Significance
Mercury is a potent neurotoxin that accumulates in food webs, posing a global threat to environmental health. Marine mammals are common sentinel species for studying marine pollution; however, their potential role as vectors of contaminants to local ecosystems has rarely been addressed. By quantifying the concentration and chemical form of mercury in seawater affected by Northern elephant seal (Mirounga angustirostris) colonization, we demonstrated here that marine mammal behavioral ecology can substantially influence nearshore mercury cycling. Elevated methylmercury (MeHg) levels in seawater adjacent to the rookery during the molting season may become bioavailable to lower trophic levels, indicating that large marine mammal assemblages represent an important source of MeHg to nearshore food chains and coastal marine fisheries, thereby threatening ecosystem health.
Keywords: mercury, biogeochemistry, marine mammals, biomagnification, environmental toxicology
Abstract
Methylmercury (MeHg) is a potent neurotoxin that is biomagnified approximately 1–10 million-fold in aquatic carnivores such as the Northern elephant seal (Mirounga angustirostris), whose excreta and molted pelage, in turn, constitute a source of environmental MeHg contamination at the base of marine food chains. The potential for this top-down contamination is greatest in coastal areas with productive marine ecosystems that provide ideal habitats for large marine mammal colonies that can number in the thousands. This recycling of MeHg was evidenced by comparing total mercury (HgT) and MeHg concentrations in seawater, and HgT in molted pelage of M. angustirostris, at the Año Nuevo State Reserve pinniped rookery with concentrations at neighboring coastal sites in Central California. Seawater MeHg concentrations around the rookery (average = 2.5 pM) were markedly higher than those at the comparison coastal sites (average = 0.30 pM), and were as high as 9.5 pM during the M. angustirostris molting season. As a consequence, excreta and molts from this marine mammal colony, and presumably other marine predator populations, constitute a major source of MeHg at the base of the local marine food chain.
Anthropogenic emissions have increased global atmospheric mercury (Hg) deposition to the oceans three- to fivefold since preindustrial times (1, 2). Although more than 95% of industrial and natural inputs are inorganic forms of Hg [e.g., Hg(0) and Hg(II)], a small fraction of inorganic Hg is converted by microbial activity to methylmercury (MeHg), an organic neurotoxin that readily bioaccumulates in marine organisms and biomagnifies in food chains (3–5). As a result, MeHg concentrations in high-trophic predators (i.e., piscivorous fish, birds, and marine mammals) can reach levels that are physiologically detrimental to them and that constitute a human health threat (6, 7).
Although most “hot spots” of contamination in coastal waters are associated with local industrial inputs, the highest Hg concentrations initially detected in California’s Mussel Watch program four decades ago (1977–1978) were at Año Nuevo State Reserve (Fig. 1) (8). Año Nuevo hosts a relatively remote, protected rookery for a multitude of pinniped species including Northern elephant seals (Mirounga angustirostris), harbor seals (Phoca vitulina), Northern fur seals (Callorhinus ursinus), California sea lions (Zalophus californianus), Steller sea lions (Eumetopias jubatus), and Guadalupe fur seals (Arctocephalus townsendi). The average (mean ± 1 SD) Hg concentration in mussels (Mytilus californianus) at Año Nuevo was 2.50 ± 0.52 µg⋅g−1 dry weight (wt.) (8). This value was 5–35 times greater than Hg concentrations in mussels collected from 42 other locations along the west coast of the continental United States (0.07–0.55 µg⋅g−1 dry wt.). The exceptionally high Hg levels in mussels were tentatively attributed to the excreta of marine mammals colonizing Año Nuevo because of the relatively high Hg concentrations found in California sea lion feces (0.80 µg⋅g−1) collected at the site (8). However, that attribution could not be substantiated because of the technical inability to accurately measure Hg concentrations in seawater in the 1970s (9).
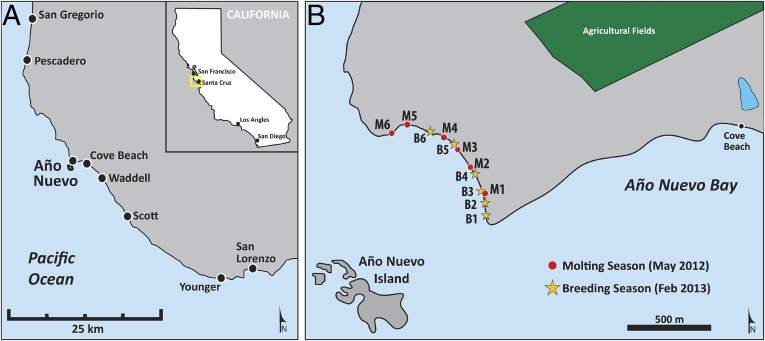
Fig. 1.
Nearshore seawater sampling locations. (A) Locations of Año Nuevo and comparison sites along the Central California coast. (B) Detailed map showing the sampling stations at the south end of the Año Nuevo mainland breeding rookery during the 2012 Northern elephant seal molting season (M1–M6) and 2013 breeding season (B1–B6), as well as the Cove Beach Año Nuevo State Reserve sampling site.
The objective of this study was to test the fundamental hypothesis of top-down Hg contamination by comparing total Hg (HgT) and MeHg concentrations in nearshore seawater at the mainland Northern elephant seal rookery at Año Nuevo South Beach with those of seawater from comparison sites not affected by large marine mammal populations (Fig. 1). In addition, Northern elephant seal molted pelage was analyzed for HgT to determine whether the sloughed-off integument represented a potential source of Hg to the local ecosystem.
Results
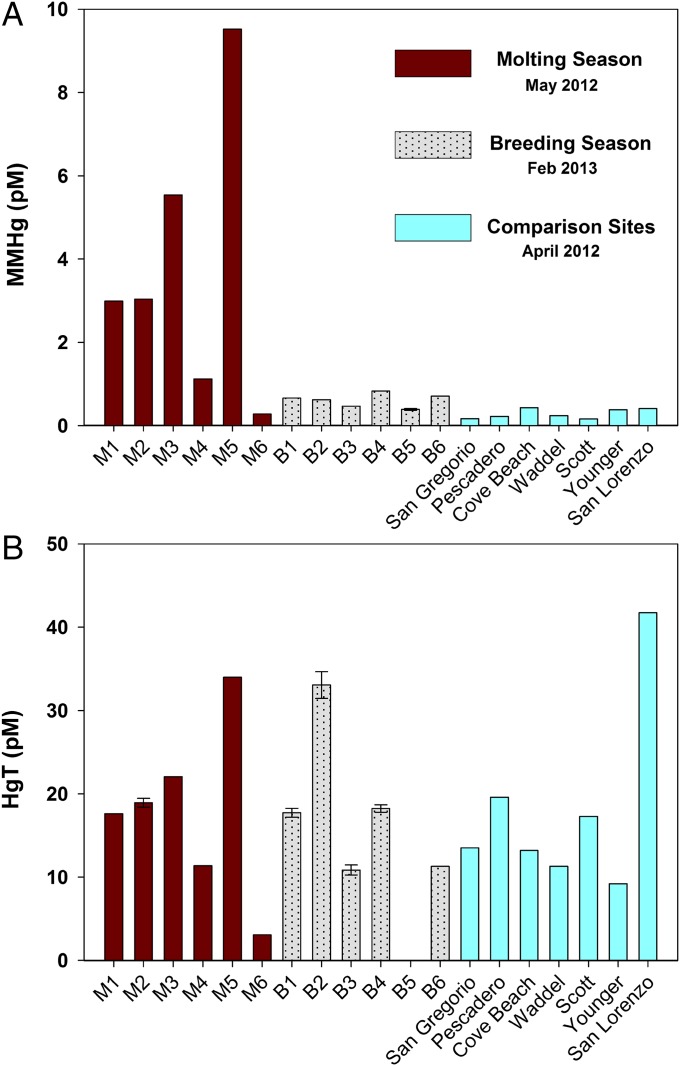
We found no measurably elevated HgT seawater concentrations at Año Nuevo (sampled in May 2012 and February 2013) compared with the nearby coastal sites that did not have large marine mammal populations (sampled in April 2012; Table 1 and Fig. 2B). Total Hg concentrations in surface seawater collected from the sound end of the Año Nuevo mainland breeding rookery, where the majority of Northern elephant seals were hauled out, ranged from 3.1 to 34.0 pM and were comparable during the breeding and molting seasons (Fig. 2B). This range was similar to concentrations observed at the comparison sites (9.2–41.7 pM), where the highest HgT value was from the San Lorenzo River, which passes through the city of Santa Cruz.
Table 1.
HgT and MeHg concentrations in unfiltered nearshore seawater and in Northern elephant seal molted pelage
| Site* | HgT, pM† | MeHg, pM† | Percentage MeHg | Date sampled |
| Año Nuevo molting season | ||||
| M1 | 17.6 | 3.0 | 17 | 5/19/2012 |
| M2 | 18.9 ± 0.5 | 3.0 | 16 | 5/19/2012 |
| M3 | 22.1 | 5.8 | 26 | 5/19/2012 |
| M4 | 11.4 | 5.3 | 46 | 5/19/2012 |
| M5 | 34.0 | 9.5 | 28 | 5/19/2012 |
| M6 | 3.1 | 0.28 | 9.1 | 5/19/2012 |
| Average‡ | 17.8 ± 10.4 | 4.5 ± 3.4 | 24 ±13 | |
| Año Nuevo breeding season | ||||
| B1 | 17.7 ± 0.5 | 0.66 | 3.7 | 2/12/2013 |
| B2 | 33.1 ± 1.6 | 0.62 | 1.9 | 2/12/2013 |
| B3 | 10.9 ± 0.6 | 0.47 | 4.3 | 2/12/2013 |
| B4 | 18.2 ± 0.5 | 0.83 | 4.6 | 2/12/2013 |
| B5 | (1,650)§ | 0.39 ± 0.02 | — | 2/12/2013 |
| B6 | 11.3 | 0.71 | 4.9 | 2/12/2013 |
| Average‡ | 18.2 ± 9.0 | 0.61 ± 0.2 | 3.9 ± 1.2 | |
| Comparison coastal sites | ||||
| San Gregorio | 13.5 | 0.17 | 1.3 | 4/15/2012 |
| Pescadero | 19.6 | 0.22 | 1.1 | 4/15/2012 |
| Cove Beach | 13.2 | 0.50 | 3.8 | 4/14/2012 |
| Waddell | 11.3 | 0.24 | 2.1 | 4/14/2012 |
| Scott | 17.3 | 0.16 | 0.9 | 4/14/2012 |
| Younger | 9.2 | 0.38 | 4.1 | 4/13/2012 |
| San Lorenzo | 41.7 | 0.41 | 0.9 | 4/13/2012 |
| Average‡ | 18.0 ± 11.0 | 0.30 ± 0.13 | 2.0 ± 1.4 | |
| HgT in elephant seal molt samples | ||||
| Molt 1 | 4.5 µg⋅g−1 dry wt. | 5/19/2012 | ||
| Molt 2 | 3.1 µg⋅g−1 dry wt. | 5/19/2012 | ||
| Molt 3 | 3.3 µg⋅g−1 dry wt. | 5/19/2012 | ||
| Average‡ | 3.6 ± 0.8 µg⋅g−1 dry wt. |
Site locations shown in Fig. 1.
Samples that were analyzed at least three times are reported as the mean ± 1 SD.
Value represents the average ± 1 SD of all sites in that sampling event.
HgT for sample B5 was contaminated and was not included in calculations or interpretations.
Fig. 2.
MeHg and HgT in nearshore unfiltered seawater samples. (A) MeHg concentrations for the Año Nuevo mainland rookery during the 2012 molting season (M1–M6), 2013 breeding season (B1–B6), and Central California comparison sites. (B) HgT concentrations for the rookery during the 2012 molting season, 2013 breeding season, and Central California comparison sites. Error bars (±1 SD) indicate the sample was analyzed at least three times. HgT for B5 (1,650 pM) was considered contaminated and is not graphed.
In contrast to the HgT concentrations at Año Nuevo that did not vary seasonally, there were pronounced variations in nearshore seawater MeHg concentrations at the Año Nuevo rookery during the molting season (0.28–9.5 pM) compared with during the breeding season (0.39–0.83 pM; Table 1 and Fig. 2A). Furthermore, the MeHg concentrations from the rookery were significantly higher during both seasons than the concentrations at the comparison sites (P < 0.05), which ranged from 0.16 to 0.41 pM during spring and summer upwelling conditions (10), excluding the Cove Beach station (0.50 pM), which is situated ∼2 km down-current from the rookery. For perspective, the average MeHg concentration of the six comparison sites located outside of Año Nuevo State Reserve (i.e., excluding Cove Beach) was 0.26 pM. This concentration is approximately half the average concentration of the six breeding season sites (0.61 pM) and more than an order of magnitude lower than the average MeHg concentration of samples collected from the rookery during the molting season (4.5 pM).
Northern elephant seal molted pelage samples (n = 3) collected concurrently with seawater during the molting season in May 2012 were analyzed for HgT. Mercury concentrations in the sloughed off material ranged from 3.1 to 4.5 µg⋅g−1 dry wt. (Table 1). These values are comparable to previously reported Hg concentrations of the hair of Pacific harbor seals (2.96–144.31 µg⋅g−1 dry wt.) (11), California sea lions (6.55–139 µg⋅g−1 dry wt.) (12), Australian fur seals (9.59 µg⋅g−1 dry wt.) (13), and Southern sea lions (19.16 µg⋅g−1 dry wt.) (14). We assume the vast majority of the HgT in molted pelage is organic Hg (e.g., MeHg), on the basis of previous studies that determined that MeHg accounts for more than 80% (wt/wt) of the HgT in both bat fur (15) and human hair (16, 17).
Discussion
In contrast to the original hypothesis that concentrations of all Hg species measured (i.e., HgT and MeHg) in seawater adjacent to the rookery would be elevated, there was no evident increase in HgT in seawater at Año Nuevo (3.1–34.0 pM) compared with the coastal waters sampled at sites not colonized by large populations of marine mammals (9.2–41.7 pM). The range of HgT concentrations for all three sampling events were comparable to previously reported HgT concentrations for the San Francisco Bay estuary (0.73–440 pM) (18), which is widely recognized for its sizable amount of historic and ongoing sources of industrial Hg, indicating that all the sampling sites in this study were relatively enriched in Hg.
Conversely, the elevated concentrations of MeHg in seawater around the rookery relative to other coastal sites were substantial. Northern elephant seals fast throughout their entire residence time at Año Nuevo; thus, organic Hg inputs from M. angustirostris feces were considered to be negligible year-round (19). Therefore, the approximately twofold enrichment of MeHg in seawater at Año Nuevo during the elephant seal breeding season was presumably a result of the high concentrations of Hg in feces excreted by the thousands of other pinnipeds and marine birds inhabiting Año Nuevo Island, which lies adjacent to the mainland elephant seal rookery across a 500-m channel (Fig. 1B) (20). MeHg has been found to account for 45% (wt/wt) of the Hg in seabird guano, which has an average HgT concentration of 0.11 µg⋅g−1 dry wt. (21). As previously stated, California sea lion excrement has ∼0.80 µg⋅g−1 HgT dry wt. (8), a substantial percentage of which is assumed to be MeHg (22). Presumably, there was also a considerable input of Hg to nearshore waters from the degradation of molted lanugo, or natal coat, shed by newborn elephant seal pups, which numbered more than 1,700 at Año Nuevo in 2010 (20). Northern elephant seal lanugo has been found to contain an average HgT concentration of 19.0 µg⋅g−1 wet wt. (23).
The even greater (∼17-fold) enrichment of MeHg in seawater at Año Nuevo during the M. angustirostris molting season (0.28–9.5 pM) was remarkable and exceeded the range of surface water MeHg concentrations observed in the highly urbanized San Francisco Bay estuary (<0.05–2.3 pM) (18). One route of elimination for MeHg is direct secretion into keratinized structures such as hair (22); therefore, we attribute the MeHg enrichment to the large flux of Northern elephant seal integument to the nearshore marine environment during the spring molting season (19). Female and juvenile Northern elephant seals migrate to rookeries each spring between April and June for an annual molt, during which the entire pelage and underlying epidermis is sloughed off in approximately 4 wk (24). The distinct increase in average percentage MeHg to HgT (wt/wt) in seawater during the molting season (24%) compared with the breeding season (3.9%) and with the comparison coastal sites (2.0%) further indicates that the molted pelage, which likely contained a high concentration of MeHg, was the primary source of that Hg enrichment (Table 1). This assertion is supported by other studies, which report an elevated MeHg percentage in sediments with biological sources of Hg (21), whereas a lower percentage of MeHg is typically associated with industrial and anthropogenic sources of Hg contamination (1, 5, 25). For example, storm water inputs containing 0.5–1.6% (wt/wt) MeHg were a main source of Hg to the local watersheds of the San Francisco Bay estuary (25).
The importance of MeHg inputs to Año Nuevo waters from Northern elephant seals was confirmed by the HgT concentrations in molted pelage samples (average = 3.6 µg⋅g−1 dry wt.), which presumably contained >80% (wt/wt) MeHg (15–17). Assuming the average mass of molted integument eliminated from a 400-kg elephant seal is ∼13.5 kg (24), the 4,000 adult seals present at the Año Nuevo rookery in 2010 (20) shed an estimated 54,000 kg of pelage. This equates to an annual per capita emission factor of 0.05 g MeHg per adult elephant seal. On the basis of this estimate, we calculate that ∼0.2 kg organic Hg entered the nearshore environment of Año Nuevo during that molting season. To put that amount in perspective, the San Francisco Bay covers ∼1,100 km2, drains 40% of the state of California (26, 27), and receives an estimated annual load of 8.0 kg MeHg from external sources such as rivers and point source discharges (25). Conversely, the Año Nuevo State Marine Conservation Area covers only 26.4 km2 (28), indicating that the magnitude of annual inputs from pinniped colonization constitutes a relevant, previously unaccounted for, source of MeHg to that marine reserve.
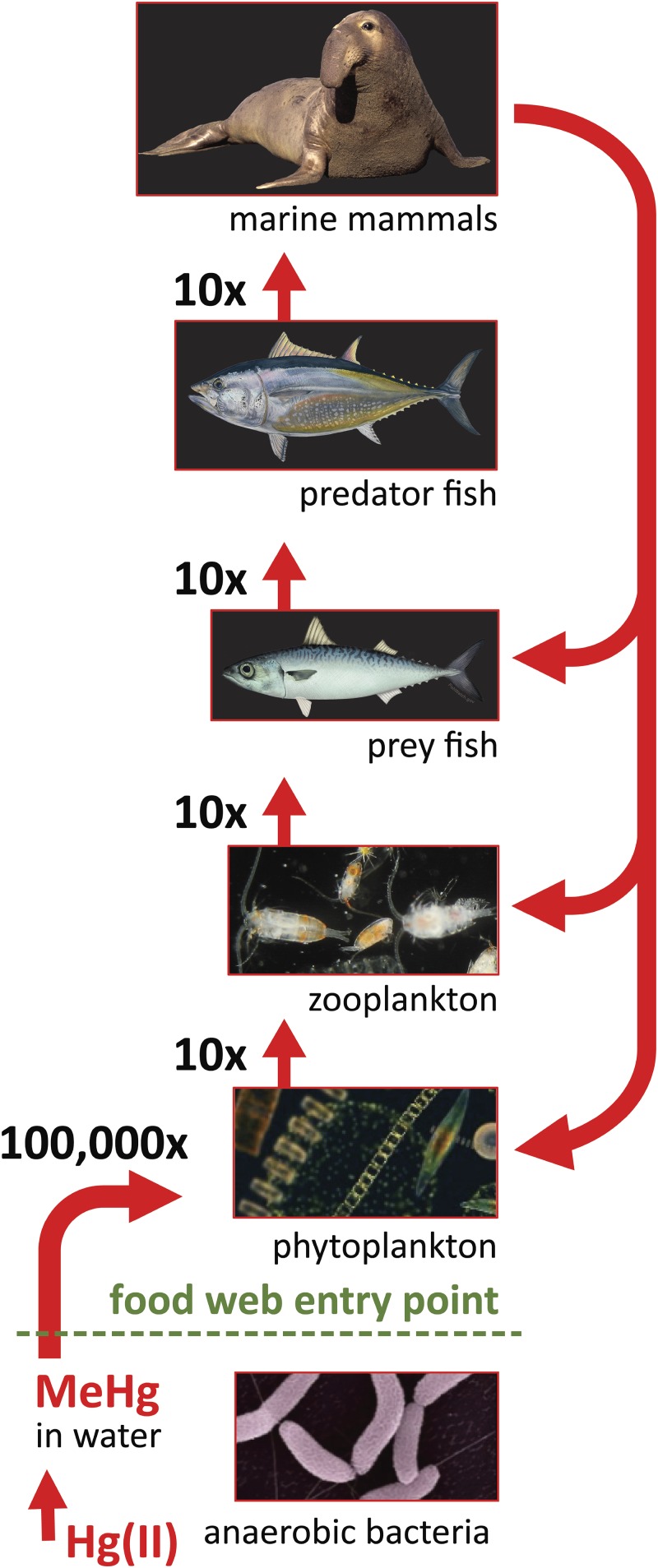
The exceptional addition of organic Hg from pinniped colonization is not unique to Año Nuevo. The 1977–1978 Mussel Watch site yielding the second-highest Hg concentration (0.55 µg⋅g−1) was at San Miguel Island, which hosted 27,000 marine mammals and 24,000 birds during the 1975–1976 breeding season (8). Therefore, it appears that similar processes are occurring at other marine mammal rookeries with large numbers of defecating and/or molting pinniped populations. In 2010, the San Miguel Island rookery experienced 16,000 M. angustirostris births, making it the largest Northern elephant seal rookery in the United States, followed by San Nicolas Island (11,000 births) and Santa Rosa Island (6,000 births; 29). The Pacific coast of the United States also hosts an estimated 238,000 California sea lions, 73,600 harbor seals, and 9,400 Northern fur seals (30), all of which aggregate in haulouts and breeding rookeries (19, 30–32), undergo an annual molt (31), and have the potential to exacerbate nearshore Hg budgets and MeHg cycling via top-down contamination. According to our preliminary calculations of Hg inputs from the molting elephant seals at Año Nuevo, we estimate that the Northern Hemisphere’s total M. angustirostris population, which was ∼210,000–239,000 in 2010 (29), is redistributing a total of 10–12 kg of Hg to the nearshore environments of the 21 total rookeries situated in the United States and Mexico (20). Most notably, the predominant proportion of that top-down contamination is MeHg that readily bioaccumulates in organisms at the base of marine food chains and then biomagnifies to potentially toxic levels in marine piscivores (Fig. 3) (3, 4, 11–14).
Fig. 3.
Illustration of the conversion of inorganic mercury [Hg(II)] to MeHg by anaerobic bacteria, biomagnification of MeHg at successive marine trophic levels, and then reintroduction of MeHg to the base of the food chain via top-down contamination. Images are courtesy of the Environmental Molecular Sciences Laboratory (EMSL), a Department of Energy Office of Science user facility at the Pacific Northwest National Laboratory (anaerobic bacteria); Richard R. Kirby, Secchi Disk project (phytoplankton); Maria Grazia Mazzocchi, Stazione Zoologica Anton Dohrn, Italy (zooplankton); and FishWatch, National Oceanic and Atmospheric Administration (prey fish).
Particle-bound MeHg in molted hair has the potential to become bioavailable to lower trophic levels through multiple pathways. We hypothesize that much of that Hg is reintroduced to the base of the food web as the molts degrade. Pinniped hair measures less than 200 µm in diameter (33) and can be directly ingested by filter-feeding and particulate-feeding invertebrates and planktivorous fish (34, 35). The digestive lining of fish has been reported to be permeable to MeHg after ingestion of prey and can then accumulate MeHg in all organs (36). Particulate MeHg is also a major source of Hg to bivalves and other benthic invertebrates because of its high assimilation efficiency from ingested sediment-associated particles (37, 38). However, the bioavailability of MeHg bound in keratinous material (e.g., hair and feathers) during digestion is largely unknown and merits future investigation because of its potential ecological relevance for nearshore environments, such as that at Año Nuevo. In addition, accumulated organic matter in bottom sediments is a main factor affecting net MeHg production (39); therefore, inputs of marine mammal excreta and sloughed epidermis may enhance Hg methylation in nearshore sediment. Resuspension of this sediment in the nearshore environment because of wave action, coastal upwelling, storm events, and/or nearshore pinniped activity may also mobilize particulate MeHg to the water column (40), which has been established as a main driver of organic Hg bioaccumulation in estuarine pelagic food webs (41).
This natural perturbation to the global Hg cycle is expected to intensify with rising Hg concentrations in pelagic food webs (2) and to geographically shift as marine mammal distributions respond to changes in ocean climate and prey availability (42, 43). Because marine mammals forage in biologically productive areas, their distribution is highly correlated with human fisheries that yield the highest catch (42). Furthermore, these regions are often developed and prone to persistent anthropogenic Hg inputs. For example, the Central Coast of California, where Año Nuevo is situated, drains rich agricultural regions, lies adjacent to a historic gold mining district where millions of kilograms of Hg were used (27), and also supports one-third of the world’s cetacean species and six pinniped species (43). This juxtaposition of economic and biologic demands makes prioritizing conservation efforts a complex challenge. Results from the Año Nuevo study suggest the phenomenon of top-down contamination at marine mammal haulouts and breeding grounds is an important mechanism of MeHg transport from open ocean prey stocks to coastal marine reservoirs. This component of coastal Hg–food web dynamics and MeHg bioavailability warrants further scrutiny as we evaluate nearshore Hg budgets and implement marine conservation strategies.
Materials and Methods
Unfiltered seawater samples were collected under a State Parks Scientific Collecting Permit, following established trace metal clean protocols (44), and then preserved with either bromine monochloride for HgT analysis, or sulfuric acid (H2SO4) for MeHg analysis, as outlined by Parker and Bloom (45). Molt samples were freeze-dried, stored at room temperature, and analyzed on a Milestone, Inc., Direct Mercury Analyzer (DMA-80) at the US Geological Survey Woods Hole Coastal and Marine Science Center. Seawater samples were analyzed for HgT in accordance with US Environmental Protection Agency Method 1631 Revision E, using a Tekran Model 2600 cold vapor atomic fluorescence spectroscopy Mercury Analyzer (46). Environment Canada’s Certified Reference Material for Hg in river water [ORMS-4 (elevated mercury in river water certified reference material)], as well as sediment reference materials [IAEA-158 (International Atomic Energy Agency marine sediment); IAEA-SL-1 (International Atomic Energy Agency lake sediment); MESS-3 (marine sediment certified reference material for trace elements and other constituents); PACS-2 (marine sediment certified reference material for trace metals and other constituents)], yielded concentrations within their certified values. MeHg was determined in accordance with US Environmental Protection Agency Method 1630 via distillation, purge and trap (tenax), and gas chromatographic cold vapor atomic fluorescence spectroscopy (47). Details regarding coastal seawater sampling and analysis are described in Ganguli et al. (48).
Acknowledgments
We are grateful to Anjali Kumar (MIT) for her help quantifying HgT concentrations in the molt samples, to Carl Lamborg for input on the manuscript, and to members of the WIGS Laboratory (esp. Jeremy Merckling) for analytical and field assistance. We also thank the Pinniped Cognition & Sensory Systems Laboratory, the California Department of Parks and Recreation, and the University of California Younger Lagoon Natural Reserve for granting access to our sampling locations. Our manuscript benefited greatly from the thoughtful input of the anonymous reviewers, and we appreciate their time. This project was supported with funding from Stevenson College (University of California, Santa Cruz), the Dr. Earl H. Myers Oceanographic and Marine Biology Trust of Pebble Beach, and the Robert & Patricia Switzer Foundation. The sampling permits to conduct our fieldwork were issued to P.M.G.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Driscoll CT, Mason RP, Chan HM, Jacob DJ, Pirrone N. Mercury as a global pollutant: Sources, pathways, and effects. Environ Sci Technol. 2013;47(10):4967–4983. doi: 10.1021/es305071v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lamborg CH, et al. Mercury in the Anthropocene Ocean. Oceanography. 2014;27(1):76–87. [Google Scholar]
- 3.Lavoie RA, Jardine TD, Chumchal MM, Kidd KA, Campbell LM. Biomagnification of mercury in aquatic food webs: A worldwide meta-analysis. Environ Sci Technol. 2013;47(23):13385–13394. doi: 10.1021/es403103t. [DOI] [PubMed] [Google Scholar]
- 4.Morel FM, Kraepiel AM, Amyot M. The chemical cycle and bioaccumulation of mercury. Annu Rev Ecol Syst. 1998;29:543–566. [Google Scholar]
- 5.Black FJ, Conaway CH, Flegal AR. Mercury in the marine environment. In: Bank MS, editor. Mercury in the Environment: Pattern and Process. Univ. of California Press; Berkeley, CA: 2012. pp. 167–220. [Google Scholar]
- 6.McKelvey W, Oken E. 2012. Mercury and public health: An assessment of human exposure. Mercury in the Environment: Pattern and Process (Univ. of California Press, Berkeley, CA), p 267.
- 7.Sunderland EM, Mason RP. Human impacts on open ocean mercury concentrations. Global Biogeochem Cycles. 2007;21(4):GB4022. [Google Scholar]
- 8.Flegal AR, Stephenson M, Martin M, Martin J. Elevated concentrations of mercury in mussels (Mytilus californianus) associated with pinniped colonies. Mar Biol. 1981;65(1):45–48. [Google Scholar]
- 9.Gill GA, Fitzgerald WF. Picomolar mercury measurements in seawater and other materials using stannous chloride reduction and two-stage gold amalgamation with gas phase detection. Mar Chem. 1987;20(3):227–243. [Google Scholar]
- 10.Graham WM, Largier JL. Upwelling shadows as nearshore retention sites: The example of northern Monterey Bay. Cont Shelf Res. 1997;17(5):509–532. [Google Scholar]
- 11.McHuron EA, Harvey JT, Castellini JM, Stricker CA, O’Hara TM. Selenium and mercury concentrations in harbor seals (Phoca vitulina) from central California: Health implications in an urbanized estuary. Mar Pollut Bull. 2014;83(1):48–57. doi: 10.1016/j.marpolbul.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 12.Elorriaga-Verplancken F, Aurioles-Gamboa D. Trace metal concentrations in the hair of Zalophus californianus pups and their relation to feeding habits. Biol Trace Elem Res. 2008;126(1-3):148–164. doi: 10.1007/s12011-008-8186-8. [DOI] [PubMed] [Google Scholar]
- 13.Bacher GJ. Mercury concentrations in the Australian fur seal Arctocephalus pusillus from SE Australian waters. Bull Environ Contam Toxicol. 1985;35(4):490–495. doi: 10.1007/BF01636543. [DOI] [PubMed] [Google Scholar]
- 14.Fossi MC, et al. Use of nondestructive biomarkers and residue analysis to assess the health status of endangered species of pinnipeds in the South-West Atlantic. Mar Pollut Bull. 1997;34(3):157–162. [Google Scholar]
- 15.Yates DE, et al. Mercury in bats from the northeastern United States. Ecotoxicology. 2014;23(1):45–55. doi: 10.1007/s10646-013-1150-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Majed NB, Preston MR. Factors influencing the total mercury and methyl mercury in the hair of the fishermen of Kuwait. Environ Pollut. 2000;109(2):239–250. doi: 10.1016/s0269-7491(99)00261-4. [DOI] [PubMed] [Google Scholar]
- 17.Magos L, Clarkson TW. The assessment of the contribution of hair to methyl mercury excretion. Toxicol Lett. 2008;182(1-3):48–49. doi: 10.1016/j.toxlet.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 18.Conaway CH, Squire S, Mason RP, Flegal AR. Mercury speciation in the San Francisco Bay estuary. Mar Chem. 2003;80(2):199–225. [Google Scholar]
- 19.Le Boeuf BJ, Laws RM. Elephant seals: Population ecology, behavior and physiology. Univ. of California Press; Berkeley, CA: 1994. pp. 1–26. [Google Scholar]
- 20.Le Boeuf BJ, Condit R, Morris PA, Reiter J. The Northern Elephant Seal (Mirounga angustirostris) rookery at Año Nuevo: A case study in colonization. Aquat Mamm. 2011;37(4):486–501. [Google Scholar]
- 21.Chen Q, et al. High levels of methylmercury in guano and ornithogenic coral sand sediments on Xisha islands, South China sea. Arch Environ Contam Toxicol. 2012;63(2):177–188. doi: 10.1007/s00244-012-9770-7. [DOI] [PubMed] [Google Scholar]
- 22.Brookens TJ, Harvey JT, O’Hara TM. Trace element concentrations in the Pacific harbor seal (Phoca vitulina richardii) in central and northern California. Sci Total Environ. 2007;372(2-3):676–692. doi: 10.1016/j.scitotenv.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Habran S, Crocker DE, Debier C, Das K. How are trace elements mobilized during the postweaning fast in Northern elephant seals? Environ Toxicol Chem. 2012;31(10):2354–2365. doi: 10.1002/etc.1960. [DOI] [PubMed] [Google Scholar]
- 24.Worthy G, Morris P, Costa D, Le Boeuf B. Moult energetics of the northern elephant seal (Mirounga angustirostris) J Zool. 1992;227(2):257–265. [Google Scholar]
- 25.Yee D, McKee LJ, Oram JJ. A regional mass balance of methylmercury in San Francisco Bay, California, USA. Environ Toxicol Chem. 2011;30(1):88–96. doi: 10.1002/etc.366. [DOI] [PubMed] [Google Scholar]
- 26.David N, et al. 2009. Mercury concentrations and loads in a large river system tributary to San Francisco Bay, California, USA. Environ Toxicol Chem 28(10):2091–2100.
- 27.Conaway CH, Black FJ, Grieb TM, Roy S, Flegal AR. 2008. Mercury in the San Francisco Estuary. Rev Environ Contam Toxicol 2008;194:29–54.
- 28.Starr RM, et al. Variation in responses of fishes across multiple reserves within a network of marine protected areas in temperate waters. PLoS One. 2015;10(3):e0118502. doi: 10.1371/journal.pone.0118502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lowry MS, et al. Abundance, distribution, and population growth of the northern elephant seal (Mirounga angustirostris) in the United States from 1991 to 2010. Aquat Mamm. 2014;40(1):20–31. [Google Scholar]
- 30.Carretta JV, et al. 2009 U.S. Pacific Marine Mammal Stock Assessments: 2009. Available at www.nmfs.noaa.gov/pr/pdfs/sars/po2009.pdf.
- 31.Riedman M. The Pinnipeds: Seals, sea lions, and walruses. University of California Press; Berkeley: 1990. pp. 222–263. [Google Scholar]
- 32.Cunningham L, et al. Harbour seal movements and haul-out patterns: Implications for monitoring and management. Aquatic Conservation: Marine and Freshwater Ecosystems. 2009;19(4):398–407. [Google Scholar]
- 33.Liwanag HE, Berta A, Costa DP, Abney M, Williams TM. Morphological and thermal properties of mammalian insulation: The evolution of fur for aquatic living. Biol J Linn Soc Lond. 2012;106(4):926–939. [Google Scholar]
- 34.Fenchel T. Marine plankton food chains. Annu Rev Ecol Syst. 1988;19:19–38. [Google Scholar]
- 35.Lazzaro X. A review of planktivorous fishes: Their evolution, feeding behaviours, selectivities, and impacts. Hydrobiologia. 1987;146(2):97–167. [Google Scholar]
- 36.Boudou A, Ribeyre F. Experimental study of trophic contamination of Salmo gairdneri by two mercury compounds - HgCl2 and CH3HgCl - analysis at the organism and organ levels. Water Air Soil Pollut. 1985;26(2):137–148. [Google Scholar]
- 37.Gagnon C, Fisher NS. Bioavailability of sediment-bound methyl and inorganic mercury to a marine bivalve. Environ Sci Technol. 1997;31(4):993–998. [Google Scholar]
- 38.Lawrence AL, McAloon KM, Mason RP, Mayer LM. Intestinal solubilization of particle-associated organic and inorganic mercury as a measure of bioavailability to benthic invertebrates. Environ Sci Technol. 1999;33(11):1871–1876. [Google Scholar]
- 39.Lambertsson L, Nilsson M. Organic material: The primary control on mercury methylation and ambient methyl mercury concentrations in estuarine sediments. Environ Sci Technol. 2006;40(6):1822–1829. doi: 10.1021/es051785h. [DOI] [PubMed] [Google Scholar]
- 40.Kim E, Mason RP, Bergeron CM. A modeling study on methylmercury bioaccumulation and its controlling factors. Ecol Modell. 2008;218(3):267–289. [Google Scholar]
- 41.Chen CY, et al. Benthic and pelagic pathways of methylmercury bioaccumulation in estuarine food webs of the northeast United States. PLoS One. 2014;9(2):e89305. doi: 10.1371/journal.pone.0089305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pompa S, Ehrlich PR, Ceballos G. Global distribution and conservation of marine mammals. Proc Natl Acad Sci USA. 2011;108(33):13600–13605. doi: 10.1073/pnas.1101525108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keiper C, Ainley D, Allen S, Harvey J. Marine mammal occurrence and ocean climate off central California, 1986 to 1994 and 1997 to 1999. Mar Ecol Prog Ser. 2005;289:285–306. [Google Scholar]
- 44.Flegal AR, et al. Dissolved trace element cycles in the San Francisco Bay estuary. Mar Chem. 1991;36(1–4):329–363. [Google Scholar]
- 45.Parker JL, Bloom NS. Preservation and storage techniques for low-level aqueous mercury speciation. Sci Total Environ. 2005;337(1-3):253–263. doi: 10.1016/j.scitotenv.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 46.US Environmental Protection Agency 2002 Method 1631, Revision E: Mercury in Water by Oxidation Purge and Trap, and Cold Vapor Atomic Fluorescence Spectrometry. Available at nepis.epa.gov/Exe/ZyPDF.cgi/P1008IW8.PDF?Dockey=P1008IW8.PDF. [PubMed]
- 47.US Environmental Protection Agency 1998 Method 1630: Methyl Mercury in Water by Distillation, Aqueous Ethylation, Purge and Trap, and CVAFS. Available at nepis.epa.gov/Exe/ZyPDF.cgi/P100IKBQ.PDF?Dockey=P100IKBQ.PDF. [PubMed]
- 48.Ganguli PM, Conaway CH, Swarzenski PW, Izbicki JA, Flegal AR. Mercury speciation and transport via submarine groundwater discharge at a southern California coastal lagoon system. Environ Sci Technol. 2012;46(3):1480–1488. doi: 10.1021/es202783u. [DOI] [PubMed] [Google Scholar]