Abstract
Background
Diabetics are considered to be at high risk for complications from influenza infection and Type 2 diabetes is a significant comorbidity of obesity. Obesity is an independent risk factor for complications from infection with influenza. Annual vaccination is considered the best strategy for protecting against influenza infection and it’s complications. Our previous study reported intact antibody responses 30 days post vaccination in an obese population. This study was designed to determine the antibody response to influenza vaccination in type 2 diabetics.
Methods
Subjects enrolled were 18 or older without immunosuppressive diseases or taking immunosuppressive medications. A pre-vaccination blood draw was taken at time of enrollment, the subjects received the influenza vaccine and returned 28–32 days later for a post-vaccination blood draw. Height and weight were also obtained at the first visit and BMI was calculated. Antibody levels to the vaccine were determined by both ELISA and hemagglutination inhibition (HAI) assays.
Results
As reported in our previous work, obesity positively correlates with the influenza antibody response (p=0.02), while age was negatively correlated with antibody response (p<0.001). In both year 1 and year 2 of our study there was no significant difference in the percentage of the type 2 diabetic subjects classified as seroprotected or a responder to the influenza vaccine compared to the non-diabetic subjects.
Conclusions
These data are important because they demonstrate that diabetics, considered a high risk group during influenza season, are able to mount an antibody response to influenza vaccination that may protect them from influenza infection.
Keywords: Obesity, Diabetes, Influenza Vaccine, Antibody and Immune Response
1. Introduction
Type 2 diabetes (T2D)1 is a significant comorbidity associated with obesity. The comorbidities associated with obesity and infection with influenza virus are significant public health concerns. Currently, greater than two-thirds of the US population is classified as overweight or obese, with 34% of the population being classified as obese[1]. Twenty nine million Americans (9.3% of the population) have diabetes with an additional 35% classified as having pre-diabetes[2]. Infection with influenza results in 3,000–49,000 deaths in non-pandemic years[3,4] and during the pH1N1 pandemic of 2009, studies suggested that diabetics were at a greater risk for hospitalization and increased complications from influenza [5–7].
Influenza vaccination remains the single most effective way to prevent serious influenza infection. The Centers for Disease Control considers diabetics to be at a higher risk for morbidity and mortality from influenza[14]. Diabetics are at greater risk for “complicated” influenza and longer hospital stays when infected, therefore, the CDC recommends that all diabetics over 6 months of age receive the trivalent inactivated form of the influenza vaccine[14, 15]. Despite this recommendation, there are very few studies that have examined the response to vaccination in T2D. A systematic review of hepatitis B vaccine studies in diabetic populations suggests that older diabetics have an impaired response to vaccine compared to older non-diabetics[16]. A small study of an adult, mixed diabetic population (both Type 1 and Type 2, n=49) showed that the antibody response to the monovalent pH1N1 vaccine suggest there was a negative correlation between HbA1c levels and seroprotection. To determine if the antibody response to the trivalent influenza vaccine is impaired in T2D subjects, we measured serum antibody titers in influenza vaccinated T2D and healthy controls. Here, we report that T2D did not affect influenza specific antibody titers 30 days post influenza vaccination.
2. Materials and methods
2.1 Study design and subjects
This is an ongoing, prospective observational study carried out at the University of North Carolina Family Medicine Center, an academic outpatient primary care facility in Chapel Hill, NC. Eligible participants were adult patients at the Center scheduled to receive the 2009–2010 or 2010–2011 seasonal trivalent influenza vaccine (TIV). Enrollment and data analysis were conducted independently for each year because of the annual change in vaccine composition. Exclusion criteria were immunosuppression, self-reported use of immunomodulator or immunosuppressive drugs, acute febrile illness, history of hypersensitivity to any influenza vaccine components, history of Guillian–Barre syndrome, or use of theophylline preparations or warfarin[17, 18]. Diabetes status (Type 2) was self-reported and confirmed from medical records (physician diagnosis, glycosylated hemoglobin (HbA1c) and fasting glucose levels). HbA1c values from within 6 months of vaccination were obtained from the medical records of subjects enrolled in the study. The medications that the diabetic subjects were taking at the time of enrollment are listed in Supplemental Table 1. These medications were not used as part of the analysis. All procedures were approved by the Biomedical Institutional Review Board at the University of North Carolina.
In year 1 of the study (September–November 2009), we enrolled 499 participants. At enrollment, informed consent, height, weight and a baseline serum sample were obtained. One dose of 2009–2010 seasonal TIV ((0.5 ml Fluzone (Sanofi Pasteur, Swiftwater, PA, USA) containing A/Brisbane/59/2007 (H1N1), A/Brisbane/10/2007 (H3N2) and B/Brisbane/60/2008)) was administered in the deltoid muscle. Participants (461, 92% completion rate) returned 28–35 days later for a post-vaccination blood draw. Pre- and post-vaccination serum samples were stored at −80 °C until analyzed.
In year 2 of the study, (September-November 2010) we enrolled 489 and 463 completed the study (94.6% completion rate). The procedures for enrollment, consent, sample collection and storage were the same as year 1. The 2010–2011 seasonal TIV contained A/California/7/2009 (H1N1), A/Perth/16/2009 (H3N2) and B/Brisbane/60/2008.
The full demographic table for year 1 of the study has been previously published[13]. The data for year 2 are in Table 1.
Table 1.
Demographic characteristics of 2010–2011 participantsa
| Underweightb | Healthy Weight | Overweight | Obese | Total | ||
|---|---|---|---|---|---|---|
| Year 2 | ||||||
| Enrolleda | 5 (1.1) | 113 (24.4) | 145 (31.3) | 200 (43.2) | 463 | |
| Agec | 62.6+/−21.4 (27–83) | 54.2 +/−15.8 (19–88) | 56.9 +/−16.4 (22–86) | 52.0 +/−13.7 (18–83) | ||
| Gendera | Male | 1 (0.2) | 43 (9.3) | 65 (14.0) | 73 (15.8) | 182 (39.3) |
| Female | 4 (0.9) | 70 (15.1) | 80 (17.3) | 127 (27.4) | 281 (60.7) | |
| Racea | White | 4 (0.9) | 87 (18.8) | 108 (23.3) | 113 (24.4) | 312 (67.4) |
| AA | 1 (0.2) | 19 (4.1) | 35 (7.6) | 84 (18.1) | 139 (30.0) | |
| Other | 0 (0) | 7 (1.5) | 2 (0.4) | 3 (0.6) | 12 (2.6) | |
| Diabetesa | Yes | 0 (0) | 9 (1.9) | 21 (4.5) | 72 (15.6) | 102 (22.0) |
| No | 5 (1.1) | 104 (22.5) | 121 (26.1) | 123 (26.6) | 353 (76.2) | |
| Unknown | 0 (0) | 0 (0) | 3 (0.6) | 5 (1.1) | 8 (1.7) |
Number (percentage, out of n=463)
BMI: Underweight (<18.5), Healthy Weight (18.5–24.9), Overweight (25–29.9), Obese (≥30)
Mean ± SD (Range)
2.2 ELISA for total anti-vaccine IgG
The method for the anti-influenza vaccine ELISA has been previously published[13]. This ELISA allows for the measurement of the total IgG made to all components of the influenza vaccine. Run in conjunction with the hemagglutination inhibition assay (described below), the combination of these two assays allows for a fuller description of antibody response to the vaccine in each subject. Briefly, vaccine was diluted and adsorbed to microtitration plates in a carbonate coating buffer. After washing, triplicate serum dilutions in PBS were allowed to react with antigen, and bound antibodies were detected by a peroxidase-conjugated goat anti-human IgG (Abcam, Cambridge, MA), followed by a chromogenic substrate. Color intensity was measured by absorbance at 450 nm. Internal control sera were included in each run. Pre- and post-vaccination sera from each subject were tested in the same run. The intra-assay coefficient of variation of the assay was 4%.
2.3 Hemagglutination inhibition assay
HAI assays were conducted to determine the level of antibodies in sera as previously described[19]. In contrast to the total vaccine component ELISA, the HAI assay measures the total antibody response to each individual strain of influenza present in the vaccine. Briefly, sera were treated with receptor destroying enzyme (RDE; Denka Seiken, Tokyo, Japan) overnight, followed by inactivation at 56°C for 1 hr, and a final dilution to 1:10 with PBS. RDE-treated sera was then incubated in duplicate with either A/Brisbane/59/2007, ABrisbane/10/2007 or the influenza B virus B/Brisbane/60/2008 for those vaccinated in year 1 of the study. For year 2, serum was incubated with A/California/4/2009 (H1N1), A/Perth/16/2009 (H3N2) influenza A viruses or the influenza B virus B/Brisbane/60/2008 for 15 min at room temperature. After a one hr incubation at 4°C with either 0.5% turkey red blood cells (A/California/4/2009 virus) or 0.5% chicken red blood cells for the other viruses, HAI titer was determined by the reciprocal dilution of the last well. Positive and negative controls as well as back titrations of virus were included on each individual plate.
An HAI titer of 1:40 is considered to be the threshold for seroprotection against influenza, while a 4-fold increase in HAI titers from pre-vaccination to post-vaccination is considered seroconversion[20].
2.4 Statistical analysis
Associations between baseline variables and antibody response at 1 month post vaccination were assessed using Spearman’s rank correlation coefficient for continuous variables (age and BMI) and the Kruskal–Wallis test for categorical variables (diabetes, race, sex and smoking status). Pre-vaccine, post-vaccine, and log fold increase (post/pre) HAI titers were modeled using linear regression, and the odds of seroprotection or seroconversion were modeled using logistic regression. No adjustments were made for multiple comparisons. All analyses were conducted in R version 2.14[21].
3. Results
3.1 Characteristics of the study population
In year 1 the study population was 29.7% healthy weight, 33.4% overweight and 35.5% obese. In addition, 19.6% of all subjects were type 2 diabetics. Within the obese group, 32.9% were type 2 diabetic, while 18.8% and 5.8% of the overweight and healthy weight subjects were type 2 diabetic, respectively[13]. In year 2 of the study, the population was 24.4% healthy weight, 31.3% overweight and 43.2% obese subjects (Table 1). Twenty-two percent of all of the subjects were type 2 diabetics. Within the obese group, 36% were type 2 diabetic, while 14.4% and 1.4% of the overweight and healthy weight subjects were type 2 diabetic, respectively.
Total anti-influenza vaccine IgG antibodies 30 days post vaccination influenced by age and BMI
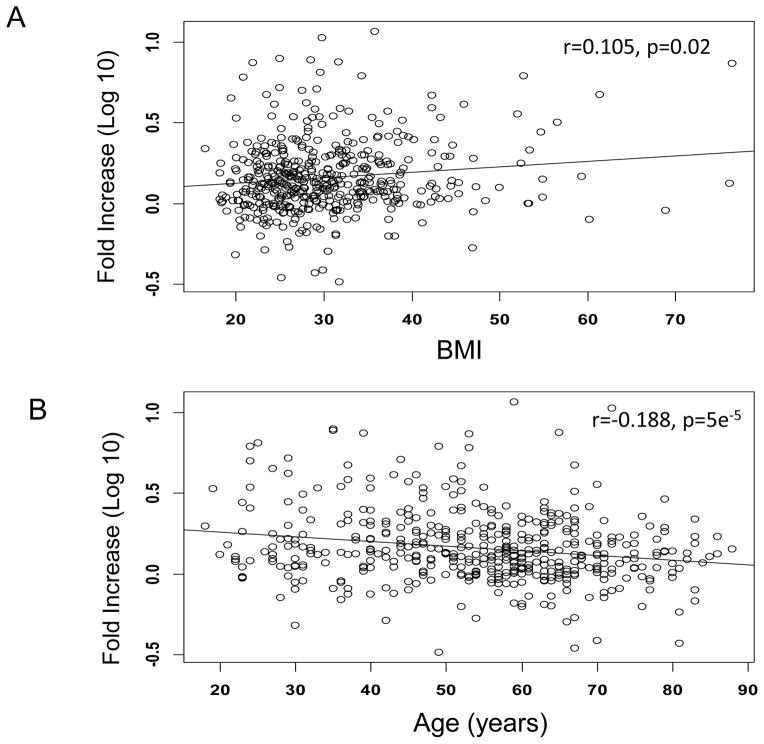
Similar to year 1 of the study, fold-increase in anti-influenza vaccine IgG levels were significantly higher in those with higher BMIs ( r=0.105, p=0.02) when measured by ELISA 30 days following vaccination. Age negatively correlated with fold-increase in anti-IgG antibodies (r=−0.188p<0.0001) (Fig. 1A and B). For individuals less than 50 years of age, there is no association between age and antibody response (p=0.93, test based on Spearman rank correlation coefficient); in contrast, for individuals 50 or older, there is a significant negative association between age and antibody response (p=0.015).
Fig. 1.
Correlation of fold increase in total anti-influenza vaccine IgG from pre-vaccination to post-vaccination with BMI (A) and Age (B). There was a significant positive association between BMI and antibody response to vaccine (A, Spearman’s rank correlation test P=0.02). There was a significant negative association between age and antibody response to vaccine (B, P<0.0001).
3.2 Age, but not BMI, decreases seroprotection and seroconversion measured by HAI
In order to determine the response to the individual influenza strains contained in the vaccine, we measured HAI titers in subject serum samples from years 1 and 2 of the study. Overall the 2009–2010 vaccine was not as effective in inducing a seroprotective HAI titer in our study population as the 2008–2009 vaccine was in year 1 of the study (Table 2). In both years, the B strain failed to induce a seroprotective GMT in the study participants. In year 2, the median post vaccination geometric mean titer (GMT) for the H1N1 strain (A/California/4/2009) was 40, while in year 1 A/Brisbane/59/2007 resulted in a post vaccination GMT of 80 (Table 2).
Table 2.
Pre and Post vaccine GMT for the total study population in each yeara
| Vaccine Strain | Prevaccine GMT | Postvaccine GMT | |
|---|---|---|---|
| Year 1 | A/Brisbane/59/2007 | 20 (±87) | 80 (±350) |
| A/Brisbane/10/2007 | 20 (±180) | 120 (±367) | |
| B/Brisbane/60/2008 | 5 (±32) | 20 (±32) | |
| Year 2 | A/California/4/2009 | 5 (±69) | 40 (±285) |
| A/Perth/16/2009 | 5 (±131) | 120 (±492) | |
| B/Brisbane/60/2008 | 5 (±70) | 20 (±63) |
Data are the Median GMT ± SD
We preformed logistic regressions for BMI and age in both years of the study. In year 1 of the study, we found that BMI was positively correlated with the odds of seroprotection and seroconversion for the A/Brisbane/59/2007, A/Brisbane/10/2007, but not influenza B/Brisbane/60/2008 (Table 3). In year 2 of the study, there was no correlation between BMI and seroprotection or conversion against any of the strains (Table 3). In contrast to BMI, age was strongly negatively correlated to seroprotection and seroconversion for most strains in both year 1 and 2 of the study (Table 4).
Table 3.
Odds ratios and 95% confidence intervals for seroprotection and seroconversion per 5 unit increase in BMIa
| Vaccine Strain | OR (95% CI) for seroprotection | P-valueb | OR (95% CI) for seroconversion | P-value | |
|---|---|---|---|---|---|
| Year 1 | A/Brisbane/59/2007 | 1.37 (1.09,1.72) | 0.01 | 1.23 (1.05,1.44) | 0.01 |
| A/Brisbane/10/2007 | 1.29 (1.04,1.58) | 0.02 | 1.32 (1.12,1.55) | <0.0001 | |
| B/Brisbane/60/2008 | 1.12 (0.97,1.30) | 0.13 | 1.15 (0.99,1.34) | 0.07 | |
| Year 2 | A/California/4/2009 | 0.95 (0.85,1.06) | 0.35 | 1.05 (0.94,1.17) | 0.38 |
| A/Perth/16/2009 | 0.89 (0.78,1.00) | 0.06 | 1.03 (0.92,1.15) | 0.61 | |
| B/Brisbane/60/2008 | 0.99 (0.89,1.11) | 0.97 | 1.10 (0.98,1.23) | 0.09 |
Data are odds ratio (95% confidence intervals).
P-values are from logistic regression model of the odds of seroconversion or seroprotection.
Table 4.
Odds ratios and 95% confidence intervals for seroprotection and seroconversion per 10 year increase in agea
| Vaccine Strain | OR (95% CI) for seroprotection | P-valueb | OR (95% CI) for seroconversion | P-value | |
|---|---|---|---|---|---|
| Year 1 | A/Brisbane/59/2007 | 0.56 (0.45,0.70) | <0.0001 | 0.90 (0.78,1.03) | 0.13 |
| A/Brisbane/10/2007 | 1.05 (0.89,1.24) | 0.55 | 0.86 (0.75,0.99) | 0.03 | |
| B/Brisbane/60/2008 | 0.84 (0.73,0.96) | 0.01 | 0.80 (0.69,0.92) | 0.002 | |
| Year 2 | A/California/4/2009 | 0.78 (0.69,0.88) | <0.0001 | 0.83 (0.74,0.94) | 0.004 |
| A/Perth/16/2009 | 0.84 (0.72,0.98) | 0.03 | 0.91 (0.81,1.03) | 0.13 | |
| B/Brisbane/60/2008 | 0.71 (0.62,0.81) | <0.0001 | 0.73 (0.64,0.83) | <0.0001 |
Data are odds ratio (95% confidence intervals).
P-values are from logistic regression model of the odds of seroconversion or seroprotection.
3.3 Diabetes does not affect the rate of seroprotection or seroconversion to influenza vaccine 30 days post vaccination
In diabetic subjects from year 1 of the study there was no significant difference between the rates of seroprotection or seroconversion against any vaccine strain compared to non-diabetics (Table 5). The groups did differ, however, in demographic characteristics. Diabetes was associated with race (p<0.001 Fisher’s exact test), BMI (p<0.001), age (p<0.001), gender (p=0.011), and smoking (p=0.025). African Americans were more likely than whites to be diabetic (37.9% versus 15.5%). Obese individuals were more likely to be diabetic than healthy weight (34.5% versus 6.1%) and diabetics tended to be older (median age 58 versus 53) and males were more likely to be diabetic than females (29.6% versus 17.2%). In addition, smokers (current or previous) were more likely to be diabetic than those who have never smoked (29.5% versus 16.8%).
Table 5.
Association of type 2 diabetes on the percentage of seroprotection and seroconversiona
| Year 1 | Diabetes | %Seroprotected | P-value | %Seroconverted | P-value |
|---|---|---|---|---|---|
| Strain | |||||
| A/Brisbane/59/2007 | No | 81.5 | 0.67 | 54.1 | 0.78 |
| Yes | 76.1 | 54.9 | |||
| A/Brisbane/10/2007 | No | 76.1 | 0.33 | 55.6 | 0.88 |
| Yes | 85.9 | 57.7 | |||
| B/Brisbane/60/2008 | No | 42.9 | 0.27 | 40.9 | 0.60 |
| Yes | 42.3 | 40.0 | |||
| Year 2 | |||||
| Strain | |||||
| A/California/7/2009 | No | 51.9 | 0.27 | 44.4 | 0.72 |
| Yes | 46.0 | 44.0 | |||
| A/Perth/16/2009 | No | 80.3 | 0.21 | 56.7 | 0.47 |
| Yes | 77.0 | 58.0 | |||
| B/Brisbane/60/2008 | No | 40.7 | 0.04 | 31.9 | 0.05 |
| Yes | 29.0 | 24.0 |
Data are the percentage of each group that demonstrated seroprotection (≥40) and the percentage that are considered responders (≥4-fold increase from baseline).
The pattern in seroconversion and seroprotection in year 2 between diabetics and non-diabetics was very similar to year 1. In year 2 there was a marginally significant decrease in seroprotection against B/Brisbane/60/2008 in the diabetic group (Table 5), however, both seroprotection and seroconversion against the other strains were similar between the diabetics and non-diabetics. In this cohort, diabetes was associated with race (p<0.001), BMI (p<0.001), age (p=0.01), but not gender (p=0.10) or smoking (p=0.39). As with year 1, diabetics were more likely to be African Americans (33.1% versus 17.8%) and obese (37.1% versus 7.1%). Diabetics tended to be older than non-diabetics (median age 59 versus 55) and were more likely to be male (26.4% versus 19.5%).
3.4 No correlation of HbA1c levels and seroprotection or seroconversion
While we did not find significant differences between diabetics and non-diabetics in their antibody response to the influenza vaccine, we were interested in determining whether glycemic control within the diabetic population is associated with the response to influenza vaccination. HbA1c levels are used to give an estimate of the blood glucose control over the previous 3 months. The American Diabetes Association recommends that diabetic patients maintain an HbA1c level of 7% or lower[22].
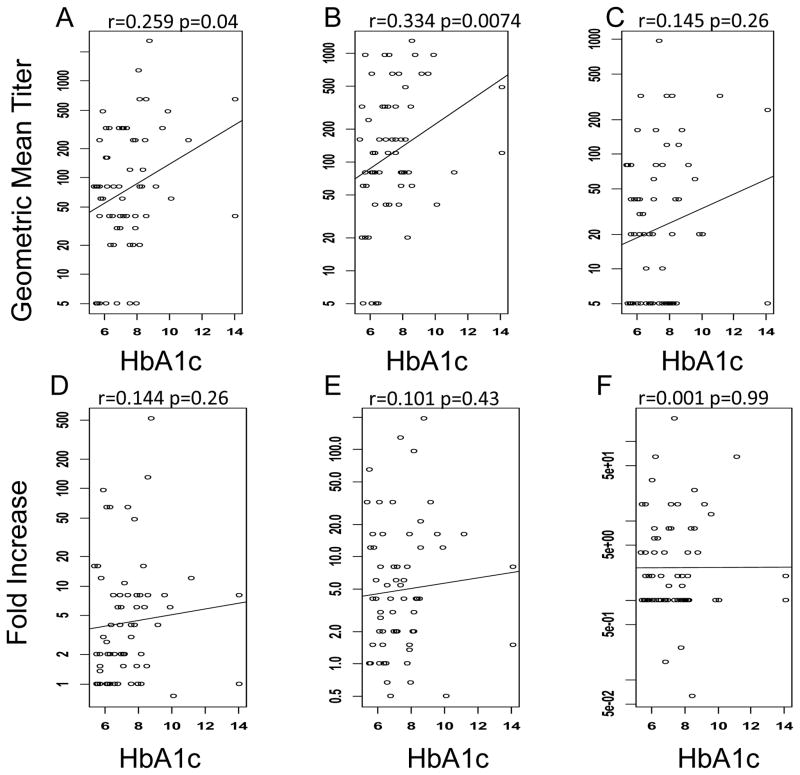
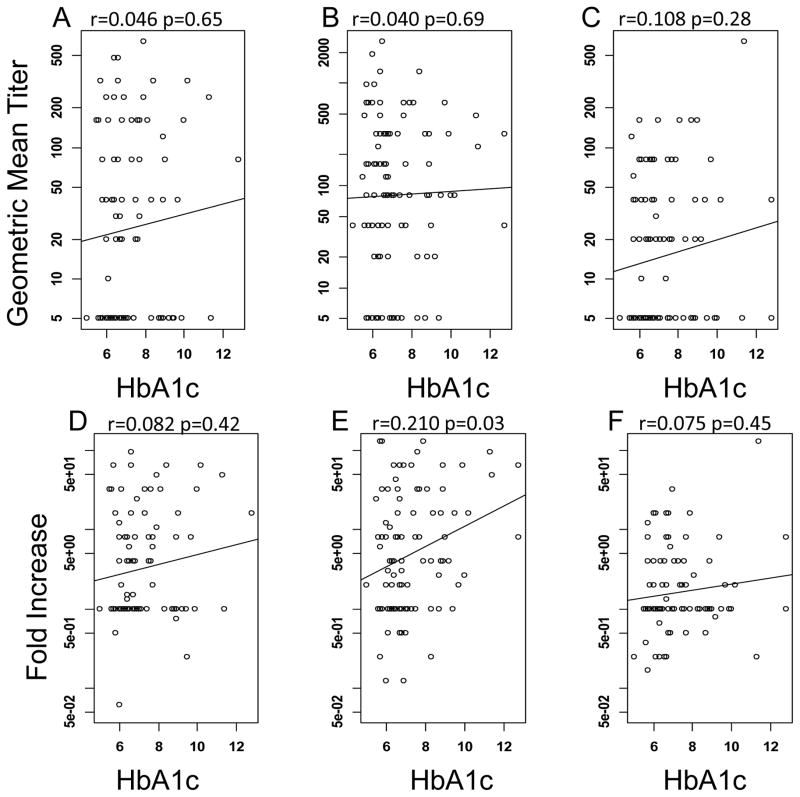
We collected HbA1c levels from the medical records of the diabetic subjects in year 1 and 2 of the study and determined if the HbA1c level correlated with seroprotection or seroconversion within the diabetic cohort. In year 1 of the study there were 63 diabetic individuals for whom we had HbA1c levels. There was no significant correlation of BMI to HbA1c in this population (p=0.64, data not shown). In addition, there was very little correlation between HbA1c levels and seroprotection and seroconversion. Linear regression analysis revealed a small, but statistically significant positive association of HbA1c and the odds of seroprotection against the A/Brisbane/10/2007 strain (Fig. 2B), however, there was no correlation for either of the other 2 strains, nor the odds of seroconversion (Fig. 2A,C and D–F). In year 2 of the study, there were 101 diabetic subjects for whom we have an HbA1c value. In this cohort, there was a marginally significant positive correlation between BMI and HbA1c values (p=0.05, data not shown). There was no significant correlation of HbA1c with seroprotection for any strain (Fig. 3A–C), while there was a small, but significant, positive correlation of HbA1c with seroconversion against A/Perth/16/2009 only (Fig. 3E).
Fig. 2.
Association of HbA1c on seroprotection and seroconversion in the year 1 cohort. HAI titers were measured in response to A/Brisbane/59/2007 (A,D), A/Brisbane/10/2007 (B,E) and B/Brisbane/60/2008 (C,F). The line shows the least squares regression of the relationship of HbA1c to HAI antibody levels seroprotection (A–C) and seroconversion (D–F). Increasing HbA1c levels only increased the odds of seroprotection against A/Brisbane/10/2007 (B).
Fig. 3.
Association of HbA1c on seroprotection and seroconversion in the year 2 cohort. Hemagglutination inhibition antibody titers were measured in response to A/California/4/2009 (A,D), A/Perth/16/2009 (B,E) and B/Brisbane/60/2008 (C,F). The line shows the least squares regression of the relationship of HbA1c to HAI antibody levels seroprotection (A–C) and seroconversion (D–F). Increasing HbA1c levels only increased the odds of seroconversion against A/Perth/16/2009 (E).
4. Discussion
Our previous studies have shown that while the initial response (30 d postvaccination) to the trivalent influenza vaccine is intact in obese humans, antibody levels fall more significantly in obese individuals compared to healthy weight individuals 11 months after vaccination[13]. In addition, influenza-specific CD8 and CD4 T cell functions are impaired in obese individuals[13, 23]. In this study, we have replicated the original findings that BMI positively correlated with the 30 d post-vaccine response in the 2010–2011 influenza season and expanded the study to include a more thorough investigation of diabetes status and vaccination response.
In year 2 of the study, there was a positive correlation with BMI and total anti-influenza vaccine IgG antibodies, like in year 1. However, while we found that in general, seroprotection and seroconversion odds were higher with increasing BMI in year 1, this was not true in year 2 of the study. It appears that overall, the 2009–2010 vaccine did not induce seroprotective responses as well as the 2008–2009 vaccine did in year 1 of the study. Other studies also found a lower immunogenicity with the 2009–2010 vaccine[24]. It is possible that while this level of immunogenicity is sufficient to induce responses in the obese that are equivalent to healthy weight individuals, more immunogenic vaccine strains induce a more robust response in the obese.
Our previous study indicated that diabetes did not alter the total IgG response when measured by the influenza vaccine ELISA in Year 1 of the study[13]. In this study, we have demonstrated that diabetes does not significantly alter the antibody response to the trivalent influenza vaccine. While there was a small and marginally significant positive correlation of HbA1c with seroconversion against A/Perth/16/2009 in the second year of the study, overall the degree of glycemic control does not affect the odds of seroconversion nor seroprotection. A study of the response to monovalent pH1N1 vaccine indicated that older individuals that had been diabetic for a long period of time may have an impaired immune response to the vaccine compared to younger (under 64) diabetics with a shorter disease period[25]. In contrast, a small study of 22 T2D subjects and 65 healthy subjects, by Frasca et al. [26] suggested that “younger” type 2 diabetics (average age 50) had an intact antibody response following influenza vaccination compared to young (average age 42) non-diabetics. In older type 2 diabetics (average age 64) the antibody response was significantly higher than non-diabetic older individuals (average age 66) and that this was likely due to intact B cell function in the diabetic groups. In both age groups the type 2 diabetics had significantly higher BMI than the non-diabetics suggesting that the effect may be driven by obesity, not diabetes itself. The Frasca et al. report therefore bolsters findings reported here that neither BMI, nor diabetes result in impairment in the initial response to the influenza vaccine. Compared with non-diabetics in the study population, the diabetic population in this study was more likely to be African-American, male, obese and older. These characteristics are consistent with diabetics in the general population[27].
During the 2009 pH1N1 pandemic, studies suggested that diabetics were at a greater risk for hospitalization and increased complications from influenza [5–7]. However, T2D is a comorbidity associated with obesity and obesity is now considered an independent risk factor for influenza morbidity and mortality[28]. In a recent study that controlled for obesity, diabetes was not associated with severe outcomes during the pandemic[29]. However, obesity remains a risk factor even when diabetes is controlled for in the analysis[28].
If diabetics are, in fact, at increased risk for morbidity and mortality from influenza, then vaccination would be the best strategy for reducing this risk. Very few studies have examined the effectiveness of the influenza vaccine in diabetic populations. A recent Canadian population-based cohort study that examined the rates of influenza like illness and hospitalization among vaccinated diabetics suggests that the vaccine is effective in this population[30]. These findings should not be surprising in light of our data that demonstrates type 2 diabetics are able to produce a seroprotective level of antibody in response to the influenza vaccine at the same rate as non-diabetics. However, there have been no randomized control trials to determine whether this level of seroprotection is effective in either diabetic or obese individuals although there has been a call for these studies to be performed[31].
Finally, this study has several strengths and limitations that should be considered. While the population in our study is racially diverse (30% African American), there was not a significant Hispanic population in the study, therefore this may limit its generalizability to the total population. Additionally, while we were able to identify the medications that the diabetics were prescribed during the study, it was not possible to examine the response to vaccine by drug type due to the small sample size in each group. Lastly, in this study, we did not examine if the vaccine was equally protective against influenza infection in diabetics vs. non-diabetics.
Supplementary Material
Acknowledgments
This work was funded by NIH grants RO1AI078090 and P30DK056350 to MAB and NIH HHSN266200700005C and ALSAC to SSC.
We thank Kim Bartholomew and Qing Shi for their help with this study. P.A.S., J.H., S.W, T.N. and M.A.B designed the studies. P.A.S. H.A.P, E.A.K, S.S-C., and M.A.B conducted the research. E.A.K and S.S-C. provided essential reagents. P.A.S and M.H. analyzed the data and performed statistical analysis. P.A.S. wrote the paper. P.A.S. and M.A.B. had primary responsibility for the final content. All authors have read and approved the final manuscript.
Appendix A. Supplementary Data
Supplemental Table 1 Diabetes-related treatments at the time of enrollment
Footnotes
Abbreviations used: HAI, hemagglutination inhibition assay; HbA1c, glycosylated hemoglobin; T2D, Type 2 diabetes; TIV, trivalent influenza vaccine
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and Trends in Obesity Among US Adults, 1999–2008. Journal of the American Medical Association. 2010;303:235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control (US) National Diabetes Statistics Report. 2014 http://wwwcdcgov/diabetes/pdfs/data/2014-report-estimates-of-diabetes-and-its-burden-in-the-united-statespdf.
- 3.Karlsson EA, Beck MA. The burden of obesity on infectious disease. Exp Biol Med (Maywood) 2010;235:1412–24. doi: 10.1258/ebm.2010.010227. [DOI] [PubMed] [Google Scholar]
- 4.Thompson MG, Shay DK, Zhou H, Bridges CB, Cheng PY, Burns E, Bresee JS, Cox NJ. Estimates of Deaths Associated with Seasonal Influenza --- United States, 1976--2007. Morbidity and Mortality Weekly. 2010;59:1057–62. [PubMed] [Google Scholar]
- 5.Allard R, Leclerc P, Tremblay C, Tannenbaum TN. Diabetes and the severity of pandemic influenza A (H1N1) infection. Diabetes care. 2010;33:1491–3. doi: 10.2337/dc09-2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Louie JK, Acosta M, Winter K, Jean C, Gavali S, Schechter R, et al. Factors associated with death or hospitalization due to pandemic 2009 influenza A(H1N1) infection in California. JAMA. 2009;302:1896–902. doi: 10.1001/jama.2009.1583. [DOI] [PubMed] [Google Scholar]
- 7.Kumar A, Zarychanski R, Pinto R, Cook DJ, Marshall J, Lacroix J, et al. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA. 2009;302:1872–9. doi: 10.1001/jama.2009.1496. [DOI] [PubMed] [Google Scholar]
- 8.CDC. Intensive-care patients with severe novel influenza A (H1N1) virus infection -Michigan, June 2009. MMWR Morb Mortal Wkly Rep. 2009;58:749–52. [PubMed] [Google Scholar]
- 9.Kwong JC, Campitelli MA, Rosella LC. Obesity and respiratory hospitalizations during influenza seasons in Ontario, Canada: a cohort study. Clin Infect Dis. 2011;53:413–21. doi: 10.1093/cid/cir442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weber DJ, Rutala WA, Samsa GP, Santimaw JE, Lemon SM. Obesity as a predictor of poor antibody response to hepatitis B plasma vaccine. JAMA. 1985;254:3187–9. [PubMed] [Google Scholar]
- 11.Weber DJ, Rutala WA, Samsa GP, Bradshaw SE, Lemon SM. Impaired immunogenicity of hepatitis B vaccine in obese persons. N Engl J Med. 1986;314:1393. doi: 10.1056/NEJM198605223142120. [DOI] [PubMed] [Google Scholar]
- 12.Eliakim A, Schwindt C, Zaldivar F, Casali P, Cooper DM. Reduced tetanus antibody titers in overweight children. Autoimmunity. 2006;39:137–41. doi: 10.1080/08916930600597326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheridan PA, Paich HA, Handy J, Karlsson EA, Hudgens MG, Sammon AB, et al. Obesity is associated with impaired immune response to influenza vaccination in humans. Int J Obes (Lond) 2012;36:1072–7. doi: 10.1038/ijo.2011.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control (US) People at High Risk of Developing Flu–Related Complications. 2013 http://wwwcdcgov/flu/about/disease/high_riskhtm.
- 15.Hong KW, Cheong HJ, Choi WS, Lee J, Wie SH, Baek JH, et al. Clinical courses and outcomes of hospitalized adult patients with seasonal influenza in Korea, 2011–2012: Hospital-based Influenza Morbidity & Mortality (HIMM) surveillance. Journal of infection and chemotherapy : official journal of the Japan Society of Chemotherapy. 2014;20:9–14. doi: 10.1016/j.jiac.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Schillie SF, Spradling PR, Murphy TV. Immune response of hepatitis B vaccine among persons with diabetes: a systematic review of the literature. Diabetes care. 2012;35:2690–7. doi: 10.2337/dc12-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patriarca PA, Kendal AP, Stricof RL, Weber JA, Meissner MK, Dateno B. Influenza vaccination and warfarin or theophylline toxicity in nursing-home residents. N Engl J Med. 1983;308:1601–2. doi: 10.1056/NEJM198306303082615. [DOI] [PubMed] [Google Scholar]
- 18.Poli D, Chiarugi L, Capanni M, Antonucci E, Abbate R, Gensini GF, et al. Need of more frequent International Normalized Ratio monitoring in elderly patients on long-term anticoagulant therapy after influenza vaccination. Blood Coagul Fibrinolysis. 2002;13:297–300. doi: 10.1097/00001721-200206000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Palmer DFDW, Coleman MT, Schild GC. Haemagglutination-inhibition test, Advanced laboratory techniques for influenza diagnosis. Atlanta, GA: US Department of Health, Education and Welfare, Public Health Service, Center for Disease Control; 1975. [Google Scholar]
- 20.Luytjes W, Enouf V, Schipper M, Gijzen K, Liu WM, van der Lubben M, et al. HI responses induced by seasonal influenza vaccination are associated with clinical protection and with seroprotection against non-homologous strains. Vaccine. 2012;30:5262–9. doi: 10.1016/j.vaccine.2012.05.060. [DOI] [PubMed] [Google Scholar]
- 21.Team RDC. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: R Foundation for Statistical Computing; 2011. [Google Scholar]
- 22.Assoc AD. Standards of Medical Care in Diabetes-2011 AMERICAN DIABETES ASSOCIATION. Diabetes care. 2011;34:S11–S61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paich HA, Sheridan PA, Handy J, Karlsson EA, Schultz-Cherry S, Hudgens MG, et al. Overweight and obese adult humans have a defective cellular immune response to pandemic H1N1 Influenza a virus. Obesity (Silver Spring) 2013 doi: 10.1002/oby.20383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xie H, Jing X, Li X, Lin Z, Plant E, Zoueva O, et al. Immunogenicity and cross-reactivity of 2009–2010 inactivated seasonal influenza vaccine in US adults and elderly. PLoS One. 2011;6:e16650. doi: 10.1371/journal.pone.0016650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nam JS, Kim AR, Yoon JC, Byun Y, Kim SA, Kim KR, et al. The humoral immune response to the inactivated influenza A (H1N1) 2009 monovalent vaccine in patients with Type 2 diabetes mellitus in Korea. Diabet Med. 2011;28:815–7. doi: 10.1111/j.1464-5491.2011.03255.x. [DOI] [PubMed] [Google Scholar]
- 26.Frasca D, Diaz A, Romero M, Mendez NV, Landin AM, Ryan JG, et al. Young and elderly patients with type 2 diabetes have optimal B cell responses to the seasonal influenza vaccine. Vaccine. 2013 doi: 10.1016/j.vaccine.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prevention CfDCa; U.S. Department of Health and Human Services CfDCaP, editor. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta, GA: 2011. p. 12. http://wwwcdcgov/diabetes/pubs/pdf/ndfs_2011pdf. [Google Scholar]
- 28.Louie JK, Acosta M, Samuel MC, Schechter R, Vugia DJ, Harriman K, et al. A novel risk factor for a novel virus: obesity and 2009 pandemic influenza A (H1N1) Clin Infect Dis. 2011;52:301–12. doi: 10.1093/cid/ciq152. [DOI] [PubMed] [Google Scholar]
- 29.Ganatra RB, McKenna JJ, Bramley AM, Skarbinski J, Fry AM, Finelli L, et al. Adults With Diabetes Hospitalized With Pandemic Influenza A(H1N1)pdm09--U.S. 2009. Diabetes care. 2013;36:e94. doi: 10.2337/dc13-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lau D, Eurich DT, Majumdar SR, Katz A, Johnson JA. Effectiveness of influenza vaccination in working-age adults with diabetes: a population-based cohort study. Thorax. 2013;68:658–63. doi: 10.1136/thoraxjnl-2012-203109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michiels B, Govaerts F, Remmen R, Vermeire E, Coenen S. A systematic review of the evidence on the effectiveness and risks of inactivated influenza vaccines in different target groups. Vaccine. 2011;29:9159–70. doi: 10.1016/j.vaccine.2011.08.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.