Abstract
Introduction
This study examined whether, among subjects with mild cognitive impairment (MCI), women progressed at faster rates than men.
Methods
We examine longitudinal rates of change from baseline in 398 MCI subjects (141 females and 257 males) in the Alzheimer's Disease Neuroimaging Initiative-1, followed for up to 8 years (mean, 4.1 ± 2.5 years) using mixed-effects models incorporating all follow-ups (mean, 8 ± 4 visits).
Results
Women progressed at faster rates than men on the Alzheimer's disease assessment scale-cognitive subscale (ADAS-Cog; P = .001) and clinical dementia rating-sum of boxes (CDR-SB; P = .003). Quadratic fit for change over time was significant for both ADAS-Cog (P = .001) and CDR-SB (P = .004), and the additional acceleration in women was 100% for ADAS-Cog and 143% for CDR-SB. The variability of change was greater in women. The gender effect was greater in apolipoprotein E (APOE) ε4 carriers.
Discussion
Women with MCI have greater longitudinal rates of cognitive and functional progression than men. Studies to confirm and uncover potential mechanisms appear to be warranted.
Trial Registration
ADNI ClinicalTrials.gov identifier: NCT00106899.
Keywords: Beta-amyloid, Apolipoprotein ε4, Activities of daily living, Gender differences, Secondary prevention
1. Introduction
Although men may have a greater risk for mild cognitive impairment (MCI) [1], women make up almost two-thirds of Alzheimer's disease (AD) patients in the United States [2]. The higher prevalence of AD in women has been attributed previously to longer female life expectancy or sociocultural detection biases, but some recent findings [1], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32] also support an alternate hypothesis that women at risk progress to AD at faster rates than men due to greater neurobiologic vulnerability. The Framingham study found that the age-specific risk of AD was almost twofold greater in women than men (17.2% vs. 9.1% at age 65 years and 28.5% vs. 10.2% at age 75 years) [3]. Holland et al. [6] reported that female gender was associated with a greater rate of cognitive change in MCI subjects than men over a 1-year period, raising further questions about what happens over longer periods.
Studies have also begun to examine underlying reasons for a possible sexual dimorphism in rates of decline. A greater potency of the AD risk associated with the apolipoprotein E4 allele and the brain derived neurotrophic factor (BDNF) Met66 allele has been noted in women [1], [5], [7], [9], [11], [12]. Other theories proposed to explain gender differences include sex hormones (such as estrogen), smaller head size, lower cognitive reserve, lower levels of exercise in women (at least in the United States) and differences in occupational or educational attainment. Gender differences in pathologic vulnerability for AD are supported by studies noting greater annual three-dimensional tensor-based magnetic resonance imaging (MRI) brain atrophy rates in women [4] and a significant association of gender with neuritic plaques and neurofibrillary tangles [5]. In one study, equivalent increases in AD pathology increased the odds of clinical AD by 20-fold for women versus threefold for men [5]. Collectively, these studies argue for more definitive long-term examination of gender differences in MCI rates of progression and pathologic vulnerability.
The aim of this report was to use 8-year longitudinal data on at-risk subjects from a national biomarker study to test the hypothesis that women progress cognitively and functionally at faster rates than men, after covarying for baseline cognition, age, and education. A second aim was to model the long-term trajectories of decline in men versus women to see if the assumptions of linear decline noted by the prior 1-year study [6] held true over a longer period of follow-up. The long-term data allowed us to test for both linear and curvilinear patterns of decline as well as acceleration over time. A third aim was to examine interactions between apolipoprotein E (APOE) genotype and gender on cognitive decline and to see if gender had an effect beyond that conferred by the E4 genotype. Finally, we examined gender differences in the variability of decline in both cognition and function, using instruments widely used in prevention trials.
2. Methods
2.1. Subjects
MCI subjects recruited in Alzheimer's Disease Neuroimaging Initiative-1 (ADNI-1; adni.loni.usc.edu) were used in our analyses. ADNI (ADNI ClinicalTrials.gov identifier: NCT00106899) is the result of efforts of many coinvestigators from a broad range of academic institutions and private corporations, and subjects have been recruited from over 50 sites across the United States and Canada. ADNI-1 originally recruited 398 MCI subjects who then had the option to be followed in ADNI-2. For up-to-date information, see www.adni-info.org. Additional details are also provided in the ADNI-1 procedures manual [33], [34].
All ADNI-1 MCI subjects were eligible for inclusion. Criteria for classification as MCI in ADNI-1 are as follows: an inclusive mini-mental state examination (MMSE) score from 24–30, subjective memory complaint, objective evidence of impaired memory calculated by scores of the Wechsler Memory Scale Logical Memory II adjusted for education, a score of 0.5 on the global CDR, absence of significant confounding conditions such as current major depressive episode, normal, or near normal daily activities, and absence of clinical dementia. For a detailed list of all selection criteria, readers are referred to the ADNI-1 procedures manual [34]. In addition, data for all the following parameters were required for subjects included for analysis: baseline age, race, gender, and years of education; baseline MMSE score; Alzheimer's disease assessment scale-cognitive subscale (ADAS-Cog) for at least two different time points, and APOE genotyping results. APOE allele genotyping of all subjects was executed using DNA extracted from peripheral blood cells, and details are provided elsewhere [34]. In total, 398 MCI subjects from ADNI-1 were included. The term “baseline” is used to indicate data collected at the subject's first visit (which may be screening or baseline).
2.2. Outcome measures
The ADAS-cog 11 is a 70-point scale designed to assess severity of cognitive impairment, and it is commonly used in MCI and Alzheimer's trials. The ADAS-Cog is composed of 11 tasks that assess learning and memory, language production and comprehension, constructional and ideational praxis, and orientation [4]. Higher scores indicate worse performance, as it is scored based on number of errors.
The clinical dementia rating-sum of boxes (CDR-SB), with a range from 0 to 18, is the sum of the ratings for the six domains of the CDR global dementia rating scale. It provides a quantitative assessment of cognitive and functional impairments based on a semi-structured interview of the subject and informant [35]. Higher scores indicate greater impairment.
2.3. Follow-up
ADNI MCI subjects were followed through ADNI-1 and then enrolled in ADNI-2. We compared ADAS-Cog and CDR-SB scores from baseline to end point (using most recent available scores at the time of our data extraction in late 2014) yielding a study duration of up to 8 years (mean duration, 4 years).
2.4. Statistical analyses (models)
We fit a quadratic model of the form (as it was found to be a better fit than just a linear model).
| (1) |
In model (1), Aj(t) is the ADAS-Cog value of subject j at follow-up time t. The model explains this in terms of μ, the ADAS-Cog value for subject j at 3.45 years, β, the rate of change in ADAS-Cog and γ, the curvature in the ADAS-Cog trajectory. Finally, εjt is the measurement error, assumed to have a zero mean Gaussian distribution with standard deviation (SD) σe. The curvature parameter captures nonlinearity of the trajectories: γ = 0 indicates linear growth, i.e. constant rate of change, γ > 0 indicates that the rate of change is increasing over time, whereas γ < 0 indicates that the rate of change is decreasing over time. The follow-up time is centered; the median follow-up time (3.45 years) is subtracted from the actual follow-up time, to allow the slope and curvature parameters to be estimated approximately independently.
To confirm our findings with regard to slopes and curvatures, a mixed-effects model was used to model the effect of gender on cognitive decline, taking into account the effect of confounders, such as baseline MMSE, age, years of education, and APOE ε4 status on baseline, with outcome being ADAS-Cog, as follows:
| (2) |
In the mentioned model, Ai(t) is the ADAS-Cog value of subject i at time t (in years). Model (2) explains this in terms of μ, the baseline ADAS-Cog value for a 75.1-year-old male with 16 years of education, MMSE of 27 and of APOE ε4 negative. The terms with α are effects of gender, APOE ε4 status, MMSE, age, and years of education on baseline ADAS-Cog values. The terms with β are effects on rates of change and β0 is the baseline rate of change. The terms with γ are effects on curvature and γ0 is the baseline curvature. We include a random effect bi and ri to account for the effect of unmeasured subject factors on the baseline ADAS-Cog value and rate of change, respectively: both are assumed to have a zero mean Gaussian distribution with SD σb. Finally, εit is the measurement error assumed to have an independent zero mean Gaussian distribution with SD σb. Note that the square root transformation was used to obtain approximate Gaussianity of estimated error terms as well as constant variance across fitted values of the response. A similar model was fit for CDR-SB. Model (0.2) was fit by the method of restricted maximum likelihood using the nlme package in the R computing platform (www.r-project.org). In our models, we used the subject's initial MMSE as a covariate to represent baseline cognition; however, gender differences in cognitive decline remained significant when the baseline ADAS-Cog was used as a covariate in the model rather than the MMSE.
3. Results
3.1. Baseline characteristics
Baseline features of the sample are summarized in Table 1. The mean baseline age and educational level in males were statistically higher than those in females (P value for both variables = .03), but the differences were small. There are no significant differences by gender for baseline ADAS, baseline MMSE, number of follow-up visits, or follow-up length.
Table 1.
Baseline characteristics and follow-up duration of MCI subjects in ADNI-1
| Variable | Male | Female | P value |
|---|---|---|---|
| n | 257 | 141 | |
| Age | 75.32 ± 7.31 | 73.67 ± 7.45 | .03 |
| Years of education | 15.87 ± 3.04 | 15.20 ± 2.99 | .03 |
| MMSE | 26.86 ± 1.78 | 27.11 ± 1.77 | .19 |
| ADAS-Cog | 11.52 ± 4.25 | 11.49 ± 4.72 | .95 |
| Follow-up visits | 8.54 ± 4.55 | 8.05 ± 4.06 | .27 |
| Follow-up length | 4.18 ± 2.56 | 4.01 ± 2.40 | .53 |
| %APOE ε4+ | 41.24 | 41.84 | NS |
Abbreviations: MCI, mild cognitive impairment; ADNI, Alzheimer's Disease Neuroimaging Initiative; n, number of subjects; MMSE, mini-mental state examination; ADAS-Cog, Alzheimer's disease assessment scale-cognitive subscale; APOE, apolipoprotein E; NS, not significant; SD, standard deviation.
NOTE. For details of selection criteria, refer to the text. Mean and SD shown in the table. Bold P values are statistically significant.
3.2. Effect of gender on ADAS-Cog change from baseline
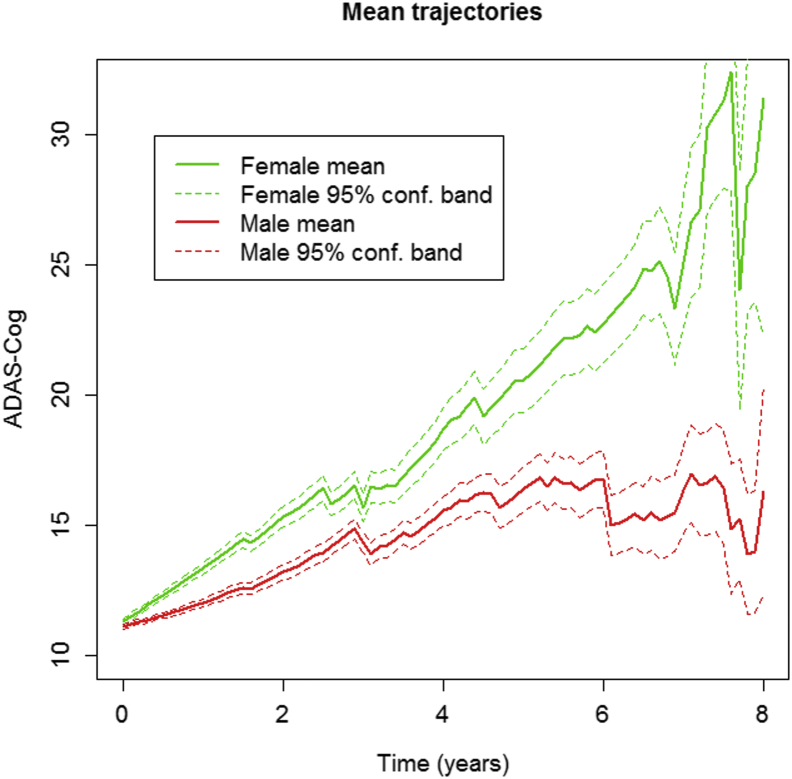
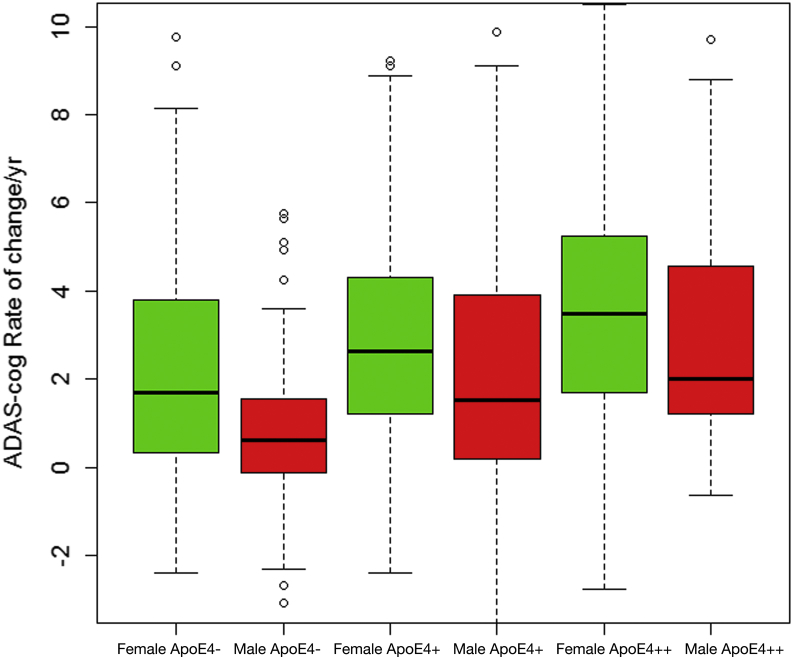
As shown in Table 2 and Fig. 1, the quadratic (curvilinear) term was significant for ADAS rate of change (P = .001). The effect of baseline cognition, APOE ε4, and years of education on ADAS-Cog change from baseline was significant, but the effect of age was not (Table 2). There was a significant effect of APOE ε4 alleles, both homozygous (carriers of two ε4 alleles) and heterozygous (one ε4 allele), for APOE ε4 on ADAS change (P < .001), and APOE ε4 influenced decline in both genders (Fig. 2). After adjusting for baseline cognition, APOE ε4, age, and education status, the effect of female gender on ADAS-Cog worsening over time was significant (P < .001). The annual change in women was 2.7 ADAS points versus 1.5 points in men (Table 2). Female gender contributed an additional 95.7% to baseline curvature. The variability of change in ADAS-Cog tended to be greater in women than in men, for both slope and curvature (Table 3).
Table 2.
Mixed-effects model of ADAS-Cog rate of change
| Term | Value | Standard error | t-value | P value |
|---|---|---|---|---|
| Baseline rate | 1.519 | 0.238 | 6.371 | <.001 |
| Baseline curvature | 0.094 | 0.028 | 3.340 | .001 |
| Female effect | 1.179 | 0.311 | 3.793 | <.001 |
| APOE ε4+ effect | 1.284 | 0.311 | 4.131 | <.001 |
| APOE ε4++ effect | 2.305 | 0.482 | 4.787 | <.001 |
| Education effect | 0.107 | 0.048 | 2.231 | .026 |
| Baseline cognition effect | −0.436 | 0.082 | −5.303 | <.001 |
| Age effect | −0.013 | 0.020 | −0.640 | .523 |
| Female effect on curvature | 0.090 | 0.040 | 2.275 | .023 |
| APOE ε4+ effect on curvature | 0.134 | 0.039 | 3.414 | .001 |
| APOE ε4++ effect on curvature | 0.276 | 0.059 | 4.646 | <.001 |
Abbreviation: ADAS-Cog, Alzheimer's disease assessment scale-cognitive subscale; APOE, apolipoprotein E.
NOTE. Values are coefficients in model 2. To avoid confusion, only the effects on rate of change, i.e. interactions with time are displayed here. Units are ADAS-Cog score change per year. Women had greater rates of decline than men. Positive changes in ADAS-Cog scores indicate worsening. Bold P values are statistically significant.
Fig. 1.
ADAS-Cog changes over time by gender. Average trajectories of ADAS-Cog scores by gender. Pointwise 95% confidence bands for the mean are based on the number of subjects at any given time point. The figure depicts time in years from baseline on the x-axis and ADAS-Cog 11 total scores on the y-axis. Increasing ADAS-Cog scores indicate worsening. Solid lines indicate mean ADAS-Cog scores, and dashed lines indicate 95% confidence intervals for these scores. The actual visit date of each subject was used (rather than pooling visits as “annual”) to give a more precise depiction of variability and progression. Abbreviation: ADAS-Cog, Alzheimer's disease assessment scale-cognitive subscale.
Fig. 2.
Median rates of ADAS-Cog change by gender and APOE ε4 status. Median rates of ADAS-Cog change per year by gender and number of APOE ε4 alleles. APOE ε4− indicates no APOE ε4 alleles, APOE ε4+ is one allele, and APOE ε4++ is two alleles. Women had higher median annual rate of change than men regardless of APOE ε4 genotype group. Abbreviation: ADAS-Cog, Alzheimer's disease assessment scale-cognitive subscale.
Table 3.
Standard deviation of subject-specific slopes and curvatures averaged by gender and APOE alleles for both ADAS-Cog and CDR-SB
| Outcome | APOE ε4 alleles | 0 | 1 | 2 |
|---|---|---|---|---|
| Slopes | ||||
| ADAS-Cog | Females | 2.38 | 3.51 | 2.26 |
| Males | 2.24 | 2.61 | 2.04 | |
| CDR-SB | Females | 0.8 | 1.09 | 1.05 |
| Males | 0.79 | 0.9 | 0.95 | |
| Curvatures | ||||
| ADAS-Cog | Females | 2.33 | 2.86 | 2.81 |
| Males | 1.32 | 1.8 | 2.5 | |
| CDR-SB | Females | 0.58 | 0.92 | 0.53 |
| Males | 0.56 | 0.56 | 0.36 | |
Abbreviations: APOE, apolipoprotein E; ADAS-Cog, Alzheimer's disease assessment scale-cognitive subscale; CDR-SB, clinical dementia rating sum of boxes.
NOTE. All numbers reported in the table are standard deviations.
3.3. Effect of gender on CDR-SB progression over time
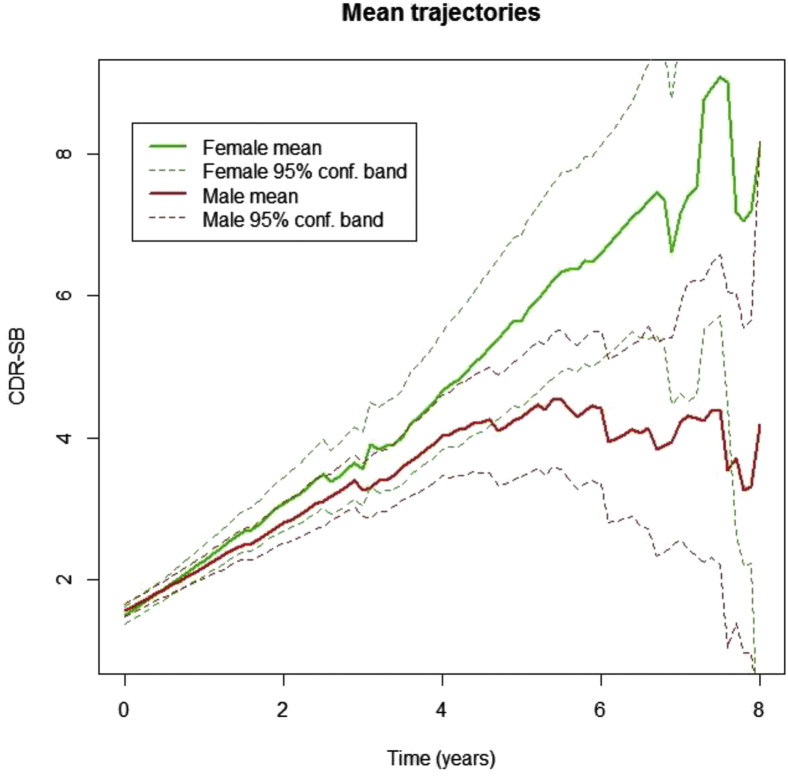
As shown in Table 4 and Fig. 3, the quadratic (curvilinear) term was significant for CDR-SB rate of change (P = .004). The effect of APOE ε4 on CDR-SB change was significant (P < .001), but the effect of age and education did not reach significance. After adjusting for these variables, the effect of female gender on CDR-SB worsening over time was significant (P = .003). The annual change in women was 0.91 CDR-SB points versus 0.59 points in men. Female gender also had a significant effect on the slope curvature (P = .004); female gender contributed an additional 143% to baseline curvature.
Table 4.
Mixed-effects model of CDR-SB rate of change over time
| Term | Value | Standard error | t-value | P value |
|---|---|---|---|---|
| Baseline rate | 0.595 | 0.085 | 7.003 | <.001 |
| Baseline curvature | 0.023 | 0.008 | 2.850 | .004 |
| Female effect | 0.325 | 0.111 | 2.934 | .003 |
| APOE ε4+ effect | 0.496 | 0.111 | 4.461 | <.001 |
| APOE ε4++ effect | 0.837 | 0.171 | 4.895 | <.001 |
| Education effect | 0.008 | 0.008 | 1.018 | .309 |
| Baseline cognition effect | 0.023 | 0.017 | 1.341 | .180 |
| Age effect | 0.010 | 0.007 | 1.331 | .183 |
| Female effect on curvature | 0.033 | 0.011 | 2.914 | .004 |
| APOE ε4+ effect on curvature | 0.017 | 0.011 | 1.486 | .138 |
| APOE ε4++ effect on curvature | 0.078 | 0.017 | 4.559 | <.001 |
Abbreviation: CDR-SB, clinical dementia rating sum of boxes; APOE, apolipoprotein E.
NOTE. Values are coefficients in model 2. For brevity, only the effects on rate of change, i.e. interactions with time are displayed here. Units are CDR-SB score units change per year. Women had greater rates of decline than men. Bold P values are statistically significant.
Fig. 3.
CDR-SB changes over time by gender. Average trajectories of CDR-SB scores by gender. Pointwise 95% confidence bands for the mean are based on the number of subjects at risk at any given time point. The figure depicts time in years from baseline on the x-axis and CDR-SB scores on the y-axis. Higher CDR-SB scores indicate worsening. Solid lines indicate mean ADAS-Cog scores, and dashed lines indicate 95% confidence intervals for these scores. The actual visit date of each subject was used (rather than pooling visits as “annual”) to give a more precise depiction of variability and progression. Abbreviations: CDR-SB, clinical dementia rating-sum of boxes; ADAS-Cog, Alzheimer's disease assessment scale-cognitive subscale.
4. Discussion
This study found a marked gender difference in longitudinal rate of change in ADAS-Cog and CDR-SB in MCI subjects and demonstrates that there is a curvilinear acceleration of rate of change over time (influenced by both gender and APOE ε4 status.) Previously, Holland et al. [6] reported a smaller gender difference in a much shorter (1 year) follow-up study of MCI subjects. We confirm and extend this to a follow-up period of up to 8 years (mean of 4 years). The baseline rate of ADAS change reported in Holland et al (0.49 points/year) was lower than that in our study—likely due to the fact that our much longer follow-up allowed us to quantify a curvilinear acceleration of change over time. The similarity of the gender effect on ADAS-Cog and CDR-SB indicates that women have a faster rate of decline in both cognitive performance and functional status. Our models adjusted for age and APOE ε4, which correlate substantially with risk for amyloid positive status. Gender differences are present in APOE ε4 carriers and noncarriers; however, gender effects appear to be greatest in E4 homozygotes, a group at greatest risk for conversion. Overall, these data confirm and extend prior findings [3], [4], [5], [6] that women with MCI may have a greater vulnerability for cognitive and functional decline.
The strengths of our study include its use of a relatively large baseline sample size (398 MCI subjects recruited nationally), the employment of specific clinical criteria for amnestic MCI with standardized data collection across multiple sites, and relatively long (mean, 4 years) follow-up duration. We focused primarily on MCI subjects originally recruited in ADNI-1 because they had a more traditional form of MCI (i.e. late MCI) whose memory criteria are well established. We did not include any subjects newly recruited in ADNI-Go or ADNI-2 with early MCI because they lacked sufficient long-term follow-up. One potential limitation is that ADNI MCI subjects are not necessarily representative of the population as a whole and as such are more representative of MCI subjects recruited at research centers or those who have been enrolled into secondary prevention trials. Although our mean follow-up time of 4 years is longer than that of others addressing this question, it may still not have been long enough to conclusively test for gender differences in rates of conversion to dementia. The rate of conversion from MCI to dementia was slightly higher among women than men but this was not statistically significant. Subjects who progressed to AD dementia were not excluded. All data points on subjects who entered the study as MCI in ADNI-1 were analyzed.
Although ADNI classified subject visits as “annual” (e.g. year 1, 2, and so forth), there was considerable variability in actual visit dates. AD does not progress in fixed annual chunks but there is a slow steady progression that is somewhat variable for each subject. Therefore, in our analyses, we used the actual date of the follow-up visit for each subject to give a more precise statistical measure of progression and variability (due to drop outs or fluctuations) over time. This is one reason why our figures show dips and peaks (e.g. around year 6). Attrition biases are possible, but the baseline cognitive status, follow-up period, and number of follow-up visits were similar between males and females. Finally, our study cannot determine causality or pathologic mechanisms because we did not look at other biomarkers or genes aside from APOE ε4. In this regard, it should be noted that amyloid positron emission tomography (PET) and cerebrospinal fluid (CSF) data are only available in a small fraction of ADNI-1 subjects.
Several different factors could underlie possible gender differences in rates of cognitive progression, including genetic, lifestyle, hormonal, psychological, and neurobiological. We found a gender effect after adjusting for the APOE ε4 effect, suggesting that additional factors may be at play. Some reports have noted an increased effect of APOE ε4 in women; for example, in women, APOE ε4 was reported to result in greater conversion risk [11], altered default mode connectivity [12], greater atrophy of hippocampus [30], and greater decrements on delayed word recall [30]. In contrast, Holland et al. [6] did not report a gender by APOE interaction on brain atrophy. The greater variability of ADAS-Cog and CDR-SB rates of decline in women suggests there could be potential undiscovered genetic causes. In this regard, it is of interest that the Met66 allele of BDNF gene, which reduces the transport of BDNF, as well as the 219K allele of the ATP Binding Cassette Transporter 1 gene have both been linked to increased risk for AD in women [1].
Further analysis should include other genetic factors besides APOE ε4 that may be significant to cognitive decline, and ADNI genetic data are now becoming available. Likewise, in a few years, we will have access to at least 4 years of ADAS and CDR data from ADNI-2 MCI subjects for our analysis to be replicated, and it should be possible to correlate gender differences in cognitive progression with various biomarkers such as amyloid deposition, cognitive reserve, and brain atrophy rates; ADNI-2 subjects receive MRI scans, florbetapir PET scans, CSF studies, and resting state functional MRI. This is highly relevant in light of a prior study which noted that women may be at much greater risk than men for cognitive decline and dementia diagnosis for every one unit of increase in global brain pathology [5]. These data raise the hypothesis that there may be gender differences in cognitive reserve (with men having higher reserve).
Our findings also have relevance for clinical treatment/prevention trials, including Dominant Inherited Alzheimer Network Treatment Units, Alzheimer's Prevention Initiative, and the Amyloid Lowering Trial in Asymptomatic Individuals (A4 trial). Despite many candidate drugs being in trials, the causes of AD are not fully known, and uncovering mechanisms underlying gender differences in cognitive progression may yield additional new treatment targets or nonpharmacologic strategies for risk modification and allowing a more personalized intervention. For example, trials of anti-amyloid therapeutics have revealed a greater vulnerability for cerebral adverse events such as amyloid-related imaging abnormalities in E4 carriers and many ongoing studies stratify enrollment by E4 and also use differential treatment dosing by E4. In a similar vein, our findings support a prior call for AD prevention trials to deliberately stratify by sex and have adequate sample size to test for a therapeutic risk-benefit in men and women separately [1]. One could conceivably also have separate thresholds for efficacy. In addition, differences in variability in the rate of change by gender suggest that unequal numbers of males and females may be required to measure the same effect size. Furthermore, the curvilinear acceleration noted in this study suggests that usual statistical approach in trials of linear models may not be optimal to model MCI disease progression over longer periods. The CDR-SB has been suggested as an acceptable single cognitive/functional end point for MCI trials, and our data suggest that lower baseline MMSE, female gender, and APOE ε4 are predictors of faster decline, and sample sizes for various specific effect sizes can be computed using the data. To our knowledge, no prior study has modeled long-term changes in cognition and function in MCI, as well as the effects of covariates, so comprehensively.
5. Conclusions
In conclusion, our results show a robust gender difference in the rate of change in ADAS-Cog and CDR-SB in MCI subjects, with women declining at much higher rates than men. These findings support prior calls [1], [3], [4], [5], [10], [11], [12], [36] to make gender-specific research in AD a priority.
Research in context.
-
1.
Systematic review: The authors reviewed the literature using traditional sources (PubMed, conference abstracts). Women have a higher prevalence of Alzheimer's than men but the reasons are not fully understood. Several recent publications have investigated gender differences in Alzheimer's risk and pathophysiology, and these are cited appropriately.
-
2.
Interpretation: Women with mild cognitive impairment have significantly greater longitudinal rates of cognitive and functional decline than men. These findings confirm and extend prior reports on gender differences in neurobiological risk for Alzheimer's.
-
3.
Future directions: Because the Alzheimer's disease assessment scale and clinical dementia rating are used as outcomes in secondary prevention trials, gender differences should be considered in the design and interpretation of such studies. Further studies to replicate and elucidate underlying gender-specific AD genetic and biomarker mechanisms are warranted.
Acknowledgments
K.A.L. and this publication were supported by the Karen L Wrenn Family trust through the Wrenn Clinical Research Scholars program gift to Duke University Medical Center. Data used in preparation of this article were obtained from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf.
The Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (U.S. Department of Defense award number W81XWH-12-2-0012) funded data collection and sharing for this project. ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Alzheimer's Association; Alzheimer's Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen Idec Inc.; Bristol-Myers Squibb Company; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; Eurolmmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; GE Healthcare; Innogenetics, N.V.; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Medpace, Inc.; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Synarc Inc.; and Takeda Pharmaceutical Company. ADNI clinical sites in Canada have been supported by funding from the Canadian Institutes of Health Research. P.M.D. received a grant from ADNI to support data collection for this study.
Footnotes
P.M.D. and J.R.P. have received research grants and/or advisory fees from several government agencies, advocacy groups, and pharmaceutical/imaging companies. P.M.D. owns stock in Maxwell, Adverse Events, and Muses Labs whose products are not discussed here.
References
- 1.Mielke M.M., Vemuri P., Rocca W.A. Clinical epidemiology of Alzheimer's disease: Assessing sex and gender differences. Clin Epidemiol. 2014;6:37–48. doi: 10.2147/CLEP.S37929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hebert L.E., Weuve J., Scherr P.A., Evans D.A. Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology. 2013;80:1778–1783. doi: 10.1212/WNL.0b013e31828726f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seshadri S., Wolf P.A., Beiser A., Au R., McNulty K., White R. Lifetime risk of dementia and Alzheimer's disease. The impact of mortality on risk estimates in the Framingham Study. Neurology. 1997;49:1498–1504. doi: 10.1212/wnl.49.6.1498. [DOI] [PubMed] [Google Scholar]
- 4.Hua X., Hibar D.P., Lee S., Toga A.W., Jack C.R., Jr., Weiner M.W. Sex and age differences in atrophic rates: An ADNI study with n1368 MRI scans. Neurobiol Aging. 2010;31:1463–1480. doi: 10.1016/j.neurobiolaging.2010.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnes L.L., Wilson R.S., Bienias J.L., Schneider J.A., Evans D.A., Bennett D.A. Sex differences in the clinical manifestations of Alzheimer disease pathology. Arch Gen Psychiatry. 2005;62:685–691. doi: 10.1001/archpsyc.62.6.685. [DOI] [PubMed] [Google Scholar]
- 6.Holland D., Desikan R.S., Dale A.M., McEvoy L.K. Higher rates of decline for women and apolipoprotein E4 carriers. AJNR Am J Neuroradiol. 2013;34:2287–2293. doi: 10.3174/ajnr.A3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farrer L.A., Cupples L.A., Haines J.L., Hyman B., Kukull W.A., Mayeux R. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: A meta-analysis: APOE and Alzheimer disease meta analysis consortium. JAMA. 1997;278:1349–1356. [PubMed] [Google Scholar]
- 8.Andersen K., Launer L.J., Dewey M.E., Letenneur L., Ott A., Copeland J.R. Gender differences in the incidence of AD and vascular dementia: The EURODEM Studies: EURODEM Incidence Research Group. Neurology. 1999;53:1992–1997. doi: 10.1212/wnl.53.9.1992. [DOI] [PubMed] [Google Scholar]
- 9.Plassman B.L., Langa K.M., McCammon R.J., Fisher G.G., Potter G.G., Burke J.R. Incidence of dementia and cognitive impairment, not dementia in the United States. Ann Neurol. 2011;70:418–426. doi: 10.1002/ana.22362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Irvine K., Laws K.R., Gale T.M., Kondel T.K. Greater cognitive deterioration in women than men with Alzheimer's disease: A meta analysis. J Clin Exp Neuropsychol. 2012;34:989–998. doi: 10.1080/13803395.2012.712676. [DOI] [PubMed] [Google Scholar]
- 11.Altmann A., Tian L., Henderson V.W., Greicius M.D. Sex modifies the APOE-related risk of developing Alzheimer's disease. Ann Neurol. 2014;75:563–573. doi: 10.1002/ana.24135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Damolseaux J.S., Seeley W.W., Zhou J., Shirer W.R., Coppola G., Karydas A. Gender modulates the APOE4 effect in healthy older adults: Convergent evidence from functional brain connectivity and spinal fluid tau levels. J Neurosci. 2012;32:8254–8262. doi: 10.1523/JNEUROSCI.0305-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spremo-Potparevic B., Zivkovic L., Djelic N., Bajic V. Analysis of premature centromere division (PCD) of the X chromosome in Alzheimer patients through the cell cycle. Exp Gerontol. 2004;39:849–854. doi: 10.1016/j.exger.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 14.Bajic V., Spremo-Potparevic B., Zivkovic L., Siedlak S.L., Casadeus G., Smith M.A. The X-chromosome instability phenotype in Alzheimer's disease: A clinical sign of accelerating aging? Med Hypotheses. 2009;73:917–920. doi: 10.1016/j.mehy.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrari R., Dawoodi S., Raju M., Thumma A., Hynan L.S., Maasumi S.H. Androgen receptor gene and gender specific Alzheimer's disease. Neurobiol Aging. 2013;34:2077.e19–2077.e20. doi: 10.1016/j.neurobiolaging.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scacchi R., Gambina G., Broggio E., Corbo R.M. Sex and ESR1 genotype may influence the response to treatment with donepezil and rivastigmine in patients with Alzheimer's disease. Int J Geriatr Psychiatry. 2014;29:610–615. doi: 10.1002/gps.4043. [DOI] [PubMed] [Google Scholar]
- 17.Brown C., Choi E., Xu Q., Vitek M.P., Colton C.A. The ApoE4 genotype alters the response of microglia and macrophages to 17B-estradiol. Neurobiol Aging. 2008;29:1783–1794. doi: 10.1016/j.neurobiolaging.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hebert L.E., Scherr P.A., McCann J.J., Beckett L.A., Evans D.A. Is the risk of developing Alzheimer's disease greater for women than for men? Am J Epidemiol. 2001;153:132–136. doi: 10.1093/aje/153.2.132. [DOI] [PubMed] [Google Scholar]
- 19.Henderson V.W. The epidemiology of estrogen replacement therapy and Alzheimer's disease. Neurology. 2007;48(5 Suppl 7):S27–S35. doi: 10.1212/wnl.48.5_suppl_7.27s. [DOI] [PubMed] [Google Scholar]
- 20.Jamshed N., Ozair F.F., Aggarwal P., Ekka M. Alzheimer disease in post-menopausal women: Intervene in the critical window period. J Midlife Health. 2014;5:38–40. doi: 10.4103/0976-7800.127791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janicki S.C., Park N., Cheng R., Lee J.H., Schupf N., Clark L.N. Estrogen receptor B variants modify risk for Alzheimer's disease in a multiethnic female cohort. J Alzheimers Dis. 2014;40:83–93. doi: 10.3233/JAD-130551. [DOI] [PubMed] [Google Scholar]
- 22.Lan Y.L., Zhao J., Li S. Update on the neuroprotective effect of estrogen receptor alpha against Alzheimer's disease. J Alzheimers Dis. 2015;43:1137–1148. doi: 10.3233/JAD-141875. [DOI] [PubMed] [Google Scholar]
- 23.Paganini-Hill A., Henderson V.W. Estrogen deficiency and risk of Alzheimer's disease in women. Am J Epidemiol. 1994;140:256–261. doi: 10.1093/oxfordjournals.aje.a117244. [DOI] [PubMed] [Google Scholar]
- 24.Skup M., Zhu H., Wang Y., Giovanello K.S., Lin J.A., Shen D. Sex differences in grey matter atrophy patterns among AD and aMCI patients: Results from ADNI. Neuroimage. 2011;56:890–906. doi: 10.1016/j.neuroimage.2011.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gibbs R.B., Aggarwal P. Estrogen and basal forebrain cholinergic neurons: implications for brain aging and Alzheimer's disease-related cognitive decline. Horm Behav. 1998;34:98–111. doi: 10.1006/hbeh.1998.1451. [DOI] [PubMed] [Google Scholar]
- 26.Yaffe K., Haan M., Byers A., Tangen C., Kuller L. Estrogen use, APOE, and cognitive decline: Evidence of gene-environment interaction. Neurology. 2000;54:1949–1953. doi: 10.1212/wnl.54.10.1949. [DOI] [PubMed] [Google Scholar]
- 27.Butler H.T., Warden D.R., Hogervorst E., Ragoussis J., Smith A.D., Lehmann D.J. Association of the aromatase gene with Alzheimer's disease in women. Neurosci Lett. 2010;468:202–206. doi: 10.1016/j.neulet.2009.10.089. [DOI] [PubMed] [Google Scholar]
- 28.Payami H., Zareparsi S., Montee K.R., Sexton G.J., Kaye J.A., Bird T.D. Gender difference in apolipoprotein E-associated risk for familial Alzheimer disease: a possible clue to the higher incidence of Alzheimer disease in women. Am J Hum Genet. 1996;58:803–811. [PMC free article] [PubMed] [Google Scholar]
- 29.Bretsky P.M., Buckwalter J.G., Seeman T.E., Miller C.A., Poirer J., Schellenberg G.D. Evidence for an interaction between apolipoprotein E genotype, gender, and Alzheimer disease. Alzheimer Dis Assoc Disord. 1999;13:216–221. doi: 10.1097/00002093-199910000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Fleisher A., Grundman M., Jack C.R., Jr., Peterson R.C., Taylor C., Kim H.T., Alzheimer’s Disease Cooperative Study Sex, apolipoprotein E epsilon 4 status, and hippocampal volume in mild cognitive impairment. Arch Neurol. 2005;62:953–957. doi: 10.1001/archneur.62.6.953. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y., Paajanen T., Westman E., Wahlund L.O., Simmons A., Tunnard C., AddNeuroMed Consortium Effect of APOE ε4 allele on cortical thicknesses and volumes: The AddNeuroMed study. J Alzheimers Dis. 2010;21:947–966. doi: 10.3233/JAD-2010-100201. [DOI] [PubMed] [Google Scholar]
- 32.Ruitenberg A., Ott A., vanSweiten J., Hofman A., Breteler M. Incidence of dementia: Does gender make a difference? Neurobiol Aging. 2001;22:575–580. doi: 10.1016/s0197-4580(01)00231-7. [DOI] [PubMed] [Google Scholar]
- 33.ADNI procedures manual. Available at: http://adni.loni.usc.edu/wp-content/uploads/2010/09/ADNI_GeneralProceduresManual.pdf. Accessed January 30, 2015.
- 34.Alzheimer's Disease Neuroimaging Initiative - Study Documents. Available at: http://adni.loni.usc.edu/methods/documents/. Accessed January 30, 2015.
- 35.Williams M.M., Storandt M., Roe C.M., Morris J.C. Progression of Alzheimer disease as measured by clinical dementia rating sum of boxes scores. Alzheimers Dement. 2013;9(1 Suppl):S39–S44. doi: 10.1016/j.jalz.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin K.A., Doraiswamy P.M. When Mars versus Venus is not a cliché: Gender differences in the neurobiology of Alzheimer's disease. Front Neurol. 2015;5:288. doi: 10.3389/fneur.2014.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]