Abstract
This study investigated the influence of a community health worker (CHW) diabetes lifestyle intervention on mental health outcomes. Our study was guided by the principles of community-based participatory research. Data were collected from 164 African American (N = 94) and Hispanic adults (N = 70) participating in a randomized, 6-month delayed intervention group design for improving glycemic control. The intervention time periods were baseline to 6 months for the treatment group and 6–12 months for the delayed group. Linear mixed models were used to conduct longitudinal analyses of the Problem Areas in Diabetes (PAID) and Patient Health Questionnaire (PHQ) scales. In the model adjusted for demographics, the PAID dropped significantly from pre-intervention to post-intervention within both the treatment and delayed groups (p < 0.05) with an average intervention effect of −6.4 (p < 0.01). The PAID dropped even further within the immediate group from 6 to 12 months. Although the PHQ did not change significantly, the PHQ-2 had an average intervention effect of −0.3 (p < 0.05) in the model adjusted for demographics. This study contributes to an understanding of how a CHW-led diabetes intervention can result in positive mental health outcomes for Latinos and African Americans with Type 2 diabetes. It also highlights the importance of further exploring what factors may contribute to racial/ethnic variation in mental health outcomes for African Americans and Latinos with diabetes and the role CHWs can play.
Keywords: Type 2 diabetes, Community health workers, Health disparities, Mental health, Depression
Introduction
In 70 % of patients with a chronic illness, behavioral health factors play a significant role in prevention, diagnosis, and treatment (Macre et al. 2011). Furthermore, 17 % of the US adult population has comorbid mental and medical conditions (Alegria et al. 2003). As the World Health Organization (WHO) states, there can be “no health without mental health,” which makes multidimensional integrated care critical for patient health (World Health Organization 2005).
Diabetes and Mental Health
Worldwide, the WHO estimates that by 2030, diabetes will affect more than twice as many people than it did in the year 2000 (Wild et al. 2004). According to the Center for Disease Control and Prevention (CDC), 25.8 million Americans (8.3 % of the US population) live with a diabetes diagnosis (CDC 2012). In both the general population and in clinical settings, depression is more common among diabetes patients (type 1 and type 2) than patients without diabetes (Eaton 2002; Anderson et al. 2001). Additionally, even when controlling for demographic, metabolic, and lifestyle factors, depression increases the risk of onset of type 2 diabetes (Golden et al. 2004; Eaton et al. 1996). Depression in diabetes patients is shown to be associated with diabetes complications including retinopathy, nephropathy, macro-vascular complications, and sexual dysfunction (Anderson et al. 2001).
Among patients with diabetes, diabetes-related emotional distress has been found to be a significant contributor both to poor adherence to diabetes self-care recommendations and to poor glycemic control (Anderson et al. 2001; Polonsky et al. 1995; Gary et al. 2000; Armstrong et al. 2011). In addition to the often burdensome daily treatment regimens, adults with diabetes must also cope with the threat of serious diabetes-related complications, including increased incidence of kidney disease, amputation, blindness, and the potential for reduced life expectancy (Anderson et al. 2001). Furthermore, adults with diabetes are more likely than those with only diabetes to live in poverty, report poor health, and lack access to health care (Anderson et al. 2001; Polonsky et al. 1995; Gary et al. 2000). In minority racial and ethnic groups and among socioeconomically disadvantaged groups, there has been a steep increase in diabetes prevalence and effects since the mid-1990s (CDC 2010; Kanjilal et al. 2006; Narayan et al. 2006; Geiss et al. 2006). When addressing diabetes in racial and ethic minority populations, a Community Health Worker model has shown significant impact on patient health (Swider 2002; Norris et al. 2006; Spencer et al. 2011).
Community Health Worker Model
In the current study, we have used a Community Health Worker (CHW) model to improve diabetes health outcomes in urban, African Americans and Latino, medically underserved community. A CHW model enlists and trains community members to work as bridges between their ethnic, cultural, or geographic communities and healthcare providers to improve health outcomes for patients. The American Public Health Association defines CHWs as “a frontline public health worker who is a trusted member of the community served. This trusting relationship enables the CHW to serve as a liaison/link/intermediary between health/social services and the community to facilitate access to services and improve the quality and cultural competence of service deliver” (Balcazar et al. 2011). CHWs have demonstrated promise in improving physical health behaviors and outcomes, particularly for racial and ethnic minority communities and in those who have traditionally lacked access to adequate health care (Swider 2002; Norris et al. 2006; Spencer et al. 2011).
REACH Detroit Partnership
Since 2000, the Racial and Ethnic Approaches to Community Health (REACH) Detroit Partnership has been conducting inter-related family, health system, and community-level interventions aimed at reducing the risk of diabetes and its complications among African American and Latino Detroit residents. Although the intervention was not targeted at reducing mental illness, there is reason to believe that the intervention could produce better mental health outcomes. Research shows that education and support are key factors in the intervention’s success (Heisler 2010). Due to the interconnectedness of diabetes and mental health, it is possible that a healthy lifestyle intervention aimed at improving diabetes health may simultaneously improve mental health (Kieffer et al. 2013). The purpose of this paper is to evaluate participants’ mental health outcomes in the current study. We hypothesize that there will be a reduction in the mental health measures: Problem Areas In Diabetes (PAID) score as well as the Patient Health Questionnaire (PHQ) score.
Methods
The CHW intervention was among several diabetes interventions conducted by REACH Detroit as part of the Centers for Disease Control and Prevention (CDC)-funded REACH 2010 Initiative and currently funded by the National Institute of Diabetes, Digestive, and Kidney Disease. Using community-based participatory research principles (Israel et al. 1998; Wallerstein and Duran 2006), community, health system, and academic partners completed a 1-year planning process to develop interventions to improve diabetes prevention and treatment in the participating communities. Using a socioecological model, family, health system, and community-level interventions were developed to address sources of diabetes disparities at each level (Two Feathers et al. 2005a, b). CHWs were central to each intervention, which were grounded in empowerment theory to emphasize a collaborative approach to facilitating the self-directed behavior change of patients (Anderson and Funnell 2005). The empowerment philosophy includes self-determination and autonomy motivation theory, which postulates that an individual will be more successful in a disease-management plan if that individual’s goals, objectives, and resources guide the development of that plan (Deci et al. 1994; Williams et al. 1998). Empowerment-based approaches have been found to be effective in improving chronic disease self-management among racial and ethnic minority patients (Funnell and Anderson 2004; Funnell et al. 2001).
We randomized African American and Latino participants with diabetes into a CHW intervention group or a 6-month delayed intervention. All participants in the study, whether in the immediate or delayed group, received information on and had access to, REACH Detroit community activities that provided free, publicly available healthy eating demonstrations, physical fitness activity (e.g., dance and exercise classes, walking clubs), and a weekly community farmers’ produce market. All participants also received health care at facilities in which healthcare providers were trained by REACH Detroit in culturally competent diabetes care through our health systems intervention.
Setting
All participants lived in either southwest Detroit, whose residents were predominantly Latino of Mexican origin (70 %) and had an annual median household income of $11,500, or eastside Detroit, which is largely African American (80 %) and had a median household income of $25,020 (US Census Bureau 2007). Participants from southwest Detroit received medical care at a federally qualified community health center, whereas participants from eastside Detroit received medical care at a major local health system.
Participants
We identified eligible participants through medical records, were at least 18 years of age, had physician-diagnosed type 2 diabetes, self-identified as African American or Latino/Hispanic, and lived in targeted zip codes. We excluded individuals who already had serious diabetes-related complications, such as blindness, amputated limbs, or kidney failure. We recruited participants from September 2004 to July 2006. Participants were stratified by race/ethnicity and healthcare site during randomization to assure that these variables were equally distributed across the two arms of the intervention. To account for possible attrition due to our delayed design, we assigned 45 % of participants to immediate and 55 % to the delayed group. Participants assigned to the delayed group were aware of the randomized structure of the study and were informed that they would receive the intervention after the 6-month control period. Because of the nature of the study design, CHWs and interviewers were not blinded to the group assignment of the participants; however, data analysts were blinded.
Out of 1,719 potentially eligible patients, 500 did not meet eligibility criteria, 395 refused to participate, and 641 could not be contacted. Of 183 randomized participants, 164 completed the baseline interview. At the 6-month follow-up, 136 participants completed the study protocols and were analyzed for the primary outcome (attrition rate = 17.1 %). Withdrawal from the study was not independently associated with treatment arm, age, gender, education, diabetes duration, baseline HbA1c, low-density lipoprotein cholesterol, or blood pressure. However, African Americans were more likely to withdraw from the study and to be missing HbA1c data.
Intervention
Trained CHWs promoted healthy lifestyle and diabetes self-management activities. We recruited CHWs from the 2 participating communities, where they were ethnically matched with their assigned participants, underwent more than 80 h of training, and conducted 3 primary activities: (1) diabetes education classes, (2) home visits (2 per month, each about 60 min in length) to address participants’ specific self-management goals, and (3) 1 clinic visit with the participant and his or her primary care provider. The diabetes education classes were culturally tailored group classes in both English and Spanish. Eleven 2-h group sessions of 8–10 participants were held every 2 weeks at community locations. The development, implementation, and evaluation of these curricula are described in depth elsewhere, but included information on stress reduction, physical activity, and healthy eating (Two Feathers et al. 2005a,b; Two Feathers et al. 2007). In home visits, CHWs assisted participants in setting patient-specific goals and supporting their progress. In addition, CHWs helped participants improve their patient–provider communication skills and facilitated necessary referrals to other service systems. CHWs also contacted participants by phone once every 2 weeks. Participants in the delayed group were contacted once a month to update contact information until they were officially enrolled in the intervention.
Community health workers also were trained in empowerment-based approaches, such as motivational interviewing, which are used to elicit participants’ goals and help participants formulate their own action plans. CHWs also used empowerment theory in the diabetes education classes by eliciting participants’ experiences and requests for information to be provided during the sessions.
Measures
The REACH Detroit baseline survey was a comprehensive, 3-h baseline survey conducted in person, usually in the household of the participant, by trained staff, in the chosen language of the participant (Spanish or English). Hemoglobin A1c measurements were abstracted from medical records. The interview consisted of items from the Behavioral Risk Factor Surveillance System, a CDC-administered survey used to track health risks in the United States (Center for Disease Control and Prevention 2004), and a battery of assessments about health, health care, behaviors and attitudes toward diabetes, quality of diabetes care, relations with healthcare providers, and dietary and physical activity practices. Respondents’ self-reported diabetes-related complications (number of complications) were also included. All instruments were translated into Spanish and pre-tested by bi-lingual interviewers as a means of testing the culturally and linguistically appropriate instruments.
Sociodemographic variables included in the analyses as predetermined control variables were race/ethnicity, sex, married/partnered versus single, age at baseline interview, and education (high school graduate yes/no). Although information on income was obtained, the excessive amount of missing values led to its exclusion from further analyses. Thus, respondents’ education (less than a high school diploma = 0, high school graduate = 1) serves as our measure of socioeconomic status.
Mental Health Outcomes
The Problem Areas in Diabetes Scale (PAID) is a well-validated assessment tool used to measure diabetes-related emotional distress (Polonsky et al. 1995). The PAID is a self-report questionnaire that consists of 20 statements measuring emotional distress in managing and dealing with diabetes and its complications. Representative items include feeling scared when you think about living with diabetes; feelings of deprivation regarding food and meals; feeling depressed when you think about living with diabetes; feeling overwhelmed by your diabetes; feeling burned out by the constant effort to manage your diabetes. Each item can be rated on a 5-point Likert scale ranging from 0 (not a problem) to 4 (serious problem). A previous study of the PAID reported a Cronbach’s alpha coefficient of 0.95.16. Also, concurrent validity has been found between the PAID and generalized distress, fear of hypoglycemia, disordered eating, and adherence to self-care behaviors with significant correlations. A Cronbach’s alpha coefficient of 0.93 was found for our sample.
Depressive severity is assessed with the Patient Health Questionnaire (PHQ-9; Kroenke et al. 2001), which is a module of the PRIME-MD and takes less than 5 min to complete. The module assesses severity of depressive symptoms over the past 2 weeks and scores each of the 9 DSM-IV criteria on a “0” (not at all) to “3” (nearly every day) scale. Good reliability for the PHQ has been reported (Cronbach’s alpha = 0.89), and we have observed a reliability coefficient of 0.83 in our REACH Detroit sample. In addition to the PHQ-9, we explored the use of the PHQ-2, which is an abbreviated 2-item version of the PHQ-9. The PHQ-2 is not intended to be used as a diagnostic tool, but to screen for depression in a first-step approach, and should be screened with the PHQ-9 to determine whether criteria for depressive disorder are met (Kroenke et al. 2003). The two items include how often in the past 2 weeks the individual has been bothered by the following: (1) little interest or pleasure in doing things and (2) feeling down, depressed, or hopeless.
Analysis
Demographic and baseline measures are displayed in Table 1. All continuous measures were compared between the immediate and delayed groups with the Student’s t test. Outcomes with skewed distributions were log-transformed to reduce skewedness. Pearson’s chi-square test was used for all categorical variables. Longitudinal analyses of the PAID and PHQ are displayed in Tables 2 and 3. These estimates were obtained by using a linear mixed model (LMM) in which baseline and follow-up values were included as the outcomes, with dummy predictor variables for each time point (baseline, month 6, month 12), group (immediate or delayed), and the interaction between group and time point. LMMs allow for correlation among observations on the same person and enable participants to be included in the analysis if they had data at one or more time points (Diggle et al. 2002; West et al. 2007). Thus, a longitudinal analysis with LMM is an intent-to-treat analysis because all available data on each participant is included. Changes in outcomes, along with 95 % confidence intervals, between two time points were estimated via post hoc contrasts. Pre-intervention was defined as baseline for the immediate arm and 6 months for the cohort 2 delayed arm. Post-intervention was 6 months for the immediate arm and 12 months for the delayed arm.
Table 1.
Baseline characteristics of REACH Detroit participants (N = 164)
| Immediate intervention (n = 72) | n | Delayed intervention (n = 92) | n | p | |
|---|---|---|---|---|---|
| Demographics | |||||
| Latino, No. (%) | 34 (47) | 36 (39) | 0.30a | ||
| African American, No. (%) | 38 (53) | 56 (61) | |||
| Female, No. (%) | 54 (75) | 72 | 62 (67) | 92 | 0.29a |
| Living alone, No. (%) | 10 (13.9) | 72 | 12 (13.0) | 92 | 0.87a |
| Married or partnered, No. (%) | 52 (72.2) | 72 | 61 (66.3) | 92 | 0.42a |
| Care at FQHCd, No. (%) | 47 (65.3) | 72 | 48 (52.2) | 92 | 0.09a |
| Age, years, (95 % CI) | 50 (47, 52) | 72 | 55 (53, 57) | 92 | 0.02b |
| High school graduate, No. (%) | 43 (60) | 72 | 54 (59) | 92 | 0.89a |
| Biological | |||||
| Hemoglobin A1c, (95 % CI) | 8.6 (8.0, 9.1) | 70 | 8.5 (8.0, 8.9) | 77 | 0.88c |
| Diabetes complications, (95 % CI) | 2.4 (2.1, 2.8) | 72 | 2.9 (2.6, 3.3) | 92 | 0.08b |
| Psycho-social | |||||
| Ever seen mental health professional, No. (%) | 23 (31.9 %) | 72 | 22 (23.9 %) | 91 | 0.25a |
| PAIDe, (95 % CI) | 23.8 (18.7, 29.0) | 71 | 25.9 (21.2, 30.6) | 92 | 0.73c |
| PHQ-9f, (95 % CI) | 5.2 (3.9, 6.5) | 72 | 5.0 (4.0, 5.9) | 92 | 0.71c |
| PHQ-2f, (95 % CI) | 1.1 (0.7, 1.5) | 72 | 1.1 (0.8, 1.4) | 92 | 0.80b |
Pearson χ2 test
Student’s t test
Student’s t test on log-transform; untransformed mean and confidence interval displayed
FQHC federally qualified health center; other site was major local health system
Problem areas in diabetes
Patient health questionnaire
Table 2.
PAID (problem areas in diabetes) across time, based on linear mixed model, estimated means, and 95 % confidence intervals
| Model | Month 6–baseline immediate |
Month 6–baseline delayed |
Month 12–baseline immediate |
Month 12–baseline delayed |
Month 12–month 6 immediate |
Month 12–month 6 delayed |
Average intervention effectc |
|---|---|---|---|---|---|---|---|
| Unadjusted | −6.3 (−11.1, 0.1) | −1.6 (−6.9, 5.4) | −12.1** (−16.3, −6.0) | −7.1 (−12.5, 0.6) | −7.8* (−12.5, −1.3) | −5.9 (−10.8, 0.6) | −6.1** (−9.7, −1.7) |
| Demographicsa,b | −6.5* (−11.2, −0.04) | −1.7 (−7.0, 5.3) | −12.3*** (−16.4, −6.3) | −7.5* (−12.7, −0.1) | −7.8* (−12.5, −1.4) | −6.2 (−11.0, 0.2) | −6.4** (−9.9, −2.0) |
| Demographicsa,b + diabetes complications | −6.3 (−11.1, 0.1) | −1.4 (−6.8, 5.6) | −12.1*** (−16.2, −6.1) | −7.3 (−12.5, 0.2) | −7.8* (−12.5, −1.3) | −6.2 (−11.0, 0.2) | −6.3** (−9.8, −2.0) |
Log-transform
Demographics included race/ethnicity (African American or Latino/a), gender (female or male), age at baseline interview, married or partnered, high school graduate
Average intervention effect = Average of (6 months–baseline for immediate group) and (12 months–6 months for delayed group)
p <0.05,
p < 0.01,
p <0.001
Table 3.
PHQ-9 and PHQ-2 (patient health questionnaire) across time, estimated means, and 95 % confidence intervals
| Model | Month 6–baseline immediate |
Month 6–baseline delayed |
Month 12–baseline immediate |
Month 12–baseline delayed |
Month 12–month 6 immediate |
Month 12–month 6 delayed |
Average intervention effectc |
|---|---|---|---|---|---|---|---|
| PHQ-9a, unadjusted | −0.2 (−1.4, 1.2) | 0.1 (−1.0, 1.4) | −0.1 (−1.3, 1.5) | −0.6 (−1.6, 0.7) | 0.1 (−1.1, 1.9) | −0.7 (−1.7, 0.7) | −0.4 (−1.2, 0.5) |
| PHQ-9a, demographicsb | −0.2 (−1.3, 1.3) | 0.1 (−1.0, 1.4) | 0.0 (−1.2, 1.6) | −0.6 (−1.6, 0.6) | 0.2 (−1.1, 1.9) | −0.7 (−1.7, 0.6) | −0.5 (−1.2, 0.5) |
| PHQ-9a, demographicsb + diabetes complications | −0.2 (−1.3, 1.3) | 0.1 (−0.9, 1.5) | 0.0 (−1.2, 1.6) | −0.6 (−1.6, 0.7) | 0.2 (−1.1, 1.9) | −0.7 (−1.7, 0.5) | −0.5 (−1.2, 0.5) |
| PHQ-2d, unadjusted | −0.3 (−0.7, 0.1) | 0.4 (0.0, 0.7) | −0.2 (−0.7, 0.3) | 0.0 (−0.4, 0.5) | 0.1 (−0.4, 0.5) | −0.3 (−0.7, 0.1) | −0.3* (−0.6, −0.04) |
| PHQ-2d, demographicsb | −0.3 (−0.7, 0.1) | 0.4 (0.0, 0.7) | −0.2 (−0.7, 0.3) | 0.0 (−0.5, 0.4) | 0.1 (−0.3, 0.5) | −0.4 (−0.8, 0.0) | −0.3* (−0.6, −0.05) |
| PHQ-2d, demographicsb + diabetes complications | −0.3 (−0.7, 0.1) | 0.4* (0.01, 0.7) | −0.2 (−0.7, 0.3) | 0.0 (−0.4, 0.5) | 0.1 (−0.3, 0.5) | −0.4 (−0.8, 0.0) | −0.3* (−0.6, −0.05) |
Log-transform
Demographics included race/ethnicity (African American or Latino/a), gender (female or male), age at baseline interview, married or partnered, high school graduate
Average intervention effect = Average of (6 months–baseline for immediate group) and (12 months–6 months for delayed group)
From baseline to 6 months, the difference between immediate and delayed arms was −0.7, significant at p < 0.05 in all three models. The delayed group increased by 0.4, while the immediate group decreased by 0.3
p < 0.05,
p < 0.01,
p < 0.001
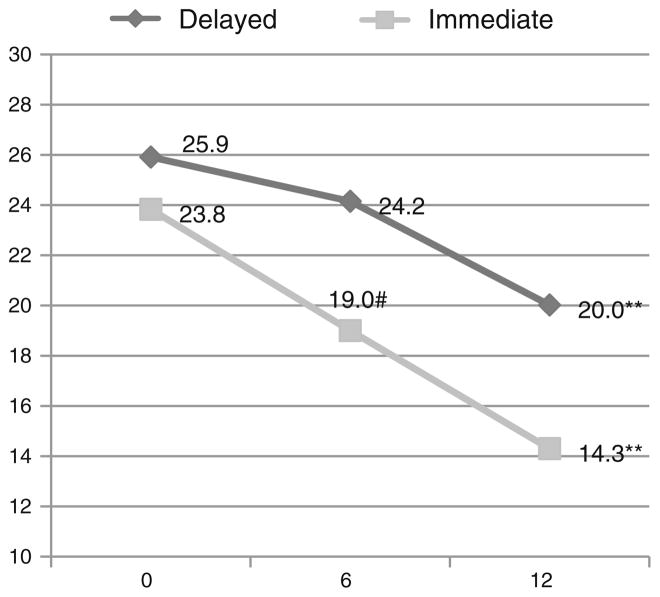
The “Intervention Effect” was estimated as a contrast between the changes in outcomes from time 1 to time 2 for the two study groups. The “Average Intervention Effect” was calculated as the average between the (6 months–baseline) change for the immediate group and the (12 months–6 months) change for the delayed group. Estimation of the “Average Intervention Effect” increases the power to detect a significant intervention effect because the entire sample is used, rather than only the immediate or delayed groups. For log-transformed outcomes, these changes and the intervention effect were exponentiated and the variances were computed with the Delta method (Casella and Berger 2002). Both Tables 2 and 3 display the PAID and PHQ longitudinal outcomes without any adjustment variables, adjusted for demographics, and adjusted further for the number of diabetes complications. These models were also run separately for Latinos and African Americans to see whether any racial/ethnic differences existed. The magnitudes of the changes were evaluated with Cohen’s d for effect size (Cohen 1988). Figure 1 shows the trajectories of the unadjusted PAID means from baseline to 12 months for the immediate and delayed groups. All analyses were conducted with SAS software, version 9.3 (SAS Institute 2012).
Fig. 1.
Unadjusted mean PAID scores at baseline, 6, 12 months by intervention group p value in reference to previous time point. #p < 0.10, *p <0.05, **p<0.01, ***p< 0.001. For immediate group, p value from baseline to 6 months was 0.052
Results
The sample consisted of 43 % Latino/a, 57 % African American, and 71 % female. The mean age was 53 years. On average, clients in the immediate intervention group were younger than clients in delayed intervention, with average ages of 50 and 55 years, respectively. Other than age, none of the other demographics differed significantly between the delayed and immediate groups. Only 13 % lived alone and 69 % were married or partnered. Fifty-eight percent received their medical care at a federally qualified health center. Approximately 59 % of the participants were high school graduates. The average number of diabetes complications was 2.7.
Overall, the baseline mental health means were 25.0 for the PAID, 5.1 for the PHQ, and 1.1 for the PHQ-2. Although neither the baseline PAID, PHQ, nor PHQ-2 scores differed by intervention group, the PAID and the PHQ-2 differed significantly between African Americans and Latino/as. The mean baseline PAID was 33.0 for Latino/as, compared to 20.3 for African Americans, p < 0.0001. Further, the baseline PHQ did not differ by race ethnicity, and the PHQ-2 means were 0.9 for African Americans and 1.5 for Latino/as, p < 0.05.
Although the unadjusted drop in the PAID within the immediate group from baseline to 6 months was not significant at the p = 0.05 level, it was borderline significant at p = 0.055. When demographics were added to the PAID model, the baseline to 6 month drop within the immediate group was significant at the p = 0.05 level and there was a drop of 6.5 on a 100-point scale. Two demographics, age and race/ethnicity, were significantly associated with the PAID. The PAID was higher among Latino/as than among African Americans and decreased with age. Further, within the immediate group, the drop in the PAID from baseline to 12 months occurred in two stages, baseline to 6 months, with another significant drop from 6 to 12 months. Within the immediate group, the total reduction in the PAID from baseline to 12 months was 12.3.
In the delayed group, the drop in the PAID from 6 to 12 months was 5.9 unadjusted (p = 0.07) and 6.2 when adjusted for demographics (p = 0.06). The borderline significance of the pre-intervention to post-intervention reduction in the PAID may have been due to low sample size. When the pre-intervention to post-intervention effects were combined for the immediate and delayed groups via “Average Intervention Effect”, the results were significant at the p < 0.05 level or greater, regardless of whether demographics were included. The result remained significant after diabetes complications was added to the model. When the PAID analyses were stratified by race/ethnicity, the outcome was significant only for Latino/as. The effect sizes for the “Average Intervention Effect” were 0.30 for African Americans and Latino/as combined, which is considered a small-to-medium effect size, but 0.53 for Latino/as, which is considered a medium effect size.
Figure 1 shows the unadjusted PAID means at baseline, 6, and 12 months. The figure illustrates that the intervention effect occurred in two stages for the immediate group, baseline to 6 months, and 6–12 months. The delayed group experienced a similar drop in the PAID from 6 to 12 months, as seen in the immediate group from baseline to 6 months.
Although the PHQ-9 was not impacted by the intervention, the PHQ-2 did drop by 0.3, as indicated by the “Average Intervention Effect” column in Table 3. The PHQ-2 had the same drop in the models with and without demographics, and maintained significance after diabetes complications was added as a covariate. The demographics significantly associated with the PHQ-2 were gender, age, and education. The PHQ-2 was higher for women than for men, lower as age increased, and lower for high school graduates.
In all three models, the baseline to 6 month difference between the immediate and delayed groups was 0.7, p < 0.05, with an effect size of 0.44. However, the PHQ-2 effect size for Latinos in the baseline to 6 month time period was 0.53 and corresponded to a difference of 1.0 instead of 0.7. The “Average Intervention Effect” had a smaller effect size than the baseline to 6 month contrast between the treatment groups, 0.21 for everyone and 0.31 for Latino/as.
Discussion
The purpose of this study was to investigate the influence of a community health worker diabetes lifestyle intervention on mental health outcomes among African Americans and Latinos with Type 2 diabetes. Specifically, this study examined diabetes-related emotional distress and depression. Consistent with the literature, participation in a lifestyle intervention significantly reduced the symptoms of mental health conditions, namely diabetes-related emotional distress, potentially reducing barriers to diabetes self-management (D’Eramo-Melkus et al. 2004; Gabbay et al. 2006). In our study, diabetes-related emotional distress dropped even further within the immediate group from 6 to 12 months.
One possible explanation for the decreases in diabetes-related emotional distress may be changes in diet and exercise REACH participants reported during the intervention (Two Feathers 2005). Regular physical activity (Fox 1999) and a healthful diet are associated with increased quality of life (Muñoz et al. 2009). Other diabetes lifestyle interventions that reported positive changes in diet and exercise have also reported decreases in diabetes-related distress (Whittemore et al. 2004; Steed et al. 2003). The change in diabetes-related emotional distress may also be explained in terms of increased social support given to participants in the intervention from healthcare staff, in this case community health workers (Gabbay et al. 2006; van Dam et al. 2005). In the REACH Detroit intervention, community health workers attended physician appointments with participants, provided a range of diabetes education and completed home visits (Two Feathers et al. 2005a,b). A diabetes diagnosis can serve as a chronic stressor in an individual because it calls for substantial changes to everyday routines, specifically adherence to treatment regimens (Connell et al. 1994). Our findings suggest that increased education and support from a CHW may have a stress-reducing effect for persons with diabetes resulting in lower levels of diabetes-related emotional distress (Wheaton 1985; Connell et al. 1994). Another possible explanation is the role of diabetes education courses in increasing the development of healthier lifestyles, for example, physical activity and healthy eating, as well as coping skills among participants leading to improved mental health outcomes (Rubin et al. 1993; Lorig snd González 2000).
Despite the reduction in the PAID, the PHQ did not change significantly; however, we observed intervention effects for the PHQ-2 after adjustment for demographics. The PHQ is a measure of depressive symptoms, and our CHW intervention was not intended to reduce depression. However, reductions in emotional distress related to diabetes combined with changes in lifestyle could account from a decrease in certain symptoms associated with depression. Thus, it may be possible that although our intervention did not impact a full measure of depression, we observed reductions in an abbreviated scale that measures two important symptoms. More research is necessary to understand how a CHW, healthy lifestyle intervention can impact depression and improve mental health outcomes for African Americans and Latinos with diabetes.
A few study limitations should also be noted. First, the findings for diabetes-related emotional distress and PHQ scale may not relate directly to other mental health outcomes that may also have an impact on diabetes-related outcomes such as anxiety (Schwartz 2002; Xu 2011). However, few studies have focused on more than one mental health outcome in these populations. Our paper contributes to the literature by examining multiple mental health outcomes, namely the diabetes-related distress and depression. More studies should be completed that examine more than one mental health outcome in order to gain a better understanding of individual and structural level factors that may influence mental health outcomes among African Americans and Latinos with diabetes.
Second, while the use of an average intervention effect by combining the results of the immediate and the delayed groups increased our sample size and our ability to detect differences, we are unable to fully understand how receiving the intervention 6 months may have impacted the intervention. In qualitative interviews with our participants, we noted that those in the delayed group expressed dissatisfaction with the randomization process and the fairness of being asked to wait for services. Future studies with larger samples from individuals who received the intervention during the same time period would be beneficial.
Third, as we have noted, our intervention was not intended to reduce symptoms of mental health problems, although our curriculum does discuss briefly the impact of stress on diabetes and largely focuses on the importance of a healthy lifestyle. It is important that future studies like ours incorporate more information on the relationship between depression and diabetes and include more mental health content in the curriculum. CHWs have played a limited role in mental health care to date. The extent to which CHWs can impact mental health outcomes, such as depression, should be further explored through training, curriculum development, and randomized controlled studies that control for intervention effects. In the era of the affordable care act and patient-centered medical homes, an integrated care team, which includes CHWs, could act as a significant line of defense for patients with comorbid chronic illness and mental health problems. CHWs could assist with screening, provide referrals, explain the nature of mental health care and what might be expected, work with clients to develop personal goals and coping strategies through home visits, and assure that clients attend treatment sessions with mental health professionals through phone calls and by accompanying clients to clinic visits. These roles are very similar to the roles that CHWs successfully achieve in diabetes care and could be extended to mental health care.
In summary, the role of CHWs in enhancing the mental health and well-being of African Americans and Latinos is an emerging field that should be further explored. CHWs have been successful in providing necessary services for patients with a range of chronic illnesses and barriers to access to care. Many of these patients also suffer from mental health problems that may currently go undetected and untreated in traditional primary care settings. With further research and development, CHWs could extend their role in multidisciplinary teams beyond chronic disease to further enhance the well-being of low-income racial and ethnic minority populations.
Acknowledgments
NIDDK R18DK0785501A1, Centers for Disease Control and Prevention (Cooperative Agreement No. U50/CCU417409), the Michigan Diabetes Research and Training Center (NIH grant 5P60-DK20572), and the Robert Wood Johnson Foundation Clinical Scholars Program.
Contributor Information
Michael S. Spencer, Email: spencerm@umich.edu, School of Social Work, University of Michigan, 1080 South University Avenue, Ann Arbor, MI 48109-1106, USA. REACH Detroit Partnership, Detroit, MI, USA
Jaclynn Hawkins, School of Social Work, University of Michigan, 1080 South University Avenue, Ann Arbor, MI 48109-1106, USA. REACH Detroit Partnership, Detroit, MI, USA.
Nicolas R. Espitia, School of Social Work, University of Michigan, 1080 South University Avenue, Ann Arbor, MI 48109-1106, USA. REACH Detroit Partnership, Detroit, MI, USA. Community Health and Social Services Center, Detroit, MI, USA
Brandy Sinco, School of Social Work, University of Michigan, 1080 South University Avenue, Ann Arbor, MI 48109-1106, USA. REACH Detroit Partnership, Detroit, MI, USA.
Tezra Jennings, School of Social Work, University of Michigan, 1080 South University Avenue, Ann Arbor, MI 48109-1106, USA. REACH Detroit Partnership, Detroit, MI, USA.
Carissa Lewis, School of Social Work, University of Michigan, 1080 South University Avenue, Ann Arbor, MI 48109-1106, USA. REACH Detroit Partnership, Detroit, MI, USA. Community Health and Social Services Center, Detroit, MI, USA.
Gloria Palmisano, REACH Detroit Partnership, Detroit, MI, USA. Community Health and Social Services Center, Detroit, MI, USA.
Edith Kieffer, School of Social Work, University of Michigan, 1080 South University Avenue, Ann Arbor, MI 48109-1106, USA. REACH Detroit Partnership, Detroit, MI, USA.
References
- Alegria M, Jackson JS, Kessler RC, Takeuchi D. National comorbidity survey replication (NCS-R), 2001–2003. Ann Arbor: Interuniversity Consortium for Political and Social Research; 2003. [Google Scholar]
- Anderson RM, Funnell MM. Art of empowerment: Stories and strategies for diabetes educators. 2. Alexandria: American Diabetes Association; 2005. [Google Scholar]
- Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes a meta-analysis. Diabetes Care. 2001;24(6):1069–1078. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- Armstrong G, Kermode M, Raja S, Suja S, Chandra P, Jorm AF. A mental health training program for community health workers in India: Impact on knowledge and attitudes. International journal of mental health systems. 2011;5(1):17. doi: 10.1186/1752-4458-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcazar H, Rosenthal L, Brownstein JN, Rush CH, Matos S, Hernandez L. Community health workers can be a public health force for the United States: Three actions for a new paradigm. American Journal of Public Health. 2011;101(12):2199–2203. doi: 10.2105/AJPH.2011.300386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casella G, Berger RL. Statistical inference. 2. Pacific Grove: Duxbury; 2002. [Google Scholar]
- Center for disease control and prevention. [Accessed April 10 2013];Behavioral risk factor surveillance system 2004. 2004 Available at: http://www.cdc.gov/brfss/annual_data/pdf-ques/2004brfss.pdf.
- Centers for disease control and prevention. National Diabetes Surveillance System. Atlanta, GA: US Department of Health and Human Services, CDC; 2010. [Accessed April 10, 2013]. Available at http://www.cdc.gov/diabetes/statistics/index.htm. [Google Scholar]
- Centers for Disease Control and Prevention. Characteristics associated with poor glycemic control among adults self-reported diagnosed diabetes—National Health and Nutrition Examination Survey United States, 2007–2010. MMWR. 2012;61(Supplement):32–37. [PubMed] [Google Scholar]
- Connell CM, Davis WK, Gallant MP, Sharpe PA. Impact of social support, social cognitive variables, and perceived threat on depression among adults with diabetes. Health Psychology. 1994;13(3):263. doi: 10.1037//0278-6133.13.3.263. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- Deci EL, Eghrari H, Patrick BC, Leone DR. Facilitating internalization: The self-determination theory perspective. Journal of Personality. 1994;62:119–142. doi: 10.1111/j.1467-6494.1994.tb00797.x. [DOI] [PubMed] [Google Scholar]
- D’Eramo-Melkus G, Spollett G, Jefferson V, Chyun D, Tuohy B, Robinson T, et al. A culturally competent intervention of education and care for black women with type 2 diabetes. Applied nursing research: ANR. 2004;17(1):10. doi: 10.1016/j.apnr.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Diggle PJ, Heagerty P, Liang K, Zeger SL. Analysis of longitudinal data. 2. New York: Oxford University Press; 2002. [Google Scholar]
- Eaton WW. Epidemiologic evidence on the comorbidity of depression and diabetes. Journal of Psychosomatic Research. 2002;53:903–906. doi: 10.1016/s0022-3999(02)00302-1. [DOI] [PubMed] [Google Scholar]
- Eaton WW, Armenian H, Gallo J, Pratt L, Ford DE. Depression and risk for onset of type II diabetes: A prospective population-based study. Diabetes Care. 1996;19(10):1097–1102. doi: 10.2337/diacare.19.10.1097. [DOI] [PubMed] [Google Scholar]
- Fox KR. The influence of physical activity on mental well-being. Public health nutrition. 1999;2(3a):411–418. doi: 10.1017/s1368980099000567. [DOI] [PubMed] [Google Scholar]
- Funnell MM, Anderson RM. Empowerment and self-management of diabetes. Clinical Diabetes. 2004;22:123–127. [Google Scholar]
- Funnell MM, Kruger DF, Spencer M. Self-management support for insulin therapy in type 2 diabetes. The Diabetes Educator. 2001;30:274–280. doi: 10.1177/014572170403000220. [DOI] [PubMed] [Google Scholar]
- Gabbay RA, Lendel I, Saleem TM, Shaeffer G, Adelman AM, Mauger DT, et al. Nurse case management improves blood pressure, emotional distress and diabetes complication screening. Diabetes Research and Clinical Practice. 2006;71(1):28–35. doi: 10.1016/j.diabres.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Gary TL, Crum RM, Cooper-Patrick L, Ford D, Brancati FL. Depressive symptoms and metabolic control in African Americans with type 2 diabetes. Diabetes Care. 2000;23(1):23–29. doi: 10.2337/diacare.23.1.23. [DOI] [PubMed] [Google Scholar]
- Geiss LS, Pan L, Cadwell B, Gregg EW, Benjamin SM, Engelgau MM. Changes in incidence of diabetes in US adults, 1997–2003. American Journal of Preventive Medicine. 2006;30(5):371–377. doi: 10.1016/j.amepre.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Golden SH, Williams JE, Ford DE, Yeh HC, Sanford CP, Nieto FJ, et al. Depressive symptoms and the risk of type 2 diabetes the atherosclerosis risk in communities study. Diabetes Care. 2004;27(2):429–435. doi: 10.2337/diacare.27.2.429. [DOI] [PubMed] [Google Scholar]
- Heisler M. Different models to mobilize peer support to improve diabetes self-management and clinical outcomes: Evidence, logistics, evaluation considerations and needs for future research. Family Practice. 2010;27(S1):i23–i32. doi: 10.1093/fampra/cmp003. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Israel BA, Schulz AJ, Parker EA, Becker AB. Review of community-based research: Assessing partnership approaches to improve public health. Annual Review of Public Health. 1998;19:173–202. doi: 10.1146/annurev.publhealth.19.1.173. [DOI] [PubMed] [Google Scholar]
- Kanjilal S, Gregg EW, Cheng YJ, Zhang P, Nelson DE, Mensah G, et al. Socioeconomic status and trends in disparities in 4 major risk factors for cardiovascular disease among US adults, 1971–2002. Archives of Internal Medicine. 2006;166:2348. doi: 10.1001/archinte.166.21.2348. [DOI] [PubMed] [Google Scholar]
- Kieffer EC, Caldwell CH, Welmerink DB, Welch KB, Sinco BR, Guzmán JR. Effect of the healthy MOMs lifestyle intervention on reducing depressive symptoms among pregnant latinas. American Journal of Community Psychology. 2013;51:76–89. doi: 10.1007/s10464-012-9523-9. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: Validity of a brief depression severity measure. Journal of General Internal Medicine. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: Validity of a two-item depression screener. Medical Care. 2003;41(11):1284–1292. doi: 10.1097/01.MLR.0000093487.78664.3C. [DOI] [PubMed] [Google Scholar]
- Lorig K, González VM. Community-based diabetes self-management education: Definition and case study. Diabetes Spectrum. 2000;13:234–238. [Google Scholar]
- Macre J, Bersani C, Khatri P, Orloff T. BPHC Enrichment Series for Grantees: The Value of Psychologists in Health Centers. Health Resources and Services Administration. 2011 Presentation: http://bphc.hrsa.gov.
- Narayan KV, Boyle JP, Geiss LS, Saaddine JB, Thompson TJ. Impact of recent increase in incidence on future diabetes burden US, 2005–2050. Diabetes Care. 2006;29:2114–2116. doi: 10.2337/dc06-1136. [DOI] [PubMed] [Google Scholar]
- Norris SL, Chowdhury FM, Van Le K, Horsley T, Brownstein JN, Zhang X, et al. Effectiveness of community health workers in the care of persons with diabetes. Diabetic Medicine. 2006;23:544–556. doi: 10.1111/j.1464-5491.2006.01845.x. [DOI] [PubMed] [Google Scholar]
- Polonsky WH, Anderson BJ, Lohrer PA, Welch G, Jacobson AM, Aponte JE, et al. Assessment of diabetes-related distress. Diabetes Care. 1995;18:754–760. doi: 10.2337/diacare.18.6.754. [DOI] [PubMed] [Google Scholar]
- Rubin RR, Peyrot M, Saudek CD. The effect of a diabetes education program incorporating coping skills training on emotional well-being and diabetes self-efficacy. The Diabetes Educator. 1993;19:210–214. [Google Scholar]
- SAS Institute. The MIXED procedure. Cary, NC: SAS Institute; 2012. [Google Scholar]
- Schwartz S. Outcomes for the sociology of mental health: Are we meeting our goals. Journal of Health and Social Behavior. 2002;43:223–235. [PubMed] [Google Scholar]
- Spencer MS, Rosland AM, Kieffer EC, Sinco BR, Valerio M, Palmisano G, et al. Effectiveness of a community health worker intervention among African American and Latino adults with type 2 diabetes: A randomized controlled trial. American Journal of Public Health. 2011;101:2253. doi: 10.2105/AJPH.2010.300106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steed L, Cooke D, Newman S. A systematic review of psychosocial outcomes following education, self-management and psychological interventions in diabetes mellitus. Patient Education and Counseling. 2003;51:5–15. doi: 10.1016/s0738-3991(02)00213-6. [DOI] [PubMed] [Google Scholar]
- Swider SM. Outcome effectiveness of community health workers: An integrative literature review. Public Health Nursing. 2002;19:11–20. doi: 10.1046/j.1525-1446.2002.19003.x. [DOI] [PubMed] [Google Scholar]
- Two Feathers J, Kieffer EC, Palmisano G, et al. Racial and Ethnic Approaches to Community Health (REACH) Detroit partnership: Improving diabetes related outcomes among African American and Latino adults. American Journal of Public Health. 2005;95(9):1552–1560. doi: 10.2105/AJPH.2005.066134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Two Feathers JT, Kieffer EC, Palmisano G, Anderson M, Sinco B, Janz N, et al. Racial and ethnic approaches to community health (REACH) Detroit partnership: Improving diabetes-related outcomes among African American and Latino adults. American Journal of Public Health. 2005a;95:1552–1560. doi: 10.2105/AJPH.2005.066134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Two Feathers JT, Kieffer EC, Palmisano G, Anderson M, Janz N, Spencer M, et al. The development, implementation and process evaluation of the REACH Detroit Partnership’s diabetes lifestyle intervention. The Diabetes Educator. 2005b;33:509–520. doi: 10.1177/0145721707301371. [DOI] [PubMed] [Google Scholar]
- Two Feathers J, Kieffer EC, Palmisano G, et al. The development, implementation and process evaluation of the REACH Detroit Partnership’s diabetes lifestyle intervention. The Diabetes Educator. 2007;33(3):509–520. doi: 10.1177/0145721707301371. [DOI] [PubMed] [Google Scholar]
- US Census Bureau American factfinder. [Accessed December 1, 2010];2007 Available at: http://factfinder.census.gov.
- van Dam HA, van der Horst FG, Knoops L, Ryckman RM, Crebolder HF, van den Borne BH. Social support in diabetes: A systematic review of controlled intervention studies. Patient Education and Counseling. 2005;59:1–12. doi: 10.1016/j.pec.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Wallerstein NB, Duran B. Using community-based participatory research to address health disparities. Health Promotion Practice. 2006;7:312–323. doi: 10.1177/1524839906289376. [DOI] [PubMed] [Google Scholar]
- West BT, Welch KB, Galecki AT. Linear mixed models: A practical guide to using statistical software. 1. Boca Raton: Chapman and Hall; 2007. [Google Scholar]
- Wheaton B. Models for the stress-buffering functions of coping resources. Journal of Health and Social Behavior. 1985;26:352–364. [PubMed] [Google Scholar]
- Whittemore R, Melkus GD, Sullivan A, Grey M. A nurse-coaching intervention for women with type 2 diabetes. The Diabetes Educator. 2004;30:795. doi: 10.1177/014572170403000515. [DOI] [PubMed] [Google Scholar]
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- Williams GC, Rodin GC, Ryan RM, Grolnick WS, Deci EL. Autonomous regulation: The motivational basis of adherence to medical regimens. Health Psychology. 1998;17:269–276. doi: 10.1037//0278-6133.17.3.269. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Mental health: facing the challenges, building solutions; Report from the WHO European Ministerial Conference; Copenhagen, Denmark: WHO Regional Office for Europe; 2005. [Google Scholar]
- Xu Y. Ethnic variations in the relationship between socioeconomic status and psychological distress among Latino adults. Race and Social Problems. 2011;3:212–224. [Google Scholar]