Abstract
Malaria is a devastating disease in sub-Saharan Africa, and current vector control measures are threatened by emerging resistance mechanisms. With the goal of developing new, selective, resistance-breaking insecticides we explored α-fluorinated methyl ketones as reversible covalent inhibitors of Anopheles gambiae acetylcholinesterase (AgAChE). Trifluoromethyl ketones 5 demonstrated remarkable volatility in microtiter plate assays, but 5c,e-h exhibited potent (1–100 nM) inhibition of wild type (WT) AgAChE and weak inhibition of resistant mutant G119S mutant AgAChE. Fluoromethyl ketones 10c-i exhibited submicromolar to micromolar inhibition of WT AgAChE, but again only weakly inhibited G119S AgAChE. Interestingly, difluoromethyl ketone inhibitors 9c and 9g had single digit nanomolar inhibition of WT AgAChE, and 9g had excellent potency against G119S AgAChE. Approach to steady-state inhibition was quite slow, but after 23 h incubation an IC50 value of 25.1 ± 1.2 nM was measured. We attribute the slow, tight-binding G119S AgAChE inhibition of 9g to a balance of steric size and electrophilicity. However, toxicities of 5g, 9g, and 10g to adult An. gambiae in tarsal contact, fumigation, and injection assays were lower than expected based on WT AgAChE inhibition potency and volatility. Potential toxicity-limiting factors are discussed. 2015 Elsevier Ltd. All rights reserved.
Keywords: Malaria, Anopheles gambiae, acetylcholinesterase, trifluoroketones, fluorinated ketones
Graphical Abstract

Malaria is a devastating disease, responsible for an estimated 584,000 deaths world-wide in 2013.1 Efforts to control the vector Anopheles gambiae have significantly reduced malaria mortality, through the use of insecticide treated nets (ITN) and indoor residual spraying (IRS).2 To date these methods rely on only two biological targets: the voltage-gated sodium ion channel and acetylcholinesterase (AChE).3 Pyrethroids modulate the sodium channel and are approved by the World Health Organization (WHO) for IRS and for use on ITNs.4 Organophosphate and carbamate AChE inhibitors (AChEI) are approved only for IRS.4 Due to the growing emergence of pyrethroid-resistant strains of An. gambiae,5 there is increased interest in developing classes of AChEI that might be safe and effective on ITNs. Our group has previously reported that α-branched 2-substituted aryl methylcarbamates (e.g. 1, Figure 1) can be highly selective for inhibition of An. gambiae AChE (AgAChE) over human AChE (hAChE).6 We have also developed five-membered ring heterocycle core carbamates and carboxamides (e.g. 2 and 3, Figure 1) that offer good toxicity against the carbamate-resistant (Akron) strain An. gambiae.7 This strain of An. gambiae is known to carry a G119S mutant AChE,7a,8 and the smaller core structure of these heterocyclic carbamates and carboxamides may partly account for their good inhibition of G119S AgAChE.
Figure 1.
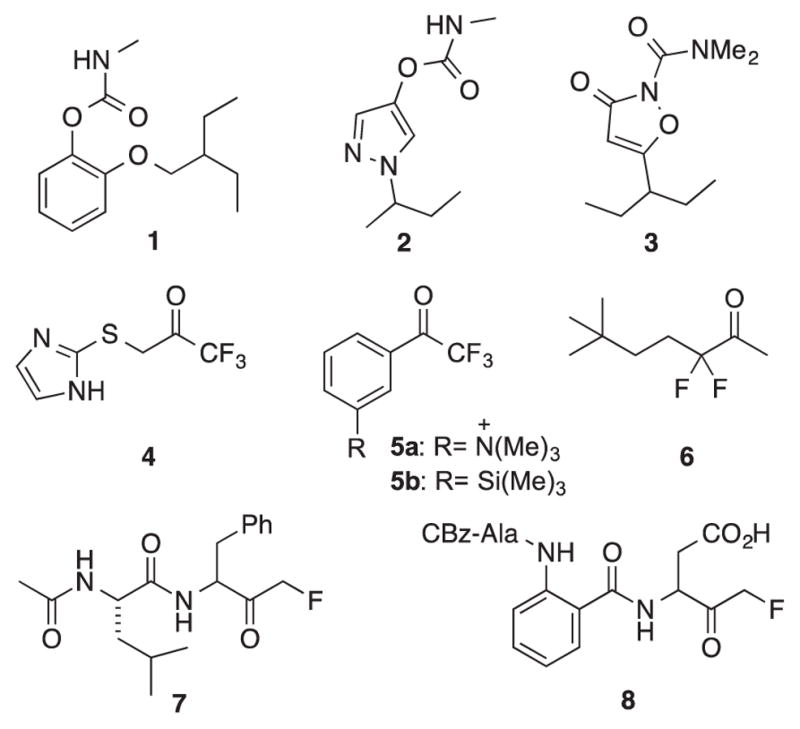
Mosquitocidal AChE inhibitors 1-3 and select fluorinated ketones (4-8) used as AChE or serine/cysteine protease inhibitors.
To further address the need for new insecticides, we sought to investigate underexplored AChEI chemotypes 4-8 (Figure 1). Trifluoromethyl ketones (e.g. 4, 5a,b) have been studied as inhibitors of AChE9 and juvenile hormone esterase.10 Despite the remarkable (picomolar) potencies that can be achieved,11 with few exceptions (e.g. 4)10b this class of compounds has received little attention as insecticides. These highly electrophilic compounds form tetrahedral intermediates with the catalytic serine of AChE, as demonstrated by X-ray crystallography.12 Zifrosilone (5b) was evaluated as an AChEI for the treatment of memory loss in Alzheimer’s disease,13 suggesting that the CNS penetrance needed for insecticidal action could be achieved with appropriate structural modification. Difluoromethyl ketones have received limited attention as AChE inhibitors, although α,α-difluoroalkyl ketone 6 was shown to be quite potent (Ki = 1.6 nM) at electric eel AChE.9b More commonly, these difluorinated ketones have been explored as inhibitors of serine proteases such as chymotrypsin,14 α-lytic protease,15 human leukocyte elastase,15 and thrombin.16 Perhaps due to the expectation that reduced electrophilicity would adversely impact inhibition potency, fluoromethyl ketones have (to our knowledge) not been reported as AChE inhibitors. Although 7 proved to be a very weak inhibitor of the serine protease chymotrypsin,14 dipeptidyl aspartyl fluoromethyl ketones such as 8 can be potent inhibitors of cysteine proteases.17
We thus synthesized a series of tri-, di-, and (mono)fluoromethyl ketones bearing substituted benzene and pyrazol-4-yl substitutents (Scheme 1).
Scheme 1.
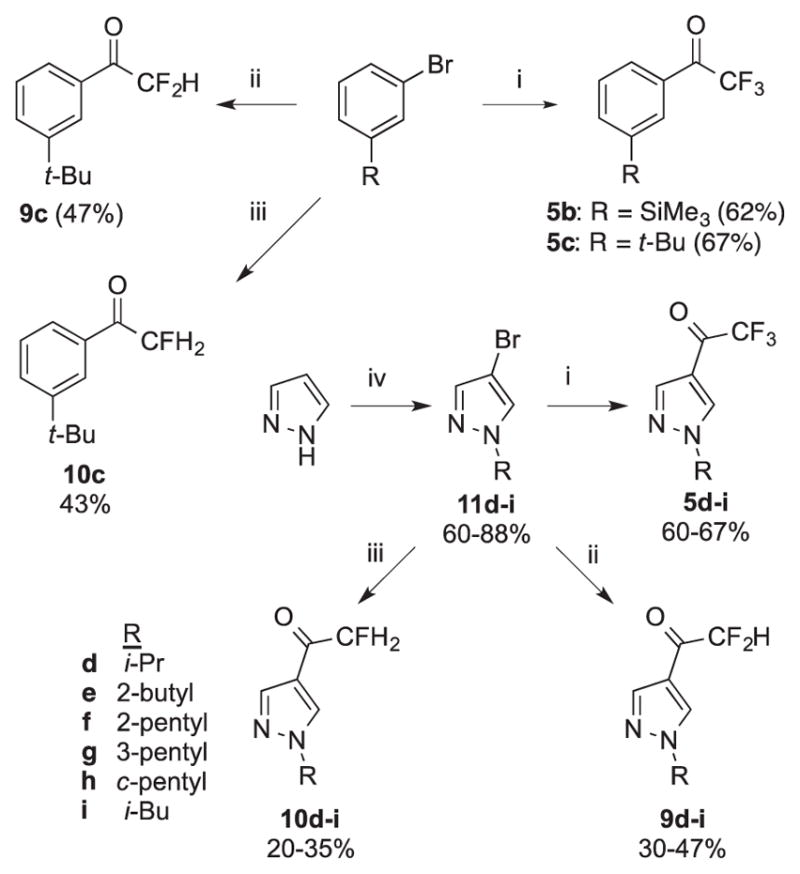
Synthesis of α-fluorinated ketones. Reagents and conditions: i) n-BuLi, THF, −78 °C, 2 h; CH3OC(O)CF3, −78 °C to RT, overnight; ii) n-BuLi, THF, −78 °C, 2 h; CH3CH2OC(O)CF2H, −78 °C, 5 min; iii) n-BuLi, THF, −78 °C, 2 h; CH3CH2OC(O)CFH2, −78 °C, 5 min. iv) NBS, H2O, 1 h; NaH, DMF, 0 °C, 1 h; R-Br, RT, overnight.
Trifluoromethyl ketones 5b-i were prepared by the literature route for 5c: metal-halogen exchange of the appropriate aryl/heteroaromatic bromide and trapping with CF3CO2Me (Scheme 1).9d The requisite N-alkyl-4-bromopyrazoles 11d-i were prepared in two steps from pyrazole.18 The preponderance of α-branched alkyl groups selected reflects the observation that these substituents increase AgAChE inhibition potency of pyrazol-4-yl7a carbamates and 3-oxoisoxazole-2(3H) carboxamides (e.g. 2, 3).7b Difluoromethyl ketones 9c-i and fluoromethyl ketones 10c-i were prepared by trapping with CF2HCO2Me and CFH2CO2Et respectively. Yields of the trifluoromethyl ketones 5b-i were moderate, and in part reflects the high volatilities of these compounds. However, yields of the difluoro- and fluoromethyl ketones 9c-i and 10c-i were only poor to fair. We attribute these poor yields to partial collapse of tetrahedral adducts 13 and 14 to the fluorinated methyl ketones 9 and 10 prior to quench, and reaction with Ar-Li (Scheme 2). Based on the relative electrophilicity of the fluorinated ketones, the extent of collapse prior to protic quench should be 14 > 13 > 12, which could account for the trend in chemical yield. Finally to assess the structure of these compounds in aqueous solution, 19F NMR spectroscopic studies9c were performed at pH 7.7. Pyrazol-4-yl trifluoromethyl ketone 5g was 67% hydrated, difluoromethyl ketone 9i was 22% hydrated, and fluoromethyl ketone 10i was < 5% hydrated (24 h, see Supplementary data).1
Scheme 2.
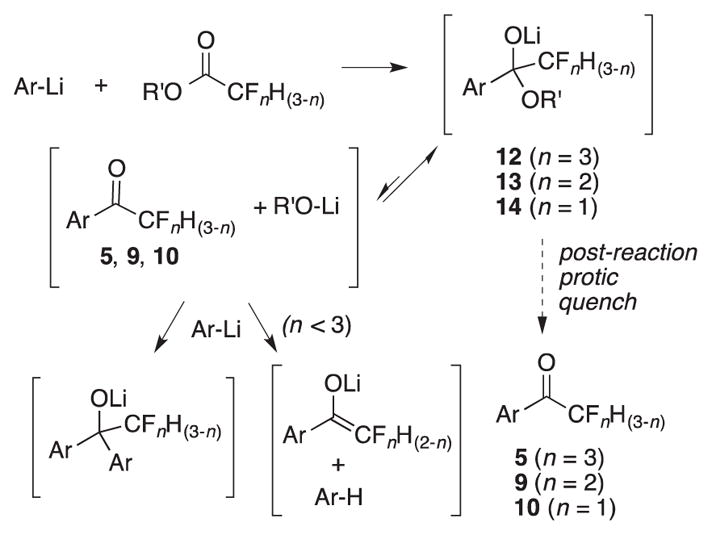
Possible side reactions in the synthesis of fluorinated ketones 5, 9, 10.
Enzyme inhibitory activity of the compounds were assessed using a modified Ellman assay20 in a 96-well microtiter plate format previously reported.7a Because time-dependent inhibition of AChE by trifluoromethyl ketones is well-documented,9a,9c,9e enzyme velocities (v/v0) were measured as a function of inhibitor concentrations [I] at incubation times of 10 min and 60 min. Sigmoidal plots of residual enzyme activity (v/v0) vs. [I] were constructed, from which the IC50 values were obtained. For the purpose of comparison we also examined the commercial carbamate propoxur, since it has excellent contact activity against susceptible (G3) strain An. gambiae, but poor toxicity against carbamate-resistant (Akron) strain An. gambiae.6b,7a Like all carbamate insecticides, propoxur carbamoylates the active site serine of AChE,21 and its time-dependent inhibition of hAChE and WT AgAChE is evident in Table 1. However this compound was a very weak inhibitor of G119S AgAChE at 10 or 60 min incubation times (IC50 > 10,000 nM), consistent with the carbamate resistance phenotype this mutation confers. The organophosphate inhibitor dichlorvos was also examined, and the potency and time-dependence of its inhibition of hAChE and WT AgAChE was similar to that of propoxur. However unlike propoxur, dichlorvos exhibited significant inhibition of G119S AgAChE, likely due to the smaller structure of its enol leaving group. The 5-fold ratio of G119S and WT IC50 values is very similar to that measured in a previous study.22
Table 1.
Inhibition IC50 values for propoxur, dichlorvos and trifluoromethyl ketones 5b-i against hAChE and AgAChE (WT & G119S).
| ||||
|---|---|---|---|---|
| Compound | Incubation time (min) | hAChE IC50 (nM)a | WT AgAChE IC50 (nM)a |
G119S AgAChE IC50 (nM)a |
| propoxur | 10 | 2,300 ± 50 | 182 ± 3 | >10,000 |
| 60 | 590 ± 19 | 43.6 ± 1.0 | >10,000 | |
| dichlorvos | 10 | 2,310 ± 60 | 324 ± 7 | 1,650 ± 44 |
| 60 | 627 ± 12 | 60.1 ± 0.9 | 300 ± 7 | |
| 5b | 10 | 285 ± 15 | 848 ± 44 | >10,000 |
| 60 | 92.9 ± 5.8 | 257 ± 15 | 10,100 ± 500 | |
| 5c | 10 | 14.6 ± 0.2 | 53.4 ± 0.8 | >10,000 |
| 60 | 5.00 ± 0.16 | 18.1 ± 0.4 | 20,800 ± 1,900 | |
| 5d | 10 | 77.5 ± 2.2 | 142 ± 5 | >10,000 |
| 60 | 121 ± 3 | 285 ± 7 | >10,000 | |
| 5e | 10 | 6.47 ± 0.15 | 2.47 ± 0.04 | 8,180 ± 450 |
| 60 | 8.84 ± 0.28 | 2.29 ± 0.17 | 2,340 ± 90 | |
| 5f | 10 | 7.15 ± 0.11 | 3.74 ± 0.08 | 19,900 ± 2,400 |
| 60 | 8.37 ± 0.16 | 2.06 ± 0.06 | 2,000 ± 140 | |
| 5g | 10 | 3.77 ± 0.06 | 2.75 ± 0.04 | >10,000 |
| 60 | 2.27 ± 0.04 | 0.68 ± 0.01 | 1,730 ± 140 | |
| 5h | 10 | 5.20 ± 0.15 | 2.87 ± 0.07 | >10,000 |
| 60 | 6.11 ± 0.18 | 1.54 ± 0.08 | 3,520 ± 140 | |
| 5i | 10 | 591 ± 11 | 950 ± 19 | >10,000 |
| 60 | 678 ± 15 | 1,040 ± 20 | >10,000 | |
Measured at 23 ± 1°C, pH 7.7, 0.1% (v/v) DMSO; all enzymes are recombinant. Average Hill slopes at WT AgAChE and hAChE are 1.0 ± 0.2.
Trifluoromethyl ketones 5b-i showed varying degrees of time-dependence to their inhibition of hAChE and WT AgAChE; those bearing a phenyl group (5b,c) showed time-dependent inhibition of both hAChE and AgAChE. However, those trifluoromethyl ketones bearing a pyrazol-4-yl substituent approach steady-state inhibition within 10 min, and 5e-h gave single-digit nanomolar IC50 values. In contrast, compound 5i, which bears a β-branched isobutyl group, was considerably less potent at hAChE and WT AgAChE than any of the pyrazol-4-yl compounds bearing α-branched substituents (5d-h). Although potent inhibition of WT AgAChE can be achieved with a pyrazol-4-yl trifluoromethyl ketone, this structure confers no inhibition selectivity against hAChE (Table 1).
In addition, none of these inhibitors offered potent inhibition of G119S AgAChE, most likely due to crowding in the oxyanion hole caused by the glycine to serine mutation. Compound 5g proved most potent at this enzyme, but its 1,730 nM IC50 value after 60 minutes incubation is roughly 2,500-fold greater than the 0.7 nM value observed for WT AgAChE. Finally 5d and 5i curiously exhibit higher hAChE and WT AgAChE IC50 values at 60 min than at 10 min. We attribute this phenomenon to rapid attainment of steady state, and evaporation of 5d and 5i out of the well plate during the longer incubation. As we will illustrate below, fluorinated compounds can be remarkably volatile, and 5d has the lowest molecular weight of all the trifluoromethyl ketones tested.
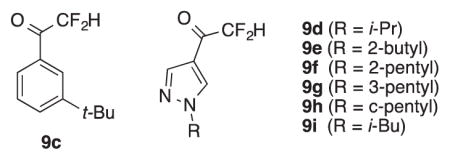
Difluoromethyl ketones 9c-i were then examined for their enzyme inhibition properties (Table 2). As expected, IC50 values for the difluoromethyl ketones at hAChE and WT AgAChE were generally higher than the values for the corresponding trifluoromethyl ketones (Table 1), with a few noteworthy exceptions. Difluoromethyl ketone 9c was more potent than trifluoromethyl analog 5c at both hAChE and WT AgAChE, and difluoromethyl ketone 9g was similar in potency to trifluoromethyl analog 5g at WT AgAChE. Both compounds have rather large alkyl substituents (t-Bu and 3-pentyl respectively), which suggests that in some cases the smaller size of the -CF2H group compared to -CF3 can compensate for its lower electron-withdrawing ability. This effect also appears to be operative in the inhibition of G119S AgAChE, which has a more crowded oxyanion hole than WT AgAChE. As can be seen in Table 2, G119S AgAChE IC50 values of difluoromethyl ketones 9c-h are uniformly lower than those of the corresponding trifluoromethyl ketones (Table 1). In addition, time-dependent inhibition is seen for the G119S enzyme: after a 60 min incubation the G119S AgAChE IC50 value of 9g is 125 nM, 13-fold lower than that of trifluoromethyl ketone 5g, and 2-fold lower than that of dichlorvos. Steady-state inhibition of G119S AgAChE by 9g is nearly attained after 330 min incubation, but after 1380 min (23 h) an IC50 value of 25.1 ± 1.2 nM was measured (Table 2). Thus in the case of 9g, the smaller size of the -CF2H group effectively compensates for its lower electron-withdrawing power, creating a slow, tight-binding inhibitor of G119S AgAChE.
Table 2.
Inhibition IC50 values for difluoromethyl ketones 9c-i against hAChE and AgAChE (WT & G119S).
| ||||
|---|---|---|---|---|
| Compound | Incubation time (min) | hAChE IC50 (nM)a | WT AgAChE IC50 (nM)a |
G119S AgAChE IC50 (nM)a |
| 9c | 10 | 6.05 ± 0.11 | 9.12 ± 0.31 | 1,650 ± 100 |
| 60 | 8.69 ± 0.18 | 9.79 ± 0.32 | 996 ± 39 | |
| 9d | 10 | 802 ± 30 | 354 ± 8 | 8,290 ± 400 |
| 60 | 869 ± 20 | 430 ± 7 | 8,380 ± 420 | |
| 9e | 10 | 110 ± 2 | 26.3 ± 0.4 | 452 ± 12 |
| 60 | 85.9 ± 1.6 | 25.2 ± 0.6 | 185 ± 7 | |
| 9f | 10 | 149 ± 2 | 23.4 ± 0.4 | 797 ± 20 |
| 60 | 158 ± 3 | 29.1 ± 0.4 | 297 ± 6 | |
| 9g | 10 | 28.8 ± 0.7 | 1.01 ± 0.02 | 680 ± 43 |
| 60 | 35.2 ± 0.7 | 1.22 ± 0.03 | 125 ± 6 | |
| 330 | ND | ND | 36.7 ± 1.7 | |
| 1380 | ND | ND | 25.1 ± 1.2 | |
| 9h | 10 | 208 ± 5 | 106 ± 2 | 3,220 ± 150 |
| 60 | 244 ± 5 | 134 ± 2 | 3,390 ± 110 | |
| 9i | 10 | 9,780 ± 470 | 3,000 ± 80 | >10,000 |
| 60 | 11,000 ± 400 | 3,950 ± 80 | >10,000 | |
Measured at 23 ± 1°C, pH 7.7, 0.1% (v/v) DMSO;. ND signifies not determined. Average Hill slopes at WT AgAChE, hAChE, and G119S AgAChE (9c-9g only for this enzyme) are 0.9 ± 0.1
To rationalize the drastically different potencies of trifluoromethyl ketone 5g at WT AgAChE and G119S AgAChE (IC50 values 0.68 and 1,730 nM respectively at 60 min), and the high potency of difluoromethyl ketone 9g for G119S AgAChE, we examined the computed structures of these compounds bound to the enzymes (Figure 2). As a starting point for docking studies we used previously developed homology models of WT and G119S AgAChE7a based on the published X-ray structure of mouse AChE complexed to 5a (PDB ID 2H9Y).12c
Figure 2.
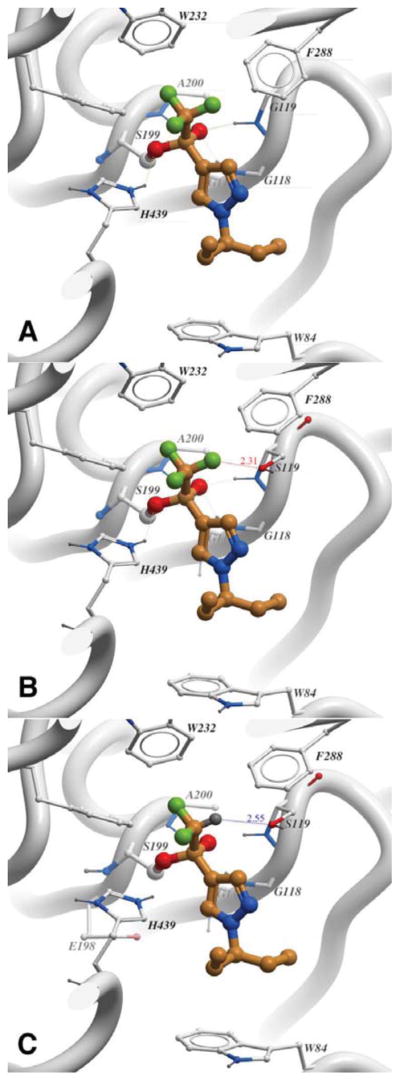
A) Trifluoromethyl ketone 5g bound to S199 of WT AgAChE. B) 5g bound to S199 of G119S AgAChE; repulsive non-bonded contact of the S119 hydroxy group with the CF3 group is evident. C) Difluoromethyl ketone 9g bound to S199 of G119S AgAChE; a potential hydrogen bond from the CF2-H of 9g to the oxygen of S119 is indicated.
Flexible ligand docking of the tetrahedral intermediates derived from 5g and WT AgAChE, and of 9g and G119S AgAChE were performed in ICM using default settings for ‘covalent’ docking mode (ICM-Docking module, Molsoft) to generate Figures 2A and 2C.23 To identify steric conflicts associated with the G119 to S119 mutation, the covalent adduct of 5g with WT AgAChE was superimposed with the G119S AgAChE apo structure (Figure 2B). As can be seen in Figure 2A, trifluoromethyl ketone 5g can easily bind to the catalytic serine (S199) of WT AgAChE, as expected from the X-ray structures of 5a bound to TcAChE and mAChE (PDB ID 1AMN12a and 2H9Y12c, respectively). However, as shown in Figure 2B, replacement of G119 with S119 causes steric repulsion between the S119 hydroxy group and one of the fluorine atoms of the CF3 group of 5g: the indicated O-F distance of 2.31 Å is significantly shorter than the sum of the respective van der Waals radii (2.99 Å). However in the complex of G119S AgAChE with the analogous difluoromethyl ketone 9g (Figure 2C), this unfavorable interaction is replaced with a potential hydrogen bond from the hydrogen of the HCF2 group to the S119 oxygen. The CF2H group is a known H-bond donor;24 in this way the excellent inhibitory potency of 9g for G119S AgAChE can be rationalized.
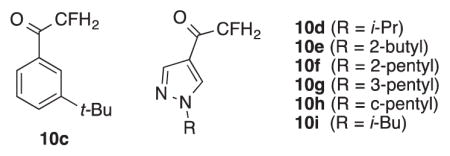
Turning to the fluoromethyl ketones 10c-i, much weaker inhibition of hAChE and WT AgAChE is seen compared to that of difluoromethyl ketones 9c-i and trifluoromethyl ketones 5c-i. (Table 3). This outcome is understandable in view of the low electrophilicity of the fluoromethyl ketones. Two results stand out, however. Firstly, fluoromethyl ketone 10g is a ~350 nM inhibitor of WT AgAChE. At a 10 minute incubation time, its IC50 value is roughly 2-fold higher than that of propoxur (Table 1). Secondly, compound 10g also displayed micromolar inhibition of G119S AgAChE. Thus given the appropriate pyrazol-4-yl substituent, fluoromethyl ketones evidence inhibition of both WT and G119S AgAChE.
Table 3.
Inhibition IC50 values for fluoromethyl ketones 10c-i against hAChE and AgAChE (WT & G119S)
| ||||
|---|---|---|---|---|
| Compound | Incubation time (min) | hAChE IC50 (nM)a | WT AgAChE IC50 (nM)a |
G119S AgAChE IC50 (nM)a |
| 10c | 10 | 578 ± 13 | 726 ± 12 | >10,000 |
| 60 | 715 ± 15 | 953 ± 18 | >10,000 | |
| 10d | 10 | >10,000 | >10,000 | >10,000 |
| 60 | >10,000 | >10,000 | >10,000 | |
| 10e | 10 | 16,900 ± 1,200 | 2,750 ± 60 | >10,000 |
| 60 | 18,000 ± 1,900 | 2,860 ± 60 | >10,000 | |
| 10f | 10 | 24,200 ± 2,700 | 3,520 ± 70 | >10,000 |
| 60 | 17,600 ± 1,800 | 3,290 ± 80 | >10,000 | |
| 10g | 10 | 5,290 ± 250 | 355 ± 11 | 2,990 ± 160 |
| 60 | 5,190 ± 270 | 337 ± 11 | 3,000 ± 160 | |
| 10h | 10 | 2,500 ± 80 | 5,860 ± 160 | >10,000 |
| 60 | 1,310 ± 30 | 1,540 ± 40 | >10,000 | |
| 10i | 10 | >10,000 | >10,000 | >10,000 |
| 60 | >10,000 | >10,000 | >10,000 | |
Measured at 23 ± 1°C, pH 7.7, 0.1% (v/v) DMSO; Average Hill slopes at WT AgAChE and hAChE are 0.9 ± 0.1 and 0.8 ± 0.1 respectively (compounds 10d, 10i excluded).
During our microtiter plate inhibition assay development we were surprised to find that trifluoroketones appeared to migrate from high [I] wells to the adjacent inhibitor-free control wells.25
We further observed that the spatial extent of this migration was significantly enhanced when the microtiter plate was covered with its accompanying “non-sealing” polystyrene lid. We believe the loose cover provided by the lid serves to direct evaporation to the neighboring wells rather than to allow it to escape to the atmosphere. Progressive “distillation” of 5g in such a loosely covered microtiter plate format is demonstrated in Figure 3: although the inhibitor was placed only in wells D6-D7, after 10 min significant enzyme inhibition is visible up to 3 wells away. Further spreading is evident at 20 and 40 min, and at 60 min nearly every well evidences contamination by 5g. In a 10 min incubation study of a homologous series of compounds, volatility was seen to decrease from 5g (CF3) to 9g (CF2H) to 10g (CFH2) (Figure 4). Whereas trifluoromethyl ketone 5g diffuses over 3 wells from D6-D7 in 10 min, difluoromethyl ketone 9g diffuses only 1 well. In contrast, no diffusion of fluoromethyl ketone 10g seen over 10 min. Thus the degree of fluorination plays a dominant role in the volatility of this series of analogs. Interestingly, no diffusion of dichlorvos was seen over 10 min, suggesting it is less volatile than 5g and 9g (see Figure S1, Supplementary data). Finally, as expected, no diffusion of the propoxur control is seen.
Figure 3.
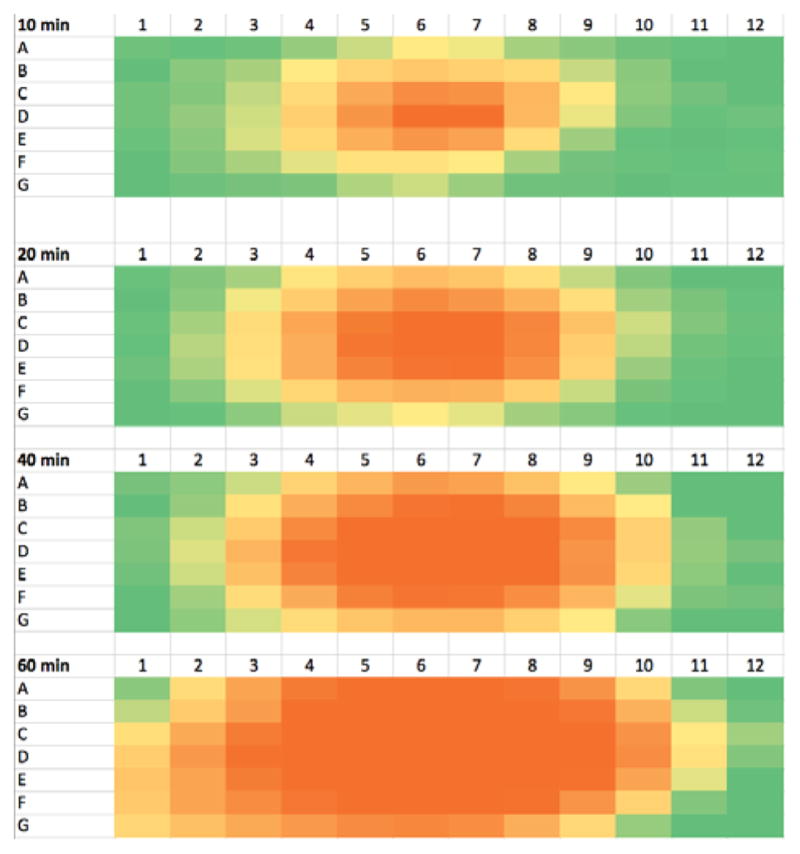
Microtiter plate heat maps of WT AgAChE residual activity in which only wells D6–D7 of the microtiter plates were charged with 10,000 nM of inhibitor 5g for the indicated incubation time (10–60 min, 23 ± 1°C) before the addition of substrate. Data for Row H (enzyme-free background wells) are not shown. Color coding: red, ≤10% residual activity; yellow, ≤ 75% residual activity; green ≥ 93% residual activity. Progressive vapor phase diffusion of 5g over 60 min is evident. See Supplementary data for residual activity values.
Figure 4.
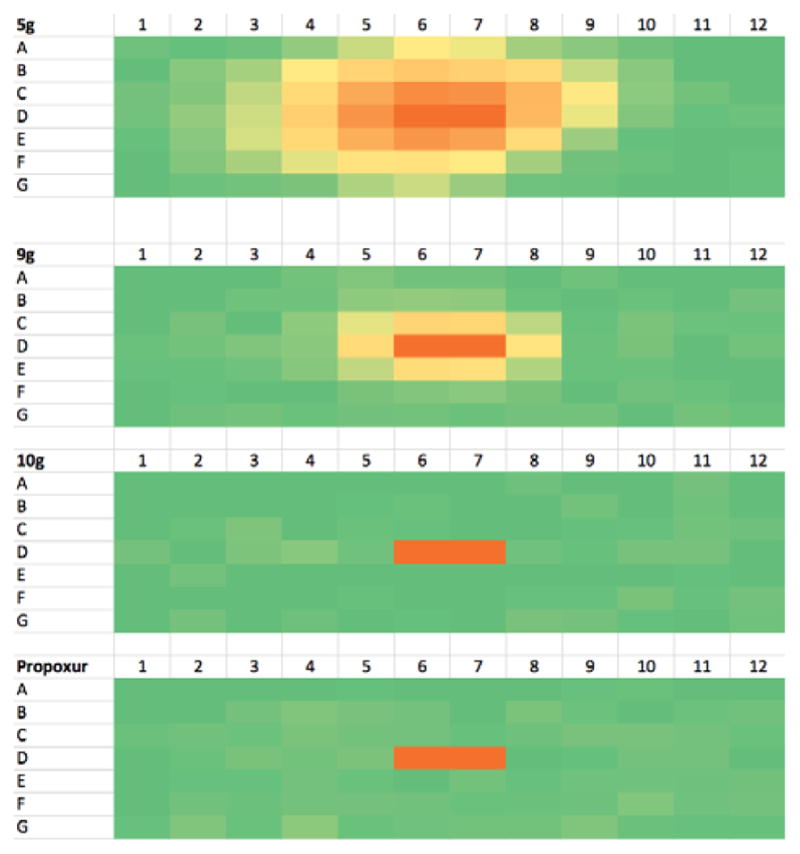
Microtiter plate heat maps of WT AgAChE residual activity (10 min incubation) in which only wells D6–D7 of the microtiter plates were charged with 10,000 nM of inhibitor (5g, 9g, 10g, propoxur: 23 ± 1°C). Data for Row H (enzyme-free background wells) are not shown. Color coding follows that of Figure 3. Vapor phase diffusion of 5g and 9g is evident. See Supplementary data for residual activity values.
As noted above, several of the fluorinated methyl ketone inhibitors (5c-h, 9e-h) demonstrated potent inhibition of WT AgAChE. We thus examined the tarsal contact toxicity of select compounds to adult susceptible (G3) strain An. gambiae, using the recommended WHO treated paper protocol26 (Table 4).
Table 4.
Tarsal contacta toxicity of select fluorinated methyl ketones to susceptible (G3) strain adult An. gambiae.
| Compound | LC50 (μg/mL) or % mortality at 1000 μg/mL |
|---|---|
| propoxurb | 39 (32–45)c |
| 5b | 10% |
| 5c | 8% |
| 5d | 20% |
| 5e | 30% |
| 5f | 0% |
| 5g | 40% |
| 5h | 40% |
| 9c | 20% |
| 9d | 50% |
| 9e | 50% |
| 9f | 0% |
| 9g | 0% |
| 9h | 0% |
| 10c | 50% |
| 10d | 85% |
| 10e | 65% |
| 10f | 20% |
| 10g | 85% |
| 10h | 0% |
| 10i | 20% |
Papers were treated with ethanolic solutions of fluorinated methyl ketones and allowed to dry overnight. Mosquitoes were exposed (1 h) and mortality was recorded after 24 h. LC50 values derive from the concentrations of inhibitor used to treat the paper; 95% confidence limits are given in parenthesis.
Data for propoxur were reported previously.7a
As can be seen none of the compounds tested approach the contact toxicity of propoxur, despite the fact that many (5c, 5e-5h, 9c, 9e-9g) are much more potent inhibitors of WT AgAChE than propoxur after a 60 min incubation. The most toxic compounds tested were fluoromethyl ketones 10d and 10g (85% mortality at 1,000 μg/mL). Yet these compounds differ dramatically in their inhibition of WT AgAChE, giving IC50 values of >10,000 nM and 337 ± 11 nM respectively (60 min incubation). Therefore there is no apparent correlation between tarsal contact % mortality and AChE inhibition potency for these compounds. The procedure for this toxicity assay26 mandates that treated papers be dried overnight to remove the solvent vehicle prior to mosquito exposure. Could extensive evaporation of tri- and difluoroketones (all liquids) from the treated papers prior to mosquito exposure account for the low and variable toxicities seen in Table 4? To assess this possibility, we measured the evaporative weight loss of 5g at room temperature, and compared it to propoxur (mp 91 °C) and dichlorvos (liquid at room
As can be seen in Table 5, water evaporated completely within 1 day, and propoxur showed no weight loss over 28 days. The liquid organophosphate inhibitor dichlorvos lost 32 ± 1% of its mass over 12 days, and 51 ± 1% over 28 days. Trifluoromethyl ketone 5g proved even more volatile than dichlorvos, losing 68 ± 1% of its mass over 12 days, and 99 ± 1 % over 28 days. However over 1 day, 5g lost only 9 ± 1 % of its mass. Thus the ~25-fold lower tarsal contact toxicity of 5g (40% mortality at 1000 μg/mL) relative to propoxur (LC50 = 39 μg/mL) cannot be attributed solely to compound evaporation.
Table 5.
Evaporative weight loss of AChE inhibitors (and water control) at room temperature.a
| Compound | % mass lost by evaporation | |||
|---|---|---|---|---|
| 1 day | 6 days | 12 days | 28 days | |
| propoxur | 0 ±1 | 0 ± 1 | 1 ± 1 | 0 ± 1 |
| dichlorvos | 2 ± 1 | 16 ± 1 | 32 ± 1 | 51 ± 1 |
| 5g | 9 ± 1 | 33 ± 1 | 68 ± 1 | 99 ± 1 |
| water | 99 ± 1 | 99 ± 1 | 100 ± 1 | 100 ± 1 |
Compounds (starting masses 8–15 mg) were placed in open 1 dram vials in a fume hood at room temperature.
Since poor penetration of the exoskeleton following tarsal contact could impede delivery of these compounds to the mosquito CNS, we explored the toxicity of the most potent trifluoromethyl, difluoromethyl and fluoromethyl ketone inhibitors of AgAChE-WT (5g, 9g, and 10g, respectively) in two other assays. First we examined the toxicity of these compounds in a fumigation assay, 27 whereby An. gambiae were placed in a sealed 1 L vessel containing a known mass of an AChE inhibitor, but prevented from direct physical contact with the compound (Table 6). As expected, the nonvolatile compound propoxur was completely non-toxic at a high nominal concentration of 1000 ng/mL.
Table 6.
Fumigation and injection toxicity of select AChE inhibitors.
| Compound | Fumigation mortality at indicated nominal concentrationa | Injection LD50 (ng/insect) or mortality at 50 ng/insectb |
|---|---|---|
| propoxur | 0% @ 1,000 ng/mL | 0.24 (0.20–0.34) |
| dichlorvos | 100% @ 10 ng/mL | ND |
| 5g | 86 ± 7% @ 1,000 ng/mL | 83 ± 6% |
| 9g | 57 ± 25% @ 1,000 ng/mL | 65 ± 4% |
| 10g | 8 ± 6% @ 1,000 ng/mL | 21 ± 8% |
Measured % mortality after 24 h in a 1 L sealed vessel, see Supplementary data for experimental details. Nominal concentration is calculated from the mass of AChE inhibitor delivered and the volume of the vessel.
See Supplementary data for protocol; the 95% confidence interval for the propoxur LD50 value is given in parenthesis. ND signifies “not determined.”
In contrast dichlorvos, which is known for its vapor phase insecticidal action,27 displayed 100% mortality at a 100-fold lower nominal concentration (10 ng/mL). However 5g, which is 100-fold more potent than dichlorvos at WT AgAChE (Table 1), and more volatile (Table 5), proved to be >100-fold less toxic than dichlorvos (86 ± 7 % mortality at 1000 ng/mL). Thus the low fumigation toxicity of 5g relative to dichlorvos must be due to some other factor. Compounds 9g and 10g had similar low toxicities.
As a final assessment of intrinsic toxicity, injection of these compounds into the mosquito thorax was performed. Propoxur was chosen as the positive control, and it gave a low LD50 value of 0.24 ng/insect. Compounds 5g, 9g, and 10g were then assessed, but significant mortality from these compounds was seen only at the 200-fold higher dose of 50 ng/insect. Since 5g and 9g are significantly more potent inhibitors of AgAChE than propoxur (Tables 1 & 2), it is again clear that factors beside exoskeleton penetrability significantly limit the toxicity of the tri- and difluoromethyl ketones. Obviously many phenomena could be at play in mitigating the toxicity of these compounds. But given the demonstrated propensity of these compounds to form hydrates, it is possible that phase II metabolism (i.e. glycosidation28 of the hydrate) and excretion is one factor that contributes to the detoxification of these potent AChE inhibitors.
Supplementary Material
Acknowledgments
We thank the MR4 as part of the BEI Resources Repository, NIAID, NIH, for providing eggs for the G3 (MRA-112) strain of Anopheles gambiae; We thank the NIH (AI082581, to PRC) and the USDA Hatch project (FLA-ENY-005237, to JRB) for financial support.
Footnotes
Supplementary data (includes experimental protocols, additional figures, synthetic procedures, analytical characterization data for the trifluoro-, difluoro-, and fluoromethyl ketones, residual activity values for Figures 3 and 4) associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.bmcl.____.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Notes
- 1.World Malaria Report. The World Health Organization; 2014. [last accessed 12/17/14]. available at http://apps.who.int/iris/bitstream/10665/144852/2/9789241564830_eng.pdf. [Google Scholar]
- 2.Mnzava AP, Macdonald MB, Knox TB, Temu EA, Shiff CJ. Trans Royal Soc Trop Med Hyg. 2014;108:550. doi: 10.1093/trstmh/tru101. [DOI] [PubMed] [Google Scholar]
- 3.Nauen R. Pest Manag Sci. 2007;63:628. doi: 10.1002/ps.1406. [DOI] [PubMed] [Google Scholar]
- 4. [Last accessed 1/21/15];The World Health Organization Pesticide Evaluation Scheme (WHOPES) http://www.who.int/whopes/en/
- 5.N’Guessan R, Boko P, Odjo A, Knols B, Akogbeto M, Rowland M. Trop Med Int Health. 2009;14:389. doi: 10.1111/j.1365-3156.2009.02245.x. [DOI] [PubMed] [Google Scholar]
- 6.(a) Hartsel JA, Wong DM, Mutunga JM, Ma M, Anderson TD, Wysinski A, Islam R, Wong EA, Paulson SL, Li J, Lam PCH, Totrov MM, Bloomquist JR, Carlier PR. Bioorg Med Chem Lett. 2012;22:4593. doi: 10.1016/j.bmcl.2012.05.103. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wong DM, Li J, Lam PCH, Hartsel JA, Mutunga JM, Totrov M, Bloomquist JR, Carlier PR. Chem Biol Interact. 2013;203:314. doi: 10.1016/j.cbi.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Wong DM, Li J, Chen QH, Han Q, Mutunga JM, Wysinski A, Anderson TD, Ding H, Carpenetti TL, Verma A, Islam R, Paulson SL, Lam PCH, Totrov M, Bloomquist JR, Carlier PR. PLOS One. 2012;7:e46712. doi: 10.1371/journal.pone.0046712. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Verma A, Wong DM, Islam R, Tong F, Ghavami M, Mutunga JM, Slebodnick C, Li J, Viayna E, Lam PCH, Totrov MM, Bloomquist JR, Carlier PR. Bioorg Med Chem. 2015;23:1321. doi: 10.1016/j.bmc.2015.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weill M, Lutfalla G, Mogensen K, Chandre F, Berthomieu A, Berticat C, Pasteur N, Philips A, Fort P, Raymond M. Nature. 2003;423:136. doi: 10.1038/423136b. [DOI] [PubMed] [Google Scholar]
- 9.(a) Brodbeck U, Schweikert K, Gentinetta R, Rottenberg M. Biochim Biophys Acta. 1979;567:357. doi: 10.1016/0005-2744(79)90122-0. [DOI] [PubMed] [Google Scholar]; (b) Gelb MH, Svaren JP, Abeles RH. Biochemistry. 1985;24:1813. doi: 10.1021/bi00329a001. [DOI] [PubMed] [Google Scholar]; (c) Nair HK, Lee K, Quinn DM. J Am Chem Soc. 1993;115:9939. [Google Scholar]; (d) Nair HK, Quinn DM. Bioorg Med Chem Lett. 1993;3:2619. [Google Scholar]; (e) Nair HK, Seravalli J, Arbuckle T, Quinn DM. Biochemistry. 1994;33:8566. doi: 10.1021/bi00194a023. [DOI] [PubMed] [Google Scholar]
- 10.(a) Hammock BD, Wing KD, McLaughlin J, Lovell VM, Sparks TC. Pestic Biochem Physiol. 1982;17:76. [Google Scholar]; (b) Szekacs A, Halarnkar PP, Olmstead MM, Prag KA, Hammock BD. Chem Res Toxicol. 1990;3:325. doi: 10.1021/tx00016a009. [DOI] [PubMed] [Google Scholar]
- 11.Based on unhydrated ketone, 5a has KD in the femtomolar range (ref. 9c).
- 12.(a) Harel M, Quinn DM, Nair HK, Silman I, Sussman JL. J Am Chem Soc. 1996;118:2340. [Google Scholar]; (b) Doucet-Personeni C, Bentley PD, Fletcher RJ, Kinkaid A, Kryger G, Pirard B, Taylor A, Taylor R, Taylor J, Viner R, Silman I, Sussman JL, Greenblatt HM, Lewis T. J Med Chem. 2001;44:3202. doi: 10.1021/jm010826r. [DOI] [PubMed] [Google Scholar]; (c) Bourne Y, Radic Z, Sulzenbacher G, Kim E, Taylor P, Marchot P. J Biol Chem. 2006;281:29256. doi: 10.1074/jbc.M603018200. [DOI] [PubMed] [Google Scholar]
- 13.Zhu XD, Giacobini E, Hornsperger JM. Eur J Pharmacol. 1995;276:93. doi: 10.1016/0014-2999(95)00014-c. [DOI] [PubMed] [Google Scholar]
- 14.Imperiali B, Abeles RH. Biochemistry. 1986;25:3760. doi: 10.1021/bi00361a005. [DOI] [PubMed] [Google Scholar]
- 15.Govardhan CP, Abeles RH. Arch Biochem Biophys. 1990;280:137. doi: 10.1016/0003-9861(90)90528-7. [DOI] [PubMed] [Google Scholar]
- 16.Jones DM, Atrash B, Teger-Nilsson AC, Gyzander E, Deinum J, Szelke M. Lett Pept Sci. 1995;2:147. doi: 10.3109/14756369509040680. [DOI] [PubMed] [Google Scholar]
- 17.(a) Wang Y, Jia S, Tseng B, Drewe J, Cai SX. Bioorg Med Chem Lett. 2007;17:6178. doi: 10.1016/j.bmcl.2007.09.030. [DOI] [PubMed] [Google Scholar]; (b) Wang Y, Huang JC, Zhou ZL, Yang W, Guastella J, Drewe J, Cai SX. Bioorg Med Chem Lett. 2004;14:1269. doi: 10.1016/j.bmcl.2003.12.065. [DOI] [PubMed] [Google Scholar]
- 18.(a) Moslin RM, Weinstein DS, Wrobleski ST, Tokarski JS, Kumar A. Patent WO 2014074661 A1. Pyridazinecarboxamide derivatives useful as modulators of IL-12, IL-23 and/or INFalpha responses and their preparation and use in the treatment and use of inflammatory and autoimmune diseases. 2014 May 15;; (b) Chen L, Dillon MP, Feng L-C, Hawley RC, Yang M-M. WO 2009077367 A1. Pyrazole-substituted arylamide derivatives and their use as P2X3 and/or P2X23 purinergic receptor antagonists and their preparation and use in the treatment of disease. 2009 Jun 25;
- 19.Note that under these conditions aryl trifluromethyl ketone 5c was 88% hydrated; the lower extent of hydration of pyrazol-4-yl trifluoromethyl ketone 5g (67%) suggests that the 1-alkylpyrazol-4-yl ring is more electron-releasing that the 3-t-butylphenyl ring.
- 20.Ellman GL, Courtney KD, Andres VJ, Featherstone RM. Biochem Pharmacol. 1961;7:88. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 21.Bar-On P, Millard CB, Harel M, Dvir H, Enz A, Sussman JL, Silman I. Biochemistry. 2002;41:3555. doi: 10.1021/bi020016x. [DOI] [PubMed] [Google Scholar]
- 22.Alout H, Berthomieu A, Hadjivassilis A, Weill M. Insect Biochem Mol Biol. 2007;37:41. doi: 10.1016/j.ibmb.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 23.(a) Totrov MM, Abagyan RA. Proteins, Suppl. 1997;1:215. doi: 10.1002/(sici)1097-0134(1997)1+<215::aid-prot29>3.3.co;2-i. [DOI] [PubMed] [Google Scholar]; (b) Neves MC, Totrov M, Abagyan R. J Comput-Aided Mol Design. 2012;26:675. doi: 10.1007/s10822-012-9547-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.(a) Erickson JA, McLoughlin JI. J Org Chem. 1995;60:1626. [Google Scholar]; (b) Meanwell NA. J Med Chem. 2011;54:2529. doi: 10.1021/jm1013693. [DOI] [PubMed] [Google Scholar]
- 25.As described in the Supplementary Data, the microtiter plate format featured inhibitor-free controls in columns 1 and 12, with 10 concentrations of inhibitor ranging from high to low in columns 2–11. The right-most inhibitor free control well (column 12) was used to calculate residual enzyme activity; the difference in enzyme activity between inhibitor-free controls in columns 1 and 12 served as a measure of the extent of migration of the inhibitor from column 2.
- 26.Guidelines for Testing Mosquito Adulticides for Indoor Residual Spraying and Treatment of Mosquito Nets. World Health Organization; Geneva: 2006. WHO/CDS/NTD/WHOPES/GCDPP/2006.3. [Google Scholar]
- 27.(a) Chaskopoulou A, Pereira RM, Scharf ME, Koehler PG. J Med Entomol. 2009;46:1400. doi: 10.1603/033.046.0621. [DOI] [PubMed] [Google Scholar]; (b) Chaskopoulou A, Nguyen S, Pereira RM, Scharf ME, Koehler PG. J Med Entomol. 2009;46:328. doi: 10.1603/033.046.0218. [DOI] [PubMed] [Google Scholar]
- 28.Yu SJ. In: Encyclopedia of Entomology. 2. Capinera JL, editor. Vol. 4. Springer; 2008. p. 1193. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


