Abstract
BACKGROUND
Despite clear recommendations and evidence linking colorectal cancer screening to lower incidence and mortality, >40% of adults are not up to date with screening. Existing domestic and international models of organized cancer screening programs have been effective in increasing screening rates. Implementing an organized, evidence-based, national screening program may be an effective approach to increasing screening rates.
METHODS
In the current study, the authors estimated the initial investment required and the cost per person screened of a nationwide fecal immunochemical test (FIT)-based colorectal cancer screening program among adults aged 50 years to 75 years.
RESULTS
The initial additional investment required was estimated at $277.9 to $318.2 million annually, with an estimated 8.7 to 9.4 million individuals screened at a cost of $32 to $39 per person screened. The program was estimated to prevent 2900 to 3100 deaths annually.
CONCLUSIONS
The results of the current study indicate that implementing a national screening program would make a substantial public health impact at a moderate cost per person screened. Results from this analysis may provide useful information for understanding the public health benefit of an organized screening delivery system and the potential resources required to implement a nationwide colorectal cancer screening program, and help guide decisions about program planning, design, and implementation.
Keywords: colorectal cancer, health economics, public health, screening, early detection
INTRODUCTION
Colorectal cancer (CRC) is the second leading cause of cancer-related death and the second most common cancer affecting both men and women in the United States. In 2010, 131,607 people were diagnosed with CRC, and 52,045 died of the disease.1 In addition, the economic burden of CRC is substantial. The national cost of CRC care was estimated to be $14.1 billion in 2010 and was projected to increase to $17.4 billion in 2020.2 Lost productivity from CRC deaths is estimated to cost $15.3 billion annually.3
Screening has been shown to reduce the incidence and mortality rates of CRC through prevention (identifying and removing premalignant polyps) and early detection,4 and screening consistently has been shown to be cost-effective or even cost-saving.5 CRC screening is a desirable approach to reduce CRC incidence and mortality rates and treatment costs.6 An investment in screening pre–Medicare-eligible individuals may result in significant savings to Medicare.7 The US Preventive Services Task Force recommends screening for CRC using fecal occult blood testing (FOBT), sigmoidoscopy, or colonoscopy in adults aged 50 years to 75 years.4
Despite this recommendation and the clear evidence linking CRC screening to lower incidence and mortality rates, many adults aged 50 years to 75 years are not receiving the recommended screenings.8 In 2010, only 58.6% of all adults and 20.8% of uninsured adults aged 50 years to 75 years were up to date with CRC screening.9 Increasing the percentage of US adults aged 50 years to 75 years screened for CRC is a leading national health objective in Healthy People 2020.10
It is well established that public health efforts to increase CRC screening would reduce the burden of the disease. Although the medical care system is an important partner in promoting and providing cancer screening services, efforts to increase screening rates in clinical settings are limited by the opportunistic nature of the provision of clinical services and suboptimal access, particularly for the uninsured. The majority of patients are offered screening tests when they visit a medical provider for unrelated reasons. As evidenced by the low CRC screening rates among the uninsured, lack of health insurance is an important barrier to screening.9 The Patient Protection and Affordable Care Act (ACA) helps to make insurance coverage more available by promoting the expansion of Medicaid programs in states, and by establishing a Health Insurance Marketplace. Likewise, for new private health insurance plans and expanded Medicaid, the ACA provides for the elimination of cost-sharing for recommended preventive services rated as “A” or “B” by the US Preventive Services Task Force, such as CRC screening.11 Even with adequate health insurance, individuals still face obstacles to obtaining cancer screening, such as lack of provider recommendation, transportation or geographic access, lack of awareness, and language barriers. Efforts to address these hurdles to CRC screening could make the increased insurance coverage made possible under the ACA even more effective in increasing screening rates. There is strong evidence for the effectiveness of interventions reducing structural barriers to screening, such as mailing FOBT cards.12 FOBT offers the advantage of being noninvasive and convenient for patients and is ideally suited for organized CRC screening programs. The fecal immunochemical test (FIT), one type of FOBT, has been shown to have improved patient acceptance and improved specificity compared with other guaiac-based tests.13 Mailed outreach invitations with FIT have been shown to result in higher screening rates compared with other approaches.14 Together, these factors suggest that implementing an organized evidence-based screening program using the FIT may be an effective and affordable approach to increase CRC screening rates in the United States.
In the current study, we modeled the initial additional investment required and the cost per person screened of implementing a nationwide FIT-based screening program for adults aged 50 years to 75 years. Although similar programs have been implemented internationally or on a smaller scale (ie, managed care system or community health center),13,15 to the best of our knowledge no study to date has examined the feasibility of a national program in the United States. Modeling offers a means of understanding the costs and potential benefits of such a program, and can help inform program implementation decisions.
MATERIALS AND METHODS
Study Design and Target Population
We examined a hypothetical CRC screening program under 2 different intervention designs. The target population included all adults aged 50 years to 75 years in the United States regardless of insurance status and CRC screening status. The target population consisted of 82.1 million adults, leading to 41.1 million being offered a FIT test each year in a biennial screening program.17 Using estimates from the literature, we examined the total initial (first 2 years) annual cost and the cost per person screened under each intervention design.
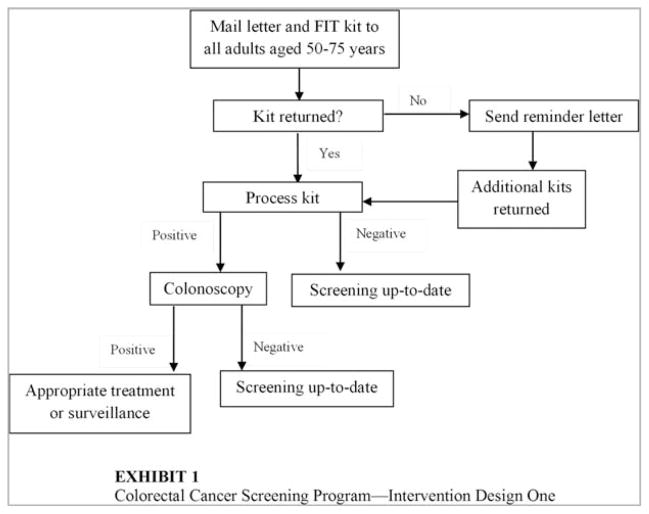
Under the first scenario, a FIT kit would be mailed to every adult aged 50 years to 75 years (Fig. 1). Participants would be asked to complete the test and return the sample in a prepaid envelope. Individuals with a personal history or family history of CRC (estimated at 11%),17 would be instructed to discuss CRC screening with a health care provider rather than completing the test kit. A follow-up educational reminder letter would be sent to individuals who did not initially return the FIT test.
Figure 1.
First intervention design of the colorectal cancer screening program is shown. FIT indicates fecal immunochemical test.
Under the second intervention scenario, all adults aged 50 years to 75 years would be mailed a postcard inviting them to participate in the screening program (Fig. 2). Individuals interested in participating would be instructed to return the postcard indicating their intent, whereas those with a personal history or family history of CRC would be instructed to discuss screening with a health care provider. All individuals returning a postcard would be sent a FIT kit and instructed to complete the test and return it in a prepaid envelope.
Figure 2.
Second intervention design of the colorectal cancer screening program is shown. FIT indicates fecal immunochemical test.
Participation Rates
Under the first scenario, in which the initial mailing included the FIT kit, we assumed participation rates based on data from 2 randomized trials.18,19 We assumed an initial FIT kit return rate of 18%,18 and a 33% increase with the use of educational reminder letters,19 resulting in a final participation rate of 24%. Under this scenario, 8.7 million individuals would be screened for CRC annually during the first 2 years (Table 1).16–29
TABLE 1.
First Intervention Design: Mail FIT Kits to Adults Aged 50 to 75 Years
| Participation and adherence | |
| 82,114,360 | US adults aged 50–75 y16 |
| 41,057,180 | No. offered screening each y (biennial screening) |
| 6,577,360 | Initial participantsa based on 18% participation rate18 |
| 33% | Increase in participation from reminder letters19 |
| 8,747,889 | Final participants, based on 24% final participation rate18,19 |
| 5.5% | Percentage with positive findings20 |
| 481,134 | Positive tests |
| 75% | Colonoscopy adherence21–23 |
| 360,850 | New diagnostic colonoscopies |
| 72% | Percentage of colonoscopies that will find adenomas or cancer and require surveillance20 |
| 259,812 | No. that will enter surveillance colonoscopy program |
| 416,277 | Annual no. of colonoscopies for program (diagnostic and surveillance) |
| Cost assumptions | |
| FIT kit costs | |
| $82,114,360 | Kit mailing fee ($2 each) |
| $17,495,778 | Kit return fee ($2 each) |
| $16,833,444 | Reminder letters ($.50 each) |
| $61,235,224 | Test processing fees ($7 each) |
| Colonoscopy and associated medical costsb | |
| $3,156,238 | Primary care visits (80% with a positive test will see a PCP; $82 per visit)24 |
| $30,223,665 | Colonoscopies ($727 cost per colonoscopy)25 |
| $259,476 | Colonoscopy complications (2.4 per 1000 colonoscopies; $6233 cost per complication)17,24,26–28 |
| Improving participation and adherence | |
| $73,613,487 | Improving participation and adherence ($153 per positive FIT)29 |
| Program administrative costs | |
| $60,000,000 | General program administrative costs |
| Cost offsets | |
| $26,700,000 | Current annual spending on screening program |
Abbreviation: FIT, fecal immunochemical test; PCP, primary care provider.
Excludes 11% of adults with a family history of colorectal cancer.17
Costs included only for the uninsured population aged 50 years to 64 years (10% of sample).
Under the second scenario, in which an advanced notification postcard would be sent followed by a FIT kit, on the basis of data from a randomized trial, we assumed that 33% of the target population would express interest in participating.30 Using results from the same trial, we assumed a 78% return rate from the FIT kit mailing,30 resulting in a final participation rate of 26%. Under this scenario, 9.4 million individuals would be screened for CRC annually in the first 2 years (Table 2).16,20–30
TABLE 2.
Second Intervention Design: Mail Advanced Notification Postcard Followed by FIT Kit to Adults Aged 50 to 75 Years
| Participation and adherence | |
| 82,114,360 | US adults aged 50 to 75 y16 |
| 41,057,180 | No. offered screening each y (biennial screening) |
| 12,058,494 | Would express interest in participatinga based on 33% participation rate30 |
| 78% | Return rate of mailed FIT kits30 |
| 9,405,625 | Final participants, based on 26% participation rate30 |
| 5.5% | Percentage with positive findings20 |
| 517,309 | Positive tests |
| 75% | Colonoscopy adherence21–23 |
| 387,982 | New diagnostic colonoscopies |
| 72% | Percentage of colonoscopies that will find adenomas or cancer and require surveillance20 |
| 279,347 | Will enter surveillance colonoscopy program |
| 447,576 | Annual no. of colonoscopies for program (diagnostic and surveillance) |
| Cost assumptions | |
| FIT kit costs | |
| $20,528,590 | Kit mailing fee ($2 each) |
| $24,116,988 | Kit return fee ($2 each) |
| $18,811,250 | Reminder letters ($.50 each) |
| $65,839,376 | Test processing fees ($7 each) |
| Colonoscopy and associated medical costsb | |
| $3,393,550 | Primary care visits (80% with a positive test will see a PCP; $82 per visit)24 |
| $32,496,121 | Colonoscopies ($727 cost per colonoscopy)25 |
| $236,428 | Colonoscopy complications (2.4 per 1000 colonoscopies; $6233 cost per complication)17,24,26–28 |
| Improving participation and adherence | |
| $79,148,336 | Improving participation and adherence ($153 per positive FIT)29 |
| Program administrative costs | |
| $60,000,000 | General program administrative costs |
| Cost offsets | |
| $26,700,000 | Current annual spending on screening program |
Abbreviation: FIT, fecal immunochemical test; PCP, primary care provider.
Excludes 11% of adults with a family history of colorectal cancer.17
Costs included only for the uninsured population aged 50 to 64 years (10% of sample).
Detection Rates
On the basis of data from a randomized trial, we assumed a positive FIT rate of 5.5% using a threshold of 100 ng/mL as a positive test.20 In addition, we assumed a 75% adherence rate to a follow-up colonoscopy, on the basis of findings from observational and randomized studies.21–23 Among those patients with a positive FIT receiving a colonoscopy, we assumed 9% would have cancer, 43% would have advanced adenomas, and 20% would have nonadvanced adenomas on the basis of results from a randomized trial.20 Screening program participants with adenomas or cancer would receive appropriate treatment and surveillance colonoscopies.31
Cost Assumptions
Costs were based on estimates from other modeling studies and from the Centers for Disease Control and Prevention (CDC) Colorectal Cancer Screening Demonstration Program. We included estimates of program administrative costs, including general program administrative costs, identifying eligible adults, public education and outreach, quality assurance, partnership development, data collection, and program evaluation. The costs associated with data collection would provide information regarding patient screening and allow for the seamless continuation of the program beyond its initial implementation. We assumed the costs of mailing the FIT kits were $2 each, reminder letters were $0.50 each, FIT kit return fees were $2 each, and test processing fees were $7 each. We assumed the costs associated with improving participation and adherence among individuals with a positive FIT to be $153 per positive FIT on the basis of data from the demonstration program.29 The cost of diagnostic colonoscopies and primary care visits were included for the uninsured population aged 50 years to 64 years; individuals aged ≥65 years were assumed to be insured through Medicare. Each colonoscopy without a polypectomy was estimated to cost $587, whereas each colonoscopy with a polypectomy was estimated to cost $76525 for the 78% of cases in which polyps are detected.20 We assumed that 80% of individuals with a positive FIT would visit a primary care provider.32 The cost of primary care visits was based on Medicare reimbursements.24 On the basis of data from an integrated health care delivery system and observational studies, colonoscopy complications were assumed to occur in 2.4 per 1000 colonoscopies, with an average cost of $6233 per complication.17,26–28,33 Among the insured patients, service delivery costs would be accounted for through the usual care system, as is currently the case. The amount spent on the CDC Colorectal Cancer Screening Demonstration Program annually was subtracted from the total gross annual cost to determine the initial additional investment required each year. The cost per person screened was determined by dividing the total gross annual cost by the number of individuals screened annually.
Mortality Reduction
We estimated the effect of the screening program on CRC mortality using estimates from the literature regarding efficacy and the estimated number of preventable CRC deaths,34 and the participation rate estimated in the current study. The efficacy of FOBT in preventing CRC mortality was estimated at 38% from 3 randomized controlled trials, 1 quasirandomized trial, and 8 case-control studies.34 Once adjusted for participation (24% and 26%, respectively), the screening program was estimated to reduce CRC deaths by 9% to 10%. Risk reductions were applied against an estimated 31,299 preventable CRC deaths per year.34
Sensitivity Analysis
We conducted several additional analyses to examine the sensitivity of the results of the current study to changes in participation rates, program costs, and anticipated increases in insurance coverage under the ACA. Results were examined by varying participation rates by 25% and administrative costs by 25%, and reducing the number of uninsured adults by 50%. To account for the potential use of cooling bags to avoid the degradation of FIT by heat, we also examined the results when varying mailing and processing costs by 25%.
RESULTS
Scenario 1
Under the first scenario design, in which the FIT kit is first mailed to adults aged 50 years to 75 years, the final participation rate of 24% would result in 8.7 million participants. Among those participating, 481,134 would have a positive FIT, with 360,850 individuals following up with a diagnostic colonoscopy (Table 1).16–29
The initial gross annual cost of a FIT-based program for those aged 50 years to 75 years was estimated at $344.9 million. The cost associated with testing is the largest component of overall costs, totaling approximately $177.7 million, which includes mailing the FIT kits, reminder letters, kit return costs, and test processing fees. The costs associated with diagnostic and surveillance colonoscopies among the uninsured population would be approximately $33.6 million, whereas administrative costs are estimated at $60.0 million and costs associated with improving participation and adherence are approximately $73.6 million.
The modeled screening program would be expected to screen 8.7 million average-risk adults aged 50 years to 75 years for CRC with an initial additional investment of $318.2 million annually, and a cost per individual screened of $39.43. The screening program was estimated to prevent 2900 deaths annually.
Scenario 2
In the second scenario, in which a postcard is first mailed to all adults aged 50 years to 75 years followed by a FIT kit to individuals expressing interest, the final participation rate of 26% resulted in 9.4 million participants. Among those participating, 517,309 would have a positive FIT each year, with 387,982 individuals following up with a diagnostic colonoscopy (Table 2).16,20–30
The initial gross annual cost of a FIT-based program for those aged 50 years to 75 years was estimated at $304.6 million. The cost associated with testing is the largest component of overall costs, totaling approximately $129.3 million, which includes mailing the initial postcards, mailing the FIT kits, kit return costs, and test processing fees. The costs associated with diagnostic and surveillance colonoscopies among the uninsured population are approximately $36.1 million. Meanwhile, administrative costs are estimated at $60.0 million, and the costs associated with improving participation and adherence are approximately $79.1 million.
The modeled screening program would be expected to screen 9.4 million average-risk adults aged 50 years to 75 years for CRC with an initial additional investment of $277.9 million annually, and a cost per individual screened of $32.38. The screening program was estimated to prevent 3100 deaths annually.
Sensitivity Analysis
Under the first scenario, varying the participation by 25% would result in a final participation rate of 18% to 30%, with 6.6 million to 10.9 million individuals screened. Variation in program costs resulted in an initial additional investment ranging from $273.8 to $362.7 million annually, and a cost per person screened ranging from $34.35 to $44.51. With a 50% reduction in rates of uninsured individuals, the initial additional investment was estimated at $301.4 million annually, with a cost per person screened of $37.51 (Table 3).
TABLE 3.
Results of Sensitivity Analyses of Varying Participation Rates, Costs Associated With FIT Kit Mailing and Processing, and Administrative Costs
| Intervention Design 1
|
Intervention Design 2
|
|||
|---|---|---|---|---|
| Total Additional Investment | Cost Per Person Screened | Total Additional Investment | Cost Per Person Screened | |
| Base case | $318.2 million | $39.43 | $277.9 million | $32.38 |
| Participation rate | ||||
| 25% increase | $364.7 million | $35.80 | $327.9 million | $30.16 |
| 25% decrease | $271.7 million | $45.49 | $227.9 million | $36.09 |
| Costs associated mailing and processing | ||||
| 25% increase | $362.7 million | $44.51 | $310.2 million | $35.82 |
| 25% decrease | $273.8 million | $34.35 | $245.5 million | $28.95 |
| Administrative costs | ||||
| 25% increase | $333.2 million | $41.14 | $292.9 million | $33.98 |
| 25% decrease | $303.2 million | $37.72 | $262.9 million | $30.79 |
| Uninsurance rates | ||||
| 50% reduction | $301.4 million | $37.51 | $259.8 million | $30.46 |
Abbreviation: FIT, fecal immunochemical test.
In the second scenario, varying the final participation rate by 25% would result in a final participation rate of 19% to 32%, with 7.1 million to 11.8 million individuals screened. Variation in program costs resulted in an initial additional investment required ranging from $245.5 to $310.2 million annually, and a cost per person screened ranging from $28.95 to $35.82. With a 50% reduction in uninsurance rates, the initial additional investment was estimated at $259.8 million annually, with a cost per person screened of $30.46 (Table 3).
DISCUSSION
In the current study, we estimated the total initial investment required and the cost per person screened in a nationwide FIT-based screening program for adults aged 50 years to 75 years. Under the first scenario, in which the initial mailing included the FIT kits, the initial additional investment required was estimated at $318.2 million annually, with an estimated 8.7 million individuals screened for CRC at a cost of $39.43 per person screened. Under this scenario, the screening program was estimated to result in 2900 fewer deaths from CRC annually. Under the second intervention scenario, in which an invitation was mailed first, the initial additional investment required was estimated at $277.9 million annually, with an estimated 9.4 million individuals screened for CRC at a cost of $32.38 per person screened. Under this scenario, the program was estimated to result in 3100 fewer deaths from CRC annually.
Organized CRC screening programs in other high-income countries have demonstrated the ability of such approaches to reach a substantial number of individuals at a modest cost per person screened. For example, a regional FOBT-based screening program in England screened 1.4 million people, and a national FOBT-based screening program in Germany screened 4.4 million people during the course of 1 year.35 The costs per person screened in organized FOBT-based screening programs are similar to those estimated in the current study. For example, a regional FOBT-based screening program in Portugal screened >6000 individuals at a cost of $29 per person screened.35 A biennial FOBT-based screening program in Australia was estimated to screen 1 million people annually, with the cost per person screened ranging from $31 to $50.32
Organized CRC screening programs have demonstrated the ability to increase screening rates and reduce health disparities, both domestically and internationally. Kaiser Permanente has implemented an organized CRC screening program by using a mailed FIT. From 2005 to 2011, they have increased screening rates from 37% to 75% among the privately insured population and from 41% to 85% among their managed Medicare population.13,36 A randomized controlled trial has shown mailed FIT outreach to be most effective in increasing CRC screening rates, compared with usual care and colonoscopy outreach.14 In Delaware, a statewide program providing coverage for CRC screening and treatment, patient navigation, and case management has increased CRC screening rates from 57% in 2002 to 74% in 2009. The program also eliminated racial disparities in screening, incidence, and advanced stage of disease at the time of diagnosis.37 In Australia, data from the FIT-based National Bowel Cancer Screening Program demonstrated that individuals screened with mailed FIT kits were diagnosed at a significantly earlier stage of disease compared with those not invited, suggesting that the program will lead to reductions in CRC mortality in Australia.38
The success of the CDC’s National Breast and Cervical Cancer Early Detection Program (NBCCEDP), which provides breast and cervical cancer screening to medically underserved low-income women, has been well documented. For example, between 1991 and 2006, 1.8 million women were screened for breast cancer in the NBCCEDP, and the program saved 100,800 life-years compared with no program.39 The cost estimates in the current study were considerably lower than those observed in the NBCCEDP. The cost per woman screened for breast cancer has been estimated at $108, whereas the cost per woman screened for cervical cancer was estimated at $65 in the NBCCEDP.40
Implementation of an organized population-based CRC screening program has the potential to make a significant public health impact by substantially increasing screening rates.13,36 Using the results from the current study, if we assume that each individual screened in the first year was not up to date with CRC screening, after the first year of program implementation, the percentage of adults aged 50 years to 75 years being up to date would increase from 59% to approximately 86% to 88%. However, if we take a more conservative approach and assume that only one-half of those newly screened were not up to date, the percentage of adults being up to date with screening would increase from 59% to approximately 72% to 73%.
Implementation of a FIT-based organized CRC screening program also has the potential to reduce CRC death rates and CRC treatment costs, at a modest cost per person screened. Consistent evidence has demonstrated that CRC screening among adults aged 50 years to 75 years is effective in reducing incidence and mortality, and cost-effective.5,34 As demonstrated, organized CRC screening programs have the potential to eliminate health disparities37 and reduce CRC death rates.38 The rising costs of treating patients with advanced-stage CRC make screening a desirable approach to reduce incidence and mortality, and to control the cost of treatment.6 Increasing screening among adults aged 50 years to 64 years, before Medicare eligibility, has the potential to substantially reduce the costs associated with CRC treatment in the Medicare population.7
The current study is subject to several limitations. First, annual program costs would likely be reduced after the initial 2 years of program implementation as individuals opt out or are up to date with screening. In addition, participants with a positive FIT receiving a normal follow-up colonoscopy would be up to date with CRC screening for the next 10 years, whereas those with abnormal results would have any polyps removed and enter a colonoscopy surveillance program. Second, these results are subject to the limitations of the data currently available and to our assumptions. For example, although our participation rate estimates were estimated based on studies conducted outside the United States, we also compared participation rates with randomized controlled trials in the United States among adults who were not up to date with CRC screening.14,41 As expected, our estimated participation rates (24%–26%) were lower than those observed in the previous studies (30%–44%) given the different study population.14,41 We examined the impact of higher participation rates in the sensitivity analyses in the current study. Third, our assumptions were based on data from the published literature; however, for several parameters, limited data were available. Fourth, we did not account for different positivity thresholds for the FIT, which can impact test results.13 Last, our modeling did not examine the cost-effectiveness; however, previous studies have shown CRC screening to be very cost-effective.5 The focus of the current study was to examine the costs of a national FIT-based CRC screening program from a program perspective. Additional modeling is warranted to examine the full costs and benefits from the societal perspective.
The substantial health and economic burden of CRC in the United States has made its prevention and early detection a public health priority in cancer prevention and control. Public health can help to guide cancer control approaches that are comprehensive, strategic, and organized to ensure that participation in cancer screening is widespread and equitable. Implementing a nationwide FIT-based CRC screening program would result in a substantial number of individuals screened at a moderate cost per person screened. Results from the current analysis may provide useful information for understanding the public health benefit of an organized screening delivery system as well as the potential resources required to implement a nationwide CRC screening program, and help guide decisions regarding program planning, design, and implementation.
Acknowledgments
FUNDING SUPPORT
No specific funding was disclosed.
Footnotes
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
CONFLICT OF INTEREST DISCLOSURES
Dr. Pignone’s work on the current study was supported by an Inter-agency Personnel Agreement with the Centers for Disease Control and Prevention.
References
- 1.US Cancer Statistics Working Group. United States Cancer Statistics: 1999–2010 Incidence and Mortality Web-based Report. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2013. [Accessed February 25, 2014]. www.cdc.gov/uscs. [Google Scholar]
- 2.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103:117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ekwueme DU, Guy GP, Li C, Rim SH, Parelkar P, Chen SC. The health burden and economic costs of cutaneous melanoma mortality by race/ethnicity–United States, 2000 to 2006. J Am Acad Dermatol. 2011;65(5 suppl 1):S133–S143. doi: 10.1016/j.jaad.2011.04.036. [DOI] [PubMed] [Google Scholar]
- 4.US Preventive Services Task Force. Screening for Colorectal Cancer. Rockville, MD: Agency for Healthcare Research and Quality; 2008. [Accessed March 2, 2013]. uspreventiveservicestaskforce.org/uspstf/uspscolo.htm. [Google Scholar]
- 5.Lansdorp-Vogelaar I, Knudsen AB, Brenner H. Cost-effectiveness of colorectal cancer screening. Epidemiol Rev. 2011;33:88–100. doi: 10.1093/epirev/mxr004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lansdorp-Vogelaar I, van Ballegooijen M, Zauber AG, Habbema JDF, Kuipers EJ. Effect of rising chemotherapy costs on the cost savings of colorectal cancer screening. J Natl Cancer Inst. 2009;101:1412–1422. doi: 10.1093/jnci/djp319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dobson A, Sen N, DaVanzo J. [Accessed March 2, 2013];Methodology for Estimating the Potential Cost Savings to Medicare from Increased Colorectal Cancer Screening of the Pre-Medicare Population. nccrt.org/about/policy-action/savings-to-medicare/
- 8.Stanley SL, King JB, Thomas CC, Richardson LC. Factors associated with never being screened for colorectal cancer. J Community Health. 2013;38:31–39. doi: 10.1007/s10900-012-9600-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention (CDC). . Cancer screening—United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:41–45. [PubMed] [Google Scholar]
- 10.US Department of Health and Human Services. [Accessed March 12, 2013];Healthy People 2020. healthypeople.gov/2020/topicsobjectives2020/default.aspx.
- 11.Koh HK, Sebelius KG. Promoting prevention through the Affordable Care Act. N Engl J Med. 2010;363:1296–1299. doi: 10.1056/NEJMp1008560. [DOI] [PubMed] [Google Scholar]
- 12.Sabatino SA, Lawrence B, Elder R, et al. Community Preventive Services Task Force. . Effectiveness of interventions to increase screening for breast, cervical, and colorectal cancers: 9 updated systematic reviews for the guide to community preventive services. Am J Prev Med. 2012;43:97–118. doi: 10.1016/j.amepre.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Levin TR, Jamieson L, Burley DA, Reyes J, Oehrli M, Caldwell C. Organized colorectal cancer screening in integrated health care systems. Epidemiol Rev. 2011;33:101–110. doi: 10.1093/epirev/mxr007. [DOI] [PubMed] [Google Scholar]
- 14.Gupta S, Halm EA, Rockey DC, et al. Comparative effectiveness of fecal immunochemical test outreach, colonoscopy outreach, and usual care for boosting colorectal cancer screening among the under-served: a randomized clinical trial. JAMA Intern Med. 2013;173:1725–1732. doi: 10.1001/jamainternmed.2013.9294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benson VS, Patnick J, Davies AK, Nadel MR, Smith RA, Atkin WS. Colorectal cancer screening: a comparison of 35 initiatives in 17 countries. Int J Cancer. 2008;122:1357–1367. doi: 10.1002/ijc.23273. [DOI] [PubMed] [Google Scholar]
- 16.US Census Bureau. [Accessed March 12, 2013];Single Years of Age and Sex: 2010. factfinder2.census.gov/faces/tableservices/jsf/pages/productview.xhtml?pid=DEC_10_SF1_QTP2&prodType=table.
- 17.Wilschut JA, Steyerberg EW, van Leerdam ME, Lansdorp-Vogelaar I, Habbema JDF, van Ballegooijen M. How much colonoscopy screening should be recommended to individuals with various degrees of family history of colorectal cancer? Cancer. 2011;117:4166–4174. doi: 10.1002/cncr.26009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cole S, Smith A, Wilson C, Turnbull D, Esterman A, Young GP. An advance notification letter increases participation in colorectal cancer screening. J Med Screen. 2007;14:73–75. doi: 10.1258/096914107781261927. [DOI] [PubMed] [Google Scholar]
- 19.Lee JK, Reis V, Liu S, et al. Improving fecal occult blood testing compliance using a mailed educational reminder. J Gen Intern Med. 2009;24:1192–1197. doi: 10.1007/s11606-009-1087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Rossum L, Van Rijn AF, Laheij R, et al. Random comparison of guaiac and immunochemical fecal occult blood tests for colorectal cancer in a screening population. Gastroenterology. 2008;135:82–90. doi: 10.1053/j.gastro.2008.03.040. [DOI] [PubMed] [Google Scholar]
- 21.Yabroff KR, Washington KS, Leader A, Neilson E, Mandelblatt J. Is the promise of cancer-screening programs being compromised? Quality of follow-up care after abnormal screening results. Med Care Res Rev. 2003;60:294–331. doi: 10.1177/1077558703254698. [DOI] [PubMed] [Google Scholar]
- 22.Myers RE, Turner B, Weinberg D, et al. Impact of a physician-oriented intervention on follow-up in colorectal cancer screening. Prev Med. 2004;38:375–381. doi: 10.1016/j.ypmed.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 23.Turner B, Myers RE, Hyslop T, et al. Physician and patient factors associated with ordering a colon evaluation after a positive fecal occult blood test. J Gen Intern Med. 2003;18:357–363. doi: 10.1046/j.1525-1497.2003.20525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centers for Medicare and Medicaid Services. [Accessed April 10, 2013];Physician Fee Schedule Search HCPCS Code 11400-11646. cms.gov/apps/physician-fee-schedule/search/search-results.aspx?Y=0&T=0&HT=2&CT=0&H1-11400&H2=11646&M=5.
- 25.Zauber AG. Cost-effectiveness of colonoscopy. Gastrointest Endosc Clin N Am. 2010;20:751–770. doi: 10.1016/j.giec.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levin TR, Zhao W, Conell C, et al. Complications of colonoscopy in an integrated health care delivery system. Ann Intern Med. 2006;145:880–886. doi: 10.7326/0003-4819-145-12-200612190-00004. [DOI] [PubMed] [Google Scholar]
- 27.Regula J, Rupinski M, Kraszewska E, et al. Colonoscopy in colorectal-cancer screening for detection of advanced neoplasia. N Engl J Med. 2006;355:1863–1872. doi: 10.1056/NEJMoa054967. [DOI] [PubMed] [Google Scholar]
- 28.Lieberman DA, Weiss DG, Bond JH, et al. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. N Engl J Med. 2000;343:162–168. doi: 10.1056/NEJM200007203430301. [DOI] [PubMed] [Google Scholar]
- 29.Subramanian S, Tangka FK, Hoover S, DeGroff A, Royalty J, Seeff LC. Clinical and programmatic costs of implementing colorectal cancer screening: evaluation of five programs. Eval Program Plann. 2011;34:147–153. doi: 10.1016/j.evalprogplan.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 30.Levi Z, Birkenfeld S, Vilkin A, et al. A higher detection rate for colorectal cancer and advanced adenomatous polyp for screening with immunochemical fecal occult blood test than guaiac fecal occult blood test, despite lower compliance rate. A prospective, controlled, feasibility study. Int J Cancer. 2011;128:2415–2424. doi: 10.1002/ijc.25574. [DOI] [PubMed] [Google Scholar]
- 31.Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR United States Multi-Society Task Force on Colorectal Cancer. . Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143:844–857. doi: 10.1053/j.gastro.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 32.Pignone MP, Flitcroft KL, Howard K, Trevena LJ, Salkeld GP, St John DJ. Costs and cost-effectiveness of full implementation of a biennial faecal occult blood test screening program for bowel cancer in Australia. Med J Aust. 2011;194:180–185. doi: 10.5694/j.1326-5377.2011.tb03766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pox C, Schmiegel W, Classen M. Current status of screening colonoscopy in Europe and in the United States. Endoscopy. 2007;39:168–173. doi: 10.1055/s-2007-966182. [DOI] [PubMed] [Google Scholar]
- 34.Maciosek MV, Solberg LI, Coffield AB, Edwards NM, Goodman MJ. Colorectal cancer screening: health impact and cost effectiveness. Am J Prev Med. 2006;31:80–89. doi: 10.1016/j.amepre.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 35.Subramanian S, Tangka FK, Hoover S, et al. International Comparison of Economic Assessments of Colorectal Cancer Screening Programs: Preliminary Results. Presented at the 2012 CDC National Cancer Conference; August 21–23, 2012; Washington, DC. [Google Scholar]
- 36.Levin TR. Optimizing colorectal cancer screening by getting FIT right. Gastroenterology. 2011;141:1551–1555. doi: 10.1053/j.gastro.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 37.Grubbs SS, Polite BN, Carney J, Jr, et al. Eliminating racial disparities in colorectal cancer in the real world: it took a village. J Clin Oncol. 2013;31:1928–1930. doi: 10.1200/JCO.2012.47.8412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cole SR, Tucker GR, Osborne JM, et al. Shift to earlier stage at diagnosis as a consequence of the National Bowel Cancer Screening Program. Med J Aust. 2013;198:327–330. doi: 10.5694/mja12.11357. [DOI] [PubMed] [Google Scholar]
- 39.Hoerger TJ, Ekwueme DU, Miller JW, et al. Estimated effects of the National Breast and Cervical Cancer Early Detection Program on breast cancer mortality. Am J Prev Med. 2011;40:397–404. doi: 10.1016/j.amepre.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ekwueme DU, Gardner JG, Subramanian S, Tangka FK, Bapat B, Richardson LC. Cost analysis of the National Breast and Cervical Cancer Early Detection Program: selected states, 2003 to 2004. Cancer. 2008;112:626–635. doi: 10.1002/cncr.23207. [DOI] [PubMed] [Google Scholar]
- 41.Jean-Jacques M, Kaleba EO, Gatta JL, Gracia G, Ryan ER, Choucair BN. Program to improve colorectal cancer screening in a low-income, racially diverse population: a randomized control trial. Ann Fam Med. 2012;10:412–417. doi: 10.1370/afm.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]