Significance
Conventional mosquito-control methods are becoming less effective in controlling mosquito-borne diseases because of widespread resistance against safe and recommended public health insecticides. Therefore innovative and effective alternatives are urgently needed. We present a novel method for exposing mosquitoes to insecticides that uses electrostatic forces to bind insecticide particles. Results show that this method increases exposure of mosquitoes to such an extent that even those that have developed high levels of resistance can be killed effectively. The ability to boost the efficacy of WHO-recommended insecticides provides a significant benefit to the field of vector-borne disease control and will further the development of novel resistance-breaking control tools.
Keywords: electrostatic coating, insecticide, resistance management, mosquito, malaria
Abstract
Insecticide resistance poses a significant and increasing threat to the control of malaria and other mosquito-borne diseases. We present a novel method of insecticide application based on netting treated with an electrostatic coating that binds insecticidal particles through polarity. Electrostatic netting can hold small amounts of insecticides effectively and results in enhanced bioavailability upon contact by the insect. Six pyrethroid-resistant Anopheles mosquito strains from across Africa were exposed to similar concentrations of deltamethrin on electrostatic netting or a standard long-lasting deltamethrin-coated bednet (PermaNet 2.0). Standard WHO exposure bioassays showed that electrostatic netting induced significantly higher mortality rates than the PermaNet, thereby effectively breaking mosquito resistance. Electrostatic netting also induced high mortality in resistant mosquito strains when a 15-fold lower dose of deltamethrin was applied and when the exposure time was reduced to only 5 s. Because different types of particles adhere to electrostatic netting, it is also possible to apply nonpyrethroid insecticides. Three insecticide classes were effective against strains of Aedes and Culex mosquitoes, demonstrating that electrostatic netting can be used to deploy a wide range of active insecticides against all major groups of disease-transmitting mosquitoes. Promising applications include the use of electrostatic coating on walls or eave curtains and in trapping/contamination devices. We conclude that application of electrostatically adhered particles boosts the efficacy of WHO-recommended insecticides even against resistant mosquitoes. This innovative technique has potential to support the use of unconventional insecticide classes or combinations thereof, potentially offering a significant step forward in managing insecticide resistance in vector-control operations.
Mosquito-borne infectious diseases continue to pose a huge public health burden worldwide. Malaria, lymphatic filariasis, dengue, Chikungunya, and West Nile virus cause significant medical and economic impacts that disproportionately affect developing countries (1–3). Because there are no commercially available vaccines against these diseases, vector control remains crucial to reduce disease transmission. Contemporary vector control focuses on the use of four classes of public health insecticides recommended by the WHO (4). However, intensive and widespread use of these insecticides induced intense selection pressure that has resulted in the development and subsequent intensification of various genetically modulated resistance mechanisms in mosquitoes (5–7). Today, insecticide resistance is regarded as the most serious threat to the control of mosquito-borne diseases. Over the last decade resistance has been reported in all three major mosquito genera and against all four classes of recommended insecticides in most disease-endemic regions where substantial progress in control was reported previously (4, 8).
Larval exposure to low residual doses of insecticides from agricultural pest control has been a major driver of resistance development in mosquito populations (9, 10). Mosquitoes can become resistant to insecticides by (over)expressing detoxifying enzymes or via genetic mutations at the location where the insecticide is active (11). Such traits might result in fitness costs for the insect because their expression can deplete energy resources, reducing the insect’s ability to compete with nonresistant counterparts (12–14). To manage resistance adequately, the WHO recommends the use of rotations, insecticide mixtures, or novel insecticide classes that have completely distinct modes of action (4), and several promising developments are aimed at facilitating these strategies (15–20). However, the selection of new active ingredients is severely restricted by the need for products that are safe for humans who come into frequent contact with nets and sprayed surfaces. Another means to improve insecticidal impact is to increase the effective target dose.
Several factors influence what dose of insecticide is effectively transferred to the target insect, including the type of formulation and substrate as well as the size and adherence properties of insecticidal particles on these substrates (21). Small flying insects such as mosquitoes are particularly difficult to target with lethal doses of insecticides. Current vector-control products use oil- or water-based formulations as carriers to achieve adherence and retention of the chemicals on vertical substrates such as walls or netting. For instance, long-lasting insecticidal nets (LLINs) and indoor residual sprays (IRS) used in malaria control deploy formulations of pyrethroids via coating, impregnation, or spraying. Absorption of the carrier and binding forces associated with oily formulations can limit the bioavailability of the active ingredient. Studies show that insecticide absorption in porous mud walls significantly decreases the long-lasting efficacy of IRS applications (22). For LLINs, impregnation methods such as insecticide incorporation within the netting fibers or slow-release coatings are being used to prolong persistence and withstand several washings, but these techniques limit the amount of insecticide that is available to the target insect upon contact. Prolonged use of long-lasting material under normal household conditions might result in mosquitoes being exposed to a progressively smaller dosage of insecticides as the chemicals dissipate (23); a consequence of such exposure might be selection for resistant vector populations.
Here we present an application method with improved insecticide bioavailability that can be used to deploy both pyrethroid and nonpyrethroid insecticides to target resistant vectors effectively with a lethal dose. This method consists of a coating that can be applied on different substrates and has an electrostatic charge that binds particles via polarity. Products that incorporate electrostatic binding forces are already being used in insect pest control, for example as aerial electrostatic-charged insecticide sprays for the control of sweet potato whitefly (Bemisia tabaci) (24) or as electrostatic mating-disruption powder against codling moth (Cydia pomonella) (25). However, this is the first time, to our knowledge, that electrostatic forces have been used in a coating that can be deployed on a variety of surfaces and that can bind different types of particles. The charged coating was developed originally for use in house screens to trap airborne pollen and is commercially available for that purpose (https://www.buypollentec.com/test-data/ accessed July 29, 2015). It is applied on netting fibers via a special process that allows fixation and the formation of a long-lasting static charge. The electrostatic charge enables the adherence of insecticide particles without the need of a carrier formulation.
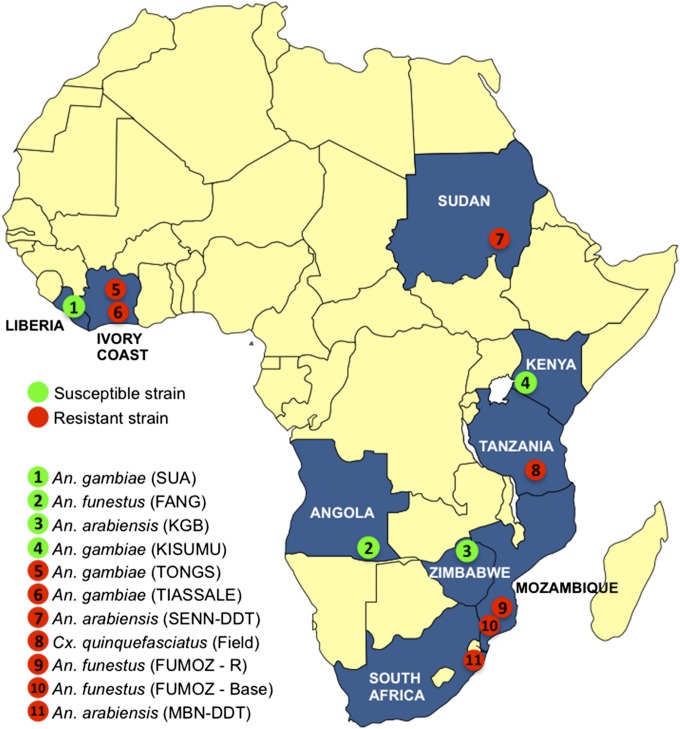
In this study, the bioavailability and impact of insecticide particles bound via polarity were measured and compared with the impact of a standard long-lasting deltamethrin-coated bednet. Multiple strains of pyrethroid-resistant Anopheles malaria vectors originating from nine different countries in Africa (Fig. 1) were exposed to electrostatic netting saturated with deltamethrin amounts similar to the insecticide dose used in standard LLINs [PermaNet 2.0; target dose, 55 mg active ingredient (AI)/m2]. The impact of the two methods was compared by measuring initial knockdown (1 h) and final mortality (24 h post exposure). Lower insecticide doses and shorter exposure times were included to demonstrate the potential vector-control impact of electrostatic netting. Three other (nonpyrethroid) public health insecticide classes were deployed, and the mortality impact against Aedes aegypti and Culex quinquefasciatus mosquitoes was tested to demonstrate the broader vector-control and resistance-management options of this innovative application technique.
Fig. 1.
Origins of the tested pyrethroid-resistant and susceptible Anopheles strains and the field-collected Culex strain.
Results
Particle Transfer.
To test the bioavailability of insecticide particles, particle transfer to mosquitoes was visualized by applying fluorescent dust on the electrostatic netting. The quantity of transferred fluorescent particles served as a visual proxy for contamination efficacy (Fig. 2A). In a standard 3-min WHO cone exposure assay (26), mosquitoes obtained fluorescent particles across the entire body including tarsi, antennae, proboscis, thorax, and lower abdomen (Fig. 2C), demonstrating that an extensive dose was transferred from the electrostatic netting to the mosquito. Shorter contact assays, using exposures of only 5 s, also resulted in effective particle transfer from the netting to mosquitoes, with visible amounts of fluorescent particles adhering to the tarsi and body (Fig. 2B). Both exposure times were included in the insecticide impact evaluations to quantify insecticidal impacts after standard and short contact duration.
Fig. 2.

(A) Photograph of electrostatic netting saturated with fluorescent dust particles lighting up orange under UV light at 50× magnification. (B) A Culex mosquito contaminated with fluorescent particles after a 5-s contact with the netting. (C) Culex female with fluorescent particles after 3-min contact with netting.
Insecticide Bioavailability.
Insecticide exposures were conducted on multiple strains of pyrethroid-resistant mosquitoes, including the major malaria vectors Anopheles arabiensis (27–29), Anopheles funestus (30, 31), and Anopheles gambiae s.s. (32, 33) with well-defined mechanisms and levels of resistance, using standard WHO resistance assays with 0.05% deltamethrin papers (Fig. S1). A detailed description of the mosquito strains used, including their origin and resistance mechanisms, is provided in the Supporting Information. Insecticide-susceptible mosquito strains were included to confirm sample quality.
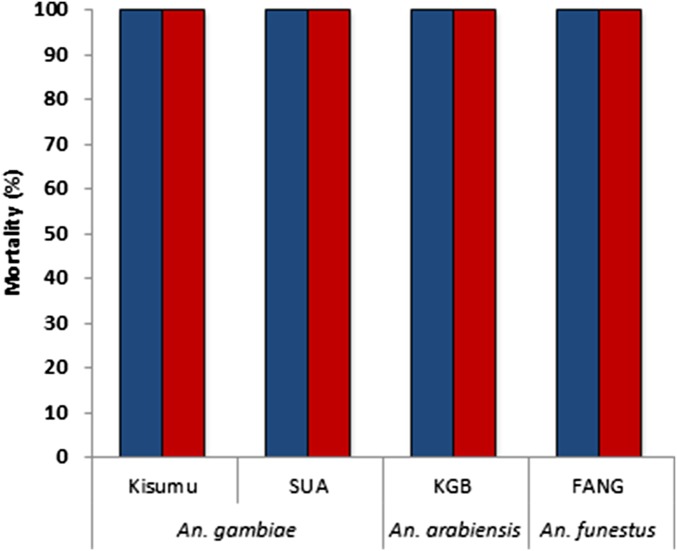
Fig. S1.
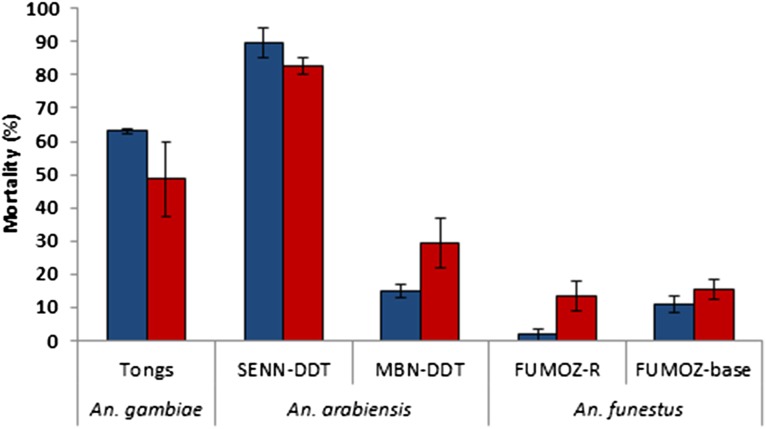
Average (± SE) mortality and knockdown rates of two groups of 25 female pyrethroid-resistant anopheline strains from the Vector Control Research Unit (VCRU) measured 1 h (blue bars) or 24 h (red bars) after 1-h exposure to 0.05% deltamethrin papers in WHO test tubes.
The insecticidal impact of deltamethrin coated on a standard polyester LLIN was compared with deltamethrin applied on electrostatic netting, keeping the amounts of AI as similar as possible. Standard 3-min WHO cone exposure assays were used to compare PermaNet 2.0 netting with deltamethrin (target dose, 55 mg AI/m2) and electrostatic netting saturated with deltamethrin (target dose, 37 mg AI/m2). Positive control tests with susceptible anopheline strains showed that both PermaNet and electrostatic netting induced 100% mortality 24 h after exposure, confirming the insecticidal quality of the tested samples (Fig. S2).
Fig. S2.
Mortality rates of susceptible anopheline strains measured 24 h after 3-min exposure to PermaNet 2.0 netting containing 55 mg/m2 deltamethrin (blue) or electrostatic netting containing 37 mg/m2 deltamethrin (red).
Results showed that for all six tested resistant Anopheles strains the adulticidal impact of deltamethrin applied on electrostatic netting was significantly higher than the impact induced by LLIN netting (P < 0.001 for all groups) (Fig. 3). PermaNet 2.0 killed only 9.6% [95% confidence interval (CI) = 1.2–18.0] of the highly resistant An. gambiae Tiassale strain, whereas electrostatic netting with a 33% lower application dose of deltamethrin induced 100% mortality. The most resistant Anopheles line tested, An. funestus FUMOZ-R, showed 10.6% mortality (CI = 1.8–19.4) after PermaNet exposure, whereas the electrostatic netting was able to knock down 96% (CI = 89.8–100) (Table S1) and kill 63% (CI = 49.1–76.9) of the exposed females (Fig. 3). The significantly higher mortality rates induced by electrostatic netting indicate that higher amounts of insecticide were transferred upon contact. The enhanced bioavailability of electrostatically bound particles thus enables effective killing of resistant mosquitoes with WHO-approved doses of public health insecticides.
Fig. 3.
Corrected mortality percentage (n = 50 mosquitoes per treatment) 24 h after pyrethroid-resistant anopheline mosquito strains were exposed to PermaNet netting (55 mg deltamethrin/m2; blue bars) or electrostatic netting (37 mg deltamethrin/m2; red bars) for 3 min. For each treatment, the mortality of mosquitoes exposed to insecticide was corrected for the mortality of counterparts exposed to control netting using Abbott’s formula. Asterisks indicate significant differences determined by χ2 test; ***P < 0.001.
Table S1.
Corrected knockdown and mortality expressed as the percentage of the total number of exposed mosquitoes after 3-min exposure to standard deltamethrin-coated polyester (PermaNet 2.0) or electrostatic gauze with two concentrations of deltamethrin
| PermaNet 2.0, DM 55 mg AI/m2 | Electrostatic gauze, DM 3.7 mg AI/m2 | Electrostatic gauze, DM 37 mg AI/m2 | |||||||||
| Mosquito | Strain | Status | N | Knockdown, % (95% CI) | Mortality, % (95% CI) | N | Knockdown, % (95% CI) | Mortality, % (95% CI) | N | Knockdown, % (95% CI) | Mortality, % (95% CI) |
| Anopheles gambiae | Kisumu | Susceptible | 40 | 97.5 (92.6–100) | 100 | 42 | 100 | 100 | 40 | 100 | 100 |
| SUA | Susceptible | 54 | 100 | 100 | 51 | 100 | 100 | 55 | 100 | 100 | |
| Tiassale | Resistant | 47 | 70.6 (62.0–79.1) | 9.6 (1.2–18.0) | 47 | 93.2 (86.3–100) | 100 | 49 | 97.9 (94.0–100) | 100 | |
| Tongs | Resistant | 55 | 60.0 (47.0–72.9) | 58.2 (24.9–51.1) | 46 | 95.7 (89.8–100) | 95.7 (89.8–100) | 57 | 100 | 100 | |
| Anopheles arabiensis | KBG | Susceptible | 45 | 100 | 100 | 52 | 100 | 100 | 53 | 100 | 100 |
| SENN_DDT | Resistant | 52 | 88.5 (79.9–97.1) | 76.9 (65.5–88.3) | 45 | 75.6 (63.1–88.1) | 82.2 (71.0–93.4) | 55 | 100 | 100 | |
| MBN-DDT | Resistant | 48 | 12.5 (3.1–21.9) | 37.5 (23.8–51.2) | 53 | 88.7 (80.1–97.3) | 96.2 (91.1–100) | 54 | 100 | 100 | |
| Anopheles funestus | FANG | Susceptible | 48 | 100 | 100 | 53 | 100 | 100 | 52 | 100 | 100 |
| Fumoz-R | Resistant | 47 | 8.5 (0.5–16.5) | 10.6 (1.8–19.4) | 46 | 17.4 (6.4–23.4) | 39.1 (25.0–53.1) | 46 | 95.7 (89.8–100) | 63.0 (49.1–76.9) | |
| Fumoz-base | Resistant | 51 | 5.9 (0.0–12.4) | 7.8 (0.4–15.2) | 51 | 13.7 (4.3–23.1) | 49.0 (35.3–62.7) | 53 | 92.5 (88.4–99.6) | 83.0 (72.8–93.2) | |
| Culex quinquefasciatus | I2C-CX | Unknown | 30 | 43.3 (25.7–60.9) | 40.0 (22.6–57.4) | 50 | 10.0 (1.8–18.2) | 82.0 (71.4–92.6) | 31 | 93.5 (86.4–100) | 100 |
| Wild-type | Unknown | 41 | 2.4 (0.0–7.1) | 4.9 (0.0–11.6) | 48 | 52.1 (38.0–66.2) | 95.8 (90.1–100) | 36 | 56.5 (42.2–70.8) | 93.5 (86.4–100) | |
| Aedes aegypti | I2C-AE | Unknown | 32 | 84.4 (71.9–96.9) | 87.5 (76.1–98.9) | 52 | 73.1 (61.0–85.3) | 92.3 (85.1–99.6) | 50 | 100 | 100 |
Knockdown and mortality rates are shown with 95% CIs calculated for each pooled sample proportion. DM, deltamethrin.
Short Contact and Low Insecticide Doses.
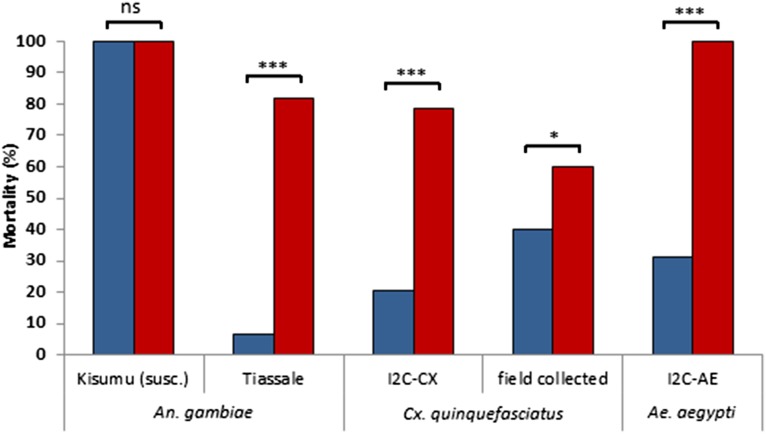
To test further the potential vector-control capacity of electrostatic netting, the exposure period was reduced, and the dose of test insecticides was lowered. A 5-s exposure time was included to mimic situations in which mosquitoes make only short and transient contact with a treated surface, for instance when netting is applied in eave screens. The impact of exposure on the highly resistant An. gambiae Tiassale strain and its susceptible counterpart (Kisumu strain) were tested, as were two strains of Cx. quinquefasciatus and one strain of Ae. aegypti. All strains except the susceptible Kisumu strain were resistant to LLIN exposure, which killed less than 40% after 24 h (Table S2). A 5-s contact resulted in overall lower knockdown and mortality impacts than the 3-min exposures but confirmed the enhanced bioavailability of deltamethrin on electrostatic netting. With a 5-s exposure, electrostatic netting induced significantly higher mortality rates than LLIN netting: 60–100% mortality (P < 0.05 for all groups) (Fig. 4). This increased impact was observed not only in the resistant laboratory strains but also in field-collected Culex specimens from the Kilombero valley in Tanzania. That mosquito contact as short as 5 s is sufficient to induce high mortality indicates that the electrostatic coating may be a useful application technique for vector-control tools for which insect–surface contact is short and transient, such as eave screens or lure-and-kill devices.
Table S2.
Corrected knockdown and mortality as a percentage of the total number of exposed mosquitoes after 5-s contact exposure to PermaNet 2.0 polyester coated with deltamethrin and deltamethrin-dusted electrostatic gauze with two concentrations of active ingredient
| PermaNet 2.0, DM 55 mg AI/m2 | Electrostatic gauze, DM 3.7 mg AI/m2 | Electrostatic gauze, DM 37 mg AI/m2 | |||||||||
| Mosquito | Strain | Status | N | Knockdown, % (95% CI) | Mortality, % (95% CI) | N | Knockdown, % (95% CI) | Mortality, % (95% CI) | N | Knockdown, % (95% CI) | Mortality, % (95% CI) |
| Anopheles gambiae | Kisumu | Susceptible | 48 | 100 | 100 | 42 | 100 | 100 | 40 | 100 | 100 |
| Tiassale | Resistant | 47 | 63.8 (50.1–76.3) | 6.4 (0.0–13.5) | 51 | 15.7 (5.7–25.7) | 37.3 (24.0–50.6) | 50 | 96.0 (90.5–100) | 82.0 (71.4–92.6) | |
| Culex quinquefasciatus | I2C-CX | Unknown | 39 | 20.7 (6.0–35.4) | 20.7 (6.0–35.4) | 38 | 2.6 | 50.0 (34.1–65.9) | 38 | 57.1 (38.7–75.5) | 78.6 (63.3–93.9) |
| Wild-type | Unknown | 50 | 0.0 | 40.0 (26.5–53.5) | 50 | 4.0 | 60.0 (46.5–73.5) | 50 | 38.0 (24.5–51.5) | 60.0 (46.5–73.5) | |
| Aedes aegypti | I2C-AE | Unknown | 42 | 31.0 | 31.0 (17.1–44.9) | 45 | 46.7 | 62.2 (48.1–76.3) | 50 | 100 | 100 |
Knockdown and mortality rates are shown with 95% confidence intervals calculated for each pooled sample proportion. DM, deltamethrin.
Fig. 4.
Corrected mortality percentage (n = 50 mosquitoes per treatment) 24 h after pyrethroid-resistant mosquito strains were exposed to PermaNet netting (55 mg deltamethrin/m2; blue bars), or electrostatic netting (37 mg deltamethrin/m2; red bars) for 5 s. For each treatment, the mortality of mosquitoes exposed to insecticide was corrected for the mortality of counterparts exposed to control netting using Abbott’s formula. Asterisks indicate significant differences determined by χ2 test; *P < 0.05; ***P < 0.001; ns, not significant.
Furthermore, experiments were conducted to test whether lower-than-standard insecticide doses can be used to target mosquitoes effectively when using electrostatic netting. A 15-fold lower target dose of deltamethrin (3.7 mg AI/m2) still was able to kill significantly more resistant mosquitoes in standard cone assays than LLIN netting coated with deltamethrin at 55 mg AI/m2 (P < 0.001; Fig. 5). This difference was less pronounced in the Ae. aegypti strain that showed minimal resistance to the LLIN. These results show that electrostatic netting can kill highly pyrethroid-resistant mosquitoes with deltamethrin concentrations 93% lower than those used on an LLIN and confirm that applying insecticide particles on an electrostatic coating significantly improves bioavailability and hence mosquito mortality.
Fig. 5.
Corrected mortality percentage (n = 50 mosquitoes per treatment) 24 h after pyrethroid-resistant mosquito strains were exposed for 3 min to PermaNet netting coated with deltamethrin (55 mg AI/m2; blue bars), a 15-fold lower dose of deltamethrin on electrostatic netting (3.7 mg AI/m2; black bars), or a similar dose of deltamethrin on electrostatic netting (37 mg AI/m2; red bars). For each treatment, the mortality of mosquitoes exposed to insecticide was corrected for the mortality of counterparts exposed to control netting using Abbott’s formula. Asterisks indicate significant differences determined by χ2 test; *P < 0.05; ***P < 0.001; ns, not significant.
Resistance-Breaking Vector-Control Options.
A range of nonpyrethroid public health insecticides was tested on electrostatic netting, including bendiocarb (a carbamate) and azamethiphos (an organophosphate). Chlorfenapyr (a pyrrole), which is a relatively new class of insecticide for malaria vector control, was evaluated also (34). With standard 3-min cone assays, all tested insecticides induced 100% mortality in Cx. quinquefasciatus and Ae. aegypti mosquitoes 24 h after exposure (Table 1). When contact time was reduced to 5 s, the fast-acting chemicals bendiocarb and azamethiphos still induced 100% mortality, whereas the slower-acting chlorfenapyr induced 97% (CI = 89.9–100) and 31% (CI = 15.5–45.7) mortality in Cx. quinquefasciatus and Ae. aegypti, respectively (Table 1). For both strains, 5-s exposures to chlorfenapyr achieved 100% killing after 48 h. These findings show that the electrostatic coating can be used to apply multiple insecticide classes onto polyester netting effectively and can achieve high mortality rates in mosquitoes from all important vector genera.
Table 1.
Corrected knockdown and mortality in exposed C. quinquefasciatus or A. aegypti mosquitoes after contact with insecticide-loaded electrostatic-coated gauze
| Mosquito genus | Strain | Exposure time | 10% azamethiphos | 20% chlorfenapyr | 1.25% bendiocarb | ||||||
| N | Knockdown, % (95% CI) | Mortality, % (95% CI) | N | Knockdown, % (95% CI) | Mortality, % (95% CI) | N | Knockdown, % (95% CI) | Mortality, % (95% CI) | |||
| Culex | I2C-CX (laboratory) | 5 s | 32 | 87.5 (76.1–98.9) | 100 | 29 | 3.4 (0.1–10.1) | 96.6 (89.9–100) | 48 | 100 | 100 |
| 3 min | 48 | 100 | 100 | 44 | 39.0 (72.7–93.9 | 100 | 47 | 100 | 100 | ||
| Aedes | I2C-AE (laboratory) | 5 s | 48 | 83.3 (72.7–93.9) | 100 | 36 | 0.0 | 30.6 (15.5–45.7) | 50 | 92.0 (84.6–99.4) | 100 |
| 3 min | 46 | 100 | 100 | 53 | 0.0 | 100 | 44 | 100 | 100 | ||
Corrected knockdown and mortality in percentage of C. quinquefasciatus or A. aegypti mosquitoes after 5-s or 3-min contact with electrostatic coated gauze loaded with public health insecticides in powder form. N indicates the total number of mosquitoes exposed per strain (in groups of five to eight mosquitoes). Knockdown and mortality rates are shown with 95% CIs calculated for each pooled sample proportion.
Discussion
For the first time, to our knowledge, these data have demonstrated a new technique to apply and retain insecticides on vertical treated surfaces without a carrier formulation. Results show that the uptake of insecticidal particles from electrostatic netting is much more efficient than the uptake from an LLIN at almost similar or lower target doses of active ingredient per unit surface area. Fluorescent dust tests provided visual support of high powder-transfer efficacy even upon short and transient contact. High insecticidal efficacy of electrostatic netting against six Anopheles mosquito strains with different mechanisms of pyrethroid resistance from across Africa was demonstrated. Even with a mere 5-s contact and at a 15-fold lower dose, the impact of deltamethrin on electrostatic netting was significantly higher than the impact of deltamethrin on an LLIN, confirming the increased bioavailability of the active ingredient. Because the active compound, deltamethrin, was kept unaltered in these comparisons and the observed increases in mortality were similar in all strains regardless of their resistance status, it is unlikely that the mosquitocidal impacts were augmented by differences in modes of action, toxicity, or resistance mechanisms. It is likely that the higher mortality observed results solely from the significant increase in the effective contamination dose, which exceeds the dose that can be tolerated by resistant strains. Similar results indicating that higher dosages of permethrin kill resistant genotypes more efficiently than lower dosages have been reported previously (35). This innovative application method presents an opportunity to improve greatly the control of malaria mosquitoes, in particular those that have become resistant to the insecticides recommended by the WHO.
Netting is a useful application surface for targeting mosquitoes because it can be deployed in house-screening tools and bednets. Currently, insecticide applications for polyester, polyethylene, or polypropylene netting fibers are limited to pyrethroids, the only class of insecticides that can withstand the high temperatures involved in the impregnation process and considered safe enough when contacted daily by humans to be used in bednets. By using the electrostatic coating, we were able to apply multiple public health insecticide classes onto polyester fibers successfully. The ability to deploy multiple insecticides effectively against all important mosquito vector genera opens a myriad of resistance-breaking opportunities to improve the impact of vector control in areas where insecticide resistance is a problem. The electrostatic coating is not limited to insecticidal applications. Previous studies show that it can be used effectively to apply novel and biological control agents such as entomopathogens and autodisseminants. Experiments with Beauveria bassiana spores and pyriproxyfen applied on electrostatic netting inside a novel Aedes ovitrap showed that effective doses of these agents were transferred to gravid aedine mosquitoes, inducing high fungus infection rates and successful larvicide dissemination (36). Electrostatic netting thus can provide a means to apply and deploy novel insecticides currently under development (16), which can further assist in the management of insecticide resistance. Further experiments will focus on using combination products, such as multiple classes of insecticides and combinations with potentially synergistic biological agents (37, 38), and on determining particle characteristics to investigate binding and retention on the electrostatic netting. In-depth knowledge of the binding strength of various types of insecticidal particles to the coating might result in further optimization of electrostatic netting for insect control.
This application technique has potential for use in a large variety of vector-control tools. The electrostatic coating can be applied effectively onto various surfaces, including walls, via spray or paint. Quality control tests with the electrostatic coating on antipollen screens have shown that the electrostatic netting fibers can be washed up to 40 times and still retain the electrostatic charge. Thus coated surfaces can remain active for long time periods and can be reloaded with insecticide at appropriate time intervals. This feature may be of particular use for IRS-like wall applications and tools that use removable inserts. However, further research and field tests will be needed to demonstrate the impact and utility of the coating on different (re)treated substrates and in novel vector-control products.
The electrostatic coating is not considered suitable for bednet treatment, because WHO approves only pyrethroid impregnations for such products (39), and reduced efficacy with direct contact and repeated handling of the netting is anticipated. Electrostatic netting can be useful for house-screening products and point-source applications such as mosquito traps (36). The use of electrostatic netting placed at the eave level of rural houses in Tanzania is currently being investigated. In these houses, the eaves are sealed, and 6-in eave tubes are inserted into the wall. Mosquitoes attracted to the human odors that pass through the tubes are blocked from house entry by electrostatic netting that covers the eave tube. Ongoing field studies indicate that bendiocarb- and deltamethrin-saturated electrostatic netting is highly effective when placed in eave tubes. Large-scale field tests in an area with high frequency of insecticide resistance and particularly multiple insecticide resistance, such as West Africa, would be a next logical step in this research.
When electrostatic netting is deployed, even 15-fold lower doses of approved public health insecticides can kill resistant mosquito strains effectively. The electrostatic mode of application thus may provide a means to lower the total amount of AI needed for effective vector control. More studies will be needed to measure the extent to which doses can be lowered for different insecticides while still achieving resistance-breaking impacts. The potential ability to reduce insecticide application doses in mosquito-control tools could help reduce negative impacts on human health and the environment and might provide more cost-effective vector-control options; such options currently are needed in disease-endemic regions where resources are limited.
In conclusion, the application of electrostatically adhered particles can boost the efficacy and provide resistance-breaking applications of currently recommended public health insecticides. Electrostatic netting offers a wide range of application options for vector control, potentially using insecticide combinations or mosaicking/rotations of multiple bioactives and/or novel classes of insecticides. Insecticide resistance is a growing problem in many countries; a new application technique that can boost insecticidal impact, reduce application doses, and expand options for using other bioactives will provide a significant step forward in vector control and resistance management.
Materials and Methods
Mosquitoes.
Mosquito strain specifications, origins, and resistance profiles are listed in Tables S3 and S4. Ae. aegypti and Cx. quinquefasciatus strains were reared and maintained under laboratory conditions at In2Care BV. An. gambiae strains were reared under laboratory conditions at the Liverpool School of Tropical Medicine or at the Vector Control Reference Laboratory in Johannesburg, South Africa. Cx. quinquefasciatus specimens were collected as larvae from septic tanks in the field in Ifakara, Tanzania (8.05592 S, 36.41001 E) in March 2014 and were kept under ambient conditions. To exclude the impact of intrinsic factors related to insecticide toxicity, all mosquito cohorts used comprised 2- to 6-d-old unfed females, according to the WHO protocol (40).
Table S3.
Origin, rearing, strain, and exposure specifics of the susceptible mosquitoes and the mosquito strains with undefined resistance used in the insecticide bioassays
| Mosquito species | Susceptible mosquitoes | Mosquitoes with undefined resistance | |||||
| Mosquito species | An. gambiae s.s. | An. gambiae s.s. | An. arabiensis | An. funestus | Ae. aegypti | Cx. quinquefasciatus | Cx. quinquefasciatus |
| Mosquito strain | Kisumu | SUA | KGB | FANG | I2C-AE | I2C-CX | Field-collected |
| Origin | Kisumu, Kenya | Suakoko, Liberia | Kanyembe, Zimbabwe | Southern Angola | Aruba | USA | Kilombero, Tanzania |
| Reared by | LSTM (LITE) | VCRU | VCRU | VCRU | In2Care | In2Care | N/A |
| Selected resistance | None | None | None | None | None (field-collected 01/2012) | None | None (field-collected 03/2014) |
| Resistance profile | Fully susceptible | Fully susceptible | Fully susceptible | Fully susceptible | Unknown | Fully susceptible | Fully susceptible |
| Exposure date | 13/03/14 | 28/11/14 | 26/11/14 | 30/11/14 | 10/02/14 | 17/02/14 | 28/03/14 |
| Mosquito age | 3–6 d | 2–4 d | 2 d | 2 d | 3–6 d | 3–4 d | 2 d |
LST (LITE), Liverpool School of Tropical Medicine (Liverpool Insect Testing Establishment).
Table S4.
Origin, rearing, strain, resistance status, and exposure specifics of mosquito strains with well-defined resistance profiles used in the insecticide bioassays
| Mosquito species | An. gambiae s.s. | An. gambiae s.s. | An. arabiensis | An. arabiensis | An. funestus | An. funestus |
| Mosquito strain | Tiassale | Tongs | SENN-DDT | MBN-DDT | Fumoz-R | Fumoz-base |
| Origin | Tiassale, Burkina Faso | Tongon, Ivory Coast | Sennar, Sudan | KwaZulu Natal, South Africa | Mozambique | Mozambique |
| Reared by | LSTM (LITE) | VCRU | VCRU | VCRU | VCRU | VCRU |
| Selected resistance | Pyrethroid resistance | Multiple, no selection since 2010 | DDT resistance (selected since 1995) | DDT resistance (selected until 2013) | Permethrin resistance (selected until 2001) | Naturally multiple resistant |
| Resistance profile | Kdr and P450s | Not known | Kdr, GSTs, P450s, esterases | Kdr, P450s, GSTs, esterases | P450s, GST | P450, GST |
| Exposure date | 13/03/2014 | 26/11/14 | 25/11/14 | 01/12/2014 | 25/11/14 | 27/11/14 |
| Mosquito age | 2–4 d | 3 d | 3 d | 2 or 3 d | 2–4 d | 2–4 d |
LST (LITE), Liverpool School of Tropical Medicine (Liverpool Insect Testing Establishment).
Anopheline Resistance Levels.
The deltamethrin-resistance status of the anopheline mosquito strains tested in the Vector Control Reference Laboratory in Johannesburg, South Africa was confirmed. Standard WHO resistance assays were performed, comprising a 1-h exposure of two replicate groups of 25 unfed females (2- to 4-d-old) per strain, using test tubes lined with 0.05% deltamethrin papers obtained by the WHO vector-control reference unit in Malaysia. The susceptible strains (SUA, TONGs, KGB, and FANG) were included to confirm the insecticidal activity of the WHO test papers (Fig. S1). The susceptible strains all showed 100% knockdown after 1 h and 100% mortality 24 h after exposure. Results of the knockdown and mortality rates of the resistant lines are shown in Tables S1 and S2.
Insecticides.
For baseline pyrethroid impacts, bioavailability tests used a standard WHO-recommended bednet, PermaNet 2.0 (Vestergaard-Frandsen) (41), a long-lasting insecticide-treated polyester net that contains coated deltamethrin (target dose, 55 mg AI/m2) obtained through the courtesy of Helen Pates, Vestergaard-Frandsen, Lausanne, Switzerland. To evaluate electrostatic netting samples, we used a deltamethrin powder (Spritex antiwasp powder containing 0.25% AI) produced by Denka International BV. Electrostatic netting was manufactured by Van Heek Textiles BV. Netting samples of 15 × 15 cm were fully saturated with deltamethrin powder by manually shaking an excess of powder on the gauze in a closed container, resulting in a target dose of 37 mg AI/m2 gauze. A dilution of deltamethrin dust was prepared by mixing 10% Spritex antiwasp powder and 90% inert dust (synthetic amorphous silica; van Eck BV). This dilution was applied onto netting by the same application method, resulting in a target dose of 3.7 mg AI/m2. Untreated polyester bednet material (Vestergaard Frandsen) and untreated electrostatic polyester netting (Van Heek Textiles BV) were included as control treatments.
Bioassays with other public health insecticide classes deployed 1.25% bendiocarb dust (Ficam D; Bayer), 10% azamethiphos wettable powder (Twenty One WP), and 20% chlorfenapyr powder (technical-grade chlorfenapyr obtained from CTF2000) mixed in inert dust. These tests included untreated electrostatic polyester netting as control treatments.
Exposure Assays.
Three-minute exposures were conducted using cone assays according to the WHO protocol (40). Short-contact assays were conducted using a similar set-up made from 1.5-L plastic bottles with the lower half removed and the open end covered with treated netting (42). In all exposures the mosquito numbers and ages were according to the WHO protocol and manual aspirators were used to remove the mosquitoes from the cones after 3-min gauze contact or from the bottles after 5-s gauze contact. After exposure, mosquito cohorts were pooled per treatment, and 1-h knockdown and 24-h mortality were recorded.
Statistical Analysis.
If the control mortality ranged between 5% and 20%, mortality data were corrected using Abbott’s formula to adjust mortality: (%) = (X − Y)/(100 − Y) × 100, where X is the percentage mortality in the treated sample and Y is the percentage mortality in the untreated control sample. Data were analyzed using SPSS 21.0 software. For each experiment, treatments were compared using χ2 tests to test for significance. 95% CIs were estimated for the pooled sample proportions, using the one-sample test of proportions formula: CI = (p̂ ± z*√(p̂(1- p̂)/n))*100, where p̂ is the sample proportion, z is the critical value for a 95% CI, and n is the sample size.
Supplementary Material
Acknowledgments
We thank our European Union project partners Matt Thomas, Andreas Rose, and Patrick Hartmann for fruitful discussions; Judit Bagi (Liverpool School of Tropical Medicine), Lizette Koekemoer and Shüné Oliver (National Institute for Communicable Diseases/National Health Laboratory Service), and the rearing team and technical staff of the Ifakara Health Institute for technical support and the rearing of mosquitoes; the staff of Centre Suisse de Recherche Scientifique for providing access to the Tiassale strain; Ole Skovmand for information on impregnation techniques; and Helen Pates (Vestergaard-Frandsen) for providing PermaNet 2.0 bednets. This work was supported by European Union Seventh Framework Programme Grant 306105, FP7-HEALTH-2012-INNOVATION-1.
Footnotes
Conflict of interest statement: R.A., J.S., R.A.S., A.J.O., B.G.J.K., and M.F. are remunerated by or receive compensation for services delivered to In2Care BV and hold shares in In2Care BV. In2Care BV has one or more patents or patent applications related to the subject of this paper.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1510801112/-/DCSupplemental.
References
- 1.Bhatt S, et al. The global distribution and burden of dengue. Nature. 2013;496(7446):504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petersen LR, Hayes EB. West nile virus in the Americas. Med Clin North Am. 2008;92(6):1307–1322, ix. doi: 10.1016/j.mcna.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 3.WHO 2014 World Malaria Report 2014 (World Health Organization, Geneva) Available at www.who.int/malaria/publications/world_malaria_report_2014/en/. Accessed June 3, 2015.
- 4.WHO 2012 Global Plan for Insecticide Resistance Management in Malaria Vectors (World Health Organization, Geneva) Available at www.who.int/malaria/publications/atoz/gpirm/en/. Accessed June 3, 2015.
- 5.Toé KH, et al. Increased pyrethroid resistance in malaria vectors and decreased bed net effectiveness, Burkina Faso. Emerg Infect Dis. 2014;20(10):1691–1696. doi: 10.3201/eid2010.140619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ranson H, et al. Pyrethroid resistance in African anopheline mosquitoes: What are the implications for malaria control? Trends Parasitol. 2011;27(2):91–98. doi: 10.1016/j.pt.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Hemingway J. The role of vector control in stopping the transmission of malaria: Threats and opportunities. Philos Trans R Soc Lond B Biol Sci. 2014;369(1645):20130431. doi: 10.1098/rstb.2013.0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brogdon WG, McAllister JC. Insecticide resistance and vector control. Emerg Infect Dis. 1998;4(4):605–613. doi: 10.3201/eid0404.980410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diabate A, et al. The role of agricultural use of insecticides in resistance to pyrethroids in Anopheles gambiae s.l. in Burkina Faso. Am J Trop Med Hyg. 2002;67(6):617–622. doi: 10.4269/ajtmh.2002.67.617. [DOI] [PubMed] [Google Scholar]
- 10.Nauen R. Insecticide resistance in disease vectors of public health importance. Pest Manag Sci. 2007;63(7):628–633. doi: 10.1002/ps.1406. [DOI] [PubMed] [Google Scholar]
- 11.Hemingway J, Ranson H. Insecticide resistance in insect vectors of human disease. Annu Rev Entomol. 2000;45(1):371–391. doi: 10.1146/annurev.ento.45.1.371. [DOI] [PubMed] [Google Scholar]
- 12.Chevillon C, Bourguet D, Rousset F, Pasteur N, Raymond M. Pleiotropy of adaptive changes in populations: Comparisons among insecticide resistance genes in Culex pipiens. Genet Res. 1997;70(3):195–203. doi: 10.1017/s0016672397003029. [DOI] [PubMed] [Google Scholar]
- 13.Raymond M, Berticat C, Weill M, Pasteur N, Chevillon C. Insecticide resistance in the mosquito culex pipiens: What have we learned about adaptation? Genetica. 2001;112-113:287–296. [PubMed] [Google Scholar]
- 14.Otali D, et al. Increased production of mitochondrial reactive oxygen species and reduced adult life span in an insecticide-resistant strain of Anopheles gambiae. Bull Entomol Res. 2014;104(3):323–333. doi: 10.1017/S0007485314000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corbel V, et al. Field efficacy of a new mosaic long-lasting mosquito net (PermaNet 3.0) against pyrethroid-resistant malaria vectors: A multi centre study in Western and Central Africa. Malar J. 2010;9:113. doi: 10.1186/1475-2875-9-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hemingway J, Beaty BJ, Rowland M, Scott TW, Sharp BL. The Innovative Vector Control Consortium: Improved control of mosquito-borne diseases. Trends Parasitol. 2006;22(7):308–312. doi: 10.1016/j.pt.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Hougard JM, et al. Efficacy of mosquito nets treated with insecticide mixtures or mosaics against insecticide resistant Anopheles gambiae and Culex quinquefasciatus (Diptera: Culicidae) in Côte d’Ivoire. Bull Entomol Res. 2003;93(6):491–498. doi: 10.1079/ber2003261. [DOI] [PubMed] [Google Scholar]
- 18.N’Guessan R, et al. Mosquito nets treated with a mixture of chlorfenapyr and alphacypermethrin control pyrethroid resistant Anopheles gambiae and Culex quinquefasciatus mosquitoes in West Africa. PLoS One. 2014;9(2):e87710. doi: 10.1371/journal.pone.0087710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pennetier C, et al. Efficacy of Olyset® Plus, a new long-lasting insecticidal net incorporating permethrin and piperonyl-butoxide against multi-resistant malaria vectors [corrected] PLoS One. 2013;8(10):e75134. doi: 10.1371/journal.pone.0075134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oxborough RM, et al. ITN mixtures of chlorfenapyr (Pyrrole) and alphacypermethrin (Pyrethroid) for control of pyrethroid resistant Anopheles arabiensis and Culex quinquefasciatus. PLoS One. 2013;8(2):e55781. doi: 10.1371/journal.pone.0055781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hadaway AB, Barlow F, Grose JE, Turner CR, Flower LS. Evaluation of compounds for insecticidal activity on adult mosquitos. 5. Toxicity to adult mosquitos and residual properties of some pyrethroids. Bull World Health Organ. 1970;42(3):387–398. [PMC free article] [PubMed] [Google Scholar]
- 22.Etang J, et al. Variations of insecticide residual bio-efficacy on different types of walls: Results from a community-based trial in south Cameroon. Malar J. 2011;10:333. doi: 10.1186/1475-2875-10-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindblade KA, et al. Evaluation of long-lasting insecticidal nets after 2 years of household use. Trop Med Int Health. 2005;10(11):1141–1150. doi: 10.1111/j.1365-3156.2005.01501.x. [DOI] [PubMed] [Google Scholar]
- 24.Latheef MA, Carlton JB, Kirk IW, Hoffmann WC. Aerial electrostatic-charged sprays for deposition and efficacy against sweet potato whitefly (Bemisia tabaci) on cotton. Pest Manag Sci. 2009;65(7):744–752. doi: 10.1002/ps.1748. [DOI] [PubMed] [Google Scholar]
- 25.Huang J, Stelinski LL, Gut LJ. Mating behaviors of Cydia pomonella (Lepidoptera: Tortricidae) as influenced by sex pheromone in electrostatic powder. J Econ Entomol. 2010;103(6):2100–2106. doi: 10.1603/ec10063. [DOI] [PubMed] [Google Scholar]
- 26.WHO 2011 Guidelines for Monitoring the Durability of Long-Lasting Insecticidal Mosquito Nets Under Operational Conditions (World Health Organization, Geneva) Available at www.who.int/malaria/publications/atoz/9789241501705/en/. Accessed June 3, 2015.
- 27.Abdalla H, et al. Insecticide susceptibility and vector status of natural populations of Anopheles arabiensis from Sudan. Trans R Soc Trop Med Hyg. 2008;102(3):263–271. doi: 10.1016/j.trstmh.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 28.Hargreaves K, et al. Anopheles arabiensis and An. quadriannulatus resistance to DDT in South Africa. Med Vet Entomol. 2003;17(4):417–422. doi: 10.1111/j.1365-2915.2003.00460.x. [DOI] [PubMed] [Google Scholar]
- 29.Nardini L, et al. Detoxification enzymes associated with insecticide resistance in laboratory strains of Anopheles arabiensis of different geographic origin. Parasit Vectors. 2012;5:113. doi: 10.1186/1756-3305-5-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brooke BD, et al. Bioassay and biochemical analyses of insecticide resistance in southern African Anopheles funestus (Diptera: Culicidae) Bull Entomol Res. 2001;91(4):265–272. doi: 10.1079/ber2001108. [DOI] [PubMed] [Google Scholar]
- 31.Hargreaves K, et al. Anopheles funestus resistant to pyrethroid insecticides in South Africa. Med Vet Entomol. 2000;14(2):181–189. doi: 10.1046/j.1365-2915.2000.00234.x. [DOI] [PubMed] [Google Scholar]
- 32.Awolola TS, Brooke BD, Hunt RH, Coetze M. Resistance of the malaria vector Anopheles gambiae s.s. to pyrethroid insecticides, in south-western Nigeria. Ann Trop Med Parasitol. 2002;96(8):849–852. doi: 10.1179/000349802125002581. [DOI] [PubMed] [Google Scholar]
- 33.Diabate A, et al. The spread of the Leu-Phe kdr mutation through Anopheles gambiae complex in Burkina Faso: Genetic introgression and de novo phenomena. Trop Med Int Health. 2004;9(12):1267–1273. doi: 10.1111/j.1365-3156.2004.01336.x. [DOI] [PubMed] [Google Scholar]
- 34.N’Guessan R, et al. Chlorfenapyr: A pyrrole insecticide for the control of pyrethroid or DDT resistant Anopheles gambiae (Diptera: Culicidae) mosquitoes. Acta Trop. 2007;102(1):69–78. doi: 10.1016/j.actatropica.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 35.Corbel V, et al. Dosage-dependent effects of permethrin-treated nets on the behaviour of Anopheles gambiae and the selection of pyrethroid resistance. Malar J. 2004;3:22. doi: 10.1186/1475-2875-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Snetselaar J, et al. Development and evaluation of a novel contamination device that targets multiple life-stages of Aedes aegypti. Parasit Vectors. 2014;7(1):200. doi: 10.1186/1756-3305-7-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farenhorst M, et al. Synergy in efficacy of fungal entomopathogens and permethrin against West African insecticide-resistant Anopheles gambiae mosquitoes. PLoS One. 2010;5(8):e12081. doi: 10.1371/journal.pone.0012081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farenhorst M, et al. Fungal infection counters insecticide resistance in African malaria mosquitoes. Proc Natl Acad Sci USA. 2009;106(41):17443–17447. doi: 10.1073/pnas.0908530106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kelly-Hope L, Ranson H, Hemingway J. Lessons from the past: Managing insecticide resistance in malaria control and eradication programmes. Lancet Infect Dis. 2008;8(6):387–389. doi: 10.1016/S1473-3099(08)70045-8. [DOI] [PubMed] [Google Scholar]
- 40.WHO 2006 Guidelines for Testing Mosquito Alduticides for Indoor Residual Spraying and Treatment of Mosquito Nets (World Health Organization, Geneva) Available at https://extranet.who.int/iris/restricted/handle/10665/69296. Accessed June 3, 2015.
- 41.WHO 2009 Report of the Twelfth WHOPES Working Group Meeting (World Health Organization, Geneva) Available at www.who.int/whopes/recommendations/wgm/en/. Accessed June 3, 2015.
- 42.Sternberg ED, Waite JL, Thomas MB. Evaluating the efficacy of biological and conventional insecticides with the new ‘MCD bottle’ bioassay. Malar J. 2014;13:499. doi: 10.1186/1475-2875-13-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.