Abstract
Introduction
Currently, the gold standard for donor organ preservation in clinical organ transplantation consists of 3 plastic bags and an ice box. The first plastic bag includes the organ itself immersed in preservation solution (e.g. Celsior). This bag is put in a second bag filled with saline, and then these two are put in a third bag filled with saline which is then put in the ice box. The disadvantage of this method is that the organ usually gets too cold. It has been shown that the theoretical perfect temperature for organ preservation is 4°C - 8°C. While higher temperatures lead to hypoxic injury of the organ because the metabolism is not decreased efficiently, lower temperatures than 4°C increase the risk of cold injury with protein denaturation. In the current study, we investigated a device that keeps the organ temperature consistently in the desired range of 4°C - 8°C and can potentially decrease cold injury to donor organs.
Methods
Three different ex vivo studies were performed with the Paragonix Sherpa Pak™ devices: 1) the temperature of the fluid-filled device was measured for up to 30 hours at an outside temperature set at 22°C, 2) the temperature of the fluid-filled device was measured for up to 30 hours at extreme outside temperatures set at -8°C and 31°C, 3) the temperature of a pig heart attached to the device was measured up to 12 hours.
Results
All studies showed that the Paragonix Sherpa Pak™ can keep the temperature of the heart consistently between 4° and 8°C.
Conclusions
The Paragonix Sherpa Pak™ device may decrease cold injury of donor organs by maintaining the temperature consistently between 4°C and 8°C and therefore may decrease primary graft failure after organ transplantation.
Keywords: hypothermic cold storage, heart transplantation, kidney transplantation, organ preservation
Introduction
Successful organ preservation is an important component of transplantationand ensures the maintenance of organ viability until implantation into the recipient. Currently, heart preservation for transplantation is limited to 4 to 6 hours of cold ischemic storage, and longer periods of ischemia are known to adversely affect survival [1,2,3]. This is in contrast to preservation of the liver, kidney, and pancreas, which have been successfully preserved for 24 to 36 hours although graft function may be transiently compromised. Following procurement of an organ, ischemia represents the immediate cause of injury. Ischemia initiates a complex injury process that is characterized by the loss of high energy phosphates, the accumulation of intracellular inosine and hypoxanthine, cessation of the Na+-K+ pump, followed by cell swelling, cytosolic calcium increase, and enzymatic degradation [4,5,6].
In general, lower organ temperatures (0°C to 4°C) maintain high energy phosphates more effectively [7,8,9,10]. However, temperatures below 2°C significantly increase the risk of cold injury with some proteins denaturing below 0°C. At higher temperatures (8°C to 12°C), organ function is maintained to a greater extent [11, 12]. However, at temperatures above 12°C the higher metabolic demand for oxygen leads to irreversible hypoxic injury and thus significantly impairs organ function. Ideally, there should be a temperature range at which these two tendencies can be balanced. The instructions for use of organ preservation solutions state a temperature range between 2-8°C or 4-8°C.
Hence, the balance seemingly forms the cornerstone of cold ischemic storage which is hypothermia at 4°C to 8°C with an appropriate cold storage solution. Hypothermia decelerates metabolism and the ionic constituents of the storage solution facilitate rapid cessation of electrical activity. The formulation of the preservation solution is based on three principles: (a) hypothermic arrest of metabolism; (b) provision of a physical and biochemical environment that maintains viability of the structural components of the tissue during hypothermic metabolic arrest; and (c) minimization of the effects of reperfusion injury.
Hypothermia does not stop metabolism but it slows down biochemical reaction rates and decreases the rate at which intracellular enzymes degrade essential cellular components necessary for organ viability [13]. Most enzymes of hypothermic animals show a 1.5 to 2.0-fold decrease in activity for every 10°C decrease in temperature. Hypothermia also retards lysis of organelles like lysosomes that, in turn, release autolytic enzymes that cause cell death. Hypothermia remains one of the most important tools used to preserve organs today.
The current gold standard for transporting a donor organ to a recipient for transplant involves storing the organ in organ preservation fluid within sequential plastic bags that are placed on ice and transported in a cooler. Some of the potential issues associated with this method of storage include (a) variability of packaging materials used; (b) bioincompatibility of the material that touches the organ; (c) tissue injury due to movement/vibration; and (d) potential uneven cooling of the organ tissue. In a multicenter study of 186 organs, recommended packaging guidelines were tested for their ability to maintain organ temperatures at acceptable temperatures [14]. The results showed that all packaging protocols maintained organ temperatures below ±0°C.
Paragonix Technologies, Inc. has developed a series of medical devices intended for transport of donor hearts and kidneys to recipients for implant: the Sherpa Pak™ Cardiac Transport System (CTS) and the Sherpa Pak™ Kidney Transport System (KTS). The Sherpa Pak™ System (Figures 1 and 2) is intended to provide a safe, consistent method for cold ischemic storage and transport of donor organs.
Figure 1.
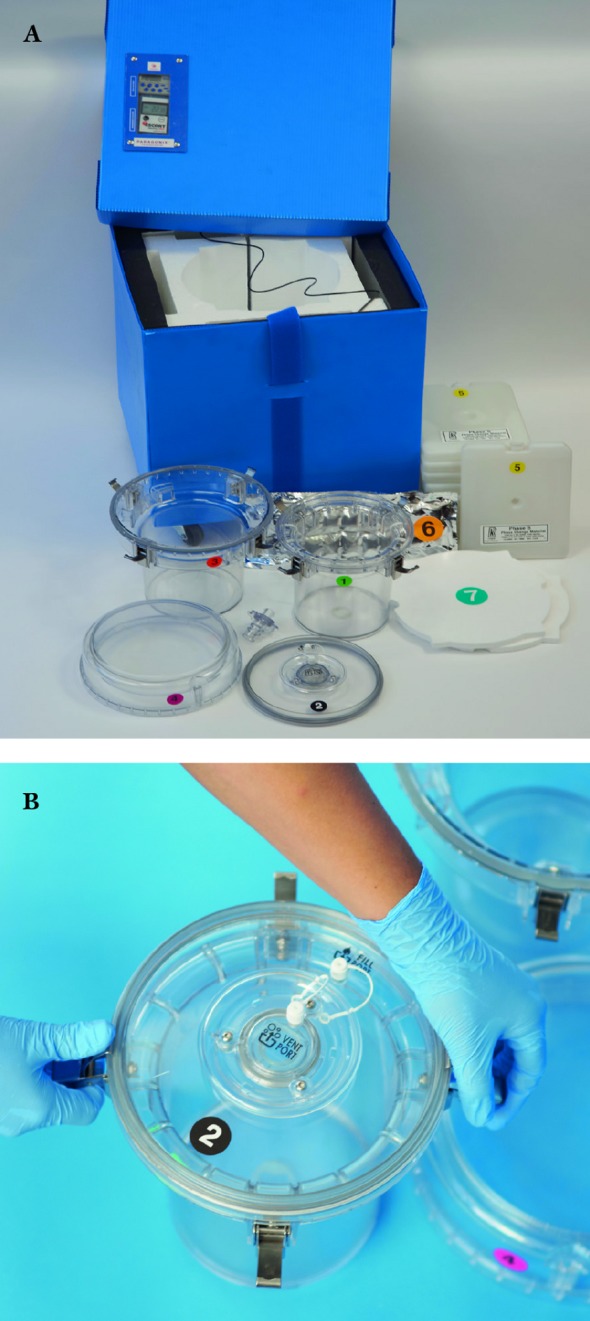
(A) Full assembly of Paragonix Sherpa Pak™ prototype used in studies. (B) Innermost container that holds the organ.
Figure 2.
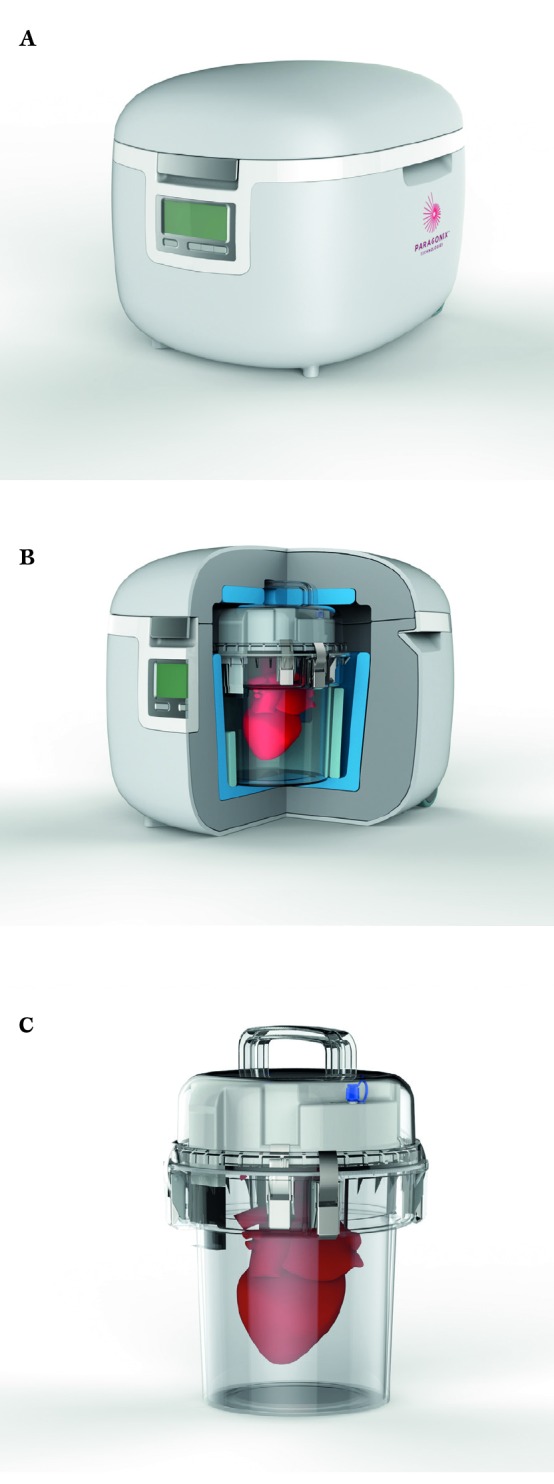
A, B,C) Future product design of Sherpa Pak™ CTS and KTS.
The Systems are designed to overcome many of the potential issues associated with the current systems used for transport.
The Sherpa Pak’s cooling mechanism is based on phase-change material (PCM), which is a substance with a high heat of fusion which is capable of storing and releasing large amounts of energy. The Sherpa Pak’s PCM panels are designed to hold 5°C longer than conventional phase change material cold packs (which undergo phase change at 0°C, and have little heat capacity at 5°C). In addition, the PCM can be placed in direct contact with temperature sensitive products because there is no risk of freezing (please note, however, that the cold panels do not come in direct contact with the donor organ).
The entire System is single-use and does not require power during operation. Management of preservation in the Sherpa Pak™ System is the same for both kidneys and hearts other than that the heart is connected to a connector in the inner hard shell assembly and the kidney is not.
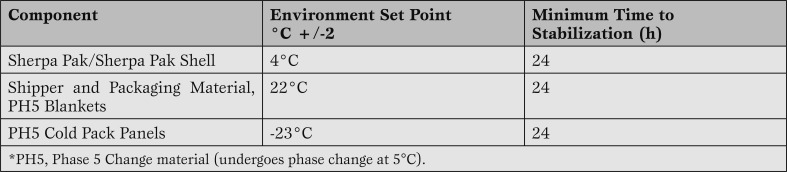
We have performed multiple temperature profile studies for varying periods of time, from 4 hours up to 30 hours of storage (Tables 1 and 2).
Table 1.
Preconditioning protocol for components of the Sherpa Pak™ Systems.
Table 2.
Performance data for static hypothermic storage between 4 and 8°C for up to 30 hours using the Sherpa Pak™ technology at ambient temperature. Sample sizes reflect the number of data points collected at regular interval.
Methods
Sherpa Pak™ storage. All protocols were in conformity with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health 86-23, revised in 1985. A total of 14 pig hearts were used in this study. Anesthesia was induced with 4.4 mg/kg Telazol, 2.2 mg/kg xylazine and 0.04 mg/kg atropine intramuscularly and maintained with isoflurane after endotracheal intubation. Median sternotomy was performed and heparin (300 units/kg) was given intravenously. An aortic root cannula was inserted and, after cross-clamping the ascending aorta, 1 L of Celsior® (Genzyme, Cambridge, MA, USA) cardioplegia was administered through the cannula. The heart was vented through the left atrial appendage, and superior and inferior vena cava, and donor cardiectomy was performed.
Complete Sherpa Pak™ Cardiac Transport Systems (CTS) and Sherpa Pak™ Kidney Transport Systems (KTS) were utilized for the thermal qualification test. Prior to initiating the testing, the components of the Sherpa Pak™ Systems were preconditioned according to the instructions for use provided with the device (Table 1).
The Sherpa Pak™ System consists of multiple components: 1) an outer transport shipper which contains various non-ice-based temperature controlled packaging elements; 2) an inner and outer hard shell assembly (i.e. Sherpa Pak™/Sherpa Pak™ Shell) which provides a double, rigid barrier shipper in which the organ is immersed and suspended in a cold storage fluid cleared for use in storing and transporting donor organs; 3) an off-the-shelf temperature data logger that monitors and displays the temperature of the organ during transport; and 4) an off-the-shelf timer that displays elapsed time during transport.
Once conditioned, the systems were assembled in accordance with the instructions for use with the exception that additional thermocouples were placed to monitor temperature at various locations within the system. These thermocouples were connected to an external display (the presence of the thermocouples did not alter the closure of the device). Temperature readings were monitored and recorded at regular intervals for the period of the experiment. Temperature measurements were taken every 5 min in Studies I.a, I.b and III.a, and every 10 s in studies II and III.b. When not otherwise indicated, the storage solution was saline or water.
In order to capture any variations in temperature within the device, thermocouples were placed in various locations of the device. Thermocouple locations are designated as follows: Center1 = One Probe (n=1) located in the center of the fluid filled Sherpa Pak™. Wetted probe = One Probe (n=1) located at the top of the fluid filled Sherpa Pak™ (within fluid).
Conventional ice storage. The organ was immersed in solution and packaged in a first plastic bag. This first bag was then placed into a second bag filled with solution. This assembly was then placed into a third solution-filled bag and placed onto crushed ice in an ice chest. When not otherwise indicated, the storage solution was saline or water.
Study design. Three different ex-vivo studies have been performed with the Paragonix Sherpa Pak™ devices:
Study I: The temperature of the fluid filled device was measured for up to 30 h at an outside temperature set at 22°C (Study I.a and Study I.b, Table 2).
Study II: The temperature of the fluid filled device was measured for up to 30 h at extreme outside temperatures set at -8°C and 31°C (Study II, Table 3).
Study III: The temperature of pig hearts attached to the device was measured for up to 12 h (study III.a and III.b; Table 4).
Table 3.
Performance data for static hypothermic storage between 4 and 8°C for up to 30 hours using the Sherpa Pak™ technology with two 1 hour excursions to extreme hot and cold temperatures.
Table 4.
Summary of animal studies of static hypothermic storage between 4 and 8°C using the Sherpa Pak™ technology.
Results
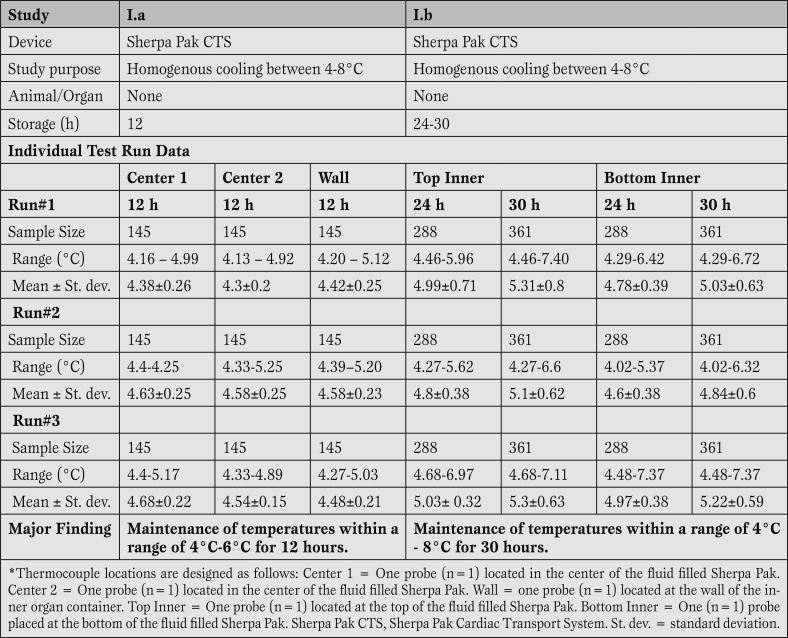
Sherpa Pak™ can keep the temperature of the preservation solution between 4-8°C for a period of up to 30 hours. The first part of study I (Table 2) demonstrated in three separate test runs that all temperatures recorded from the individually placed temperature probes remained below the 8°C upper limit of the design specification through four hours. Temperatures were recorded every 5 min, providing 145 readings. Moreover, all recorded temperatures from all individually placed temperature probes remained below the 8°C upper limit through 12 hours of experimentation.
Most importantly, the experiment verified that the fluid within the Sherpa Pak™ (in which the donor heart is immersed) was maintained between 4°C and 6°C (as measured by the three probes placed within the fluid; two at center of the container, one against the side wall) which is within the accepted 4°C - 8°C.
The second part of study I (Table 2) demonstrated that all temperatures recorded from the individually placed temperature probes remained below the 8°C upper limit through 30 hours. Temperatures were recorded every 5 min, providing up to 361 readings. Most importantly, the experiment verified that the fluid within the Sherpa Pak™ (in which the donor organ is immersed) was maintained between 4°C and 8°C (as measured by the temperature probes placed within the fluid).
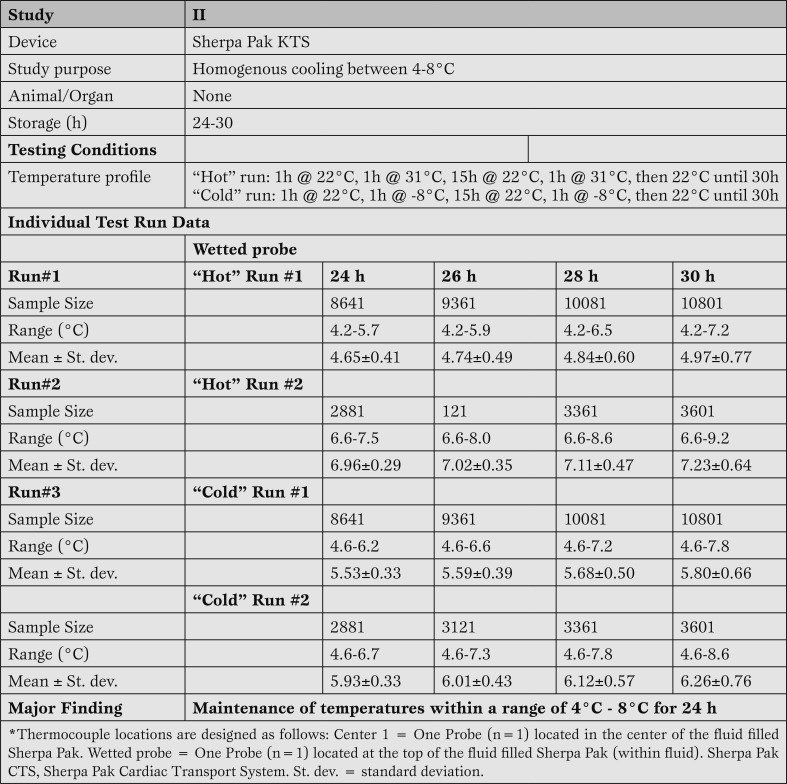
Sherpa Pak™ consistently maintains fluid temperature at 4-8°C despite extreme fluctuations in ambient temperature. Results of the four separate test runs (two test runs each at cold or hot temperatures) of the experiment (study II: Table 3) demonstrated that the storage solution remained below the 8°C upper limit of the design specification through 24 hours. Temperatures were recorded every 10 s, providing up to 10801 readings. Interestingly, in the second warm temperature test run, the cold storage solution wasn't preconditioned according to its instructions for use and the starting temperature of the fluid was 7ºC (versus 6ºC according to manufacturer IFUs). Even with this slightly elevated temperature, the Sherpa Pak™ system was capable of maintaining temperature throughout the 24 hour period.
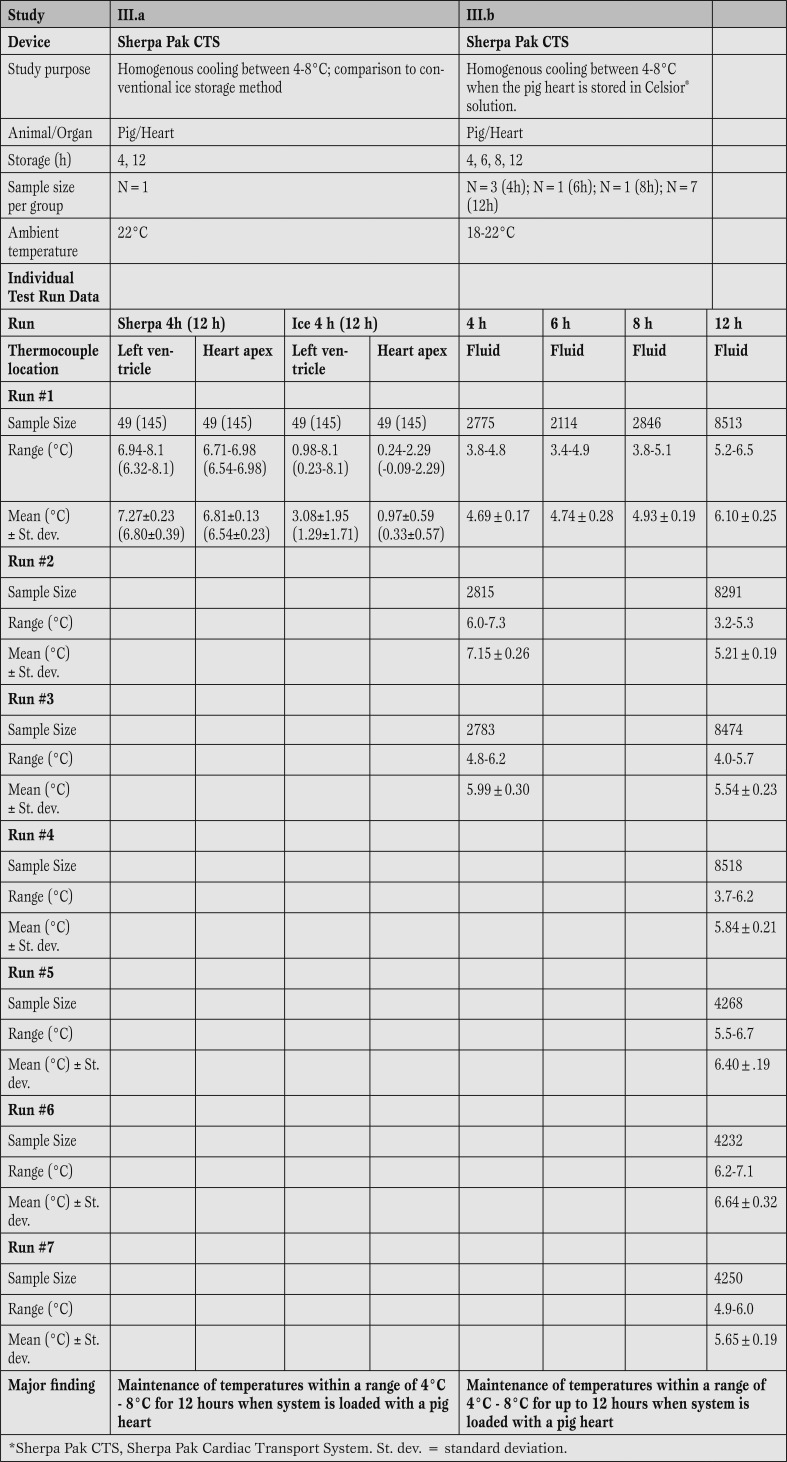
Sherpa Pak™ can maintain the temperature at 4-8°C when the system is loaded with a pig heart. Both the Sherpa Pak™ CTS as well as the conventional ice storage demonstrated their ability to cool and maintain temperature throughout the experimental period. However, the “ice system” cooled the heart based on the two thermocouples to temperatures as low as 0°C at the apex closest to the ice, and < 1°C inside the left ventricle of the pig heart. The experiment verified that the fluid within the Sherpa Pak™ (in which the donor organ is immersed) was maintained between 4°C and 8°C (study III, table 4). Temperatures were recorded every 5 min, providing up to 145 readings in study III.a, and every 10 s, providing up to 10801 readings in study III.b
Results of 12 temperature test runs during pig heart storage demonstrated that the Sherpa Pak™ CTS was able to maintain fluid temperatures within a range of 4-8°C when fully loaded with a pig heart (Table 4).
Discussion
The purpose of this study was to verify whether the Paragonix Sherpa Pak™ device was capable of maintaining the desired temperature for organ storage which lies between 4 and 8°C. Several studies were performed during which all system components including the cold storage solution were preconditioned according to the instructions for use. In certain studies, exterior conditions remained constant as the System will always be under the direct supervision of medical personnel during transport and not anticipated to be exposed to uncontrolled temperatures or environmental conditions. The purpose of study II, in which exterior conditions were changed to either high temperatures (31°C) or low temperatures (-8°C) for 1 hour twice during 30 hours of monitoring, was to challenge the system at two extreme temperatures.
The results of the testing verified that the design of the Sherpa System was able to maintain the fluid in which the donor organ is contained within the clinically acceptable range of 4°C - 8°C through 24 hours, even when challenged to high and low temperatures. When ambient temperatures were kept constant (at 22°C) the System was able to maintain temperatures within a range of 4°C - 8°C throughout 30 hours of monitoring. Based upon the results of the testing, we conclude that the Sherpa Pak™ devices are designed such that they can reliably maintain a clinically acceptable storage temperature for the donor organ.
It is commonly accepted that the 4°C - 8°C temperature range is the best balance for preservation of high energy phosphate stores, minimization of cold injury, and preservation of post-transplant function [15]. Although temperatures below 4°C offer superior maintenance of high energy phosphates, temperatures below 2°C significantly increase the risk of cold injury and frostbite [8, 9].
The results of the studies demonstrated that the Paragonix Sherpa Pak™ device was capable of maintaining a very consistent temperature within the 4°C - 8°C temperature range through twelve hours. The conventional ice storage was also very effective at cooling and maintaining temperature of the heart through six hours; however, the data show that temperatures encountered with this system approached 0°C for the last few hours of the experiment, thus increasing the potential for issues with the donor heart. These data compared well to a multicenter study on transport temperature of organs [14]. Studying 186 organs, the average organ temperature during transportation was below 2°C, and after 6 hours below 0°C. Based upon these test results, it appears that the Paragonix Sherpa Pak™ Transport System is effective at maintaining the temperature of the organ in the optimal temperature range.
While the study investigated ambient temperatures that might be expected in a clinical situation and temperature excursion that may occur during organ transport, a limitation of the study is that measurements were taken in a research setting, which may not entirely reflect the clinical setting.
Conclusion
The Paragonix Sherpa Pak™ device may decrease cold injury of donor organs by maintaining the temperature consistently between 4°C and 8°C and therefore may decrease primary graft failure after organ transplantation.
Footnotes
Source of Support This work was in supported by a Phase I SBIR grant (1R43HL115852-01) awarded to Paragonix Technologies Inc.
Disclosures L.M. Anderson is an employee of Paragonix Technologies, Inc.
Cite as: Michel SG, LaMuraglia II GM, Madariaga MLL, Anderson LM. Innovative cold storage of donor organs using the Paragonix Sherpa Pak™ devices. Heart, Lung and Vessels. 2015; 7(3): 246-255.
References
- Keck B M, White R, Breen T J, Daily O P, Hosenpud J D. Thoracic organ transplants in the United States: a report from the UNOS/ISHLT Scientific Registry for Organ Transplants. United Network for Organ Sharing. International Society for Heart and Lung Transplantation. Clin Transpl. 1994;37:46–46. [PubMed] [Google Scholar]
- Stehlik J, Edwards L B, Kucheryavaya A Y, Benden C, Christie J D, Dipchand A I. et al. The Registry of the International Society for Heart and Lung Transplantation: 29th official adult heart transplant report--2012. J Heart Lung Transplant. 2012;31:1052–1064. doi: 10.1016/j.healun.2012.08.002. [DOI] [PubMed] [Google Scholar]
- Minasian S M, Galagudza M M, Dmitriev Y V, Karpov A A, Vlasov T D. Preservation of the donor heart: from basic science to clinical studies. Interactive cardiovascular and thoracic surgery. 2015;20:510–519. doi: 10.1093/icvts/ivu432. [DOI] [PubMed] [Google Scholar]
- Weinberg J M. The cell biology of ischemic renal injury. Kidney Int. 1991;39:476–500. doi: 10.1038/ki.1991.58. [DOI] [PubMed] [Google Scholar]
- Belzer F O, Southard J H. Principles of solid-organ preservation by cold storage. Transplantation. 1988;45:673–676. doi: 10.1097/00007890-198804000-00001. [DOI] [PubMed] [Google Scholar]
- Taylor M J, Baicu S C. Current state of hypothermic machine perfusion preservation of organs: The clinical perspective. Cryobiology. 2010;60:20–35. doi: 10.1016/j.cryobiol.2009.10.006. [(3 Suppl)] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard M, Cartoux C, Caus T, Sciaky M, Cozzone P J. The influence of temperature on metabolic and cellular protection of the heart during long-term ischemia: a study using P-31 magnetic resonance spectroscopy and biochemical analyses. Cryobiology. 1998;37:309–317. doi: 10.1006/cryo.1998.2126. [DOI] [PubMed] [Google Scholar]
- Mollhoff T, Sukehiro S, Flameng W, Van Aken H. [Catabolism of high-energy phosphates during the long-term preservation of explanted donor hearts in a dog model]. Anasthesie, Intensivtherapie, Notfallmedizin. 1990;25:399–404. [PubMed] [Google Scholar]
- Tian G, Smith K E, Biro G P, Butler K W, Haas N, Scott J. et al. A comparison of UW cold storage solution and St. Thomas' solution II: a 31P NMR and functional study of isolated porcine hearts. J Heart Lung Transplant. 1991;10:975–985. [PubMed] [Google Scholar]
- Keon W J, Hendry P J, Taichman G C, Mainwood G W. Cardiac transplantation: the ideal myocardial temperature for graft transport. Ann Thorac Surg. 1988;46:337–341. doi: 10.1016/s0003-4975(10)65939-5. [DOI] [PubMed] [Google Scholar]
- Ingemansson R, Budrikis A, Bolys R, Sjoberg T, Steen S. Effect of temperature in long-term preservation of vascular endothelial and smooth muscle function. Ann Thorac Surg. 1996;61:1413–1417. doi: 10.1016/0003-4975(96)00109-9. [DOI] [PubMed] [Google Scholar]
- Mankad P, Slavik Z, Yacoub M. Endothelial dysfunction caused by University of Wisconsin preservation solution in the rat heart. The importance of temperature. J Thorac Cardiovasc Surg. 1992;104:1618–1624. [PubMed] [Google Scholar]
- Buckberg G D. Myocardial temperature management during aortic clamping for cardiac surgery. Protection, preoccupation, and perspective. J Thorac Cardiovasc Surg. 1991;102:895–903. [PubMed] [Google Scholar]
- Horch D F, Mehlitz T, Laurich O, Abel A, Reuter S, Pratschke H. et al. Organ transport temperature box: multicenter study on transport temperature of organs. Transplant Proc. 2002;34:2320–2320. doi: 10.1016/s0041-1345(02)03253-0. [DOI] [PubMed] [Google Scholar]
- Jahania M S, Sanchez J A, Narayan P, Lasley R D, Mentzer R M Jr. Heart preservation for transplantation: principles and strategies. Ann Thorac Surg. 1999;68:983–983. doi: 10.1016/s0003-4975(99)01028-0. [DOI] [PubMed] [Google Scholar]