Abstract
Recovery from severe immunosuppression requires hematopoietic stem cell reconstitution and effective thymopoiesis to restore a functional immune cell repertoire. Herein, a model of immune cell reconstitution consequent to potentially lethal doses of irradiation is described which may be valuable in evaluating potential medical countermeasures. Male rhesus macaques were total body irradiated by exposure to 6.00 Gy 250 kVp x-radiation (midline tissue dose, 0.13 Gy min-1) resulting in an approximate LD10/60 (n = 5/59). Animals received medical management and hematopoietic and immune cell recovery was assessed (n ≤ 14) through 370 days post exposure. A subset of animals (n ≤ 8) was examined through 700 days. Myeloid recovery was assessed by neutrophil and platelet-related parameters. Lymphoid recovery was assessed by the absolute lymphocyte count and FACS-based phenotyping of B- and T-cell subsets. Recent thymic emigrants were identified by T cell receptor excision circle quantification. Severe neutropenia, lymphopenia and thrombocytopenia resolved within 30 days. Total CD3+ cells μL-1 required 60 days to reach values 60% of normal, followed by subsequent slow recovery to approximately normal by 180 days post irradiation. Recovery of CD3+4+ and CD3+8+ cell memory and naïve subsets were markedly different. Memory populations were ≥ 100% of normal by day 60; whereas, naïve populations were only 57% normal at 180 days and never fully recovered to baseline post irradiation. Total (CD20+) B cells μL-1 were within normal levels by 77 days post exposure. This animal model elucidates the variable T- and B-cell subset recovery kinetics after a potentially lethal dose of total-body irradiation that are dependent on marrow-derived stem and progenitor cell recovery, peripheral homeostatic expansion and thymopoiesis.
Keywords: whole body irradiation, x rays, laboratory animals, blood
INTRODUCTION
Immune reconstitution following cytotoxic therapy, conditioning for stem cell transplant and potentially lethal doses of radiation in the accident or terrorist scenario remains a serious challenge. The significant delay in regeneration of CD4+ T cells, marked imbalance in the CD4/CD8 ratio and limited T cell repertoire leave the patient at risk for infectious complications, viral disease and compromised ability to mount an effective immune response to vaccines.
Thymopoiesis is dependent on continuous seeding of bone marrow-derived hematopoietic stem cells (HSC) and/or early T-lineage progenitors (ETP) into a functional thymic niche. Regeneration of the B cell compartment relies upon recovery of the HSC and B cell lineage specific hematopoietic progenitor cells (HPC) within the respective bone marrow niche (LeBien et al. 2008). The prolonged kinetics associated with long-term immune reconstitution, particularly the T cell repertoire, reflects the requisite regeneration of hematopoietic stem cells (HSC) to a threshold level compatible with long-term survival and definitive hematopoietic recovery of functional neutrophils and platelets. Recently defined assays for assessing naïve T-cell subsets and bone marrow-derived output of naïve B-cells may aid in further definition of the recovery kinetics for these two cellular subsets (Haines et al. 2009; Kohler et al. 2009; Mensen et al. 2013; Sottini et al. 2010).
The ultimate goal is to define an “optimum” therapeutic protocol for treatment of the hematopoietic syndrome in severely irradiated personnel following a nuclear terrorist event. The use of leucocyte growth factors and medical management will likely enhance survival through recovery of hematopoietic progenitor cells and increased production of neutrophils (Monroy et al. 1988; Schuening et al. 1993; MacVittie et al. 2005; Farese et al. 2013; Farese et al. 2012b; Dainiak et al. 2011; Plett et al. 2012; Herodin F et al. 2007; Yu et al. 2011; Armstrong et al. 2012; Hankey et al. 2015; Amgen 2015). However, there have been no studies that suggest stem cells and associated immune reconstitution are affected through the use of leukocyte growth factors. Furthermore, there are no medical countermeasures (MCM) available to mitigate the prolonged T cell deficiencies or the severe depletion of hematopoietic stem cells required for effective thymopoiesis.
The lack of relevant large animal models of long-term immune cell recovery hinders the ability to assess efficacy of MCM that may stimulate HSC renewal and immune reconstitution. A nonhuman primate model has been described that used partial-body irradiation of significantly higher doses in an effort to link multiple organ injury (MOI) and delayed immune cell recovery (MacVittie et al. 2012; MacVittie et al. 2014). The use of low-lethal total body irradiation (TBI) with administration of medical management will provide a relevant model of hematopoietic myelosuppression and long-term immune suppression without confounding, overt MOI to assess the treatment efficacy of candidate MCM to enhance recovery of a functional immune system.
Methods
Animal selection, husbandry and care
Male, young, sexually mature, rhesus macaques (n = 14), Macaca mulatta, [4.7 ± 0.2 kg body weight (bw)] were in good health, sero-negative for simian immunodeficiency virus, simian T cell leukemia virus type 1, and herpes B virus, and tuberculosis. The animals described herein served as irradiation controls for several experiments over a period of 5 years. The in-life phase of the various experiments ranged between 370 days to 700 days, therefore data for all parameters was not collected for the entire control population at every time point through 700 days. A pool of donor nonhuman primate(s) (NHP) (bw ≥ 7 kg) was used for blood donation. All animal procedures performed and blood volumes withdrawn were detailed in a study protocol approved by the Institutional Animal Care and Use Committee. Animal husbandry and care has been previously described in detail (Farese et al. 2012b). Briefly, animal housing and care was performed in accordance with the Animal Welfare Act (7 U.S.C. 2131 et. seq.) and the Guide for the Care and Use of Laboratory (Committee for the Update of the Guide for the Care and Use of Laboratory Animals 2011).
Radiation
Radiation Exposure rationale for radiation dose
This is a well-codified model of 6.00 Gray (Gy) total-body x-radiation-induced myelo- and immuno- suppression in the rhesus macaque (Eldred et al. 1954; Stanley et al. 1966; Henschke et al. 1957; Haigh et al. 1956; Schlumberger et al. 1954; Allen et al. 1966; Farese et al. 2012b; MacVittie et al. 2000). The rationale for the radiation dose was previously described (Farese et al. 2012b). Briefly, the radiation dose selected was based on published studies that determined the lethality DRR for male rhesus macaques exposed to uniform, bilateral exposure to 250 kVp x-radiation with or without benefit of medical management (Farese et al. 2012b; Neelis et al. 1997; MacVittie et al. 2015).
Radiation exposure and dosimetry
The x-ray machine was calibrated in the geometry used for NHP irradiation. Depth dose measurements were made at the center of a cylindrical phantom that approximates the mean diameter of the experimental rhesus macaque. Three phantoms of increasing size were available. The phantom was made of 0.32 cm Lucite and filled with water. Dose measurements were performed with ion chambers. TBI was delivered to each NHP with a 250 kVp x-radiation (1.5 mm Cu HVL; 1 mm Al, 0.5 mm Cu) at 0.13 Gy min-1 in the posterior-anterior position, rotated 180° at mid-dose (3.00 Gy) to the anterior-posterior position for completion of the total 6.00 Gy midline tissue dose (MLTD) exposure. Dosimetry was performed using paired 0.5 cm3 ionization chambers, with calibration factors traceable to the National Institute of Standards and Technology.
Animal Irradiation Procedures
Healthy animals previously acclimated to a Lucite chair restraint were selected for the study. Approximately 18 hours prior to exposure, food but not water was removed from each NHP. On the day of irradiation, NHP were administered an antiemetic, Zofran (GlaxoSmithKline, Durham, NC 27713) or Ondansetron (Hospira, Inc., Lake Forest, IL 60045) [1-2 mg kg-1 intravenous (IV) or intramuscular (IM)], 45-90 minutes prior to and following irradiation exposure. Following transport to the x-radiation facility at the University of Maryland, Department of Radiation Oncology the sedated NHP was secured in the restraint chair and allowed to awaken prior to TBI. An in-room camera permitted observation of the NHP during the irradiation procedure. The transport procedure was reversed when TBI was completed. The NHP were administered Lactated Ringer’s Solution (LRS) (10-15 mL kg-1 IV), returned to their home cage and recovery from sedation was monitored.
Medical Management
Antibiotics, fresh irradiated whole blood and fluids were administered as previously described (Farese et al. 2012b). Some animals (n = 5) were administered Rocephin (Roche Laboratories Inc., Nutley, NJ 07110) (50 mg kg-1 IM, QD) or Claforan (Aventis Pharmaceuticals, NJ 08807) [50 mg kg-1 IM, BID (twice daily)] in lieu of gentamicin. Zofran or Ondansetron [(0.1-0.2 mg kg-1 IV or IM, QD or BID] was administered when evidence of emesis was observed. Antidiarrheal treatment was administered if diarrhea was observed as previously described (Farese et al. 2012a). All animals were provided additional fluid support ad libitum (1 L bottles attached to the front of the cage) in the form of a commercial hydration fluid (Shaklee Corp., Pleasanton, CA 9458).
Clinical Observations
Hematologic Evaluations
Serial hematopoietic evaluations were performed on rhesus macaques (n=12 to 14 on days 0 through 370, n = 4 to 8 on days 400 through 550, n = 3 on days 580 through 700). Peripheral blood was obtained by venipuncture at selected time points from the saphenous vein to assay complete blood count (CBC) (Sysmex K-4500, Long Grove, IL, 60047 or Beckman Coulter Ac·T diff, Beckman Coulter, Inc., Miami, FL, 33196) including manual WBC differential performed on blood film (Hematek II™, Bayer Corp., Diagnostic Division, Tarrytown, NY, 10591) stained with Wright-Giemsa using a stain pack (Fisher Scientific, Pittsburgh, PA, 16275).
Flow Cytometric Studies
Rhesus macaque lymphocyte subset analysis was performed on EDTA-anticoagulated, peripheral blood [(n ≤ 14), Figs. 3-6, Tables 2-3]. The monoclonal antibodies and isotype controls, blood processing method, and cell acquisition and analysis have been previously described (MacVittie et al. 2014). Briefly, a whole blood lysing method recommended by BD Biosciences was used (BD Biosciences, 2350 Qume Drive, San Jose, CA 95131) and samples were acquired on a Becton Dickinson FACSCalibur™ flow cytometer and analyzed using CellQuest Pro software (BD Biosciences). The standard panels of antibodies used to differentiate T and B or NK cells were CD3/CD20/CD8/CD4 and CD3/CD8/CD16, respectively. Due to the lack of cross-reactivity between the available human CD45RO monoclonal antibodies (mAb) with rhesus cells, CD45RA and CD62L mAb were used to differentiate between naïve and memory T cell subsets. Because there is evidence that CD45RO+ memory cells can re-acquire CD45RA without losing CD45RO (Arlettaz et al. 1999), T cell naïve or memory phenotyping herein was designated as CD45RA+/CD62L+/CD4 or CD8 and CD45RA-/CD62L+/CD4 or CD8, respectively. The normal, baseline (BL) values for these subsets in rhesus macaques were similar to that seen in normal humans. Another consideration in developing reliable phenotypic definitions of T cell subsets in the macaque was that macrophages can express CD4 and natural killer cells can express CD8 (Carter et al. 1999). Therefore, only cells positive for CD3 in addition to CD4 or CD8 were designated as T cells. Additionally, because antiCD8 coated microbeads were used to separate the CD8+ cells for T cell receptor excision circle (TREC) analysis this sample also contained NK cells. Therefore, NK cells (CD3-CD8+CD16+) were identified by flow cytometric methods. This value was subtracted from the reported CD8+ cells μL-1 and used to determine the number of TREC+ CD8+ T cells μL-1.
Figure 3.
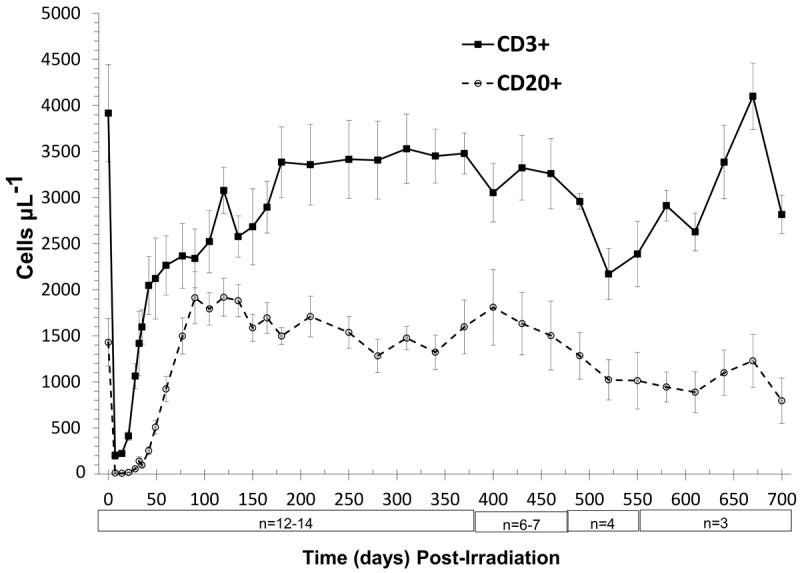
Time course of peripheral T- and B-lymphocyte loss and recovery in peripheral blood of rhesus macaques following 6.00 Gy total body x-irradiation (mean values ± standard error). Samples were obtained on selected days (n = 12-14 between days 0 to 370, n = 6-7 between days 385 to 460, n = 3-4 between days 475 to 700) following TBI. Whole blood was stained with fluorescently tagged antiCD3, antiCD20 antibodies, red blood cells were lysed, then T- and B-lymphocytes were identified using a flow cytometer, quantified and presented as cells μL-1.
Figure 6.
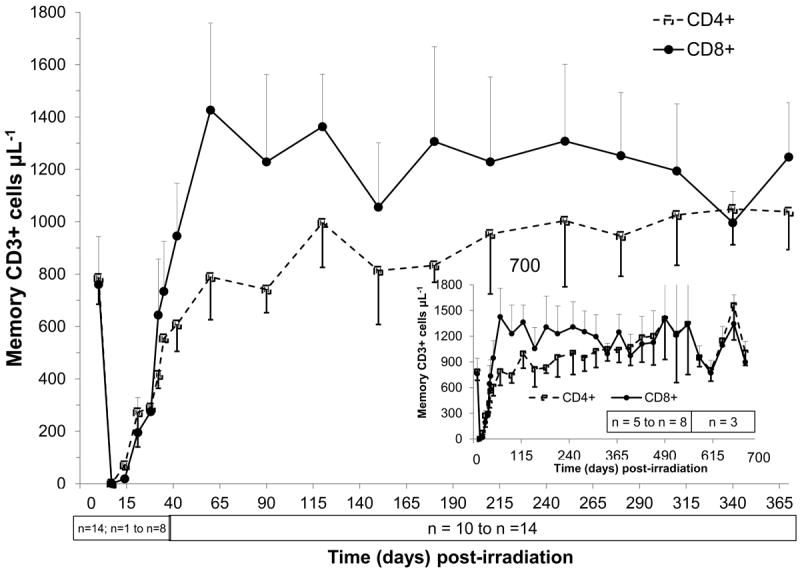
Time course of peripheral blood memory T-cell recovery in rhesus macaques following 6.00 Gy total-body x-irradiation (mean values ± standard error). Samples were obtained on selected days (n = 14 on day 0, n = 1-8 day 7 to day 35, n = 10-14 day 42 to day 370, n = 5-8 on day 400 to day 550, n = 3 on day 580 to day 700) following TBI. Whole blood was stained with fluorescently tagged antiCD3, antiCD45RA, antiCD62L and either antiCD4 or antiCD8 antibodies; red blood cells were lysed, and T cell memory subsets (CD3+CD4+CD45RA-CD62L+ cells μL-1 or CD3+CD8+CD45RA-CD62L+ cells μL-1) were identified using a flow cytometer, quantified and plotted.
Table 2. Radiation-induced B and T cell loss and recovery in rhesus macaques exposed to x-irradiation.
Animals were total body x-irradiated at 6.00 Gy. Baseline and nadir absolute CD20+ CD3+, CD3+4+ and CD3+8+ cells μL-1 [mean values, standard error (SEM)] are enumerated and the observed days of nadir are presented. The recovery points over approximately 2 years post-TBI (days 60, 120, 180, 370, 700) for each cell type are presented as %BL values.
| Cell Type | Baseline a (cells μL-1) | Observed Nadir (cells μL-1) (day) | Days Post-Irradiation | |||||
|---|---|---|---|---|---|---|---|---|
| 60 b (%BL) | 120 a (%BL) | 180 c (%BL) | 370 c (%BL) | 700 d (%BL) | ||||
| B Cell (CD20+) | 1430 ± 259 | 8 ± 2 b (d14) | 64.5 | 134.2 | 104.8 | 111.7 | 55.6 | |
| T cell (CD3+) | 3916 ± 528 | 202 ± 43 b (d7) | 57.8 | 78.6 | 86.4 | 88.8 | 71.9 | |
| CD3+4+ | 2165 ± 283 | 93 ±12 b (d7) | 38.9 | 69.5 | 72.4 | 83.2 | 71.2 | |
| CD3+8+ | 1858 ± 270 | 102 ± 19 c (d14) | 88.2 | 106.1 | 111.4 | 106.8 | 102.1 | |
| CD3+4+:CD3+8+ Ratio | 1.2 | 0.4/0.5 (d32e, d42 a) | 0.5 | 0.8 | 0.8 | 0.9 | 0.8 | |
n=14.
n=12.
n=13.
n=3.
n=4.
Table 3. T-cell memory and naive subset loss and recovery in the peripheral blood of total body x-irradiated rhesus macaques.
Rhesus macaques were total body x-irradiated at 6.00 Gy. Absolute cell numbers [mean values, standard error (SEM)] and percent of baseline (BL) values for naïve and memory CD3+, CD3+4+ and CD3+CD8+ populations from rhesus macaque peripheral blood are enumerated. Number of animals, cells counts and % BL values through 700 days post irradiation for each T cell subset are presented.
| Day (n) | Total CD3+ | CD3+4+ | CD3+8+ | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Naïve | Memory | Naïve | Memory | Naïve | Memory | |||||||
| cells μL-1 | % BL | cells μL-1 | % BL | cells μL-1 | % BL | cells μL-1 | % BL | cells μL-1 | % BL | cells μL-1 | % BL | |
| BL (n=14) | 2152±374 | 100 | 1652±244 | 100 | 1253±215 | 100 | 785±101 | 100 | 746±142 | 100 | 760±142 | 100 |
|
| ||||||||||||
| 60 (n=12) | 155±24 | 7 | 2224±356 | 135 | 74±15 | 6 | 789±163 | 100 | 55±10 | 7 | 1427±333 | 188 |
|
| ||||||||||||
| 90 (n=14) | 378±74 | 18 | 2040±356 | 123 | 206±50 | 16 | 741±88 | 94 | 125±22 | 17 | 1229±22 | 162 |
|
| ||||||||||||
| 120 (n=14) | 779±167 | 36 | 2354±240 | 143 | 451±110 | 36 | 996±170 | 127 | 277±64 | 37 | 1363±64 | 179 |
|
| ||||||||||||
| 150 (n=14) | 887±175 | 41 | 1779±303 | 108 | 545±119 | 43 | 813±205 | 104 | 287±53 | 38 | 1056±53 | 139 |
|
| ||||||||||||
| 180 (n=13) | 1294±196 | 60 | 2254±368 | 136 | 712±123 | 57 | 833±65 | 106 | 425±59 | 57 | 1306±59 | 172 |
|
| ||||||||||||
| 280 (n=14) | 1350±260 | 63 | 2190±268 | 133 | 742±150 | 59 | 946±154 | 120 | 528±111 | 71 | 1252±111 | 165 |
|
| ||||||||||||
| 370 (n=13) | 1362±234 | 63 | 2218±194 | 134 | 774±89 | 62 | 1038±145 | 132 | 518±81 | 69 | 1247±81 | 164 |
|
| ||||||||||||
| 550 (n=5) | 830±191 | 39 | 2159±776 | 131 | 494±150 | 39 | 1340±588 | 171 | 223±150 | 30 | 1344±150 | 177 |
|
| ||||||||||||
| 700 (n=3) | 832±156 | 39 | 1952±184 | 118 | 480±41 | 38 | 1005±147 | 128 | 255±13 | 34 | 897±13 | 118 |
T cell Receptor Excision Circle (TREC) quantitation in lymphocyte subsets
Peripheral blood collection
Peripheral blood (7 to 10 mLs) was obtained from rhesus macaques (n ≤ 12) by venipuncture and drawn into a syringe containing K2-EDTA anticoagulant. During the in life phase of the study, an animal’s absolute lymphocyte count (ALC) was required to be approximately 2,000 cells μL-1 in order to yield an adequate numbers of T cells to process for TREC content within the approved blood volume. Therefore, TREC analysis was not performed on all animals during the early time points post-irradiation (Fig. 7). Additionally, only a few animals [(n ≤ 5), Fig. 7] were observed between days 400 through 700.
Figure 7.
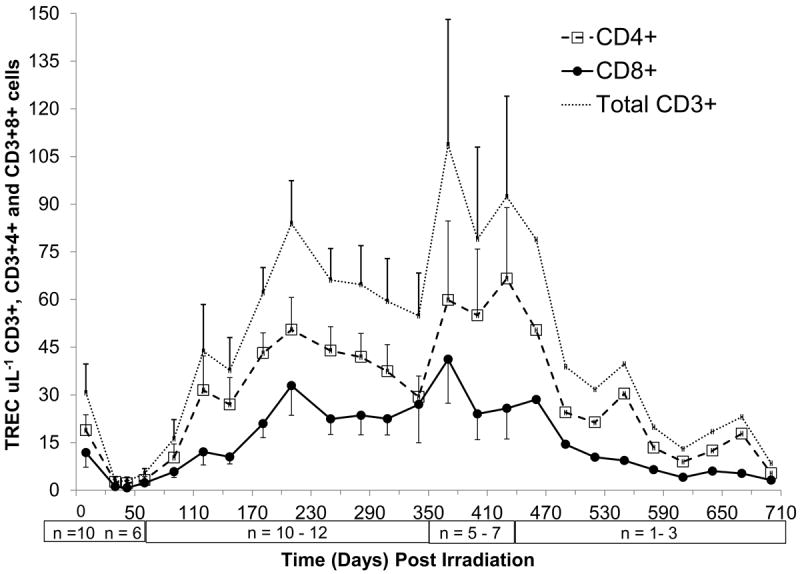
Time course of peripheral blood T cell receptor excisions circles (TREC) + recovery in rhesus macaques following 6.00 Gy total-body x-irradiation (mean values ± standard error). Samples were obtained on selected days (n = 9 on day 0, n = 6-10 on days 30 to 90, n = 11-12 on days 120 to 340, n = 5-7 on days 370 to 430, n = 1-3 on days 460 to 700) following TBI. Mononuclear cells (MNC) were collected by density gradient centrifugation and incubated with microbeads coated with antiCD4 or antiCD8 then cells and separated on a positive selection column. DNA was extracted and PCR analysis performed and the absolute levels of RTE CD4+ cells μL-1 or CD8+ T cells μL-1 were quantified and plotted.
Isolation of Peripheral Blood Mononuclear Cells and CD4 and CD8 T Cell Purification
Peripheral blood (PB) mononuclear cells (MNC) were separated from PB samples by density gradient centrifugation using Histopaque 1077, (Sigma-Aldrich, 3050 Spruce St., St. Louis, MO, 63103) according to the manufacturer’s recommended protocol. The MNC were divided into two samples and incubated with either antiCD4+ or antiCD8+ MACs™ MicroBead and separated on positive selection columns (Miltenyi Biotec, 2303 Lindbergh Street, Auburn, CA 95602) according to the manufacturer’s protocol. The selected cells were counted, pelleted by centrifugation, and frozen at -80°C.
DNA Extraction
DNA was extracted from cell pellets using 100 μg mL-1 of Proteinase K (Roche Molecular Biochemicals, address Mannheim, Germany) in 10 mM Tris-HCL. Cell pellets were stored at -80°C.
PCR
The primers and probe were developed using Primer Express software version 1.0 (Applied Biosystems, Foster City, CA, USA). A forward primer 5’-cacatccctttcaaccatgct-3’ and reverse primer 5’-gccagctgcagggtttagg-3’ together with the probe, 5’-FAM-acgcctctggtttttgtaaaggtgctcact-TAMRA-3’ (Applied Biosystems) were used. Real-time PCR was performed using the ABI Prism® 7700 Sequence Detection System and software (Applied Biosystems). Samples, including negative controls and samples previously analyzed, were analyzed in duplicate. A standard curve, used to quantify TRECs, was created from PCR analysis of serial dilutions of a plasmid containing a signal-joint breakpoint (generously provided by Dr. Daniel Douek) (Sodora et al. 2000; Douek et al. 1998; Douek et al. 2000).
RESULTS
Mortality, hematopoietic suppression and recovery
Mortality
Radiation exposure of rhesus macaques to 6.00 Gy x-ray TBI is an approximate LD10/60 with medical management and results in severe, acute myelosuppression and prolonged immune cell suppression. Using this model, 59 animals were designated as irradiation controls for a multitude of experiments conducted over a 12 year period. Survival and hematopoietic recovery were evaluated. The overall mortality was 8.5% (5/59). A subset of animals that were TBI during the final five years of these studies were evaluated for relatively long-term lymphocyte recovery based on cell surface antigen expression (n ≤ 14) and TREC recovery (n ≤ 12).
Neutrophil and platelet response following TBI
The acute myelosuppression was characterized by a significant reduction in neutrophil-related parameters. Neutropenia (ANC < 500 cells μL-1) began on day 5.5 ± 0.2 and persisted for 15.1 (± 0.6) days. The neutrophil nadir was 51 (± 17) cells μL-1 and the time to recovery to ANC > 500 or 1,000 cells μL-1 was 20.9 (± 0.6) days and 22.6 (± 0.7) days, respectively (Fig. 1, Table 1). Antibiotic support was required for 15.1 (± 0.8) days. Febrile neutropenia defined as a concurrent ANC < 500 cells μL-1 and body temperature ≥ 103.0°F (39.4°C), occurred in 50% of the NHP and the first day was observed on day 9.1 (± 1.3). Platelet-related values were also reduced. The duration of thrombocytopenia (platelet count < 20,000 platelets μL-1) was 5.4 (± 0.5) days. The mean, median and range of the platelet nadir were 6,929 (± 2,093) platelets μL-1, 4500 platelets μL-1 and 0-31,000, respectively. The first day of thrombocytopenia occurred on day 11.0 (± 0.3) and the range was from day 9 to day 12 post TBI. The mean, median and range of the time to recovery to a platelet count ≥ 20,000 platelets μL- 1 were 17.0 (± 0.4) days, 17 days and day 15 to day 20, respectively (Fig. 1). Only 21% of the animals (3/14) required a transfusion (average 1.6 transfusions per NHP).
Figure 1.
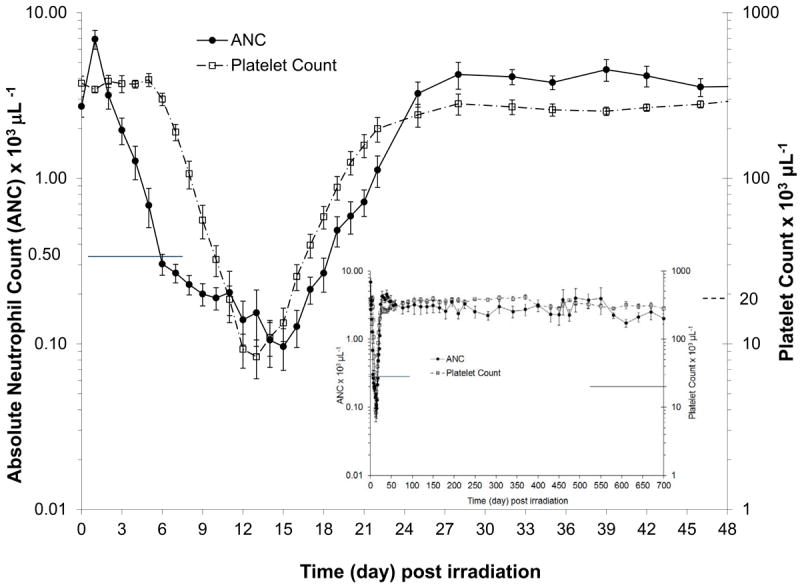
Absolute neutrophil count (ANC) and platelet count (PLT) in rhesus macaques (n = 14) exposed to 6.00 Gy x-irradiation (mean values ± standard error). Animals received medical management following total body irradiation (TBI). Parameters were monitored pre irradiation (day 0) and on selected days through the in life phase of each animal (n = 14 on days 0-1, n = 4 on days 2-3, n = 7 on day 5, n = 11-14 on days 4, 6 to 370, n = 5-8 between days 400 to 460, n = 3-4 between days 475 to 700) post TBI and are presented as cells μL-1. The ANC and PLT recovery was within baseline values by approximately 24 days post TBI (full screen graph) and continued in all animals monitored through 700 days (insert).
Table 1. Neutrophil-related parameters for rhesus macaques following x-irradiation.
Animals were exposed to 250 kVp x-radiation at 6.00 Gy total body irradiation. The mean, standard error (SEM), median and range for neutrophil-related parameters are reported. The day of the occurrence of neutropenia [absolute neutrophil count (ANC) < 500 cells μL-1 or < 100 cells μL-1, respectively] is shown. The duration of neutropenia was estimated as the number of days that a subject had an observed or extrapolated ANC < 500 cells μL-1 or < 100 cells μL-1. Any single observed ANC that was ≥ 500 cells μL-1 and was immediately preceded and followed by ANC < 500 cells μL-1 was counted as a day of neutropenia. The time to recovery was determined as the number of days from study day 1 until the first 2 consecutive days observed or extrapolated to an ANC ≥ 500 cells μL-1 or 1,000 cells μL-1 after the nadir.
| First day (d) of Neutropenia (cells μL-1) | Duration (d) of Neutropenia (cells μL-1) | Day of Recovery a ANC (cells μL-1) (day) | Nadir a (cells μL-1) | ||||
|---|---|---|---|---|---|---|---|
| ANC<500a | ANC<100b | ANC<500a | ANC<100b | ANC≥500 | ANC≥1,000 | ANC | |
| Mean ± SEM | 5.5 ± 0.2 | 10.5 ± 0.8 | 15.1 ± 0.6 | 6.3 ± 1.2 | 20.9 ± 0.6 | 22.6 ± 0.7 | 51 ± 17 |
| Median | 6.0 | 11.0 | 15.0 | 6.5 | 21.5 | 23.0 | 31 |
| Range | d4 - 7 | d6 - 18 | d12 - 20 | d0 - 15 | d18 - 25 | d19 - 28 | 0 - 210 |
n = 14
n = 12
Lymphoid suppression and recovery
Absolute lymphocyte counts (ALC)
Exposure to 6.00 Gy TBI caused a characteristic, significant, early decrease in the ALC from a mean pre irradiated value of 5,509 (± 698) cells uL-1 to a nadir of 167 (± 27) cells uL-1 (3.0% of BL) (Fig. 2). ALC recovery to within non irradiated levels occurred slowly over time to levels approximately 25%, 60% and 100% of BL by 28 days, 60 days and 105 days, respectively.
Figure 2.
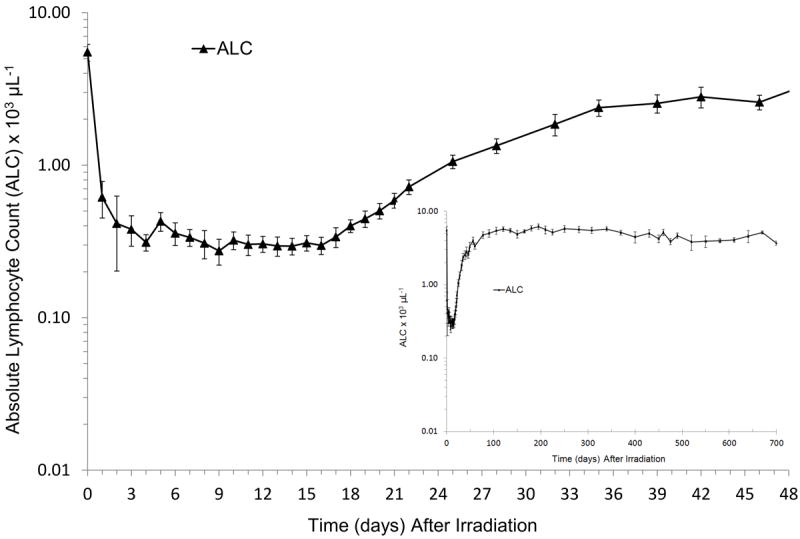
Absolute lymphocyte count (ALC) in rhesus macaques exposed to 6.00 Gy x-irradiation (mean values ± standard error). Animals received medical management following total body irradiation (TBI). Parameters were monitored pre irradiation (day 0) and on selected days through the in life phase of each animal (n = 14 on days 0-1, n = 4 on days 2-3, n = 7 on day 5, n = 11-14 on days 4, 6 to 370, n = 5-8 between days 400 to 460, n = 3-4 between days 475 to 700) post TBI and presented as cells μL-1. The ALC did not return to within baseline values until approximately 100 days post-TBI (full screen graph) and continued in all animals monitored through 700 d (insert).
Peripheral Blood B and T cells, T cell memory and naïve subsets, recent thymic emigrants, and double positive cells (CD4+CD8+) post TBI
Total peripheral blood B cells
Recovery of the T and B cell populations and respective subsets were markedly different (Figs. 3 and 4, Table 2). The peripheral blood B cell count in BL NHP was 1430 ± 259 cells μL-1. At 7 days post irradiation, B cells were profoundly depleted to 0.8% of BL B cells (12.0 ± 5.5 B cells uL-1). However, recovery was rapid, achieving 3.9%, 17.8%, and 64.5% of BL at 28, 42, and 60 days post TBI, respectively. By approximately 77 days, the B cell levels remained at or above BL levels throughout 370 days post TBI. A gradual decline of B cells was noted in the small subset of animals (n ≤ 7) observed through 700 days (Fig. 3, Table 2).
Figure 4.
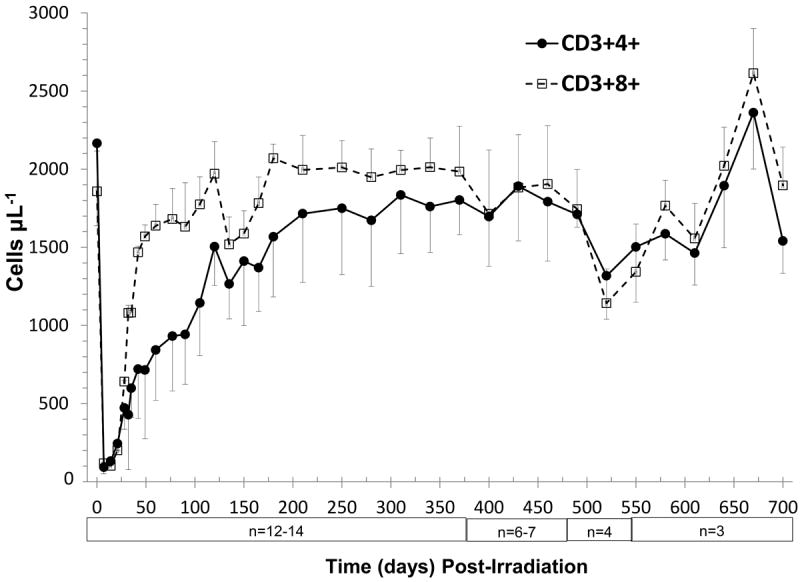
Time course of peripheral blood T cell loss and recovery in rhesus macaques following 6.00 Gy total body x-irradiation (mean values ± standard error). Samples were obtained on selected days (n = 14 on day 0 through day 370, n = 6 or 7 on day 385 to day 475, n = 3 or 4 on day 490 to day 700) following TBI. Whole blood was stained with fluorescently tagged antiCD3, antiCD4 and antiCD8 antibodies, red blood cells were lysed, and total T cells (CD3+ cells μL-1) and T cell subsets (CD3+4+cells μL-1 and CD3+8+ cells μL-1) were identified using a flow cytometer, quantified and plotted.
Total peripheral blood T cells
The BL total T cell count in rhesus macaques was 3916 ± 528 cells μL-1 with a corresponding T cell subset ratio of 1.2 (2165 CD3+4+ cells μL-1 to 1858 CD3+8+ cells μL-1). Total T cell depletion was also severe (Fig. 4, Table 2). T cells decreased to 5.1% of BL (202 ± 43 CD3+ cells μL-1) at 7 days post TBI. Total T cell recovery was rapid through 60 days post TBI, reaching approximately 60% of BL levels (2,265 ± 322 CD3+ cells μL-1) with a T cell subset ratio of 0.5. The recovery of the total T cell population was ≥ 80% of BL at 180 days through 460 days post TBI at 6.00 Gy (Fig. 4, Table 2).
T cell Naïve and Memory Subsets
Naïve and memory total T cell levels at BL (n = 14) were 2152 ± 374 cells μL-1 and 1652 ± 244 cells μL-1, respectively (Figs. 5-6, Table 3). The recovery of CD3+4+ and CD3+8+ naïve cell levels to pre irradiated values was similar. The maximum level of both CD3+ naïve subsets was approximately 68% of BL at 300 ± 30 days post TBI and remained constant through 370 days post exposure (Fig. 5, Table 3). In the cohort followed during the remaining time post TBI (n ≤ 8), naïve cells in both T cell subsets declined by approximately 65% of BL.
Figure 5.
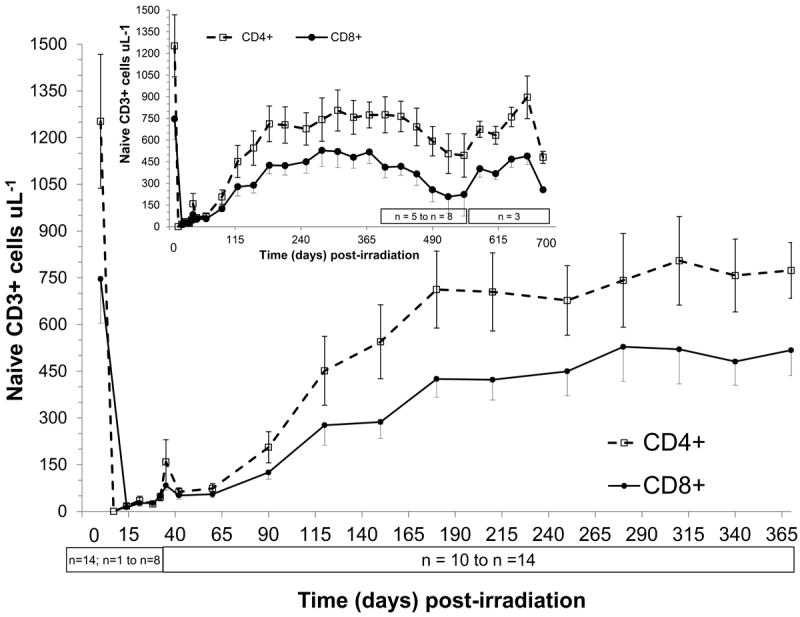
Time course of peripheral blood naïve T cell recovery in rhesus macaques following 6.00 Gy total-body x-irradiation (mean values ± standard error). Samples were obtained on selected days (n = 14 on day 0, n = 1-8 day 7 to day 35, n = 10-14 day 42 to day 370, n = 5-8 on day 400 to day 550, n = 3 on day 580 to day 700) following TBI. Whole blood was stained with fluorescently tagged antiCD3, antiCD45RA, antiCD62L and either antiCD4 or antiCD8 antibodies, red blood cells were lysed, and T cell naïve subsets (CD3+CD4+CD45RA+CD62L+ cells μL-1 or CD3+CD8+CD45RA+CD62L+ cells μL-1) were identified using a flow cytometer quantified and plotted.
In contrast, both the CD3+4+ and CD3+8+ memory cell populations recovered levels at or above BL values by 60 days post exposure (Fig. 6, Table 3). The maximum increase from BL levels in memory CD3+4+ cells was 134% at 340 days, whereas memory CD3+8+ cells were 188% by 60 days post irradiation. Thereafter, the memory cell populations in both subsets (CD3+4+ cells and CD3+8+ cells) remained above BL levels in all animals through day 370 (n=12-14), as well as in the animals (n ≤ 8) followed for 700 days post TBI. The early increase in memory subsets reflected the functional and kinetic differences between peripheral homeostatic expansion and thymopoiesis in restoring respective T cell compartments.
Recent thymic emigrants (RTE)
RTE absolute levels in peripheral blood were enumerated as total TREC+ T cells μL-1. The earliest time point RTE analysis was feasible based on ALC recovery was 30 days post TBI (n=6). The total TREC+ CD3+ cell nadir, 3 ± 1 cells μL-1 occurred on days 30 to 42 (n = 6) (Fig. 7). The recovery of total TREC+ T cells μL-1 to within the mean BL value of 31 ± 9 μL-1 in all animals studied (n = 12) required approximately 110 days. Thereafter, the total TREC+ T cells μL-1 were sustained at or above BL levels through 340 days post TBI. In animals followed through 700 days (n ≤ 7) the TREC+ T cells μL-1 remained elevated up to 460 days post TBI then gradually returned to within BL values.
Analysis of the recovery kinetics of TREC+CD4+ and TREC+CD8+ cells μL-1 was equally dramatic. The BL levels of TREC+CD4+ and TREC+CD8+ cells μL-1 were 18.9 ± 4.9 cells μL-1 and 11.8 ± 4.6 cells μL-1, respectively. The recovery of both TREC+ T cell subsets following TBI was similar in that recovery to levels greater than BL was gradual. TREC+CD4+ cells μL-1 hovered within 20% of BL through 60 days, then increased to 50% of BL at 90 days and eventually surpassed BL levels by 120 days. TREC+CD8+ cells μL-1 levels were approximately 10% of BL through 42 days, but then mirrored the recovery pattern of TREC+CD4+ cells. The recovery of TREC+ T cell subsets was striking and remained ≥ 200% of BL levels up to 340 days in all animals and the trend continued in animals observed through 460 days post TBI (n ≤ 7). The ratio of absolute number of TREC+CD4+ to TREC+CD8+ cells μL-1 was 1.6 for BL values. Following TBI, this ratio remained above 1.0 and the highest ratios were noted on days 42 (3.0) and then again on days 120 and 147 (2.6).
Recovery of Double Positive (DP) T cells
CD4+8+ T progenitor cells plummeted following TBI. DP T cells decreased from BL levels, 226 ± 58 cells μL-1 to 14.8 ± 3.3 cells μL-1 on day 14 post irradiation (data not shown). Recovery improved quickly reaching 57% of BL at 35 days to 103% of BL at 60 days. CD4+8+ T progenitor cells remained above BL through 370 days in all animals (n = 13) and continued in a subset of animals (n ≤ 7) until 700 days post TBI.
DISCUSSION
The rhesus macaque has been an important model for elucidation of immune suppression associated with chemotherapy-induced toxicity, HIV/SIV infection, stem cell transplant conditioning, chronic infections, hematology, virology and immunology (Nikolich-Zugich 2007; Herodin et al. 2005; Bosco et al. 2010). A valid model is essential to the development of MCM against the radiation-induced myelosuppression and mortality associated with the H-ARS (Monroy et al. 1988; Farese et al. 2013; Farese et al. 2012b; MacVittie et al. 2014; Hankey et al. 2015; Thrall et al. 2015; Monroy et al. 1988; Farese et al. 2013; Farese et al. 2012b; MacVittie et al. 2014; Hankey et al. 2015; Thrall et al. 2015). NHP models for radiation-induced immune cell kinetics and immunosuppression associated with the potentially lethal exposure that results in the H-ARS are not well-defined. A recent report has described the immune cell depletion and recovery consequent to high-dose partial-body irradiation with an approximate 5% sparing of bone marrow (PBI/BM) (MacVittie et al. 2014). This model requires HSC mobilization from the spared BM to reconstitute the hematopoietic system and support immune reconstitution via HSC seeding of the thymus with consequent thymopoiesis. The PBI/BM model permitted high-dose radiation exposure, 10 Gy to 12 Gy, to approximately 95% of the body that included the thymus and majority of secondary lymphoid tissue. Immune cell recovery was assessed through 180 days post exposure. The recovery kinetics demonstrated the significant and disparate roles of B cell recovery, homeostatic peripheral expansion (HPE) of T cells, HSC and HPC recovery, memory T cell expansion, and recovery of thymopoiesis and generation of RTE and naïve T cells. An additional model was developed to assess prolonged immune cell recovery consequent to 6.00 Gy uniform, bilateral TBI, a low-lethal but highly myelo- and immuno-suppressive dose. Clinically relevant, medical management administered post TBI resulted in low mortality despite significant myelosuppression.
Immune cell reconstitution is dependent upon the radiation dose- and time-dependent kinetics of BM-derived HSC recovery and generation of early thymic progenitors with subsequent seeding in a functional thymic niche. TBI of 6.00 Gy significantly reduced the number of surviving HSC. HSC must recover to threshold levels to ensure adequate production of B cell progenitors as well as effective HSC mobilization and subsequent seeding of a receptive, post-irradiation thymus for generation of new T cells. These coordinated requirements predict a prolonged recovery period post high-dose TBI (Foss et al. 2001; Foss et al. 2002; Dudakov et al. 2010; Goldschneider 2006; Donskoy et al. 2003; Thiebot et al. 2005; Zlotoff et al. 2011a; Zlotoff et al. 2011b; MacVittie et al. 2014). Hematopoiesis and B cell regeneration occur concurrently with HPE and generation of thymus-independent memory T cell subsets (Mackall et al. 1996; Mackall et al. 1995).
The radiation-induced lymphopenic environment provides the critical cytokine milieu for the initial expansion of surviving memory subsets shown to occur relatively rapidly within 60 days post TBI. B cell recovery required approximately 75 days post TBI, significantly longer than the approximate 30 days required for BM-derived myelopoietic recovery of ANC and platelets. The B cell kinetics were similar to that noted in the PBI/BM sparing model (MacVittie et al. 2014). Unfortunately this report is without a more definitive analysis of total B cell recovery. Recent protocols have been developed to assess recent BM-derived emigrants, defined as kappa-depleting recombinant excision circles (KREC) as a counterpart to thymic-derived emigrants as TRECs (Mensen et al. 2013; Sottini et al. 2010). The ability to assess KRECs in the NHP would permit a correlation to immature transitional, naïve and mature B cells.
Total CD3+ T cells required approximately 60 days, for the initial phase of recovery to 60% of BL and gradually increased to within BL values by approximately 180 days post TBI. Similarly, the initial recovery of total CD3+ T cells to an equivalent level (60% of BL) in the PBI/BM sparing model occurred within 77 days. However, T cells remained below BL values through 180 following PBI between 9.0 to 12.0 Gy with BM sparing relative to full recovery noted in the 6.00 Gy TBI model (MacVittie et al. 2014).
The HPE is dominated by expansion of CD4+ and CD8+ memory T cell subsets which recovered to within BL values by 60 days and 42 days post TBI, respectively and remained at or above BL values throughout the 370 days study duration. The recovery of memory subsets within the dose range used for TBI and PBI/BM5 models appears to be dependent on the surviving memory cell subsets within the respective acute lymphopenic, humoral environments (Mackall et al. 1997; Mackall et al. 1996; Mackall et al. 1995). The study herein, regretfully, precluded the functional assessment of the CD20+ B cells or CD3+ T cells.
Thymopoiesis and de novo production of naïve T cells requires the BM-derived mobilization of HSC and/or early thymic progenitors and successful seeding into a receptive thymic niche (Zlotoff et al. 2011a; Zlotoff et al. 2011b; Bhandoola et al. 2012; Goldschneider 2006). The prolonged recovery of thymopoiesis, as evidenced by the relative delay in TREC+ and naïve CD3+CD4+ and CD3+CD8+ cell recovery, is a consequence of the requisite coordinated and chance events critical to production of naïve T cells in the post irradiation microenvironment. Indeed, preclinical and clinical studies suggested a complex sequence of biologic events in the post irradiation, myelosuppressed BM, coupled with an irradiated, dysfunctional thymus that contributes to the long recovery of naïve T cell production (Chung et al. 2001; Foss et al. 2001; Thiebot et al. 2005; Dudakov et al. 2010; Kenins et al. 2008; Zubkova et al. 2005). There was a significant early data base on the efficacy of in vivo administration of IL-7 in rodent models of immunosuppression or BMT as well as in NHP models of SIV-infected rhesus macaques and autologous BMT in baboons (Abdul-Hai et al. 1996; Mackall et al. 2001; Fry et al. 2003; Storek et al. 2003). Unfortunately IL-7 did not progress beyond preclinical efficacy. This study was also limited in the amount of plasma analysis for relevant cytokines such as IL-7 and/or histochemical identification of IL-7 in the epithelial cells of the thymic niche.
Recent investigations into thymic regeneration after high-dose irradiation in several mouse models have suggested an interesting interaction between depletion of double-positive thymocytes, IL-23 release from thymic dendritic cells and stimulation of IL-22 production by a subset of RORγt+ innate lymphoid cells (Dudakov et al. 2012). Endogenous IL-22 was increased in the thymus and interacts with IL-22 receptor+ thymic epithelial cells to promote their survival and proliferation. Furthermore, IL-22 administered exogenously, pre and post irradiation and transplant increased thymic cellularity. Additional studies have demonstrated the efficacy of reversible sex steroid blockade with luteinizing hormone-releasing hormone antagonist (LHRH-Ant) to enhance thymopoiesis in sublethally irradiated mice (Velardi et al. 2014). The enhanced recovery of thymic cellularity was also noted in young and aged male as well as female mice. Lai and colleagues, using a mouse model of allogeneic BM transplantation, demonstrated the efficacy of a recombinant hybrid cytokine of IL-7 and hepatocyte growth factorβ (IL-7/HGFβ) to increase thymic reconstitution and functional activities of peripheral T cells (Jin et al. 2011; Lai et al. 2013). These studies provide important insights into potential MCM to stimulate regeneration of functional thymopoiesis.
The kinetics of cell recovery provided an interesting parallel between the uniform irradiation in both the TBI model and the PBI/BM5 model that essentially myeloablates 95% of active BM yet spares approximately 5% of tibial BM. Hematopoiesis in the 6.00 Gy TBI model is initiated from surviving stem and progenitor cells diluted throughout the entire marrow space. In contrast, hematopoiesis, to include stem cell and T cell progenitors and B cell lymphopoiesis in the PBI/BM5 model initiates recovery from marrow located in the tibiae that received an approximate 0.50 Gy dose of radiation. It is important to note that the thymus received a uniform dose of 6.00 Gy x-radiation or 9.00-12.00 Gy LINAC-derived photons in the TBI and PBI/BM5 models, respectively.
Regrettably, the time frame for the conduct of this study precluded the use of additional, newly defined markers for naïve T cells and recent bone marrow-derived, naïve B cell emigrants. A recent marker, CD31, has been promoted to differentiate between recent thymic-derived CD4+CD31+ naïve T cells and a more mature CD4+CD31- naïve T cells in peripheral blood (Kohler et al. 2009; Mensen et al. 2013). The analysis of B cell lymphopoiesis has been aided by the recent identification of kappa (K) depleting recombinant excision circles (KRECs) formed during the production of marrow-derived naïve B cells. KRECs are reported in approximately 50% of newly produced B cells (Mensen et al. 2013; Sottini et al. 2010; Frankova et al. 2008). Mensen et al. showed that the CD31+ subset of naïve T cells was better correlated with TREC level post allogeneic HSC transplant than the CD31- subset. They further demonstrated that KREC levels correlated with transitional as well as naïve B cell recovery (Mensen et al. 2013). These new markers, including protein tyrosine kinase 7 (PTK7), another marker for CD4+ RTE, and CD103 (αEβ7 integrin) a marker of CD8+ RTE may help elucidate the time course of hematopoietic and immune reconstitution after high-dose irradiation-induced myelo- and immunosuppression (Haines et al. 2009; McFarland et al. 2000).
These NHP models provide the means to investigate the efficacy of MCM developed to mitigate the acute radiation-induced ARS and the delayed effects of acute radiation exposure (DEARE). Radiation-induced immune suppression is a key component of both acute and delayed effects. The entire time to onset and progression of radiation-induced delayed organ injury will occur in the context of severe immunosuppression. There are no MCM that can mitigate the key cellular events required for stimulating effective thymopoiesis when administered as a mitigator within 24 to 72 hours post exposure. The model described herein may provide a means for the assessment of cellular and functional immune reconstitution consequent to acute high-dose TBI.
Acknowledgments
This project has been funded in whole or in part with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under R01 A167503-1. Authors gratefully acknowledge the superb technical assistance of Ms. Carrie Redinger, Mr. Michael E. Flynn and all the members of our Preclincial Radiobiology Laboratory for their dedication to the health and well-being of our animals.
Footnotes
The authors declare no conflict of interest.
References
- Abdul-Hai A, Or R, Slavin S, Friedman G, Weiss L, Matsa D, Ben-Yehuda A. Stimulation of immune reconstitution by interleukin-7 after syngeneic bone marrow transplantation in mice. Experimental Hematology. 1996;24:1416–1422. [PubMed] [Google Scholar]
- Allen JR, Van Lancker JL, Wolf RC. Pathologic alterations procuded by total body X-irradiation in monkeys. American Journal of Pathology. 1966;48:317–331. [PMC free article] [PubMed] [Google Scholar]
- Amgen, Inc. [16 June 2015];Neupogen Prescribing Information, ver 13. http://pi.amgen.com/united_states/neupogen/neupogen_pi_hcp_english.pdf.
- Arlettaz L, Barbey C, Dumont-Girard F, Helg C, Chapuis B, Roux E, Roosnek E. CD45 isoform phenotypes of human T cells: CD4(+)CD45RA(-)RO(+) memory T cells re-acquire CD45RA without losing CD45RO. European Journal of Immunology. 1999;29:3987–3994. doi: 10.1002/(SICI)1521-4141(199912)29:12<3987::AID-IMMU3987>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Armstrong AC, Gidding S, Gjesdal O, Wo C, Bluemke DA, Lima JAC. LV Mass Assessed by Echocardiography and CMR, Cardiovascular Outcomes, and Medical Practice. JACC: Cardiovascular Imaging. 2012;5(8):837–848. doi: 10.1016/j.jcmg.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandoola A, Artis D. Rebuilding the thymus. Science. 2012;336:40–41. doi: 10.1126/science.1221677. [DOI] [PubMed] [Google Scholar]
- Bosco N, Swee LK, Benard A, Ceredig R, Rolink A. Auto-reconstitution of the T-cell compartment by radioresistant hematopoietic cells following lethal irradiation and bone marrow transplanatation. Exp Hematol. 2010;38(3):222–232. doi: 10.1016/j.exphem.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Carter DL, Shieh TM, Blosser RL, Chadwick KR, Margolick JB, Hildreth JEK, Clements JE, Zink MC. CD56 identifies monocytes and not natural killer cells in rhesus macaques. Cytometry. 1999;37:41–50. [PubMed] [Google Scholar]
- Chung B, Barbara-Burnham L, Barsky L, Weinburg K. Radiosensitivity of thymic interleukin-7 production and thymopoiesis after bone marrow transplantation. Blood. 2001;98:1601–1606. doi: 10.1182/blood.v98.5.1601. [DOI] [PubMed] [Google Scholar]
- Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the care and use of laboratory animals. Washington, D.C.: National Academies Press; 2011. [PubMed] [Google Scholar]
- Dainiak N, Gent RN, Carr Z, Schneider R, Bader J, Buglova E, Chao N, Coleman CN, Ganser A, Gorin C, Hauer-Jensen M, Huff LA, Lillis-Hearne P, Maekawa K, Nemhauser J, Powles R, Schunemann H, Shapiro A, Stenke L, Valverde N, Weinstock D, White D, Albanese J, Meineke V. First global consensus for evidence-based management of the hematopoietic syndrome resulting from exposure to ionizing radiation. Disaster med public health prep. 2011;5:202–212. doi: 10.1001/dmp.2011.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donskoy E, Foss D, Goldscheider I. Gated importation of prothymocytes by adult mouse thymus is coordinated with their periodic mobilization from bone marrow. J Immunol. 2003;171:3568–3575. doi: 10.4049/jimmunol.171.7.3568. [DOI] [PubMed] [Google Scholar]
- Douek DC, McFarland RD, Keiser PH, Gage EA, Massey JM, Haynes BF, Polis MA, Haase AT, Feinberg MB, Sullivan JL, Jamieson BD, Zack JA, Picker LJ, Koup RA. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–695. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- Douek DC, Vescio RA, Betts MR, Brenchley JM, Hill BJ, Zhang L, Berenson JR, Collins RH, Koup RA. Assessment of thymic output in adults after haematopoietic stem cell transplant and prediction of T-cell reconstitution. Lancet. 2000;355:1875–1881. doi: 10.1016/S0140-6736(00)02293-5. [DOI] [PubMed] [Google Scholar]
- Dudakov JA, Hanash AM, Jenq RR, Young LF, Ghosh A, Singer NV, West ML, Smith OM, Holland AM, Tsai JJ, Boyd RL, van den Brink MRM. Interleukin-22 drives endogenous thymic regeneration in mice. Science. 2012;336:91–95. doi: 10.1126/science.1218004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudakov JA, Khong DMP, Chidgey AP. Feeding the fire: The role of defective bone marrow function in exacerbating thymic involution. Trends in Immunology. 2010;31:191–198. doi: 10.1016/j.beha.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Eldred E, Trowbridge WV. Radiation sickness in the monkey. Radiology. 1954;62:65–73. doi: 10.1148/62.1.65. [DOI] [PubMed] [Google Scholar]
- Farese AM, Cohen MV, Katz BP, Smith CP, Gibbs A, Cohen DM, MacVittie TJ. Filgrastim improves survival in lethally irradiated nonhuman primates. Radiat Res. 2013;179:89–100. doi: 10.1667/RR3049.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farese AM, Cohen MV, Katz BP, Smith CP, Jackson W, III, Cohen DM, MacVittie TJ. A nonhuman primate model of the hematopoietic acute radiation syndrome plus medical management. Health Phys. 2012a;103:367–382. doi: 10.1097/HP.0b013e31825f75a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farese AM, Cohen MV, Stead RB, Jackson W, III, MacVittie TJ. Pegfilgrastim administered in an abbreviated schedule, significantly improved neutrophil recovery after high-dose radiation-induced myelosuppression in rhesus macaques. Radiat Res. 2012b;178:403–413. doi: 10.1667/RR2900.1. [DOI] [PubMed] [Google Scholar]
- Foss DL, Donskoy E, Goldschneider I. The importation of hematogenous precursors by the thymus is a gated phenomenon in normal adult mice. J Exp Med. 2001;193:365–374. doi: 10.1084/jem.193.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foss DL, Donskoy E, Goldschneider I. Functional demonstration of intrathymic binding sites and microvascular gates for prothymocytes in irradiated mice. Int Immunol. 2002;14:331–338. doi: 10.1093/intimm/14.3.331. [DOI] [PubMed] [Google Scholar]
- Frankova E, Muzikova K, Mejstrikova E, Kovac M, Formankova R, Sedlacek P, Hrusak O, Stary J, Trka J. B cell reconstitution after allogeneic SCT impairs minimal residual disease monitoring in children with ALL. Bone Marrow Transplant. 2008;42:187–196. doi: 10.1038/bmt.2008.122. [DOI] [PubMed] [Google Scholar]
- Fry TJ, Moniuszko M, Creekmore S, Donohue SJ, Douek DC, Giardina S, Hecht TT, Hill BJ, Komschlies K, Tomaszewski J, Franchini G, Mackall CL. IL-7 therapy dramatically alters peripheral T-cell homeostasis in normal and SIV-infected nonhuman primates. Blood. 2003;101:2294–2299. doi: 10.1182/blood-2002-07-2297. [DOI] [PubMed] [Google Scholar]
- Goldschneider I. Cyclical mobilization and gated importation of thymocyte progenitors in the adult mouse: evidence for a thymus-bone marrow feedback loop. Immunological Reviews. 2006;209:58–75. doi: 10.1111/j.0105-2896.2006.00354.x. [DOI] [PubMed] [Google Scholar]
- Haigh MV, Paterson E. Effects of a single session of whole body irradiation in the rhesus monkey. British Journal of Radiology. 1956;29:148–157. doi: 10.1259/0007-1285-29-339-148. [DOI] [PubMed] [Google Scholar]
- Haines CJ, Giffon TD, Lu LS, Lu X, Tessier-Lavigne M, Ross DT, Lewis DB. Human CD4+ T cell recent thymic emigrants are identified by protein tyrosine kinase 7 and have reduced immune function. J Exp Med. 2009;206:275–285. doi: 10.1084/jem.20080996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankey KG, Farese AM, Blaauw E, Gibbs AM, Smith CP, Katz B, Tong Y, MacVittie TJ. Pegfilgrastim administration significantly improves survival in an LD50/60 model of total-body irradiation (TBI) in nonhuman primates. Radiat Res. 2015;183(6):643–655. doi: 10.1667/RR13940.1. [DOI] [PubMed] [Google Scholar]
- Henschke UK, Morton JL. Mortality of Rhesus monkeys after single total body irradiation. American Journal of Roentgenol. 1957;77:889–909. [PubMed] [Google Scholar]
- Herodin F, Roy L, Grenier N, Delaunay C, Bauge S, Vaurijoux A, Gregoire E, Martin C, Alonso A, Mayol J-F, Drouet M. Antiapoptotic cytokines in combination with pegfilgrastim soon after irradiation mitigates myelosuppression in nonhuman primates exposed to high radiation dose. Exp Hematol. 2007;35:1172–1181. doi: 10.1016/j.exphem.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Herodin F, Thullier P, Garin D, Drouet M. Nonhuman primates are relevant models for research in hematology, immunology and virology. Eur Cytokine Netw. 2005 Jun;16(2):104–116. [PubMed] [Google Scholar]
- Jin J, Goldschneider I. In vivo administration of the recombinant Il-7/hepatocyte growth factor beta hybrid cytokine efficiently restores thymopoiesis and naive T cell generation in lethally irradiated mice after syngeneic bone marrow transplantation. J Immunol. 2011;186:1915–1922. doi: 10.1371/journal.pone.0085789. [DOI] [PubMed] [Google Scholar]
- Kenins L, Gill JW, Boyd RL. Intrathymic expression of flt3 ligand enhances thymic recovery after irradiation. J Exp Med. 2008;205:523–531. doi: 10.1084/jem.20072065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler S, Thiel A. Life after the thymus: CD31+ and CD31- human naive CD34+ T cell subsets. Blood. 2009;113:769–774. doi: 10.1182/blood-2008-02-139154. [DOI] [PubMed] [Google Scholar]
- Lai L, Zhang M, Song Y, Rood D. Recombinant IL-7/HGFb hybrid cytokine enhances T-cell recovery in mice following allogeneic bone marrow transplantation. PLos One. 2013;8(12):1–11. doi: 10.1172/JCI46055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBien TW, Tedder TF. B lymphocytes: how they develop and function. Blood. 2008;122(September 1):1570–1580. doi: 10.1182/blood-2008-02-078071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackall CL, Bare CV, Granger LA, Sharrow SO, Titus JA, Gress RE. Thymic-independent T-cell regeneration occurs via antigen-driven expansion of peripheral T cells resulting in a repertoire that is limited in diversity and prone to skewing. Journal of Immunology. 1996;156:4609–4616. [PubMed] [Google Scholar]
- Mackall CL, Fleisher TA, Brown MR, Andrich MP, Chen CC, Feuerstein IM, Horowitz ME, Magrath IT, Shad AT, Steinberg SM, Wexler LH, Gress RE. Age, Thymopoiesis, and CD4+ T-Lymphocyte Regeneration after Intensive Chemotherapy. The New England Journal of Medicine. 1995;332:143–149. doi: 10.1056/NEJM199501193320303. [DOI] [PubMed] [Google Scholar]
- Mackall CL, Fleisher TA, Brown MR, Andrich MP, Chen CC, Feuerstein IM, Magrath IT, Wexler LH, Dimitrov DS, Gress RE. Distinctions between CD8+ and CD4+ T-cell regeneration pathways result in prolonged T-cell subset imbalance after intensive chemotherapy. Blood. 1997;89:3700–3707. [PubMed] [Google Scholar]
- Mackall CL, Fry TJ, Bare C, Morgan P, Galbraith A, Gress RE. IL-7 increases both thymic-dependent and thymic-independent T-cell regeneration after bone marrow transplantation. Blood. 2001;97:1491–1497. doi: 10.1182/blood.v97.5.1491. [DOI] [PubMed] [Google Scholar]
- MacVittie TJ, Bennett A, Booth C, Garofalo M, Tudor G, Ward A, Shea-Donohue T, Gelfond D, Mcfarland E, Jackson W, III, Lu W, Farese AM. The prolonged gastrointestinal syndrome in rhesus macaques: the relationship between gastrointestinal, hematopoietic, and delayed multi-organ sequelae following acute, potentially lethal, partial-body irradiation. Health Phys. 2012;103:427–453. doi: 10.1097/HP.0b013e318266eb4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacVittie TJ, Bennett AW, Cohen V, Farese AM, Higgins A, Hankey KG. Immune cell reconstitution after exposure to potentially lethal doses of radiation in the nonhuman primate. Health Phys. 2014;106:84–96. doi: 10.1097/HP.0b013e3182a2a9b2. [DOI] [PubMed] [Google Scholar]
- MacVittie TJ, Farese AM, Jackson W., III Defining the full therapeutic potential of recombinant growth factors in the post radiation-accident environment: the effect of supportive care plus administration of G-CSF. Health Phys. 2005;89:546–555. doi: 10.1097/01.hp.0000173143.69659.5b. [DOI] [PubMed] [Google Scholar]
- MacVittie TJ, Farese AM, Jackson WI. The hematopoietic syndrome of the acute radiation syndrome in rhesus macaques: A systematic review of the lethal dose response relationship. Health Phys. 2015;109(5):XXX–XXX. doi: 10.1097/HP.0000000000000352. [DOI] [PubMed] [Google Scholar]
- MacVittie TJ, Farese AM, Smith WG, Baum CM, Burton E, McKearn JP. Myelopoietin, an engineered chimeric IL-3 and G-CSF receptor agonist, stimulates multilineage hematopoietic recovery in a nonhuman primate model of radiation-induced myelosuppression. Blood. 2000;95:837–845. [PubMed] [Google Scholar]
- McFarland RD, Douek DC, Koup RA, Picker LJ. Identification of a human recent thymic emigrant phenotype. PNAS. 2000;97:4215–4220. doi: 10.1073/pnas.070061597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mensen A, Ochs C, Stroux A, Wittenbecher F, Szyska M, Imberti L, Fillatreau S, Uharek L, Arnold R, Dorken B, Thiel A, Scheibenbogen C, Na IK. Utilization of TREC and KREC quantitation for the monitoring of early T- and B-cell neogenesis in adult patients after allogeneic hematopoietic stem cell transplantation. J Translational Medicine. 2013;11(188):1–9. doi: 10.1186/1479-5876-11-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroy RL, Skelly RR, Taylor P, Dubois A, Donahue RE, MacVittie TJ. Recovery from severe hematopoietic suppression using recombinant human granulocyte-macrophage colony-stimulating factor. Exp Hematol. 1988;16:344–348. [PubMed] [Google Scholar]
- Neelis KJ, Dubbelman YD, Qingliang L, Thomas GR, Eaton DL, Wagemaker G. Simultaneous administration of TPO and G-CSF after cytoreductive treatment of rhesus monkeys prevents thrombocytopenia, accelerates platelet and red cell reconstitution, alleviates neutropenia, and promotes the recovery of immature bone marrow cells. Experimental Hematology. 1997;25:1084–1093. [PubMed] [Google Scholar]
- Nikolich-Zugich J. Review: Non-Human primate model of T-cell reconstitution. Seminars in Immunology. 2007;19:310–317. doi: 10.1016/j.smim.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plett PA, Sampson CH, Chua HL, Joshi M, Booth C, Gough A, Johnson CS, Katz BP, Farese AM, Parker J, MacVittie TJ, Orschell CM. Establishing a murine model of the hematopoietic syndrome of the acute radiation syndrome. Health Phys. 2012;103:343–355. doi: 10.1097/HP.0b013e3182667309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlumberger HG, Vazquez JJ. Pathology of total body irradiation in the monkey. American Journal of Pathology. 1954;30:1013–1047. [PMC free article] [PubMed] [Google Scholar]
- Schuening FG, Appelbaum FR, Deeg HJ, Sullivan-Pepe M, Grahm R, Hackman R, Zsebo KM, Storb R. Effects of recombinant canine stem cell factor, a c-kit ligand and recombinant granulocyte colony stimulating factor on hematopoietic recovery after otherwise lethal total body irradiation. Blood. 1993;81:20–26. [PubMed] [Google Scholar]
- Sodora DL, Douek DC, Silvestri G, Montgomery L, Rosenzweig M, Igarashi T, Bernacky B, Johnson RP, Feinberg MB, Martin MA, Koup RA. Quantification of thymic function by measuring T cell receptor excision circles within peripheral blood and lymphoid tissues in monkeys. European Journal of Immunology. 2000;30:1145–1153. doi: 10.1002/(SICI)1521-4141(200004)30:4<1145::AID-IMMU1145>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Sottini A, Ghidini C, Chiarini M, Caimi L, Lanfranchi A, Moratto D, Porta F, Imberti L. Simultaneous quantitation of recent thymic T-cell and bone marrow B-cell emigrants in patients with primary immunodeficiency undergone to stem cell transplantation. Clin Immun. 2010;136:217–227. doi: 10.1016/j.clim.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Stanley RE, Seigneur LJ, Strike TA. The acute mortality response of monkeys (Macaca mulatta) to mixed gamma-neutron radiations and 250 kVp X rays. Armed Forces Radiobiology Research Institute; Bethesda, MD: 1966. SR66-23. [Google Scholar]
- Storek J, Gillespy T, Lu H, Joseph A, Dawson MA, Gough M, Morris J, Hackman RC, Horn PA, Sale GE, Andrews RG, Maloney DG, Kiem HP. Interleukin-7 improves CD4 T-cell reconstitution after autologous CD34 cell transplantation in monkeys. Blood. 2003;101:4209–4218. doi: 10.1182/blood-2002-08-2671. [DOI] [PubMed] [Google Scholar]
- Thiebot H, Vaslin B, Derdouch S, Bertho JM, Mouthon F, Prost S, Gras G, Ducouret P, Dormont D, LeGrand R. Impact of bone marrow hematopoiesis failure on T-cell generation during pathogenic simian immunodeficiency virus infection in macaques. Blood. 2005;105:2403–2409. doi: 10.1182/blood-2004-01-0025. [DOI] [PubMed] [Google Scholar]
- Thrall K, Love R, O’Donnell K, Farese AM, Manning R, MacVittie T. An inter-laboratory validation of the radiation dose response relationship (DRR) for the H-ARS in the rhesus macaque. Health Phys. 2015;109(5):XXX–XXX. doi: 10.1097/HP.0000000000000339. [DOI] [PubMed] [Google Scholar]
- Velardi E, Tsai JJ, Holland AM, Wertheimer T, Yu VWC, Zakrzewski JI, Tuckett AZ, Singer NV, West ML, Smith OM, Young LF, Kreines FM, Levy ER, Boyd RL, Scadden DT, Dudakov JA, va den Brink MRM. Sex steroid blockade enhances thymopoiesis by modulating notch signaling. J Exp Med. 2014;211(12):2341–2349. doi: 10.1084/jem.20131289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu ZY, Li M, Han AR, Xing S, Ou HL, Xiong GL, Xie L, Zhao YF, Xiao H, Shan YJ, Zhao ZH, Liu XL, Cong YW, Luo QL. RhG-CSF improves radiation-induced myelosuppression and survival in the canine exposed to fission neutron irradiation. J Radiat Res. 2011;52:472–480. doi: 10.1269/jrr.10103. [DOI] [PubMed] [Google Scholar]
- Zlotoff DA, Bhandoola A. Hematopoietic progenitor migration to the adult thymus. Ann N Y Acad Sci. 2011a;1217:122–138. doi: 10.1111/j.1749-6632.2010.05881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotoff DA, Zhang SL, De Obaldia ME, Hess PR, Todd SP, Logan TD, Bhandoola A. Delivery of progenitors to the thymus limits T-lineage reconstitution after bone marrow transplantation. Blood. 2011b;118:1962–1970. doi: 10.1182/blood-2010-12-324954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubkova I, Mostowski H, Zaitseva M. Upregulation of IL-7, stromal-derived factor-1alpha, thymus-expressed chemkine, and secondary lymphoid tissue chemokine gene expression in the stromal cells in response to thymocyte depletion: implication for thymus reconstitution. J Immunol. 2005;175:2321–2330. doi: 10.4049/jimmunol.175.4.2321. [DOI] [PubMed] [Google Scholar]


