the baseline blood flow. The H2S-induced vasodilatation appeared to be limited to the mesenteric micro-vascular district for two reasons: 1) it did not result in any clinically significant systemic hemodynamic effect; 2) it did not directly affect the cutaneous microcirculation, suggesting a tissue-selectivity. Although H2S is known to have negative inotropic and chronotropic effects, the absence of a significant systemic hemodynamic change in our experiments can be explained by the acute administration of the compound, whereas the absence of metabolic modifications is in line with previous findings on the porcine ischemia/reperfusion model [18].
The H2S-induced improvement in mesenteric microcirculation perfusion was confirmed in PEEP-ischemic animals. PEEP produced a mesenteric hypo-perfusion, cutting down 66% of the mesenteric blood flow, consistent with previous studies [13, 14]. In this condition, the H2S-induced vascular relaxation was associated with a 70% restoration of the pre-ischemic mesenteric blood flow at NaHS dose of 1000 µg/kg (Figure 3, panel C). As could be expected, in the ischemic group, the mesenteric mucosa seemed to be more H2S-responsive than in the control group, especially at elevated doses (Figure 3, panel B and D). Skin perfusion did not significantly change, suggesting that the effects of H2S were exerted mostly in the central blood compartment rather than in the peripheral circulation (Figure 3, panel D). Our findings confirmed the property of exogenous H2S in the mesenteric circulation of pigs, which was previously shown in small animal models [7]. As shown in Figure 3 (panels D), there is a dip in the dose/effect curve. This seems to be far from a pure sigmoidal dose/effect curve, and is probably caused by the limited sample.
Limitations of this study must be mentioned. Although our study provided proof of the concept of H2S-related marked improvement of mesenteric perfusion, it does not allow quantitative conclusions to be drawn about the effective increases in blood flow, not having an untreated ischemia group. Also, the decrease in splanchnic perfusion induced by PEEP may differ from other pathophysiology conditions resulting in splanchnic ischemia and may, therefore, show different reactions on the infusion of H2S-donors. Finally, we were not able to dose H2S, and its plasma concentration was only mathematically deduced.
Conclusion
Exogenous H2S-donor produced a tissue-selective dilatation of the microcirculation capable of increasing blood flow in mesenteric mucosa both in normal and ischemic conditions, without affecting systemic hemodynamic parameters. Thus, we think that H2S could be a potentially useful compound available in critical conditions to reverse mesenteric hypoxia-reperfusion injury. Studies are needed to assess its effects on cell metabolism, its precise pharmacodynamics and pharmacokinetics, and to assess the safety of possible future clinical use.
Footnotes
Source of Support This study was supported in part by the Ministry for University and Scientific Research (MiUR) Rome, Italy. PRIN 2010-2011 to S.M.
Disclosures None declared.
Cite as: Pavoni V, Nicoletti P, Benemei S, Materazzi S, Perna F, Romagnoli S, Chelazzi C, Zagli G, Coratti A. Effects of hydrogen sulfide (H2S) on mesenteric perfusion in experimental induced intestinal ischemia in a porcine model. Heart, Lung and Vessels. 2015; 7(3): 231-237.
References
- Kimura H. Hydrogen Sulfide and Polysulfides as Biological Mediators. Molecules. 2014;19:16146–16157. doi: 10.3390/molecules191016146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore P K, Bhatia M, Moochhala S. Hydrogen sulfide: from the smell of the past to the mediator of the future? Trends Pharmacol Sci. 2003;24:609–611. doi: 10.1016/j.tips.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Chen X, Jhee K H, Kruger W D. Production of the neuromodulator H2S by cystathionine beta-synthase via the condensation of cysteine and homocysteine. J Biol Chem. 2004;279:52082–52086. doi: 10.1074/jbc.C400481200. [DOI] [PubMed] [Google Scholar]
- Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J. 2001;20:6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoki R, Matsuki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun. 1997;237:527–531. doi: 10.1006/bbrc.1997.6878. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Ndisang J F, Tang G, Cao K, Wang R. Hydrogen sulfide-induced relaxation of resistance mesenteric artery beds of rats. Am J Physiol Heart Circ Physiol. 2004;287:2316–2323. doi: 10.1152/ajpheart.00331.2004. [DOI] [PubMed] [Google Scholar]
- Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K. et al. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stechmiller J K, Treloar D, Allen N. Gut dysfunction in critically ill patients: a review of the literature. Am J Crit Care. 1997;6:204–209. [PubMed] [Google Scholar]
- Ortiz-Diaz E, Lan C K. Intra-abdominal hypertension in medical critically ill patients: a narrative review. Shock. 1997;41:175–180. doi: 10.1097/SHK.0000000000000100. [DOI] [PubMed] [Google Scholar]
- Kolkman J J, Mensink P B. Non-occlusive mesenteric ischaemia: a common disorder in gastroenterology and intensive care. Best Pract Res Clin Gastroenterol. 2003;17:457–473. doi: 10.1016/s1521-6918(03)00021-0. [DOI] [PubMed] [Google Scholar]
- Lehtipalo S, Biber B, Frojse R, Arnerlov C, Johansson G, Winso O. Effects of positive end-expiratory pressure on intestinal circulation during graded mesenteric artery occlusion. Acta Anaesthesiol Scand. 2001;45:875–884. doi: 10.1034/j.1399-6576.2001.045007875.x. [DOI] [PubMed] [Google Scholar]
- Snygg J, Aneman A, Pettersson A, Fandriks L. Jejunal mucosal nitric oxide production and substrate dependency during acute mesenteric hypoperfusion in pigs. Crit Care Med. 2000;28:2563–2566. doi: 10.1097/00003246-200007000-00063. [DOI] [PubMed] [Google Scholar]
- Fujita Y. Effects of PEEP on splanchnic hemodynamics and blood volume. Acta Anaesthesiol Scand. 1993;37:427–431. doi: 10.1111/j.1399-6576.1993.tb03742.x. [DOI] [PubMed] [Google Scholar]
- Kotzampassi K, Paramythiotis D, Eleftheriadis E. Deterioration of visceral perfusion caused by intra-abdominal hypertension in pigs ventilated with positive end-expiratory pressure. Surg Today. 2000;30:987–992. doi: 10.1007/s005950070018. [DOI] [PubMed] [Google Scholar]
- Lee R D, Choe E, Flint L, Steinberg S. Neither dopamine nor dobutamine corrects mesenteric blood flow depression caused by positive end-expiratory pressure in a rat model of acute lung injury. Crit Care Med. 1998;26:1875–1880. doi: 10.1097/00003246-199811000-00032. [DOI] [PubMed] [Google Scholar]
- Johnson D J, Johannigman J A, Branson R D, Davis K Jr., Hurst J M. The effect of low dose dopamine on gut hemodynamics during PEEP ventilation for acute lung injury. J Surg Res. 1991;50:344–349. doi: 10.1016/0022-4804(91)90201-v. [DOI] [PubMed] [Google Scholar]
- Woolsey C A, Coopersmith C M. Vasoactive drugs and the gut: is there anything new? Curr Opin Crit Care. 2006;12:155–159. doi: 10.1097/01.ccx.0000216584.72427.e4. [DOI] [PubMed] [Google Scholar]
- Simon F, Giudici R, Duy C N, Schelzig H, Oter S, Groger M. et al. Hemodynamic and metabolic effects of hydrogen sulfide during porcine ischemia/reperfusion injury. Shock. 2008;30:359–364. doi: 10.1097/SHK.0b013e3181674185. [DOI] [PubMed] [Google Scholar]