Significance
In this paper we present results on 3D, multiscale laser machining of soft, transparent biomaterials suited for cellular growth and/or implantation. We use an ultrafast laser to generate high-resolution, 3D structures within the bulk of a transparent soft-biomaterial formulation that can support cell growth and allow cells to penetrate deep within the material. The structure is created by multiphoton absorption which, thanks to the clarity of the silk gels, is possible nearly 1 cm below the surface of the material. This depth represents an ∼10× improvement over other materials. The ability to create micrometer-scale voids over such a large volume has promising applications in the biomedical field and its efficacy was demonstrated both in vitro and in vivo.
Keywords: ultrafast lasers, biomaterials, silk, micromachining, tissue engineering
Abstract
Light-induced material phase transitions enable the formation of shapes and patterns from the nano- to the macroscale. From lithographic techniques that enable high-density silicon circuit integration, to laser cutting and welding, light–matter interactions are pervasive in everyday materials fabrication and transformation. These noncontact patterning techniques are ideally suited to reshape soft materials of biological relevance. We present here the use of relatively low-energy ( 2 nJ) ultrafast laser pulses to generate 2D and 3D multiscale patterns in soft silk protein hydrogels without exogenous or chemical cross-linkers. We find that high-resolution features can be generated within bulk hydrogels through nearly 1 cm of material, which is 1.5 orders of magnitude deeper than other biocompatible materials. Examples illustrating the materials, results, and the performance of the machined geometries in vitro and in vivo are presented to demonstrate the versatility of the approach.
The ability to controllably shape biomaterials on the microscale in two, and especially three, dimensions is important given the utility of these structures in guiding cellular growth, differentiation, gene expression, and regeneration (1–4). The use of soft, biocompatible materials, however, poses challenges in fabrication due to their mechanical characteristics. Widely adopted biomaterial microfabrication techniques such as soft- and photolithography are largely limited to two dimensions. The recent advent of 3D printing technology has exploited the interaction of light with materials to rapidly prototype parts for a variety of industries, and has expanded to significantly impact the biomedical field (5–7). Microscale 3D printing has also shown promise for tissue engineering and regenerative medicine applications (8, 9). Here we will present a technique for generating voids as small as 5 μm in diameter within a biocompatible hydrogel using multiphoton absorption (MPA) of light that shares many similarities with 3D printing. Furthermore, we demonstrate that this technique functions in the absence of exogenous photoinitiators or chemical cross-linkers, thereby avoiding potentially biologic incompatibility that can otherwise limit the utility of such processes.
MPA is a process that occurs under extremely intense illumination where two or more low-energy photons are absorbed simultaneously by a material (10). To achieve photon densities high enough for MPA, very short laser pulses must be tightly focused within a material. If the material is transparent to the low-energy photons, very little of the light is absorbed at the surface, allowing a focal spot to be formed, and MPA to occur, deep within the material. Multiphoton-induced structural modification leading to void formation has been investigated in a variety of biocompatible materials including collagen, poly(vinyl-alcohol) (PVA), poly(methyl methacrylate), and gelatin hydrogels (11–13). Poly(ethylene glycol) hydrogels cross-linked with a photolabile bond can be selectively degraded to induce 3D structures (14). Collagen, due to its turbidity, is unsuitable for 3D patterning with features limited to a few tens of micrometers below the surface (15). Transparent materials such as PVA have very high threshold power requirements necessitating the use of high numerical aperture objectives, or amplified femtosecond pulses to initiate MPA for void formation. Extremely high light intensities found in these amplified pulses can locally change a material’s refractive index, resulting in self-focusing of the beam. Self-focusing limits the depth at which a tight focal spot can be formed and has limited MPA-induced void formation to less than 200 μm below the surface of the material (12). Some natural proteins including amyloid (16) and silk fibroin (17) are much more efficient multiphoton absorbers than their amino acid composition would suggest. The hypothesis here was that the large multiphoton cross-section of these natural materials will allow the initiation of MPA at low threshold powers, potentially reducing the effects of self-focusing.
Silk fibroin collected from the domesticated Bombyx mori silkworm has been under steady investigation for decades because of its suitability as a material for biomaterials and tissue engineering. Silk is cytocompitable, biodegradable, and able to stabilize labile compounds such as enzymes and drugs (18). Silk fibroin has also been studied as an optical material due to its transparency to visible light and low surface roughness, giving it the ability to conform to nanoscale structures such as diffraction gratings (19–21) or to generate 3D photonic crystals (22). Previous work involving photomodification of silk has thus far only considered surface modification of dried films (23). Extending this work into the third dimension requires silk to take on a different form. Recently, a highly transparent elastomeric silk fibroin hydrogel has been developed, which is ideally suited to multiphoton laser micromachining (Fig. 1A, Inset) (24). These gels are robust enough to be easily handled, amenable to cell growth, and well tolerated upon implantation. Importantly, these gels are greater than 90% water, which allows material disrupted during MPA to be deposited around the outside of the machined region without fouling.
Fig. 1.
Overview of the micromachining process. (A) Schematic of the multiphoton micromachining workstation. (Inset) Photograph of the transparent silk hydrogel. (B) Three-dimensional AFM image of one of the lines in D. (C) Graph relating line dimensions with pulse energy. Error bars represent 1 SD (n = 4). (D) Microphotograph of lines machined into the upper surface of a silk gel at pulse energies ranging from 0.25 nJ (Bottom) to 5 nJ (Top). (E) End-on view of 30-μm-wide lines machined into a silk gel. Light was incident from the bottom of the image. Ruler on right side measures depth from the surface of the sample. Due to the large area involved, this image was stitched together from a series of microphotographs. (Inset) Detail of the cross-section of one line.
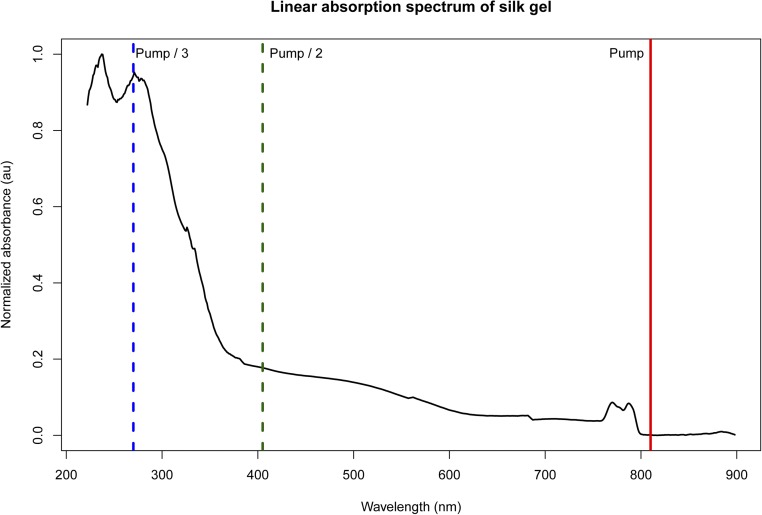
Here we present the exploration of laser-induced void formation in silk hydrogels (hereafter referred to as multiphoton micromachining). We find that relatively low-energy (sub-2 nJ per pulse) infrared (λ = 810 nm) pulses at a high repetition rate (80 MHz) can be used to form voids within the hydrogels in three dimensions. The gels have a linear absorption peak at 270 nm, suggesting this to be a three-photon absorption process (Fig. S1). The short time between pulses (12.5 ns) implies that the heat deposited by the first pulse that arrives does not have time to diffuse away before another pulse hits, leading to thermal accumulation at the focus of the beam which disrupts the silk structure forming voids. The voids formed survive the rigors of handling, cell growth, and subdermal implantation. We further find that it is possible to form voids within the gels nearly 1 cm below the gel surface. To our knowledge, this represents the greatest depth of multiphoton-induced void generation reported, exceeding by 1.5 orders of magnitude the deepest ablation in any material yet tested (12).
Fig. S1.
Linear absorption spectrum of silk gel with background subtracted. The vertical line at 810 nm indicates the wavelength used for multiphoton micromachining. Pump/2 and Pump/3 lines indicate the wavelengths associated with a two-photon and three-photon absorption process, respectively. Linear absorption is driven by tyrosine and tryptophan residues in the silk. Spectrum was filtered by a five-point moving average to reduce noise.
A custom-built 3D laser writing workstation was constructed to study multiphoton micromachining (Fig. 1A). Ultrashort ( 100 fs) laser pulses at a pulse repetition frequency of 80 MHz were focused into the bulk of a silk hydrogel using a 10× (N.A. = 0.3) microscope objective (Fig. S2). The sample was mounted on a three-axis micropositioning stage. The sample could then be moved so the beam was focused in different locations within the material. Generation of complex 3D patterns within the material was achieved by computer control over the stage translation.
Fig. S2.
Results from the knife edge measurement of the laser spot size. (Top) Results in the X direction. (Bottom) Results from the Y direction. Propagation was in the Z direction. The full width at half maximum spot size was calculated to be 5 μm in the X direction and 6 μm in the Y direction.
The relationship between pulse energy and void size was characterized by micromachining a series of lines on the top surface of a gel ∼1 mm thick (Fig. 1D). Each line was made by a single pass of the laser at a constant speed of 50 μm/s with varying pulse energies. After machining, the lines were imaged via atomic force microscopy (AFM). We found the minimum pulse energy necessary to observe structural changes in the silk gel to be ∼0.25 nJ per pulse. At this power, the average trench dimensions were 1.5-μm full width at half maximum (FWHM) in width and 100 nm in depth. These dimensions increased to 2.5-μm FWHM and 600 nm in depth when the pulse energy was raised to 5 nJ (Fig. 1C). AFM measurements confirmed that the change in appearance of the machined region was due to material removal and not local changes in refractive index (Fig. 1B).
The depth at which features could be micromachined was tested by forming a gel inside a plastic fluorescence cuvette. Features were micromachined inside the gel at various depths and subsequently imaged by rotating the cuvette 90° and examining the features using bright-field microscopy. Visible features were found in the gel up to 8 mm below the surface (Fig. 1E and Fig. S3). Deeper features should be possible using a longer working distance objective with a similar numerical aperture. We attribute this large maximum machining depth to the clarity of the silk and the large multiphoton cross-section of the protein, which allows low-powered pulses to be used to initiate MPA without significant self-focusing (Fig. S3). We estimated the critical power for self-focusing to be greater than 6 MW, which is more than 100× more power than is found in the pulses used for micromachining (Fig. S4). This combination of qualities is, to the best of our knowledge, unique to silk and enables multiphoton micromachining to occur at such large depths. Deep, high-resolution features such as these, combined with the ability to dope the silk with growth factors and other compounds, could be used for the generation of complex 3D patterned cell scaffolds to form microenvironments for different cell types within the same scaffold.
Fig. S3.
Light incident from the left of the image was used to micromachine features at various depths in a gel. Each feature was made by a single pass of the laser scanning into the page at ∼10 nJ per pulse of power and a rate of 75 μm/s. Large boxes show a zoomed-in view of the outlined area around each individual feature. The shape of each feature remains constant at each depth, indicating that self-focusing is not significantly deflecting the beam in the material. Due to the large area involved, this image was stitched together from several different photomicrographs.
Fig. S4.
Amplified laser pulses incident from the bottom were used to create voids in the silk hydrogels at various pulse energies. Symmetrical features using 1-μJ pulses (Left) indicate that self-focusing is negligible. Strong self-focusing effects are evident in the asymmetrical shape from 10-μJ pulses (Center) and 20-μJ (Right) pulse energies. All features were made by a single pulse at each location.
With maximum penetration depth of nearly 1 cm and a lateral resolution on the order of 5 μm, silk hydrogels are an excellent substrate for multiphoton micromachining. Given the limits of travel of the micropositioning stage, the total addressable volume of our workstation was greater than 100 cm3. Within this volume, individual voxels as small as 125 μm3 could be removed at will, with the removed material deposited along the outer edges of the machined regions.
To explore the practicality of this technique to generate complex 3D structures, test patterns were micromachined into the bulk of the silk gel. The first was a helix consisting of two turns with an outer diameter of 200 μm (Fig. 2A). The structure started roughly 500 μm below the surface and extended 400 μm further into the gel. The second pattern chosen was a blood-vessel–like branching pattern (Fig. 2E). This structure was situated 300 μm from the surface and had a vertical extent of 100 μm. To image these patterns, the silk was stained with Rhodamine B after multiphoton micromachining and tomographic images were collected using confocal microscopy. The Rhodamine-stained silk fluoresced brightly whereas the machined regions were dark, indicating removal of the hydrogel in these regions. In most cases, the edges of the machined features showed evidence of greater material removal than the bulk of the features. This pattern was due to the control program, which paused lateral motion of the micropositioning stage at the end of each line before closing the shutter so the edges of the features were always exposed to more pulses than the center. Increased fluorescence was also visible around the edges of the features, which we attribute to the deposition of removed material along the borders. We also observed this phenomenon when imaging using the autofluorescence of silk for contrast rather than exogenous stains (Fig. S5).
Fig. 2.
Overview of two test patterns machined into the gel. (A) Three-dimensional model of a helical pattern input into the control program. (B) Confocal microscope image of a cross-section of the helix showing the machined region in black. (Scale bar, 100 μm.) Please see video reconstruction in Movie S1. (C) Reslice of the confocal stack along the dashed line in B. (Scale bar, 100 μm.) (D) Three-dimensional reconstruction of the segmented confocal data showing the machined feature. (E) Three-dimensional model of a branching pattern input into the machining control program. (F) Three-dimensional reconstruction of resulting machined region made by segmenting the confocal images. (G) Confocal slice showing a cross-section of the micromachined region. (Scale bar, 100 μm; same for H and I.) (H and I) Cross-sections of the confocal volumes at the indicated lines.
Fig. S5.
Confocal cross-section (Left) of the vascular-like pattern described in Fig. 2 using only the autofluorescence of silk fibroin for contrast. An increase in autofluorescence can be appreciated immediately surrounding the machined region which suggests that an increased density of silk is present in those locations. (Right) The two panels are reslices of the confocal stack along the indicated lines. (Scale bar applies to all panels.)
To be useful in biomedical applications, a material must be nontoxic and support cell growth. To ensure that the machined regions were not harmful to cells in culture, we prepared sterile gels by filtering the silk through a 0.22-μm pore filter and mixed the solution in a 35-mm-diameter plastic Petri dish under sterile conditions for gelation. Before removing the dishes from the hood the lids were covered with parafilm to maintain sterility. All machining of the gels was done within the sealed Petri dishes in ambient conditions.
Parallel lines ∼3 μm in width separated by about 20 μm were micromachined onto the top surface of a gel through the bulk. Human foreskin fibroblasts were seeded on the surface and observed using phase contrast microscopy as they attached and spread over the dish. We observed that the cells tended to align with the grooves machined into the gel and grew parallel with these surface features (Fig. 3 A–D). This contact guidance phenomenon is well-known and has previously been used to induce alignment of various cell types (25, 26). Because features can be machined onto the gel through a sealed dish, we hypothesize that this could be a convenient method to reorient or disrupt already established cell cultures.
Fig. 3.
Micromachined features in vitro. (A–D) Machined lines on the surface of a gel at day 1, 3, 5, and 8, respectively. Arrows indicate cells growing along the machined lines. D shows a fluorescently labeled cells growing in the lines. (Scale bar, 100 μm long.) D shows a slightly different region of the gel as high cell density obscured the features at the location of the other images. (E) Cartoon showing micromachining of a gel laden with hMSCs. (Inset) Bright-field image of the machined region. (Scale bar, 250 μm.) (F–H) Confocal images of the cell-laden gel following live/dead staining 76 μm below, 62 μm above, and in the plane of machining, respectively. Dashed lines outline the micromachined region. (Scale bar, 250 μm.) (I and J) Close-up of living cells irradiated by the beam above (I) and below (J) the focal plane.
In tissue engineering, access to oxygen and nutrients within an artificial tissue is a major challenge that limits cell density within tissue engineered constructs (27). To address this issue, researchers have generated scaffolds with interconnected porous networks (28). However, such pores are randomly distributed, limiting the amount of control of cell growth and infiltration that is possible. Multiphoton micromachining allows fully predetermined micrometer-scale features to be generated within a construct, allowing spatial control over cell infiltration. To test whether micromachined features within the silk hydrogels could be used to direct cell growth in three dimensions, Y-shaped branching patterns were machined into the gels such that the main branch intersected the surface, allowing cells and media to penetrate the bulk of the gel (Fig. 4A). Cells were stained with a fluorescent dye and confocal images were taken of each feature at days 5, 9, and 14 postseeding. Cell density was assessed at three locations within each feature: the main branch, the transition region, and the lower branch. By day 9 and continuing to day 14, cells were observed in all three regions in 100% of the small features. The larger features were less well populated with cells found in 100% of the main branches, 86% of the transition regions, and only 14% of the lower branches by day 9. On day 14, 71% of the large features had cells in the lower branches. One of the large features did not intersect the surface of the gel and was omitted from this analysis. No subsurface cells were observed in areas that were not laser machined (Fig. 4A).
Fig. 4.
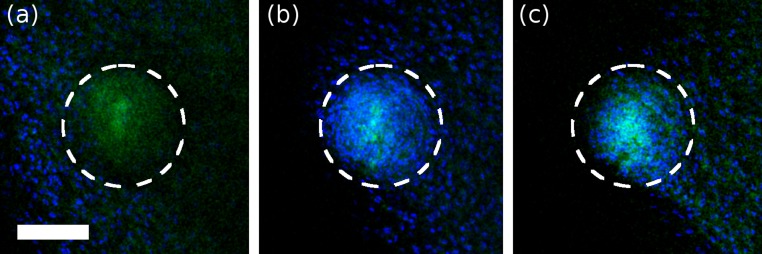
Cell infiltration into machined features. (A, Top) Three-dimensional model of the pattern machined into hydrogels that were subsequently seeded with cells in vitro. (A, Bottom) Series of confocal images of fibroblasts growing within a Y-shaped machined feature on day 9 after seeding. Each image is separated by 10 μm in the Z direction. (Scale bar, 100 μm.) (B, Top) Three-dimensional model of the pattern machined into a hydrogel that was subsequently implanted s.c. in mice. Lines marked “(i–iii)” indicate the confocal cross-sections shown in the panels below. The white circles in (B, i) and (B, ii) correspond to the main branch diameter and approximate location in the construct. The smaller circles in (B, iii) correspond to the secondary branch diameters. Cells had infiltrated to the bottom of the main branch (B, ii) and had begun extending down one of the secondary branches (B, iii). (All scale bars, 100 μm.)
Rather than providing a means for cells to infiltrate a material from the surface, it is often easier to encapsulate cells within the material itself. It has been shown that human mesenchymal stem cells (hMSCs) can be encapsulated within this type of silk hydrogel (24). When cells are encapsulated in this way, the concentrations of oxygen, nutrients, and growth factors are governed by diffusion, limiting the size of such constructs. Three-dimensional patterned cell-laden hydrogels would have more surface area for the diffusion of oxygen and well-defined patterns could provide an artificial microvasculature, greatly increasing the maximum size at which cell growth could be supported. To investigate the ability of multiphoton micromachining to pattern cell-laden hydrogels, we embedded hMSCs in the bulk of a thin gel. The word “Tufts” was micromachined into the gel (Fig. 3E) and, less than 4 h after machining, cells were stained with a live/dead fluorescence assay. Following staining the dishes were examined using confocal microscopy. We found dead cells in the plane of micromachining with living cells present both directly above and below the machined volume (Fig. 3 F–J). This was expected as cells are largely transparent to 810-nm light so they should be unaffected by the beam far from the focus. The high temperatures at the focus of the beam are likely responsible for the dead cells found in the micromachined regions.
Finally, we conducted a pilot in vivo study in which three mice were implanted with two machined gels each. One gel contained a branching pattern with a main branch diameter of 200 μm; the second gel contained a branching pattern with a main branch diameter of 400 μm. One mouse was killed at 2, 3, and 4 wk. Upon subsequent imaging we were able to identify the machined features in four of the six samples with at least one feature identified at each timepoint. Cells were found to penetrate the gels via the machined features in the 2- and 3-wk sample (Fig. 4 and Figs. S6 and S7). In the 4-wk sample, cells were found to have overgrown the machined feature and not penetrate into the gel. It is likely that the overgrowth in the 4-wk case was not due to the extra time of implantation as no cells were seen to penetrate the gel, but rather occurred relatively soon after implantation.
Fig. S6.
Confocal images of micromachined gel containing a feature with main branch diameter of 400 μm. A is on the surface of the gel and B is 74 μm below the gel surface. Dashed white lines indicate the diameter and approximate location of the main branch of the feature. No cells are present in the micromachined region on the surface, but are found in high concentrations at the bottom of the main branch. This sample was recovered 3 wk after implantation.
Fig. S7.
Confocal images of micromachined gel containing a feature with main branch diameter of 200 μm. A is taken from the top surface of the gel, B is 50.5 μm below the surface, and C is 70 μm below the surface of the gel. A relative absence of cells can be seen at the surface whereas cells conforming to the shape of the feature are visible below the surface of the gel. This sample was recovered 3 wk after implantation. (Scale bar, 100 μm.)
These results are significant as they show that multiphoton micromachining in silk fibroin hydrogels was capable of directing cell growth and speeding infiltration into an artificial construct. Patterned biocompatible constructs are of great interest in the field of tissue engineering, which seeks to artificially recapitulate natural structures in the body. One promising avenue to do so is the use of decellularization as a means to replace damaged organs (29). This technique involves the harvest of a healthy organ and the removal of all cellular material, leaving behind a structured extracellular matrix. The resulting decellularized scaffold acts as a template for new cell growth. However, this technique requires access to a healthy organ as well as time for cell culture. Whereas this method could be used to reduce rejection of donated organs, it does little to help those who are still waiting for an organ transplant. Whereas the micropatterning described here is too small-scale to be used to replicate an entire organ, it provides a unique combination of high-resolution (micrometer-scale) structuring with the possibility of generating large (nearly millimeter-scale) features. We believe this combination of high resolution with large volume of modification could prove useful to link large-scale 3D patterning of biological materials using techniques like 3D bioprinting (8), with techniques to produce random voids in a material on the 0.1-m scale (28).
In conclusion, silk hydrogels were found to be an attractive substrate for photoinitiator-free multiphoton micromachining. Using only moderate laser power it was possible to generate voids within the bulk material at depths of nearly 1 cm. This approach enabled rapid formation of high-resolution structures over multiple length scales in three dimensions and could be carried out in cell-laden hydrogels without damage to living cells in the volume immediately adjacent to the micromachined region. The features are formed in a soft, biocompatible matrix which enables the guidance of cells in three dimensions and appears to promote infiltration of cells in vivo without loss of the pattern’s structural integrity. All-aqueous processing of the material and machining at ambient temperatures without harsh solvents or toxic photoinitiators should make it possible to further promote cell infiltration and differentiation using growth factors or other chemical signals. Whereas there are many options to improve the resolution and utility of the micromachining workstation, the technique described here allows for rapid prototyping of mesoscale features in a robust, simple to use, biocompatible substrate. Three-dimensional patterns that are suitable for guiding cell growth can be produced over large volumes with high resolution. Such patterned gels allow control over cell growth and implantation on the 10-μm scale, allowing the recapitulation of native micrometer-scale structures in tissue engineering scaffolds. This approach for the generation of programmable structures using multiphoton micromachining in biocompatible silk hydrogels is virtually impossible to produce using any other method, opening numerous new avenues of investigation into the 2D and 3D patterning of soft materials.
Materials and Methods
Multiphoton Micromachining Workstation.
Approximately 100-fs pulses of 810-nm light from a titanium sapphire oscillator (Tsunami, Spectra Physics) at a repetition rate of 80 MHz were passed through a computer-controlled shutter and directed into the rear accessory port of an inverted microscope. The light was focused to an 5 μm spot through a 0.3-N.A. microscope objective with a working distance of 1.03 cm onto the sample, which was placed on a computer-controlled XYZ translation stage (Ludl Electronics). Using a custom LabView application, complex patterns could be micromachined by inputting stacks of binary images into the program. Pulse energies were manually adjusted via a half-wave plate and polarizer giving continuous control of pulse energy from 0.1 to ∼10 nJ per pulse.
Hydrogel Preparation.
Silk fibroin was extracted as described in ref. 30 with a degumming time of 60 min. Gels were prepared by adding 10 units/mL type VI horseradish peroxidase and 10 μL/mL 1% hydrogen peroxide (24). To facilitate fluorescence imaging, Rhodamine B-stained gels were prepared after the desired pattern had been micromachined. These gels were soaked in a solution of 0.1 mM Rhodamine B for 4 h and then were rinsed in 10 changes of deionized water over the following 24 h to remove any Rhodamine not bound to the silk.
Gel Micromachining.
Lines were micromachined on the top surface of a thin gel at pulse energies ranging from 0.25 to 5 nJ per pulse and were imaged on an MFP-3D-Bio AFM (Asylum Research). The samples were scanned in contact mode under PBS solution using TR800PSA cantilevers with a calibrated spring constant of 0.4 N/m.
Maximum depth of machining was determined by forming a silk gel inside a plastic fluorescence cuvette. Thirty-μm-thick lines were micromachined in the silk at regular depth intervals. Translation speed varied between 100 and 25 μm/s depending on the depth. A side view of the lines was obtained by rotating the cuvette 90° and imaging via bright-field microscopy.
Two-Dimensional Contact Guidance.
Silk solutions were filtered through a 0.22-μm filter and gelled in a 35-mm Petri dish. Before removal from the hood, dishes were sealed with parafilm to maintain sterility. Lines were then micromachined onto the top surface of the gel. Human foreskin fibroblasts were seeded onto the gel and cultured in DMEM with 10% FBS at 37 °C, 5% CO2. Gels were imaged via phase contrast microscopy at day 1, 3, and 5 postseeding. On day 8 the cells were stained with a Live/DeadViability/Cytotoxicity kit (Molecular Probes, Inc.) fluorescence assay and imaged via fluorescence microscopy.
Cell-Laden Hydrogels.
Human mesenchymal stem cells (hMSCs) were isolated from fresh bone marrow aspirate (Lonza) as previously described (31). hMSCs were gently mixed into a partially gelled silk hydrogel at a rate of 1,000 cells per mm3. One hundred μL of the silk/cell mixture was added to each glass-bottomed Petri dish (24). After gelation, micromachining was performed on the cell-laden hydrogels. Within 4 h of machining the cells were stained with a Live/DeadViability/Cytotoxicity kit (Molecular Probes, Inc.) and examined via confocal fluorescence microscopy.
Three-Dimensional Cell Guidance.
Sterile gels were prepared as described in 2D Contact Guidance above. Three-dimensional Y-shaped branching patterns were machined into the gel, with the main branch of the Y intersecting the top surface of the gel. Main branch diameters were 200 μm and 400 μm in the small and large features, respectively. Human foreskin fibroblasts were seeded onto the surface of the gel after micromachining. Gels were examined via confocal microscopy on day 5, 9, and 14 postseeding. The day before each imaging session dishes were stained with CytoTraker Green (Molecular Probes).
Implantation.
Sterile gels were prepared in 35-mm Petri dishes as described above and a 4-mm biopsy punch was used to remove cylinders of gel. Branching patterns (Fig. 4B) were machined into each cylinder. All procedures involving mice were approved by the Tufts University Institutional Animal Care and Use Committee. Animals were anesthetized by isoflurane inhalation during the procedure. Machined gels were implanted s.c. into the lumbar region of three mice. Silk implants and adjacent tissues were extracted following euthanasia (carbon dioxide asphyxiation) at 2-, 3-, and 4-wk postimplantation. Gels were recovered from the mice and fixed in 10% formalin and stained with Phalloidin and DAPI.
Scattering Loss Measurements
Silk solution was filtered through a 0.22-μm pore size filter to remove dust and other scatterers present in the solution. This solution was formed into a gel in a plastic semimicro fluorescence cuvette with 5- and 10-mm path lengths. A green laser was propagated through both the long and short paths and the transmitted intensity measured. From these measurements we were able to calculate scattering losses in the gel to be 0.6 0.3 dB/cm. This translates to a 1/e scattering length of between 5 and 14 cm.
Spot Size Measurement
The spot size at the focus of the microscope objective was measured via the knife edge technique. The beam was focused in the plane of a razor blade which was mounted on a micropositioning stage. A second microscope objective was used to collect light transmitted past the razor blade. During the measurement the razor blade was moved through the focus of the beam and the transmitted intensity measured using a photodiode. The derivative of the measured intensity was fit to a Gaussian function to calculate the spot size of the beam. This measurement was repeated in both the X and Y directions. The beam profile was found to be a good fit for the Gaussian function with a spot size of 5 μm in the X direction and 6 μm in the Y direction (Fig. S2). This spot size is larger than what would be expected from a diffraction-limited system because the beam did not fill the rear aperture of the objective.
Self-Focusing
The symmetrical shape of the features along the optical axis indicates that self-focusing effects are not present using low-energy pulses. To obtain an estimate of the critical power for self-focusing (), we used an amplified laser system to examine the shape of the features micromachined by a single pulse using the same 10×, 0.3-N.A. objective used for oscillator-only micromachining. Asymmetrical features consistent with self-focusing were observed at pulse energies above 1 μJ (Fig. S3). Assuming a 150-fs pulse, this translates to on the order of 6 MW. The peak power of the pulse during micromachining is no more than 60 kW, which is far below the threshold value and explains why self-focusing is avoided.
In Vivo Implantation
Gels with branching features machined into them were implanted in mice for up to 4 wk. Two sizes of branching features were formed, one with a main branch diameter of 200 μm and the other with a main branch diameter of 400 μm. In addition to the figure in the main text (Fig. 4), additional confocal stacks were collected and are presented here (Figs. S4 and S5).
Supplementary Material
Acknowledgments
The authors acknowledge Dr. Elise Spedden for help with the AFM. The authors acknowledge funding from the Office of Naval Research (N00014-13-1-0596). M.B.A. acknowledges support from the Stern Fellowship at Tufts University. M.B.A. and B.P.P. received support from the National Defense Science and Engineering Graduate Fellowship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1509405112/-/DCSupplemental.
References
- 1.Lapointe VLS, Fernandes AT, Bell NC, Stellacci F, Stevens MM. Nanoscale topography and chemistry affect embryonic stem cell self-renewal and early differentiation. Adv Healthc Mater. 2013;2(12):1644–1650. doi: 10.1002/adhm.201200382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi X, et al. Directing osteogenesis of stem cells with drug-laden, polymer-microsphere- based micropatterns generated by teflon microfluidic chips. Adv Funct Mater. 2012;22(18):3799–3807. [Google Scholar]
- 3.Jeon O, Alsberg E. Regulation of stem cell fate in a three-dimensional micropatterned dual-crosslinked hydrogel system. Adv Funct Mater. 2013;23(38):4765–4775. doi: 10.1002/adfm.201300529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mandal BB, Kundu SC. Cell proliferation and migration in silk fibroin 3D scaffolds. Biomaterials. 2009;30(15):2956–2965. doi: 10.1016/j.biomaterials.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Giannatsis J, Dedoussis V. Additive fabrication technologies applied to medicine and health care: A review. Int J Adv Manuf Technol. 2007;40:116–127. [Google Scholar]
- 6.Rengier F, et al. 3D printing based on imaging data: Review of medical applications. Int J CARS. 2010;5(4):335–341. doi: 10.1007/s11548-010-0476-x. [DOI] [PubMed] [Google Scholar]
- 7.Cohen A, Laviv A, Berman P, Nashef R, Abu-Tair J. Mandibular reconstruction using stereolithographic 3-dimensional printing modeling technology. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108(5):661–666. doi: 10.1016/j.tripleo.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 8.Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32(8):773–785. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 9.Peltola SM, Melchels FPW, Grijpma DW, Kellomäki M. A review of rapid prototyping techniques for tissue engineering purposes. Ann Med. 2008;40(4):268–280. doi: 10.1080/07853890701881788. [DOI] [PubMed] [Google Scholar]
- 10.Goppert-Mayer M. Elementary processes with two quantum jumps. Ann Phys. 1931;9:273–294. [Google Scholar]
- 11.Liu Y, Sun S, Singha S, Cho MR, Gordon RJ. 3D femtosecond laser patterning of collagen for directed cell attachment. Biomaterials. 2005;26(22):4597–4605. doi: 10.1016/j.biomaterials.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 12.Oujja M, et al. Three dimensional microstructuring of biopolymers by femtosecond laser irradiation. Appl Phys Lett. 2009;95:263703. [Google Scholar]
- 13.Day D, Gu M. Microchannel fabrication in PMMA based on localized heating by nanojoule high repetition rate femtosecond pulses. Opt Express. 2005;13(16):5939–5946. doi: 10.1364/opex.13.005939. [DOI] [PubMed] [Google Scholar]
- 14.Kloxin AM, Kasko AM, Salinas CN, Anseth KS. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science. 2009;324(5923):59–63. doi: 10.1126/science.1169494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith NI, Fujita K, Nakamura O, Kawata S. Three-dimensional subsurface microprocessing of collagen by ultrashort laser pulses. Appl Phys Lett. 2001;78:999. [Google Scholar]
- 16.Hanczyc P, Samoc M, Norden B. Multiphoton absorption in amyloid protein fibres. Nat Photonics. 2013;7:969–972. [Google Scholar]
- 17.Applegate MB, Marelli B, Kaplan DL, Omenetto FG. Determination of multiphoton absorption of silk fibroin using the Z-scan technique. Opt Express. 2013;21(24):29637–29642. doi: 10.1364/OE.21.029637. [DOI] [PubMed] [Google Scholar]
- 18.Tao H, Kaplan DL, Omenetto FG. Silk materials--a road to sustainable high technology. Adv Mater. 2012;24(21):2824–2837. doi: 10.1002/adma.201104477. [DOI] [PubMed] [Google Scholar]
- 19.Omenetto FG, Kaplan DL. A new route for silk. Nat Photonics. 2008;2:641–643. [Google Scholar]
- 20.Amsden JJ, et al. Rapid nanoimprinting of silk fibroin films for biophotonic applications. Adv Mater. 2010;22(15):1746–1749. doi: 10.1002/adma.200903166. [DOI] [PubMed] [Google Scholar]
- 21.Brenckle MA, et al. Protein-protein nanoimprinting of silk fibroin films. Adv Mater. 2013;25(17):2409–2414. doi: 10.1002/adma.201204678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim S, Mitropoulos A, Spitzberg J. Silk inverse opals. Nat Photonics. 2012;6:2–7. [Google Scholar]
- 23.Lazare S, et al. Bombyx mori silk protein films microprocessing with a nanosecond ultraviolet laser and a femtosecond laser workstation: Theory and experiments. Appl Phys, A Mater Sci Process. 2011;106:67–77. [Google Scholar]
- 24.Partlow BP, et al. Highly tunable elastomeric silk biomaterials. Adv Funct Mater. 2014;24(29):4615–4624. doi: 10.1002/adfm.201400526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dunn GA, Ebendal T. Contact guidance on oriented collagen gels. Exp Cell Res. 1978;111(2):475–479. doi: 10.1016/0014-4827(78)90196-9. [DOI] [PubMed] [Google Scholar]
- 26.Gomez N, Chen S, Schmidt CE. Polarization of hippocampal neurons with competitive surface stimuli: Contact guidance cues are preferred over chemical ligands. J R Soc Interface. 2007;4(13):223–233. doi: 10.1098/rsif.2006.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sachlos E, Czernuszka JT. Making tissue engineering scaffolds work. Review: The application of solid freeform fabrication technology to the production of tissue engineering scaffolds. Eur Cell Mater. 2003;5:29–39, discussion 39–40. doi: 10.22203/ecm.v005a03. [DOI] [PubMed] [Google Scholar]
- 28.Bellan LM, et al. Fabrication of an artificial 3-dimensional vascular network using sacrificial sugar structures. Soft Matter. 2009;5:1354–1357. [Google Scholar]
- 29.Badylak SF, Taylor D, Uygun K. Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds. Annu Rev Biomed Eng. 2011;13:27–53. doi: 10.1146/annurev-bioeng-071910-124743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rockwood DN, et al. Materials fabrication from Bombyx mori silk fibroin. Nat Protoc. 2011;6(10):1612–1631. doi: 10.1038/nprot.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Altman GH, et al. Cell differentiation by mechanical stress. FASEB J. 2002;16(2):270–272. doi: 10.1096/fj.01-0656fje. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.