Significance
Every autumn across the North Atlantic, large numbers of zooplankton copepods migrate from the surface waters into the ocean's interior to hibernate at depths of 600–1,400 m. Through this migration, they actively transport lipid carbon to below the permanent thermocline, where it is metabolized at a rate comparable to the carbon delivered by sinking detritus. This “lipid pump” has not been included in previous estimates of the deep-ocean carbon sequestration, which are based on either measurements of sinking fluxes of detritus, or estimates of new primary production. Unlike other components of the biological pump, the lipid pump does not strip the surface ocean of nutrients, and decouples carbon sequestration from nutrient replenishment, a process we term the “lipid shunt.”
Keywords: lipid pump, lipid shunt, carbon sequestration, Calanus, annual migration
Abstract
Estimates of carbon flux to the deep oceans are essential for our understanding of global carbon budgets. Sinking of detrital material (“biological pump”) is usually thought to be the main biological component of this flux. Here, we identify an additional biological mechanism, the seasonal “lipid pump,” which is highly efficient at sequestering carbon into the deep ocean. It involves the vertical transport and metabolism of carbon rich lipids by overwintering zooplankton. We show that one species, the copepod Calanus finmarchicus overwintering in the North Atlantic, sequesters an amount of carbon equivalent to the sinking flux of detrital material. The efficiency of the lipid pump derives from a near-complete decoupling between nutrient and carbon cycling—a “lipid shunt,” and its direct transport of carbon through the mesopelagic zone to below the permanent thermocline with very little attenuation. Inclusion of the lipid pump almost doubles the previous estimates of deep-ocean carbon sequestration by biological processes in the North Atlantic.
Understanding the dynamics of deep-ocean carbon sequestration is fundamental to estimating global carbon budgets and its response to anthropogenic emissions. An important element of these dynamics is the uptake of CO2 in the surface ocean by phytoplankton to produce organic carbon, a proportion of which is subsequently transported to the deep ocean by several processes collectively known as the “biological pump” (1). The main vehicle for this transport is thought to be the passive sinking of organic detritus (2) which, on a global scale, is estimated to sequester 1–4 g C⋅m−2⋅y−1 at around 1,000-m depth (3, 4). Zooplankton are important players in the biological pump (5), feeding on the primary production, which they repackage into fast sinking fecal pellets (6, 7). Other zooplankton-mediated processes include the feeding and disruption of particle fluxes (8, 9), and their active transport by vertical migrations (10, 11). In particular, active transport to the deep ocean by annual ontogenic migrations of various copepod species (12–15) has been shown to be potentially important in sequestering carbon in different locations. Less well documented is how carbon sequestration associated with these annual overwintering migrations compares to other processes at the scale of ocean basins. More importantly, the biological pump is as much about nutrient cycling as it is about carbon export and sequestration (16, 17). In this, the biochemical makeup of the compounds that are transported and respired by zooplankton at depth can have profound implications for nutrient cycling and the efficiency of the oceanic carbon pump.
Every year at the end of summer, trillions of copepods descend into the deep-ocean basins of the North Atlantic to overwinter in a state of diapause (hibernation). Copepods of the genus Calanus provide a particularly striking example of organisms exhibiting this life history strategy (18). The species Calanus finmarchicus, Calanus helgolandicus, Calanus glacialis, and Calanus hyperboreus in the polar and temperate North Atlantic, and species with similar functional roles in the Pacific and Southern Ocean (19, 20), form a vital trophic link between primary producers and higher trophic levels (21, 22). In terms of distribution, C. finmarchicus is the most cosmopolitan of these and is found in high abundances from the Gulf of Maine to north of Norway (23). This species has a 1-y life cycle from eggs through six naupliar and six copepodite development stages, similar to insect instars. Reproduction, feeding, and growth occur during spring and summer in the surface waters. However, during copepodite stages 3–5 (C3–C5), assimilated food is increasingly channeled toward lipid production. These lipids form the energy reserves required to sustain the animals through the winter. In the autumn, development pauses at copepodite C5, and the population descends en masse to depths of 600–1,400 m in the ocean to overwinter in a state of diapause (24), where temperatures are between −1 and 8 °C (25). Diapause depth varies between regions but it must a priori be below the depth of the permanent thermocline to prevent the torpid animals from being prematurely returned to the surface waters. In the spring, the survivors migrate back to the surface and develop into the adults that initiate the next generation.
Depending on region, the diapause duration of C. finmarchicus varies from 4 to 9 mo. Buoyancy control (26) and survival through this long period leaving sufficient reserves to support reproduction the following spring (27), are sustained by the lipids accumulated during the previous summer in the surface waters. These reserves, which may account for over 50% of dry weight, are made up of wax esters (WEs), long-chain carbon and energy-rich compounds that include omega-3 fatty acids. The fraction of lipid that is metabolized at depth to sustain the copepodites through the period of fasting represents a sequestration flux of carbon from the ocean surface.
Here, we provide estimates for the carbon flux associated with the annual overwintering migrations of the copepod Calanus finmarchicus across the basins of the North Atlantic. In particular, we focus on the role of lipids in the annual migration of these animals and introduce two concepts, the “lipid pump” and the “lipid shunt,” both of which have important implications for our understanding of ocean carbon cycling.
Results
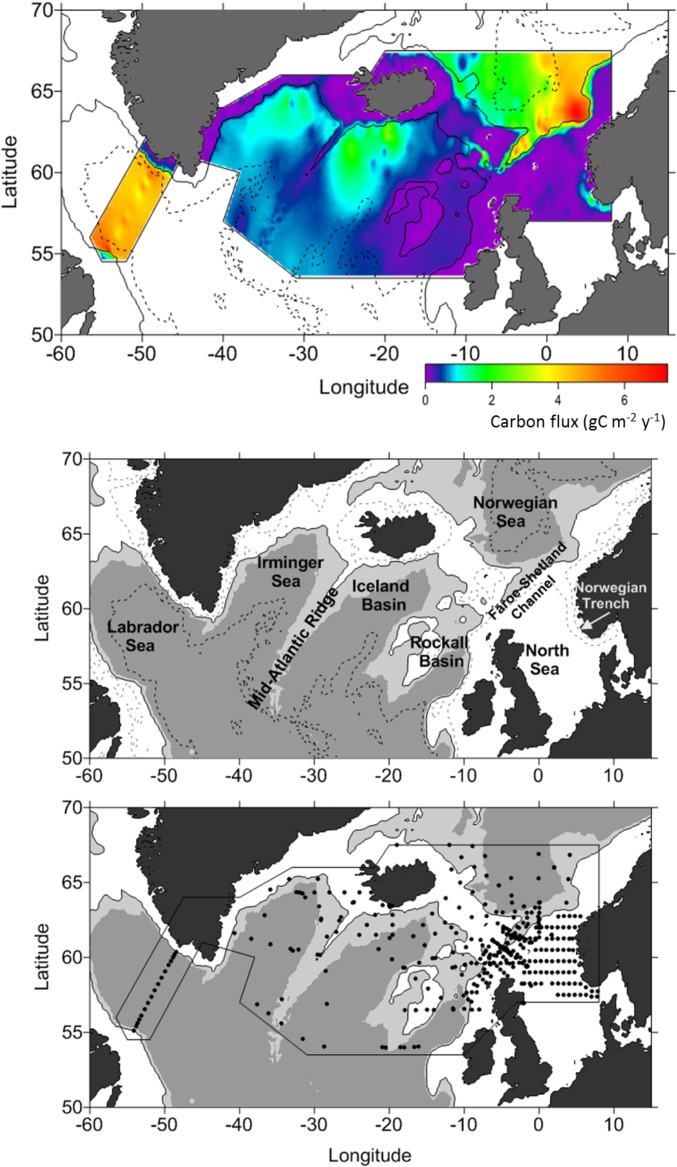
Winter surveys (25, 28) have revealed prodigious numbers [15,000–40,000 individuals (ind) per m2; Table 1] of diapausing C. finmarchicus copepodites (C5) in the various deep-ocean basins across the North Atlantic. Although each individual contains only about 200 µg of lipids, the numbers and spatial extent (Fig. 1) represent a large integrated mass of carbon (Table 1). Averaged across the geographic range of the species we estimate a vertical flux of 2–6 g C⋅m−2⋅y−1 in the form of lipids, actively transported to depth by the annual C. finmarchicus migration (Table 1).
Table 1.
Regional breakdown of the lipid pump calculations
| Regional variables | Labrador Sea | Irminger Sea | Iceland Sea | Iceland Basin | Western Norwegian Sea | Eastern Norwegian Sea | |
| Mean overwintering abundance, m−2 | 18,000 | 15,000 | 18,000 | 20,000 | 15,000 | 40,000 | |
| Mean WE content, µg ind−1 | 296 | 136 | 328 | 167 | 190 | 198 | |
| Mean carbon content, µg C⋅ind−1 | 237 | 109 | 262 | 134 | 152 | 158 | |
| Annual carbon transport, g C⋅m−2⋅y−1 | 4.3 | 1.6 | 4.7 | 2.7 | 2.3 | 6.4 | |
| Area, m2 | 3.94E+11 | 1.6E+11 | 1.51E+11 | 2.92E+11 | 1.86E+11 | 1.82E+11 | |
| Overwintering depth, m | 600 | 600 | 400 | 1,400 | 600–1,100 | 600–1,100 | |
| Temperature at overwintering depth, °C | 3.5 | 3.8 | −0.2 | 4.2 | −0.2 | −0.4 | |
| Respiration rate during diapause, µg C⋅ind−1⋅d−1 | 0.80 | 0.39 | 0.60 | 0.55 | 0.39 | 0.40 | |
| Prosome length during diapause, mm | 2.54 | 2.16 | 2.6 | 2.25 | 2.31 | 2.33 | |
| Length of diapause, d | a) | 275 | 235 | 310 | 205 | 245 | 235 |
| b) | 136 | 124 | 195 | 125 | 197 | 202 | |
| Annual respired carbon, µg C⋅ind−1 | a) | 220 | 91 | 186 | 114 | 96 | 93 |
| b) | 109 | 48 | 117 | 69 | 77 | 80 | |
| Total respired carbon, g C⋅m−2⋅y−1 | a) | 4.0 | 1.1 | 3.4 | 2.3 | 1.4 | 3.7 |
| b) | 2.0 | 0.6 | 2.1 | 1.4 | 1.1 | 3.2 | |
| Carbon remaining, µg C⋅ind−1 | a) | 76 | 45 | 142 | 53 | 94 | 105 |
| b) | 187 | 88 | 211 | 98 | 113 | 118 |
Abundance, overwintering depth, and temperatures (25, 28, 47) are shown. Prosome lengths for the Labrador Sea are published values (47), whereas the other length measurements are the authors’ own unpublished data. Area of the different ocean basins was estimated using MathWorks mapping tool box. WE content is estimated from the length measurements (30), and for length of diapause we present two different estimates, “a” (31) and “b” (51). See Materials and Methods for details. The respective annual respiration for the two different models is based on published respiration regressions (29, 30). Carbon remaining is the carbon not used over the overwintering period and can be brought back to the surface during spring ascent.
Fig. 1.
Lipid sequestrated carbon flux. (Top) A map of carbon flux (in grams of carbon per square meter per year) associated with lipid sequestration (respiration) of overwintering Calanus finmarchicus in the North Atlantic. The carbon flux “hot spots” in the eastern Norwegian Sea is due to the high copepod abundance but in the Labrador Sea due to larger copepod size and higher respiration due to higher temperatures. (Middle) Names of the ocean basins referred to in Table 1. (Bottom) Sampling locations of Calanus finmarchicus during winter for abundance, length, overwintering depths, and temperatures.
The proportion of this transported carbon that remains at depth (i.e., sequestered) depends on mortality and respiration rates. Overwintering temperatures and lipid content determine respiration rates and hence the time that individual copepods can survive in diapause (29–31). Estimated respiration rates of 0.4–0.8 µg C⋅ind−1⋅d−1 over diapause intervals of 120–300 d, result in 44–93% of the lipid reserves being respired at depth, depending on location (Table 1). The sequestration flux that penetrates below the permanent pycnocline, associated with respiration of overwintering C. finmarchicus, is thus estimated to be 1–4 g C⋅m−2⋅y−1.
Mortality of overwintering copepods, although potentially important to the survival of the Calanus population, will in all likelihood augment the fraction of transported carbon remaining at depth, making our sequestration estimate conservative. A portion of the nonrespired carbon returns to the surface the following spring with the ascending population. Based on initial values and our estimated respiration loss, the lipid content of individuals at the end of overwintering is about 100 µg C⋅ind−1 (Table 1), which is consistent with actual measurements in spring in the North Atlantic (32).
Discussion
The deep oceans are an important repository for carbon, and the North Atlantic appears to be particularly active in atmospheric CO2 drawdown through both physical and biological processes (4). Estimates of the biological pump for the entire North Atlantic range from 1.0 to 2.7 Gt C⋅y−1, constituting 10–25% of the global biological pump (4). The large variations in these estimates stem from the application of different observational methods (satellite mounted optical sensors, nutrient budgets, sediment traps, radio nuclides, models, and combinations thereof) in their derivation (33). Each of these methods captures some, but not all, of the various processes involved in transporting biogenic carbon to the deep ocean, and this makes the closure of the oceanic carbon budget notoriously difficult (33).
To compare the magnitude of the carbon sequestration flux due to the seasonal Calanus migration with the other established components of the biological pump in the North Atlantic, we need estimates of both the amount of particulate organic carbon (POC) leaving the euphotic zone (the export flux) (4, 34, 35) and the proportion of this export production sinks to depths below the permanent thermocline where it can be sequestered (36–38). Of the annual mean primary production, a fraction (5–15%) (34, 35) is typically exported out of the euphotic zone (∼50–100 m), mainly in the form of sinking particulate organic material (fecal pellets and aggregate detritus). A recent review for the North Atlantic (4) provides estimates of 29 ± 10 g C⋅m−2⋅y−1 for this aspect of the regional export flux. There is considerable attenuation of this export flux as it sinks through the mesopelagic zone to below the permanent thermocline as particles are consumed, fragmented, and remineralized. At depths of 600–1,400 m, only 10–20% of the export flux remains (38, 39), i.e., on the order of 2–8 g C⋅m−2⋅y−1. Thus, the lipid pump we identify here in association with the seasonal migration of a single zooplankton species, C. finmarchicus, sequesters a similar quantity of biogenic carbon (1–4 g C⋅m−2⋅y−1) as the sinking of POC.
Most marine organisms execute shallow diel vertical migrations, and these are known to regulate trophic interactions and vertical fluxes of carbon (11, 40), nutrients, and oxygen (11, 41). However, these are very different from the deep seasonal vertical migrations that enable some zooplankton species to synchronize their life cycles with the seasonal periodicity of primary production in temperate and boreal latitudes. A few previous studies have estimated the net vertical flux associated with such zooplankton ontogenic migrations. Most of these have focused on only single study sites. For example, a study on C. finmarchicus at Ocean Weathership India in the Iceland Basin (59°N, 19°W) estimated the migration flux to be about 0.4 g C⋅m−2⋅y−1 (12). In our basin-scale study, we show that the sequestration flux is patchy and related to abundance and temperature. C. finmarchicus is found in very low abundance at Weathership India compared with other regions of the North Atlantic (25), and our analysis yields comparable flux estimates for the same location (Fig. 1). Two other studies on ontogenic migration fluxes by Calanus both estimated about 3 g C⋅m−2⋅y−1, comparable to the export flux of detritus in the same areas. One from the Greenland Sea was based on mortality and metabolism of C. hyperboreus during diapause (>1,000 m) (42), and the other in a shallow gulf in the Arctic Ocean was based on community respiration measures of mainly C. glacialis, but only at 200-m depth and therefore not in the zone where sequestration can occur (15). In our study, we show that the lipid pump phenomenon is not limited to shallow-shelf seas, but is a widespread phenomenon across all of the deep basins of the North Atlantic and penetrates below the depth of the permanent thermocline, thereby contributing strongly to the oceans sequestration of carbon.
The map of carbon sequestered shown in Fig. 1 indicates that the strength of the lipid pump is quite variable in space controlled by the abundance and overwintering strategies of resident populations. Although our estimates are fairly robust across the northern basins of the North Atlantic, we cannot extrapolate to subtropical and equatorial latitudes. Looking further afield, there are two estimates of migration flux in the Pacific Ocean by Neocalanus spp., a copepod that has a similar ontogenic strategy as Calanus. One off Japan estimated the flux to be similar to the POC flux at 1,000 m (40); the other covered three areas in the subpolar Pacific where the estimates ranged from 1 to 9 g C⋅m−2⋅y−1 (13). In these cases, as with the C. hyperboreus in the Arctic (42), the active carbon transport includes not only the respired carbon during overwintering but also mortality as the spent females expire at depth after egg production. The lipid pump can thus be seen as a globally distributed process, albeit with regional variations characterized by resident communities of zooplankton and their life history strategies.
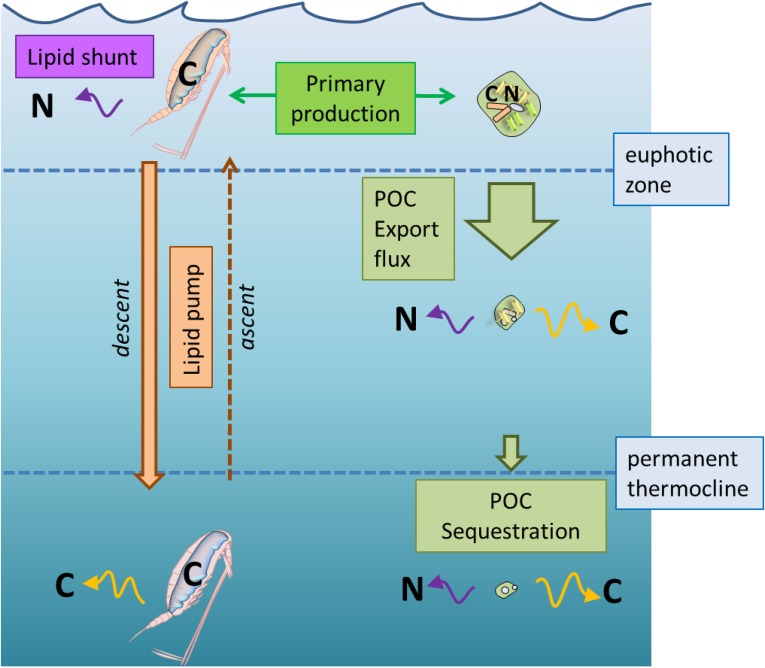
The strength and efficiency of the biological pump is as much about the cycling of nutrients as it is about carbon and productivity (16, 17). The important difference between the lipid pump and the passive sinking of POC is that the elemental ratios of nitrogen, phosphorus, silicon, and iron to carbon are extremely low or zero in lipids (lipid C:N:P = 1:<0.0001:<0.0001), compared with the Redfield ratios in typical organic matter (C:N:P = 1.00:0.15:0.01). To achieve this, copepodite stages that are synthesizing WEs in the surface waters must excrete excess nitrogen and phosphorus assimilated from their food intake back into the environment. This means that, in effect, the synthesis and transport of lipids to depth occurs without any net consumption of the essential limiting nutrients in the surface ocean (Fig. 2). Conversely, metabolism of lipids at depth will produce few waste products other than CO2 and H2O. This results in a “lipid shunt,” similar in effect (but much more pronounced) to the C enrichment of POC that occurs in the so-called “microbial shunt” (43). An important implication of the lipid shunt is that it obscures the lipid pump from other empirical estimates of the strength of the biological pump (i.e., the export flux) that are based on new production (44, 45). Specifically, any amount of lipid can be exported from the surface ocean with essentially no impact on the nutrient budget. This, together with the fact that large vertically migrating zooplankton tend to avoid sediment traps (13, 40), means that the lipid pump has not as yet entered into any of the global estimates of the biogenic carbon flux to the deep ocean.
Fig. 2.
The lipid pump component of the biological pump and the lipid shunt. The export flux of POC is much greater than the active transport of lipids by overwintering copepods. However, much of the POC flux is attenuated as it sinks to depth. At overwintering depths (greater than permanent pycnocline; 400–1,400 m), the lipid transport and the POC flux are comparable (2–8 g C⋅m−2⋅y−1). A significant portion of transported lipid is respired at depth (44–93%). The lipid pump, representing the difference between what descends in the autumn and what ascends to the surface in spring, is conservatively estimated at 1–4 g C⋅m−2⋅y−1, other sources of loss such as predation notwithstanding. The decoupling of nutrient (N) and carbon (C) transport represents a shunt in the biological pump; the nutrients associated with lipid accumulation remain in surface waters, whereas respired carbon associated with their use over winter is sequestered at depth.
This study demonstrates that the active vertical transport of lipids by overwintering zooplankton potentially contributes significantly to the ocean’s ability to sequester carbon and act as a sink in the global carbon cycle. The arguments presented here are based on data for a single species and hence present a very conservative estimate of the potential importance of this process in carbon cycling. The distribution of C. finmarchicus is limited to the North Atlantic. However, ecologically comparable species found in other ocean basins also undertake lipid-fueled seasonal migrations in their life cycle (20, 46). The seasonal migration of Neocalanus spp. in the Pacific (13, 40) contribute as much if not more to the flux of biogenic carbon to the deep oceans, at least on regional scales. The potential influence of the lipid pump identified here on global carbon cycling remains to be quantified.
Materials and Methods
Abundances of C. finmarchicus in diapause across the North Atlantic, Labrador Sea, and Iceland Sea were taken from the original datasets (25, 28). The values from the Iceland Sea were published as dry weight but the absolute numbers were provided by the authors. Body size (prosome length) of overwintering copepods from >300-m depth were obtained from 13 winter cruises in the North Atlantic basins (Fig. 1), but published values were used from the Labrador Sea (47). Mean depth and temperature during diapause were calculated as the abundance weighted depth and the corresponding temperature at each sampling site (25).
Storage lipid content of C. finmarchicus was taken from the relationship between oil sac volume (OSV) and copepod prosome length (30). We used the mean OSV value for the corresponding size to avoid overestimation in our calculations. We used 0.90 g WE⋅mL−1 to convert OSV to individual micrograms of WEs (48). The carbon weight of WE depends on the fatty acid and alcohol composition, and we based our value on observed relative compositions (49, 50) resulting in 79% of the WE weight being carbon.
Respiration rate estimates (micromoles of oxygen per gram of carbon per hour) were based on the temperature the copepods experience at depth during diapause and equations established for overwintering Calanus (30). Oxygen consumption rates were converted to carbon utilization (micrograms of carbon per individual) by using the length-to-dry weight relationship for winter and a value of 62% carbon per dry weight (29). Two different model estimates are used to calculate duration of diapause. One is based on respiration and the lipid content (OSV) of the copepods (30) [corrected equation (31)] and is length and temperature dependent. The other estimate is based on demographic time series of entry and emergence into diapause from the Labrador Sea, and surface temperatures at the onset of diapause (51).
Acknowledgments
This work was funded by the Danish Council for Strategic Research under the North Atlantic–Arctic Coupling in a Changing Climate: Impacts on Ocean Circulation, Carbon Cycling and Sea-Ice, and European Union Seventh Framework Programme Basin Scale Analysis, Synthesis and Integration (ENV.2010.2.2.1-1; www.euro-basin.eu). M.R.H. was supported by the Marine Science and Technology Scotland pooling scheme (Scottish Funding Council Grant Reference HR09011).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Longhurst AR, Harrison WG. The biological pump: Profiles of plankton production and consumption in the upper ocean. Prog Oceanogr. 1989;22(1):47–123. [Google Scholar]
- 2.Buesseler KO, et al. Revisiting carbon flux through the ocean’s twilight zone. Science. 2007;316(5824):567–570. doi: 10.1126/science.1137959. [DOI] [PubMed] [Google Scholar]
- 3.De La Rocha CL, Passow U. Factors influencing the sinking of POC and the efficiency of the biological carbon pump. Deep Sea Res Part II Top Stud Oceanogr. 2007;54(5-7):639–658. [Google Scholar]
- 4.Sanders R, et al. The biological carbon pump in the North Atlantic. Prog Oceanogr. 2014;129:200–218. [Google Scholar]
- 5.Turner JT. Zooplankton fecal pellets, marine snow, phytodetritus and the ocean’s biological pump. Prog Oceanogr. 2015;130:205–248. [Google Scholar]
- 6.Ducklow HW, Steinberg DK, Buesseler KO. Upper ocean carbon export and the biological pump. Oceanography (Wash DC) 2001;14(4):50–58. [Google Scholar]
- 7.Turner JT. Zooplankton fecal pellets, marine snow and sinking phytoplankton blooms. Aquat Microb Ecol. 2002;27(1):57–102. [Google Scholar]
- 8.Alldredge AL, Silver MW. Characteristics, dynamics and significance of marine snow. Prog Oceanogr. 1988;20(1):41–82. [Google Scholar]
- 9.Koski M, Kiørboe T, Takahashi K. Benthic life in the pelagic: Aggregate encounter and degradation rates by pelagic harpacticoid copepods. Limnol Oceanogr. 2005;50(4):1254–1263. [Google Scholar]
- 10.Zhang X, Dam HG. Downward export of carbon by diel migrant mesozooplankton in the central equatorial Pacific. Deep Sea Res Part II Top Stud Oceanogr. 1997;44(9):2191–2202. [Google Scholar]
- 11.Steinberg DK, et al. Zooplankton vertical migration and the active transport of dissolved organic and inorganic carbon in the Sargasso Sea. Deep Sea Res Part I Oceanogr Res Pap. 2000;47(1):137–158. [Google Scholar]
- 12.Longhurst A, Williams R. Carbon flux by seasonal vertical migrant copepods is a small number. J Plankton Res. 1992;14(11):1495–1509. [Google Scholar]
- 13.Bradford-Grieve JM, Nodder SD, Jillett JB, Currie K, Lassey KR. Potential contribution that the copepod Neocalanus tonsus makes to downward carbon flux in the Southern Ocean. J Plankton Res. 2001;23(9):963–975. [Google Scholar]
- 14.Kobari T, Shinada A, Tsuda A. Functional roles of interzonal migrating mesozooplankton in the western subarctic Pacific. Prog Oceanogr. 2003;57(3-4):279–298. [Google Scholar]
- 15.Darnis G, Fortier L. Zooplankton respiration and the export of carbon at depth in the Amundsen Gulf (Arctic Ocean) J Geophys Res. 2012;117(C4):C04013. [Google Scholar]
- 16.Falkowski PG. Evolution of the nitrogen cycle and its influence on the biological sequestration of CO2 in the ocean. Nature. 1997;387(6630):272–275. [Google Scholar]
- 17.Sarmiento JL, Gruber N, Brzezinski MA, Dunne JP. High-latitude controls of thermocline nutrients and low latitude biological productivity. Nature. 2004;427(6969):56–60. doi: 10.1038/nature02127. [DOI] [PubMed] [Google Scholar]
- 18.Fleminger A, Hulsemann K. Geographical range and taxonomic divergence in North Atlantic Calanus. Mar Biol. 1977;40(3):233–248. [Google Scholar]
- 19.Conover RJ. Comparative life histories in the genera Calanus and Neocalanus in high latitudes of the northern hemisphere. Hydrobiologia. 1988;167-168(1):127–142. [Google Scholar]
- 20.Lee R, Hagen W, Kattner G. Lipid storage in marine zooplankton. Mar Ecol Prog Ser. 2006;307(1):273–306. [Google Scholar]
- 21.Beaugrand G, Brander KM, Alistair Lindley J, Souissi S, Reid PC. Plankton effect on cod recruitment in the North Sea. Nature. 2003;426(6967):661–664. doi: 10.1038/nature02164. [DOI] [PubMed] [Google Scholar]
- 22.Michaud J, Taggart C. Spatial variation in right whale food, Calanus finmarchicus, in the Bay of Fundy. Endanger Species Res. 2011;15(3):179–194. [Google Scholar]
- 23.Melle W, et al. The North Atlantic Ocean as habitat for Calanus finmarchicus: Environmental factors and life history traits. Prog Oceanogr. 2014;129:244–284. [Google Scholar]
- 24.Heath MR, et al. Winter distribution of Calanus finmarchicus in the Northeast Atlantic. ICES J Mar Sci. 2000;57(6):1628–1635. [Google Scholar]
- 25.Heath MR, et al. Comparative ecology of over-wintering Calanus finmarchicus in the northern North Atlantic, and implications for life-cycle patterns. ICES J Mar Sci. 2004;61(4):698–708. [Google Scholar]
- 26.Visser AW, Jónasdóttir SH. Lipids, buoyancy and the seasonal vertical migration of Calanus finmarchicus. Fish Oceanogr. 1999;8(Suppl 1):100–106. [Google Scholar]
- 27.Richardson K, Jónasdóttir SH, Hay SJ, Christoffersen A. Calanus finmarchicus egg production and food availability in the Faroe–Shetland Channel and northern North Sea: October–March. Fish Oceanogr. 1999;8(Suppl 1):153–162. [Google Scholar]
- 28.Gislason A, Silva T. Abundance, composition, and development of zooplankton in the Subarctic Iceland Sea in 2006, 2007, and 2008. ICES J Mar Sci. 2012;69(7):1263–1276. [Google Scholar]
- 29.Ingvarsdóttir A, Houlihan DF, Heath MR, Hay SJ. Seasonal changes in respiration rates of copepodite stage V Calanus finmarchicus (Gunnerus) Fish Oceanogr. 1999;8(Suppl 1):73–83. [Google Scholar]
- 30.Saumweber WJ, Durbin EG. Estimating potential diapause duration in Calanus finmarchicus. Deep Sea Res Part II Top Stud Oceanogr. 2006;53(23-24):2597–2617. [Google Scholar]
- 31.Pierson JJ, Batchelder H, Saumweber W, Leising A, Runge J. The impact of increasing temperatures on dormancy duration in Calanus finmarchicus. J Plankton Res. 2013;35(3):504–512. [Google Scholar]
- 32.Jónasdóttir SH. Lipid content of Calanus finmarchicus during overwintering in the Faroe–Shetland Channel. Fish Oceanogr. 1999;8(Suppl 1):61–72. [Google Scholar]
- 33.Burd AB, et al. Assessing the apparent imbalance between geochemical and biochemical indicators of meso- and bathypelagic biological activity: What the @$#! is wrong with present calculations of carbon budgets? Deep Sea Res Part II Top Stud Oceanogr. 2010;57(16):1557–1571. [Google Scholar]
- 34.Laws EA, Falkowski PG, Smith WO, Ducklow H, McCarthy JJ. Temperature effects on export production in the open ocean. Global Biogeochem Cycles. 2000;14(4):1231–1246. [Google Scholar]
- 35.Henson SA, et al. A reduced estimate of the strength of the ocean’s biological carbon pump. Geophys Res Lett. 2011;38(4):L04606. [Google Scholar]
- 36.Lampitt RS, et al. 2008. Ocean fertilization: A potential means of geoengineering? Philos Trans A Math Phys Eng Sci 366(1882):3919–3945.
- 37.Passow U, Carlson CA. The biological pump in a high CO2 world. Mar Ecol Prog Ser. 2012;470(2):249–271. [Google Scholar]
- 38.Giering SLC, et al. Reconciliation of the carbon budget in the ocean’s twilight zone. Nature. 2014;507(7493):480–483. doi: 10.1038/nature13123. [DOI] [PubMed] [Google Scholar]
- 39.Marsay CM, et al. Attenuation of sinking particulate organic carbon flux through the mesopelagic ocean. Proc Natl Acad Sci USA. 2015;112(4):1089–1094. doi: 10.1073/pnas.1415311112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kobari T, et al. Impacts of ontogenetically migrating copepods on downward carbon flux in the western subarctic Pacific Ocean. Deep Sea Res Part II Top Stud Oceanogr. 2008;55(14-15):1648–1660. [Google Scholar]
- 41.Bianchi D, Galbraith ED, Carozza DA, Mislan KAS, Stock CA. Intensification of open-ocean oxygen depletion by vertically migrating animals. Nat Geosci. 2013;6(7):545–548. [Google Scholar]
- 42.Hirche J. Life cycle of the copepod Calanus hyperboreus in the Greenland Sea. Mar Biol. 1997;128(February):607–618. [Google Scholar]
- 43.Suttle CA. Marine viruses—major players in the global ecosystem. Nat Rev Microbiol. 2007;5(10):801–812. doi: 10.1038/nrmicro1750. [DOI] [PubMed] [Google Scholar]
- 44.Eppley RW, Peterson BJ. Particulate organic matter flux and planktonic new production in the deep ocean. Nature. 1979;282:677–680. [Google Scholar]
- 45.Siegel DA, et al. Global assessment of ocean carbon export by combining satellite observations and food web models. Global Biogeochem Cycles. 2014;28(3):181–196. [Google Scholar]
- 46.Pond DW, Tarling GA, Ward P, Mayor DJ. Wax ester composition influences the diapause patterns in the copepod Calanoides acutus. Deep Sea Res Part II Top Stud Oceanogr. 2012;59-60:93–104. [Google Scholar]
- 47.Pepin P, Head EJH. Seasonal and depth-dependent variations in the size and lipid contents of stage 5 copepodites of Calanus finmarchicus in the waters of the Newfoundland Shelf and the Labrador Sea. Deep Sea Res Part I Oceanogr Res Pap. 2009;56(6):989–1002. [Google Scholar]
- 48.Miller C, Morgan C, Prahl F, Sparrow M. Storage lipids of the copepod Calanus finmarchicus from Georges Bank and the Gulf of Maine. Limnol Oceanogr. 1998;43(3):488–497. [Google Scholar]
- 49.Falk-Petersen S, Sargent JR, Tande KS. Lipid composition of zooplankton in relation to the sub-arctic food web. Polar Biol. 1987;8(2):115–120. [Google Scholar]
- 50.Kattner G, Krause M. Seasonal variations of lipids (wax esters, fatty acids and alcohols) in calanoid copepods from the North Sea. Mar Chem. 1989;26(3):261–275. [Google Scholar]
- 51.Johnson CL, et al. Characteristics of Calanus finmarchicus dormancy patterns in the Northwest Atlantic. ICES J Mar Sci. 2008;65(3):339–350. [Google Scholar]