Significance
We discovered an improved hydroxamic acid-based small-molecule N-hydroxy-4-[(N(2-hydroxyethyl)-2-phenylacetamido)methyl)benzamide)] that selectively inhibits histone deacetylase 6 catalytic activity in vivo and in vitro. This can have important anticancer activity.
Keywords: HDAC, tubulin, cancer
Abstract
We report the development of a potent, selective histone deacetylase 6 (HDAC6) inhibitor. This HDAC6 inhibitor blocks growth of normal and transformed cells but does not induce death of normal cells. The HDAC6 inhibitor alone is as effective as paclitaxel in anticancer activity in tumor-bearing mice.
There are 11 zinc-dependent histone deacetylases (HDACs) in humans (1). All HDACs are nuclear proteins except for HDAC6. HDAC6 is unique among HDACs in being a cytoplasmic protein with two catalytic sites and a ubiquitin binding site (2). HDAC6 is a deacetylase whose substrates include tubulin, peroxidases, and certain DNA repair proteins, but not histones (2). HDAC6 has a role in the cellular response to accumulation of misfolded and aggregated proteins that are catalysts of certain neurological disorders such as Alzheimer’s, Parkinson’s, and Huntington’s diseases.
We have previously reported the development of an HDAC6 selective inhibitor (3), as have others (4–6). This paper describes the development of a new potent, selective HDAC6 inhibitor, N-hydroxy-4-[(N(2-hydroxyethyl)-2-phenylacetamido)methyl) benzamide)] (HPB). HPB inhibits growth of normal and transformed cells but does not induce death of these cells. HPB alone induces tumor shrinkage in tumor-bearing mice without apparent significant side effects.
Synthesis of the HDAC6 Selective Inhibitor
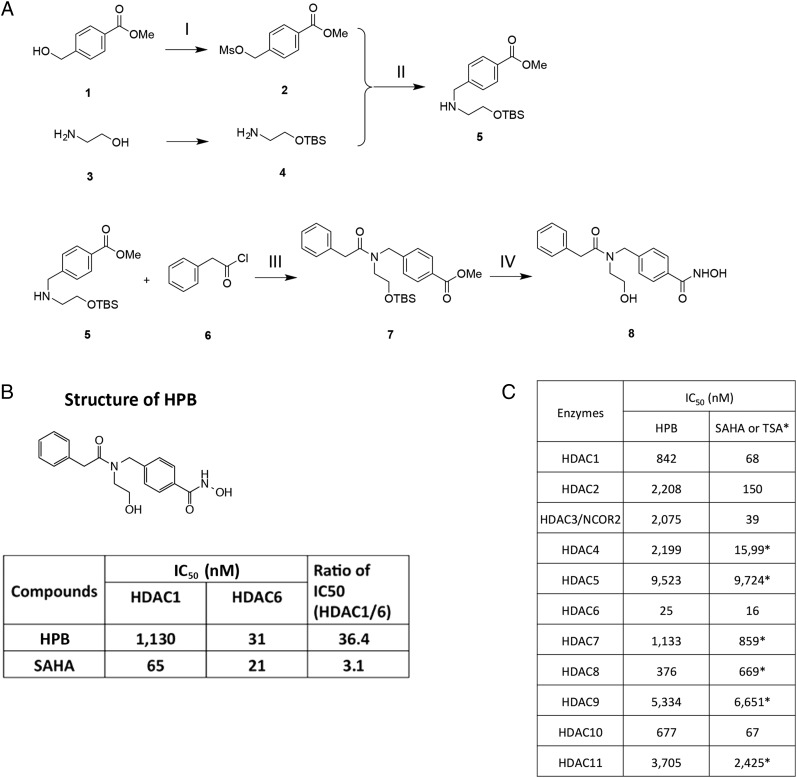
HPB was synthesized as shown in Fig. 1A. The intermediates and the final product in this synthesis were characterized by mass and NMR spectra.
Fig. 1.
HPB is a novel HDAC6 selective inhibitor. (A) HPB (8) was synthesized from commercially available materials in four steps with an overall yield of 17%. (I) Methyl 4-(hydroxymethyl)benzoate (1) was mesylated with mesylchloride to give methyl 4-(((methylsulfonyl)oxy)methyl)benzoate (2). (II) Reaction of compound 2 and TBS protected 2-aminoethanol (4) gave methyl 4-(((2-((tert-butyldimethylsilyl)oxy)ethyl)amino)methyl)benzoate (5). (III) Compound 5 was coupled with 2-phenylacetyl chloride (6) to give methyl 4-((N-(2-((tert-butyldimethylsilyl)oxy)ethyl)-2-phenylacetamido)methyl)benzoate (7). (IV) Compound 7 was reacted with hydroxyl amine in the presence of a catalytic amount of KCN to introduce hydroxamic acid functional group and finally TBS group was removed with 2% HCl in methanol to yield final compound 8, HPB. Intermediates and the final compounds were characterized by mass and NMR spectra. (B) IC50 values for HDAC6 selective inhibitor. HPB and nonselective inhibitor, SAHA. (C) IC50 values for HPB and SAHA/TSA for the 11 zinc-dependent HDAC enzymes.
HPB Is a Selective Inhibitor of HDAC6
To determine whether HPB is a selective inhibitor of HDAC6, it was assayed for inhibition of recombinant HDAC1 compared with HDAC6. HPB has an IC50 inhibitory activity for HDAC6 of 31 nM, compared with that for HDAC1 of 1,130 nM (Fig. 1B). HPB inhibition of the 11 zinc-containing HDACs showed HPB to be a 15- to almost 400-fold more potent inhibitor of HDAC6 than of other zinc-dependent HDACs (Fig. 1C). The inhibitory activity of HPB for the 11 zinc-containing HDACs was compared with suberoylanilide hydroxamic acid (SAHA) or trichostatin A (TSA) (Fig. 1C). The selectivity for HPB inhibition of HDAC6 among the 11 zinc-containing HDACs was comparable to that of SAHA and TSA (Fig. 1C).
HPB Inhibits Growth, but Not Viability, of Normal and Transformed Cells
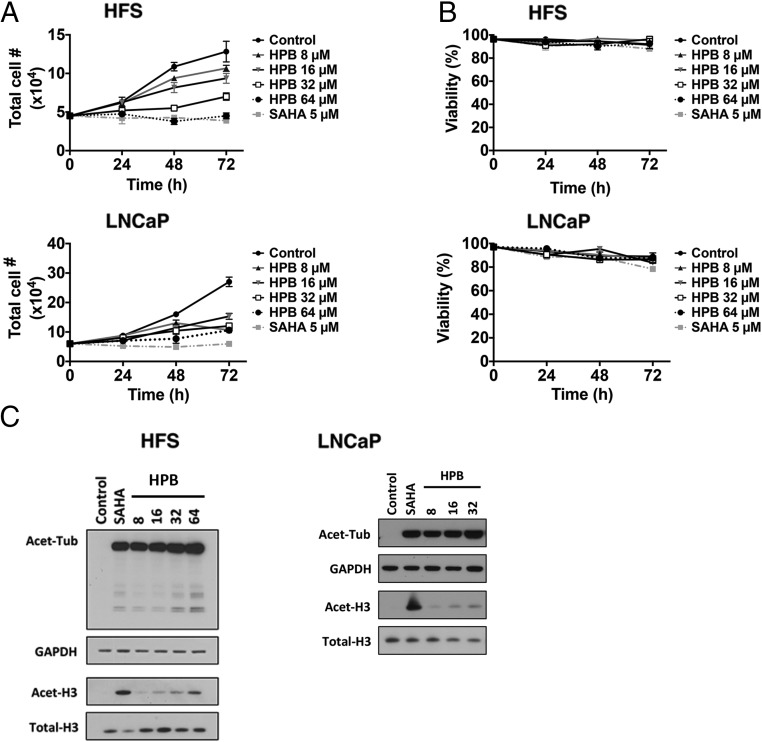
We next determined the effect of HPB on the cell growth and viability of normal (HFS, human foreskin fibroblast) and transformed (LNCaP, human prostate adenocarcinoma) cells cultured with 8, 16, 32, or 64 μM HPB for up to 72 h. HPB inhibited cell growth of normal and transformed cells in a concentration-dependent manner (Fig. 2A) but did not induce cell death of normal or transformed cells (Fig. 2B).
Fig. 2.
Effects of HPB on cell growth and viability and acetylated patterns of proteins and histones in normal and transformed cells in culture. Normal (HFS) and transformed (LNCaP) cells were cultured with indicated concentrations of HPB for 72 h. Five micromolar SAHA is a positive control. (A) Cell growth. (B) Cell viability. Inhibition of cell growth of normal and transformed cells is concentration-dependent. Viable cells were evaluated by trypan blue staining. Data are represented as mean ± SD (P < 0.001). The P value was derived from the two-way ANOVA. (C) HPB causes accumulation of acetylated alpha-tubulin but not acetylated histone H3 in normal (HFS) and transformed (LNCaP) cells. Cells were cultured with 5 µM SAHA or 8, 16, 32, or 64 µM HPB for 24 h as indicated. SAHA, the pan-HDAC inhibitor, was the control. Cell lysates were prepared for immunoblot analysis of acetylated alpha-tubulin (Acet-Tub) and acetylated histone H3 (Acet-H3). GAPDH and total H3 are loading controls.
HPB Induces Acetylation of Alpha-Tubulin, but Not Histones, in Normal and Transformed Cells
In normal (HFS) and transformed (LNCaP) cells, HPB (8 to 16 μM) caused accumulation of acetylated alpha-tubulin and acetylated peroxiredoxin, substrates of HDAC6 (7, 8) but not of acetylated histones (Fig. 2C). As previously reported (9–11), SAHA induced the accumulation of acetylated alpha-tubulin and histone H3, and tubacin induced accumulation of acetylated alpha-tubulin but not of histone H3 in normal and transformed cells (Fig. 2C).
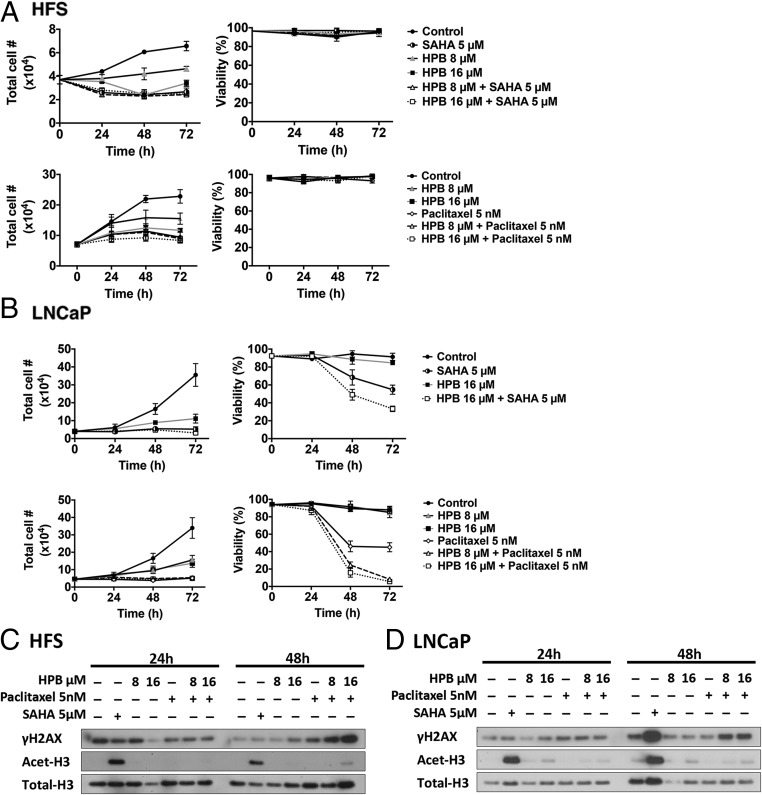
HPB Enhances Transformed Cell Death Induced by the Anticancer Drugs Paclitaxel or SAHA
To assess whether selective inhibition of HDAC6 by HPB enhances cell death of normal and transformed cells in culture with anticancer agents, cells were cultured with HPB and the mitotic inhibitor paclitaxel, or the pan-HDAC inhibitor SAHA, for 72 h. In HFS cells, HPB alone or in combination with paclitaxel or SAHA inhibited cell growth but did not induce loss of cell viability (Fig. 3A). LNCaP cell death was enhanced in cultures with HPB and 5 μM SAHA compared with cultures with SAHA alone (Fig. 3B). Combined treatment with paclitaxel and HPB also caused increased cell death in LNCaP cells compared with either drug alone (Fig. 3B). The effect of paclitaxel on tubulin acetylation is antagonistic against HDAC6 inhibition.
Fig. 3.
HPB enhances paclitaxel and SAHA induced transformed cell death but not normal cell death. Cell growth and viability of (A) HFS and (B) LNCaP cells cultured with HPB with 5 nM paclitaxel or 5 µM SAHA alone and in combination with HPB for 72 h. Cell growth and viability were determined as previously described. Data are represented as mean ± SD (P < 0.001). The P value was derived from the two-way ANOVA. (C) HFS and (D) LNCaP cell lysates prepared at 24 and 48 h after treatment for immunoblot analysis of γH2AX and acetylated histone H3 (Acet-H3). Total H3 is a loading control.
Effect of HPB Without and With Paclitaxel on Proteins Involved in DNA Repair
HPB (8 and 16 µM) with paclitaxel (5 nM) increased accumulation of γH2AX after 48 h in culture in HFS and LNCaP cells in culture. Neither agent alone altered the level of γH2AX (Fig. 3 C and D). HPB up to 16 µM did not alter the expression of RAD50, MRE11, PARP, or BCL-2 in HFS or LNCaP cells cultured for up to 48 h.
HPB Is Well Tolerated in Mice and Is As Cytotoxic As Paclitaxel, an Anticancer Drug
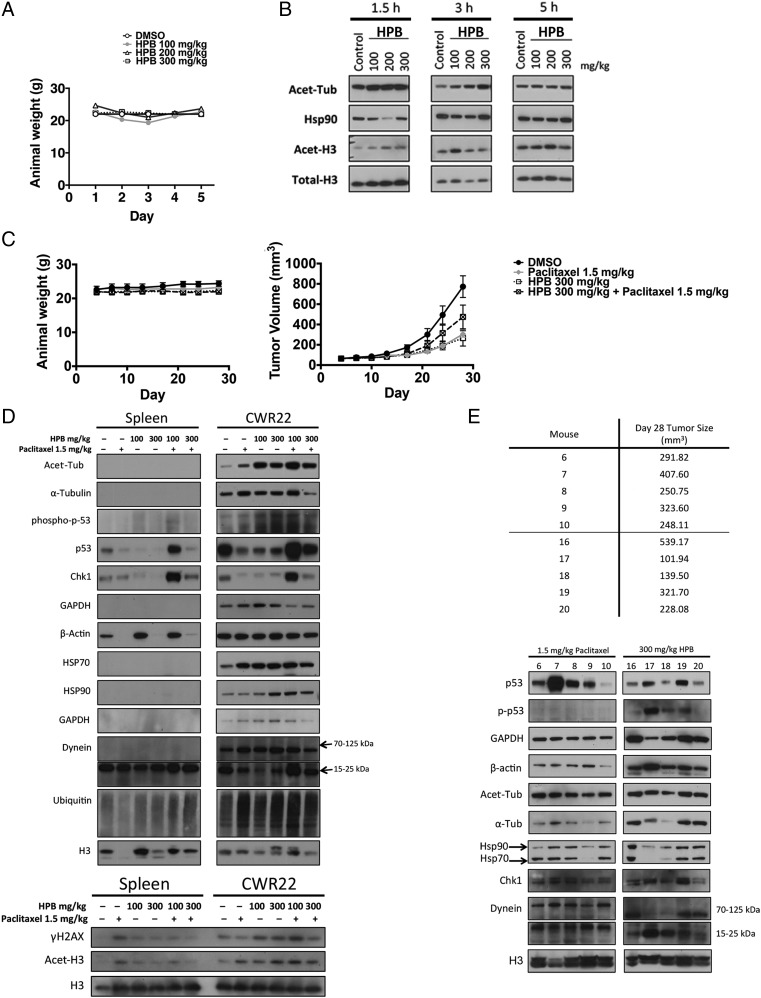
We next determined the toxicity of HPB in mice in vivo. Mice were intraperitoneally injected daily for 5 d with 100, 200, or 300 mg/kg HPB. The dose of 300 mg/kg HPB was the maximum concentration attainable without drug precipitation. There was no detectable weight loss in these mice (Fig. 4A). The effects of HPB on the acetylation of alpha-tubulin and histones in the spleen isolated from mice treated with HPB were analyzed at three time points after the administration of the drug. At 1.5 h after injection of HPB, an increased accumulation of acetylated tubulin was found in the spleen (Fig. 4B). By 5 h after injection of HPB, the accumulation of acetylated tubulin was reduced to the level seen in vehicle-treated controls. There was no detectable accumulation of acetylated histones in the spleen from the mice receiving HPB.
Fig. 4.
HPB enhances anticancer effects of SAHA in mice bearing human prostate cancer CWR22 xenograft. (A) HPB is well tolerated in mice. Mice were injected with indicated doses of HPB i.p. daily for 5 d. Data are represented as mean ± SD (P < 0.001). The P value was derived from the two-way ANOVA. (B) HDAC6 selectivity of HPB in spleen isolated from mice. Spleens were isolated from mice injected with indicated drugs at 1.5, 3, and 5 h after last injection on day 5. Immunoblots for acetylated tubulin (Acet-Tub) and acetylated histone H3 (Acet-H3). HSP90 and total H3 are loading controls. (C) Weights and tumor volumes of mice were injected with HPB (300 mg/kg) i.p. daily for 5 d a week, paclitaxel (1.5 mg/kg) i.p. once a week, or combination treatment over 4 wk. (D) Immunoblots of mice treated with DMSO vehicle solvent, 1.5 mg/kg paclitaxel, HPB (100 or 300 mg/kg), and combination treatment. Total H3 is a loading control. (E) Immunoblots of all mice treated with 300 mg/kg HPB alone or 1.5 mg/kg paclitaxel alone. Total H3 is a loading control.
We examined the effects of HPB alone or in combination with the anticancer drug paclitaxel in nude mice implanted with the androgen-dependent CWR22 human prostate cancer xenograft grown s.c. Daily administration five times a week of either 300 mg/kg HPB or of 1.5 mg/kg paclitaxel alone for 28 d caused significant suppression of the growth of established CWR22 tumors without weight loss (Fig. 4C). The combination of daily administration of 300 mg/kg HPB and of 1.5 mg/kg paclitaxel five times a week caused modest suppression of the growth of established CWR22 tumors (Fig. 4C).
Tumor and spleen were removed from the animals, and histones and proteins were extracted for the analysis of acetylated lysine patterns. There was increased accumulation of acetylated alpha-tubulin in CWR22 tumors from mice treated with HPB, paclitaxel, or a combination of HPB and paclitaxel (Fig. 4D). Increased levels of accumulation of histones were found in tumors of mice injected with paclitaxel or combination of paclitaxel and HPB, but not with HPB alone. These data indicate that HPB is a selective inhibitor of HDAC6 and can enhance the antitumor effect of paclitaxel in vivo.
HPB Alone Modulates Cytoskeletal Proteins
Where tumor shrinkage was greatest in mice treated with HPB alone at 300 mg/kg, levels of beta-actin were markedly increased (Fig. 4E). Chaperone proteins HSP90 and HSP70 decreased in the smallest tumors (Fig. 4E). Treatment with HPB alone or in combination with paclitaxel did not change levels of DNA repair proteins.
Discussion
We report the development of a novel HDAC6 selective inhibitor, HPB, and its biological effects in normal and transformed cells. HPB inhibits HDAC6 in vitro with ∼36-fold selectivity against HDAC6 over HDAC1 enzyme. Concentrations as high as 16 µM of HPB selectively induce accumulation of acetylated alpha-tubulin and other protein substrates of HDAC6, but not acetylated histones, in both normal and transformed cells. Histones are not a substrate of HDAC6. HPB in concentrations ≤16 µM do not induce normal cell death. This selectivity of HPB as an inhibitor of HDAC6 exceeds or is comparable to previously reported HDAC6-selective inhibitors (3–6). HPB enhances paclitaxel or SAHA-induced transformed cell death. Culture of transformed cells with HPB enhances the cytotoxicity of anticancer drugs, at least in part, through increased accumulation of DNA damage.
HPB is well tolerated in animals. HPB treatment alone at 300 mg/kg is as effective as paclitaxel treatment at 1.5 mg/kg in nude mice with androgen-dependent CWR22 human prostate cancer xenograft.
These findings suggest that selective inhibition of HDAC6 may be an important avenue to treat human cancers with less toxicity than currently available treatments.
Experimental Procedures
Cell Lines, Reagents, and Antibodies.
HFS was purchased from Yale Skin Diseases Research Center Core. LNCaP cells (prostate cancer cell line) were purchased from American Type Culture Collection and cultured per directions of the supplier. SAHA (vorinostat) was synthesized as reported (12) and was dissolved in DMSO. Paclitaxel was purchased from ChemiTek and was dissolved in DMSO. Antibodies used were as follows: anti-phosphorylated histone H2AX and anti-chk1 (Abcam), anti-acetylated histone H3 and total histone H3 (Active Motif), anti-acetylated alpha-tubulin and anti-dynein (Sigma), anti-p53 (Santa Cruz Biotechnology), anti-HSP90 and anti-HSP70 (Enzo), anti-ubiquitin (Enzo Biomolecule), anti-GAPDH (Thermo Scientific), anti-total alpha-tubulin (Calbiochem), and anti–phospho-p53 and anti–β-actin(Cell Signaling).
In Vitro Enzymatic Assay for Histone Deacetylases.
Histone deacetylase enzymatic activity was assayed as previously described (3). In vitro activities of the 11 recombinant human zinc-dependent HDAC enzymes (BPS Biosciences) were detected by fluorigenic release of 7-amino-4-methylcoumarin from substrate upon deacetylase enzymatic activity. A series of dilutions of the novel HDAC6 compound, tubacin, and SAHA were prepared with 10% (vol/vol) DMSO in HDAC assay buffer and 5 µL of the dilution was added to a 50-µL reaction so that the final concentration of DMSO was 1% in all of reactions. The enzymatic reactions were conducted in duplicate at 37 °C for 30 min in a 50-µL mixture containing HDAC assay buffer, 5 µg BSA, an HDAC substrate, an HDAC enzyme, and a test compound. After enzymatic reactions, 50 μL of 2× HDAC developer was added to each well and the plate was incubated at room temperature for an additional 15 min. Fluorescence intensity was measured at an excitation of 360 nm and an emission of 460 nm using a Biotek Synergy microplate reader. Negative (no enzyme, no inhibitor, a drug with no HDAC inhibition activity) and positive controls (known HDAC inhibitor SAHA) were included in the assays. Half maximal inhibitory concentration (IC50) is determined at the drug concentration that results in 50% reduction of HDAC activity compared with control.
Cell Growth and Viability.
Each cell culture of HFS and LNCaP was performed in triplicate and cell growth and viability were determined as previously described (10). Graphs were constructed using Prism 5 (GraphPad). Data were expressed as mean ± SD.
Immunoblotting Analysis.
Analyses of pattern of proteins were performed as previously described (10).
Histone Extraction.
Histone proteins were isolated from cell lysates as previously described (10).
Animal Study.
Animal studies were carried out under protocol 12-02-003, approved by the Memorial Sloan–Kettering Cancer Center Institutional Animal Care and Use Committee. Institutional guidelines for the proper, humane use of animals in research were followed.
Animal Study for Toxicity.
For administration to mice, HPB was dissolved and diluted in a vehicle of DMSO. Each group of mice received 100, 200, or 300 mg/kg HPB or 100 mg/kg SAHA daily by i.p. injection for 5 d. A control group of five animals was injected with vehicle only (DMSO). The injection volume was kept constant at 1 μL/g body weight. The mice were weighed every day during the experimental period to assess toxicity of the treatments. On the final day of the study, at 1.5, 3, or 5 h after last injection, mice were killed by carbon dioxide inhalation. The spleens and tumors were collected and were prepared for immunoblot analysis as described in previous study (13).
Animal Study with CWR22 Xenograft.
The CWR22 human prostate cancer xenograft was prepared in nude mice as previously described (13). Mice with palpable CWR22 tumors were divided into four groups (five mice per group) for the treatment study. All mice in each treatment group had tumors of similar size (5 × 5 mm) at the start of treatment. For administration to mice, HPB and paclitaxel were dissolved and diluted in a vehicle of DMSO. Each group of mice received paclitaxel, HPB, or combination of paclitaxel and HPB at doses indicated, daily five times a week by i.p. injection for 28 d. A control group of five animals was injected with vehicle only (DMSO). The injection volume was kept constant at 1 μL/g body weight. The mice were weighed three times during the experimental period to assess toxicity of the treatments, and the tumors were measured twice weekly using calipers. Tumor volume was calculated from the 2D caliper measurements using the following formula: tumor volume = length × (width)2 × π/6. The treatment period was completed after 28 d. On the final day of the study, all mice were killed by carbon dioxide inhalation. The s.c. tumors and spleens were collected and prepared for immunoblot analysis as described in previous study (13).
Statistical Analyses.
Data are expressed as mean ± SD derived minimally from three independent experiments. Statistical significance was calculated by using the one-tailed Student's t test or two-way ANOVA test.
Acknowledgments
We thank Joann Perrone for her assistance in the preparation of this manuscript. These studies were supported, in part, by National Institute of Cancer Grant P30CA08748-44, the David Koch Foundation, and a grant from Servier.
Footnotes
Conflict of interest statement: Memorial Sloan Kettering Cancer Center and Columbia University hold patents on suberoylanilide hydroxamic acid (vorinostat) and related compounds that were exclusively licensed in 2001 to ATON Pharma, a biotechnology start-up that was wholly acquired by Merck, Inc., in April 2004, and hold patents on N-hydroxy-4-[(N(2-hydroxyethyl)-2-phenylacetamido)methyl) benzamide)].
References
- 1.Marks PA. Histone deacetylase inhibitors: A chemical genetics approach to understanding cellular functions. Biochim Biophys Acta. 2010;1799(10-12):717–725. doi: 10.1016/j.bbagrm.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Y, Shin D, Kwon SH. Histone deacetylase 6 plays a role as a distinct regulator of diverse cellular processes. FEBS J. 2013;280(3):775–793. doi: 10.1111/febs.12079. [DOI] [PubMed] [Google Scholar]
- 3.Lee JH, et al. Development of a histone deacetylase 6 inhibitor and its biological effects. Proc Natl Acad Sci USA. 2013;110(39):15704–15709. doi: 10.1073/pnas.1313893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santo L, et al. Preclinical activity, pharmacodynamic, and pharmacokinetic properties of a selective HDAC6 inhibitor, ACY-1215, in combination with bortezomib in multiple myeloma. Blood. 2012;119(11):2579–2589. doi: 10.1182/blood-2011-10-387365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergman JA, et al. Selective histone deacetylase 6 inhibitors bearing substituted urea linkers inhibit melanoma cell growth. J Med Chem. 2012;55(22):9891–9899. doi: 10.1021/jm301098e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inks ES, Josey BJ, Jesinkey SR, Chou CJ. A novel class of small molecule inhibitors of HDAC6. ACS Chem Biol. 2012;7(2):331–339. doi: 10.1021/cb200134p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kovacs JJ, et al. HDAC6 regulates Hsp90 acetylation and chaperone-dependent activation of glucocorticoid receptor. Mol Cell. 2005;18(5):601–607. doi: 10.1016/j.molcel.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 8.Parmigiani RB, et al. HDAC6 is a specific deacetylase of peroxiredoxins and is involved in redox regulation. Proc Natl Acad Sci USA. 2008;105(28):9633–9638. doi: 10.1073/pnas.0803749105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marks PA, Breslow R. Dimethyl sulfoxide to vorinostat: Development of this histone deacetylase inhibitor as an anticancer drug. Nat Biotechnol. 2007;25(1):84–90. doi: 10.1038/nbt1272. [DOI] [PubMed] [Google Scholar]
- 10.Lee JH, Choy ML, Ngo L, Foster SS, Marks PA. Histone deacetylase inhibitor induces DNA damage, which normal but not transformed cells can repair. Proc Natl Acad Sci USA. 2010;107(33):14639–14644. doi: 10.1073/pnas.1008522107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Namdar M, Perez G, Ngo L, Marks PA. Selective inhibition of histone deacetylase 6 (HDAC6) induces DNA damage and sensitizes transformed cells to anticancer agents. Proc Natl Acad Sci USA. 2010;107(46):20003–20008. doi: 10.1073/pnas.1013754107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richon VM, et al. A class of hybrid polar inducers of transformed cell differentiation inhibits histone deacetylases. Proc Natl Acad Sci USA. 1998;95(6):3003–3007. doi: 10.1073/pnas.95.6.3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butler LM, et al. Suberoylanilide hydroxamic acid, an inhibitor of histone deacetylase, suppresses the growth of prostate cancer cells in vitro and in vivo. Cancer Res. 2000;60(18):5165–5170. [PubMed] [Google Scholar]