Diverse seasonal flowering behaviors drive global adaption of bread wheat (Triticum aestivum), the major crop grown in temperate zones worldwide. Many wheats are sown in autumn and flower only after experiencing the prolonged cold of winter (vernalization). By delaying flowering until spring, the requirement for vernalization minimizes the risk that frost-sensitive flowers and developing grain will be damaged by freezing. This flowering behavior is important in regions where crops are sown in autumn and experience cold winters. In other regions, warm climates, or where crops are sown in spring, the need for cold to stimulate flowering limits wheat cultivation. To adapt wheats to these regions, genes that reduce the vernalization requirement have been used to breed varieties that flower without vernalization. Previous studies have identified the sequences of three of these genes: VERNALIZATION1 (VRN1), VRN2, and VRN3 (1–5). In PNAS, Kippes et al. (6) identify the gene sequence of VRN4, a fourth gene controlling the vernalization requirement of wheat. The authors show that VRN4 is a translocated copy of the VRN1 gene.
VRN1 encodes a MADS box (MCM1/AGAMOUS/DEFICIENS/SRF) transcription factor (1–3). In vernalization-requiring “winter wheats,” VRN1 is transcriptionally activated by prolonged cold to trigger flowering. “Spring wheats” that flower without vernalization typically carry alleles of VRN1 that are actively transcribed without cold, which reduce or eliminate the requirement for vernalization. Kippes et al. (6) show that VRN4 is a variation on this theme: an additional copy of VRN1 that is transcribed without prior cold treatment (Fig. 1). A loss-of-function mutation in the extra copy of VRN1 restores the vernalization requirement, demonstrating that this additional copy of VRN1 corresponds to the VRN4 gene (6). VRN4 occurs widely in an ancient South Asian wheat subspecies, T. aestivum ssp. sphaerococcum, where the extra copy of VRN1 might have originated (6). The reason why the extra copy of VRN1 is active without prior cold exposure is not clear. One possibility is that mutations in the extra copy of VRN1 disrupt the binding site of a RNA binding repressor protein (7). Another possibility is that the new chromosomal location might influence transcriptional activity of the translocated gene copy. Further studies are needed to resolve these possibilities.
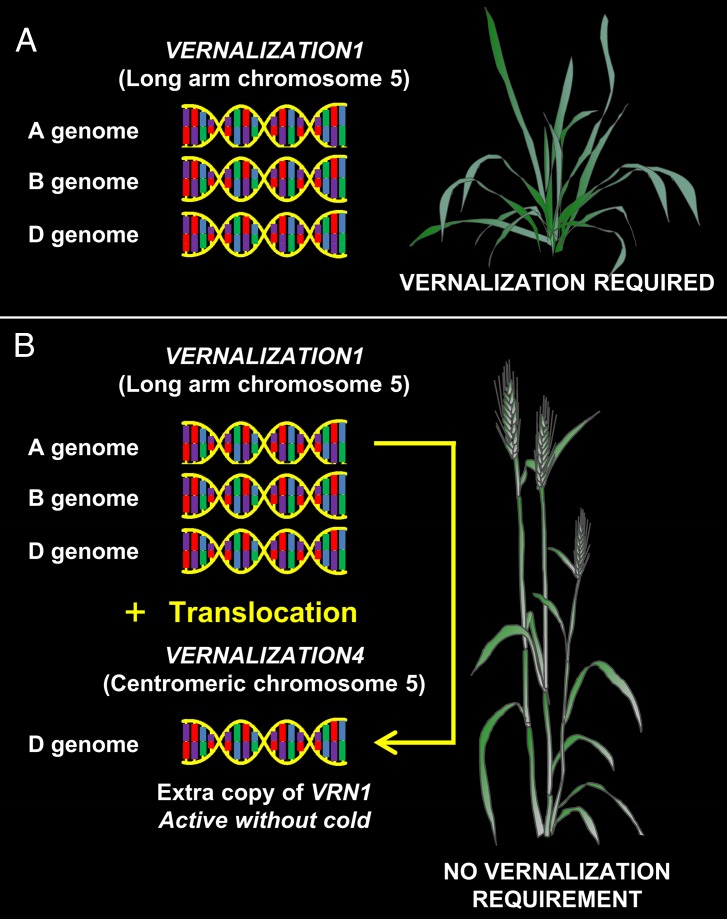
Fig. 1.
Origin of the wheat VRN4 gene that reduces the vernalization requirement. (A) Ancestral hexaploid genome of bread wheat (T. aestivum) had three homeologous VRN1 genes, one on the long arm of chromosome 5 for each of the A, B, and D genomes. VRN1 is a promoter of flowering, but the VRN1 genes are not transcribed until plants experience prolonged cold (vernalization), so flowering is delayed. In this figure, the haploid equivalent is shown, and, normally, there are six copies per nucleus (i.e., AABBDD). (B) VRN1 gene translocated from the long arm of chromosome 5 (A genome) to the centromeric region of chromosome 5 (D genome, proximal region of short arm). The additional VRN1 gene on chromosome 5D has increased transcriptional activity and triggers flowering without vernalization. This extra copy of the VRN1 gene is the basis for the VRN4 gene.
The study by Kippes et al. (6) further highlights the importance of VRN1 as a regulator of vernalization and as a major gene controlling adaptation and life cycle strategies of wheat. A range of different alleles of VRN1 have been identified in cereal breeding programs (8). It seems likely that further diversity in VRN1 awaits characterization and that this diversity can be harnessed for wheat breeding. Screening for direct regulatory targets of VRN1 has provided insights into a broader genetic network controlling flowering in wheat (9). Similarly, transcriptional regulation of VRN1 activity has been investigated (10–12). There is now great potential to investigate how VRN1 is activated by the cold of winter, which is the key to understanding the molecular basis for the vernalization response in cereals. Perhaps the most basic questions are how cereals sense cold and measure the passage of time during winter. The study of Kippes et al. (6) suggests one avenue to investigate these questions: RNA processing pathways.
The discovery that VRN4 is an additional copy of VRN1 raises interesting questions about how natural variation has been harnessed to breed modern wheat cultivars. For example, why is activation of VRN1 the major mechanism for reducing the vernalization requirement in bread wheat? Part of the answer is that bread wheat is a hexaploid crop, and recessive genes that reduce the vernalization requirement are less likely to be deployed. Another explanation is that alternative genes that reduce the vernalization requirement might compromise other important traits (e.g., grain yield), and so are not used in wheat breeding. A final explanation is that active alleles of VRN1 (and VRN4) might have been common in wild wheats at the dawn of agriculture, and so were easily incorporated into cultivated varieties (13). The vernalization requirement is an important trait, so it will be worthwhile to explore other ways to breed wheats that flower without vernalization. Efforts to map other vernalization genes will be of great interest (14).
The findings of the study by Kippes et al. (6) will have an impact on crop improvement. The identification of the VRN4 gene sequence extends the complement of “perfect markers” for wheat vernalization genes. These markers can be used for marker-assisted selection in breeding populations. Perfect markers are also useful for parent selection, allowing wheat breeders to minimize the genetic complexity of polyploid traits in breeding crosses. Marker information for VRN1 (and
The findings of the study by Kippes et al.will have an impact on crop improvement.
other genes) is already being used by breeders in this manner. VRN4 might also allow breeders to avoid ancestral linkage relationships, such as between VRN1 and other genes controlling flowering, for example (15), because VRN4 is located at a new chromosomal address.
A final perspective on the study by Kippes et al. (6) is that it provides an excellent example of current progress in crop genomics. The grand challenge for crop genomics is to harness ever-increasing sequence information to predict crop performance under field conditions. The past decade has seen rapid advances toward this objective, and our understanding of genes controlling the vernalization requirement in wheat is a leading example. More broadly, knowledge of genes controlling flowering behavior of wheat has progressed to the point where it will soon be possible to predict crop heading dates at different locations and sowing dates based on genotype data and climate information, with a high degree of accuracy. This ability to predict flowering dates will impact both crop improvement and crop management.
Footnotes
The author declares no conflict of interest.
See companion article on page E5401.
References
- 1.Yan L, et al. Positional cloning of the wheat vernalization gene VRN1. Proc Natl Acad Sci USA. 2003;100(10):6263–6268. doi: 10.1073/pnas.0937399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Danyluk J, et al. TaVRT-1, a putative transcription factor associated with vegetative to reproductive transition in cereals. Plant Physiol. 2003;132(4):1849–1860. doi: 10.1104/pp.103.023523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trevaskis B, Bagnall DJ, Ellis MH, Peacock WJ, Dennis ES. MADS box genes control vernalization-induced flowering in cereals. Proc Natl Acad Sci USA. 2003;100(22):13099–13104. doi: 10.1073/pnas.1635053100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yan L, et al. The wheat VRN2 gene is a flowering repressor down-regulated by vernalization. Science. 2004;303(5664):1640–1644. doi: 10.1126/science.1094305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yan L, et al. The wheat and barley vernalization gene VRN3 is an orthologue of FT. Proc Natl Acad Sci USA. 2006;103(51):19581–19586. doi: 10.1073/pnas.0607142103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kippes N, et al. Identification of the VERNALIZATION 4 gene reveals the origin of spring growth habit in ancient wheats from South Asia. Proc Natl Acad Sci USA. 2015;112:E5401–E5410. doi: 10.1073/pnas.1514883112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiao J, et al. O-GlcNAc-mediated interaction between VER2 and TaGRP2 elicits TaVRN1 mRNA accumulation during vernalization in winter wheat. Nat Commun. 2014;5:4572. doi: 10.1038/ncomms5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eagles HA, Cane K, Vallance N. The flow of alleles of important photoperiod and vernalisation genes through Australian wheat. Crop Pasture Sci. 2009;60(7):646–657. [Google Scholar]
- 9.Deng W, et al. Direct links between the vernalization response and other key traits of cereal crops. Nat Commun. 2015;6:5882. doi: 10.1038/ncomms6882. [DOI] [PubMed] [Google Scholar]
- 10.Li C, Dubcovsky J. Wheat FT protein regulates VRN1 transcription through interactions with FDL2. Plant J. 2008;55(4):543–554. doi: 10.1111/j.1365-313X.2008.03526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oliver SN, Finnegan EJ, Dennis ES, Peacock WJ, Trevaskis B. Vernalization-induced flowering in cereals is associated with changes in histone methylation at the VERNALIZATION1 gene. Proc Natl Acad Sci USA. 2009;106(20):8386–8391. doi: 10.1073/pnas.0903566106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oliver SN, Deng W, Casao MC, Trevaskis B. Low temperatures induce rapid changes in chromatin state and transcript levels of the cereal VERNALIZATION1 gene. J Exp Bot. 2013;64(8):2413–2422. doi: 10.1093/jxb/ert095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Golovnina KA, Kondratenko EY, Blinov AG, Goncharov NP. Molecular characterization of vernalization loci VRN1 in wild and cultivated wheats. BMC Plant Biol. 2010;10:168. doi: 10.1186/1471-2229-10-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saisho D, Ishii M, Hori K, Sato K. Natural variation of barley vernalization requirements: Implication of quantitative variation of winter growth habit as an adaptive trait in East Asia. Plant Cell Physiol. 2011;52(5):775–784. doi: 10.1093/pcp/pcr046. [DOI] [PubMed] [Google Scholar]
- 15.Chen A, et al. Phytochrome C plays a major role in the acceleration of wheat flowering under long-day photoperiod. Proc Natl Acad Sci USA. 2014;111(28):10037–10044. doi: 10.1073/pnas.1409795111. [DOI] [PMC free article] [PubMed] [Google Scholar]