Significance
In drylands worldwide, where plant cover is sparse, large amounts of the ground surface are covered by specialized organisms that form biological soil crusts (biocrusts). Biocrusts fix carbon and nitrogen, stabilize soils, and influence hydrology. Extensive physical disturbance from livestock/human trampling and off-road vehicles is known to destroy biocrusts and alter ecosystem function. More recent work also indicates that climate change can affect biocrust communities. Contrary to our expectations, experimental climate change and physical disturbance had strikingly similar impacts on biocrust communities, with both promoting a shift to degraded, early successional states. These results herald ecological state transitions in drylands as temperatures rise, calling for management strategies that consider risks from both physical disturbances and climate change.
Keywords: alternate states, biocrusts, community structure, secondary succession, warming
Abstract
Biological soil crusts (biocrusts)—communities of mosses, lichens, cyanobacteria, and heterotrophs living at the soil surface—are fundamental components of drylands worldwide, and destruction of biocrusts dramatically alters biogeochemical processes, hydrology, surface energy balance, and vegetation cover. Although there has been long-standing concern over impacts of physical disturbances on biocrusts (e.g., trampling by livestock, damage from vehicles), there is increasing concern over the potential for climate change to alter biocrust community structure. Using long-term data from the Colorado Plateau, we examined the effects of 10 y of experimental warming and altered precipitation (in full-factorial design) on biocrust communities and compared the effects of altered climate with those of long-term physical disturbance (>10 y of replicated human trampling). Surprisingly, altered climate and physical disturbance treatments had similar effects on biocrust community structure. Warming, altered precipitation frequency [an increase of small (1.2 mm) summer rainfall events], and physical disturbance from trampling all promoted early successional community states marked by dramatic declines in moss cover and increases in cyanobacteria cover, with more variable effects on lichens. Although the pace of community change varied significantly among treatments, our results suggest that multiple aspects of climate change will affect biocrusts to the same degree as physical disturbance. This is particularly disconcerting in the context of warming, as temperatures for drylands are projected to increase beyond those imposed as treatments in our study.
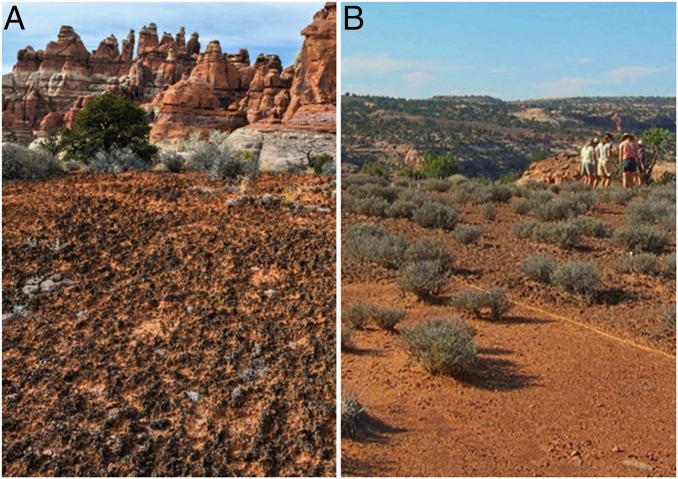
The potential for ecological state transitions in response to global change, particularly transitions that promote feedbacks to terrestrial biogeochemical cycling, is a growing concern (1–6). Anticipating state transitions in terrestrial ecosystems largely hinges on understanding the response of primary producers to warming temperatures, altered precipitation patterns, and novel disturbance regimes (4–7). In arid and semiarid ecosystems (drylands), a substantial portion of primary production can take place in biological soil crusts (biocrusts; Fig. 1) (8, 9), which are communities of mosses, lichens, cyanobacteria, and heterotrophs living at the soil surface that can constitute up to 70% of dryland ground cover (10–12).
Fig. 1.
Biocrusts can locally regulate ecosystem processes and cover large portions of dryland ecosystems as in A (photo by Bill Bowman). Biocrusts are sensitive to physical disturbances from vehicles and trampling by livestock or people as depicted in B, which shows an experimentally trampled plot (foreground) bordered by undisturbed biocrust (background).
Biocrust organisms are adapted to limited moisture and low nutrient conditions and respond rapidly to pulsed, dynamic environmental conditions (13–16). Due to their extensive cover and rapid responses to even small inputs of moisture and nutrients, biocrusts often locally regulate soil hydrology and the cycling of soil carbon (9, 12, 17–21) and nitrogen (9, 22–27). However, the same traits that adapt biocrust organisms to pulsed environmental conditions also make them potentially vulnerable to anticipated changes in climate (28–30). Given their significant influence on ecosystem processes, understanding how biocrust communities will respond to changing climate and disturbance regimes is essential for predicting ecological state changes in drylands—which cover roughly 40% of the Earth’s terrestrial surface and hold an estimated 25% of global organic soil carbon (31).
Biocrusts are bound together by filamentous strands of cyanobacteria, which glue soil particles together to form the characteristic soil shields for which the communities are renowned (10). Although this structure results in resistance to wind shear stress, it does not provide much resistance to physical disturbance. Not surprisingly, both the physical and biotic structures of biocrust are highly sensitive to a range of disturbances, such as vehicle traffic and trampling by humans and livestock (32). Disturbance-induced changes in biocrust community structure and subsequent variation across successional states are well-characterized and are remarkably similar globally: Physical disturbance typically transforms later-successional communities of lichens and mosses to early-successional communities dominated by cyanobacteria (10, 32). This successional resetting significantly impacts ecosystem processes, including large changes in carbon and nitrogen cycling (11, 24, 25, 33). Alarmingly, some experimental work suggests biocrust communities may also be highly sensitive to changing climate. Specifically, increased temperatures have been reported to reduce lichen cover in semiarid Spain (12, 34), and altered precipitation patterns promoted rapid moss mortality (35) and greater variability in cyanobacterial species composition and abundance (36, 37) in a cool desert of the Colorado Plateau. These changes in biocrusts due to climate manipulations also impact ecosystem processes (12, 35, 38).
Despite concern over potential climate change-induced shifts in biocrust community structure and the impacts on ecosystem processes, available reports of biocrust responses to climate manipulations are based on relatively short experimental time spans, often less than 3 y in duration (e.g., 12, 34–36, 39). In addition to the lack of long-term data on biocrust responses to climate change, it is not yet known how climate change impacts on biocrust communities will compare with large, continued threats from physical disturbances due to development, agriculture, and other human activities (32, 40, 41). Understanding the magnitude of threats to biocrusts from both climate change scenarios and novel disturbance regimes is thus a necessary step for predicting future ecological states and developing comprehensive management plans in drylands.
We compared the effects of warming temperatures and altered precipitation patterns on biocrust community structure using data from 10 y (autumn 2005 to autumn 2014) of biocrust community surveys, completed in a full-factorial climate manipulation experiment (control, warming, watering, warming + watering) on the Colorado Plateau, Utah. We simultaneously examined the impact of 15 y annual physical disturbance on biocrust community structure within a replicated human-trampling experiment in a site with vegetation and biocrust communities similar to those in our climate manipulation study (Fig. 1). Finally, we compared the effects of climate manipulation treatments and physical disturbance from trampling on the relative cover of three biotic groups that typically dominate biocrusts of our study region: cyanobacteria, mosses, and lichens. Our goals were, first, to compare the responses of biocrust communities to increased temperature versus increased frequency of small precipitation events, both of which are forecast by climate models for the Colorado Plateau (28, 29, 42) and, second, to compare the effects of climate manipulations to the effects of physical disturbance on biocrust community structure, with a focus on understanding potential variation in responses across different fractions of the community.
Results
Climate manipulation treatments had significant effects on biocrust community structure (P < 0.001; Table 1), particularly when the interaction of treatment and time and the interaction of treatment and experimental blocks were considered (P < 0.001 for both; Table 1 and Fig. 2). Variance partitioning from principle response curves (PRCs) (43, 44) indicated that interannual variation accounted for 17% of the changes seen in biocrust community structure, whereas treatment × time interactions accounted for 45% of the variation in community structure. Dunnett’s comparison of treatments to the control [completed on principle component analysis (PCA) axis scores] also indicated that watering and warming + watering treatments (intended to represent model predictions of more frequent, small-volume rain events) had rapid effects on community structure, with both treatments leading to a biocrust community structure significantly different from control plots within 1 y of the start of the experiment (P < 0.05; Fig. 2). Warming alone also altered biocrust community structure, but at a slower rate than watering treatments, with warmed and control communities first differing significantly nearly 6 y after the start of the experiment and roughly 3 y after warming treatments were increased from +2 °C to +4 °C (P < 0.05; Fig. 2).
Table 1.
Two-way PERMANOVA test of the effects of climate manipulation treatments and time (year of experiment) on biocrust community structure (2005–2014)
| Source* | df | SS | Pseudo-F | P |
| Treatment | 3 | 64,912 | 11.5 | 0.001 |
| Block | 4 | 7,820 | 18.0 | 0.001 |
| Time | 18 | 43,097 | 13.6 | 0.001 |
| Treatment × block | 12 | 22,609 | 17.4 | 0.001 |
| Treatment × time | 54 | 20,396 | 3.5 | 0.001 |
| Block × time | 72 | 12,693 | 1.6 | 0.001 |
| Residual | 216 | 23,407 | — | — |
First biocrust surveys were completed in Autumn 2005, and treatments were initiated in December 2005 and included controls (no climate manipulations), warming, watering (frequent, small-volume water additions), and warming + watering. SS, sums of squares from PERMANOVA.
Fig. 2.
PRCs (Left) showing temporal responses of biocrust communities (log scale) to climate manipulation treatments relative to controls (green, zero line). Taxon weights (Right; log scale) indicate the relative contribution of taxa to community shifts: Weight >0 indicates increased abundance, and weight <0 indicates decreased abundance. Overall, climate treatments are moving biocrusts away from moss-dominated (S. caninervis) to cyanobacteria-dominated (M. vaginatus) communities. Symbols above the x axis indicate when communities within a treatment first differed (P < 0.05) from controls; * and † indicate a shift in biocrust communities of watered and warmed + watered treatments, respectively, and ‡ indicates a shift in the warmed biocrust community.
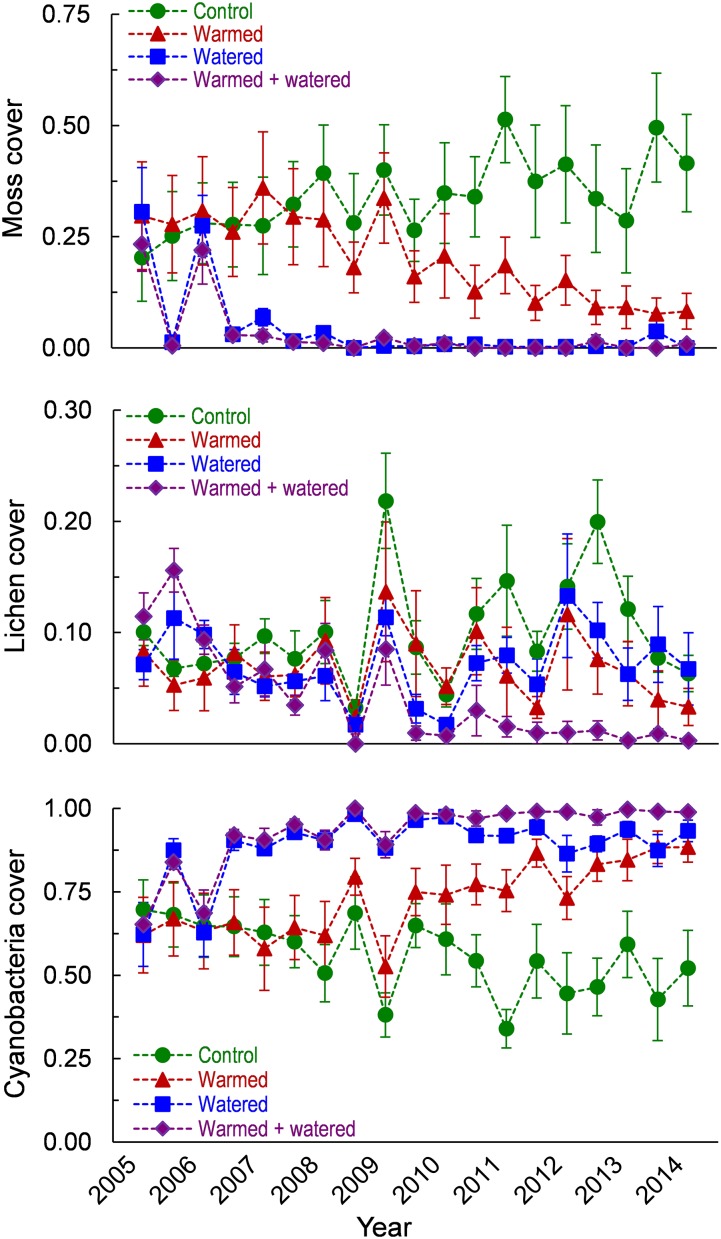
Climate manipulations had significant yet variable effects across different fractions of the biocrust community. In particular, warming, watering, and warming + watering all led to a dramatic decrease in relative cover of mosses over time (P = 0.016; Table 2 and Fig. 3) and a dramatic increase in the relative cover of cyanobacteria, which filled areas previously occupied by mosses and lichens (P < 0.0001; Table 2 and Fig. 3). Lichen responses to treatments varied over time, often mirroring natural fluctuations in lichen relative cover seen in control plots (Fig. 3), but an apparent effect of warming and warming + watering treatments emerged after treatment temperatures were increased from +2 °C to +4 °C (Table 2 and Fig. 3). Mixed-effects modeling indicated that experimental blocks (considered as a random effect) accounted for 15.3% and 13.5% of the variation in the cover of cyanobacteria and mosses, respectively, but accounted for only 1.4% of the variation in lichen cover.
Table 2.
Mixed-effects model of the impacts of climate manipulation treatments and time since the start of the experiment on the proportional cover of cyanobacteria, moss, and lichen in biocrusts
| Group | Source* | df | F | P |
| Cyanobacteria | Treatment | 3 | 0.33 | 0.8026 |
| Time | 18 | 4.34 | <0.0001 | |
| Treatment × time | 54 | 2.32 | <0.0001 | |
| Moss | Treatment | 3 | 0.58 | 0.6301 |
| Time | 18 | 2.12 | 0.0055 | |
| Treatment × time | 54 | 1.52 | 0.0161 | |
| Lichen | Treatment | 3 | 0.55 | 0.6487 |
| Time | 18 | 4.83 | <0.0001 | |
| Treatment × time | 54 | 1.52 | 0.0166 |
Treatment blocks were considered random effects and contributed 15.3%, 13.5%, and 1.4% of the variation in cyanobacterial, moss, and lichen cover, respectively. Treatments included controls (no climate manipulations), warming, watering (frequent, small-volume water addition), and warming + watering.
Fig. 3.
Temporal changes in the relative cover of moss (Top), lichen (Middle), and cyanobacteria (Bottom) in response to climate manipulation treatments. Values shown are means ± 1 SE. Repeated-measures, mixed-effect models (treatment, time, and treatment × time as fixed effects, and blocks as a random effect) revealed significant effects of time and treatment × time (P < 0.05) on all groups.
Physical disturbance from human trampling significantly altered biocrust community structure (P = 0.001; Table S1), with significant interaction effects among trampling treatment and sample year (P = 0.009; Table S1). Similar to climate manipulations, physical disturbance dramatically decreased the relative cover of mosses (F = 30.4, df = 1.12, P = 0.0001) and lichens (F = 28.4, df = 1.12, P = 0.0002), while increasing the cover of cyanobacteria, which filled in areas where mosses and lichens declined (F = 31.4, df = 1.12, P = 0.0001). Overall, mosses and lichens comprised ≤ 0.5% of cover in biocrust communities in trampled plots, compared with 18% and 1.5% of cover in biocrust communities, respectively, in undisturbed control plots. Cyanobacteria cover increased from an average of 81% of the community in control plots to an average of 99% of the community in trampled plots. Mixed-effects modeling indicated that experimental blocks accounted for <1% of the variation in the cover of cyanobacteria and mosses across the trampling experiment but accounted for 27% of the variation in lichen cover.
Table S1.
Two-way PERMANOVA test of the effects of physical disturbance (trampling) on biological soil crust community structure
| Source* | df | SS | Pseudo-F | P |
| Treatment | 1 | 3,332 | 88.3 | 0.001 |
| Year | 1 | 524 | 13.9 | 0.001 |
| Treatment × year | 1 | 206 | 5.5 | 0.009 |
| Residual | 16 | 603 | — | — |
First biocrust surveys were completed in 2007 and repeated in 2011, and treatments were initiated in 1996 and included controls (no disturbance) and human trampling (completed annually).
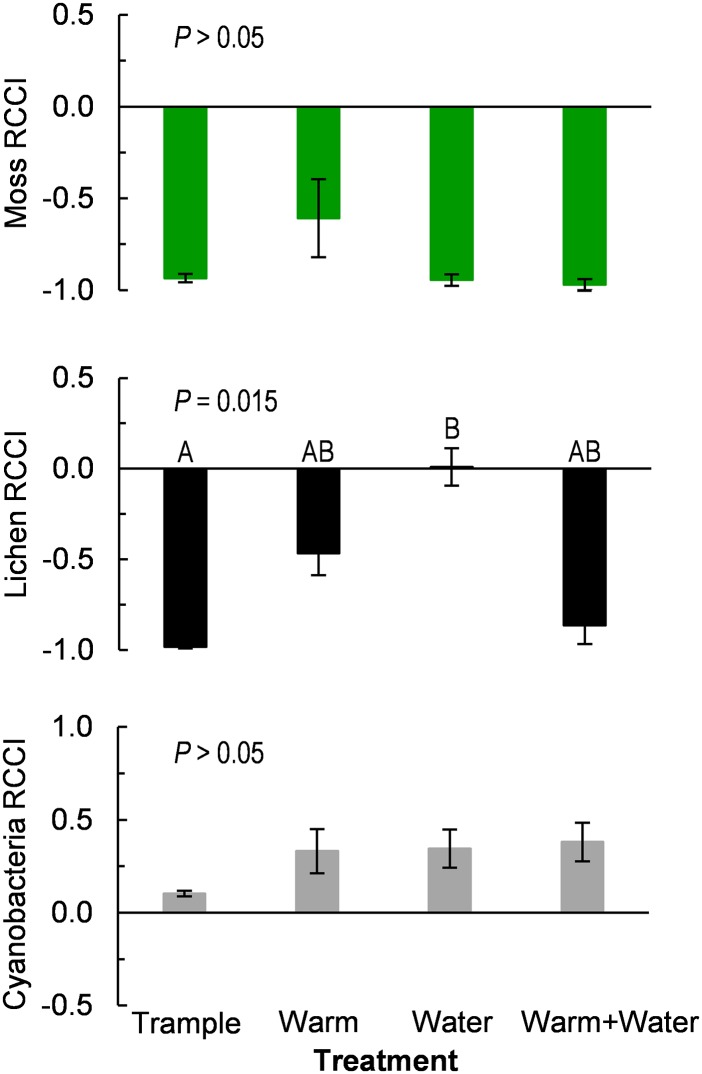
Comparing the long-term effects from physical disturbance (community survey data from 11 and 15 y after the start of trampling) to those from climate manipulation treatments (community survey data from 10 y after the start of warming and watering treatments) revealed similar treatment impacts on the cover of cyanobacteria, mosses, and lichens (Fig. 4). In all cases, treatments drastically reduced moss cover and dramatically increased cyanobacteria cover in soil crusts (Fig. 4). Climate manipulations and trampling treatments all had negative effects on lichen cover (Fig. 4), yet disturbance by trampling led to an almost complete loss of lichen cover over time, whereas climate manipulations had less drastic effects on lichens. Considered across treatments, there were no significant differences in the relative effects of trampling disturbance, warming, watering, or warming + watering on changes in cover of mosses and cyanobacteria (P > 0.05)—in other words, physical disturbance and climate manipulations caused similar changes in the abundances of mosses and cyanobacteria compared with their respective controls (Fig. 4). Although lichens did not differ in their negative responses to physical disturbance versus warming and warming + watering treatments, lichens did differ in their responses to physical disturbance versus watering (χ2 = 4.9, df = 3, P < 0.05; Fig. 4).
Fig. 4.
RCCI for moss (Top), lichen (Middle), and cyanobacteria (Bottom) in biocrusts subjected to climate manipulations or physical disturbance from repeated human trampling. RCCI shows changes in biotic cover relative to controls (Methods). The RCCI value ranges from +1 (100% increase in cover in response to treatment) to –1 (100% decrease in cover in response to treatment). Bars are means ± 1 SE, P values are probability of type I error (Kruskal–Wallis tests), and lettering indicates significant differences via Steel–Dwass nonparametric pairwise comparisons.
Discussion
We compared responses of biological soil crust communities of the Colorado Plateau to 10 y of warming and altered precipitation experiments and 15 y of physical disturbance from repeated human trampling (Fig. 1). We found that experimental warming, augmented precipitation (i.e., increased frequency of small watering events representing model predictions for the region), warming + watering, and disturbance from trampling significantly altered biocrust community structure and led to a similar early successional community state (Table 1, Table S1, and Figs. 2 and 4). The strong similarity of climate-induced changes to biocrust communities and the effects of physical disturbance were unexpected and suggest climate change has the potential to alter biocrust community structure as much as the dramatic mortality caused by physical disturbance. Specifically, climate manipulations and trampling resulted in decreased cover of mosses and increased cover of cyanobacteria, which rapidly fill areas opened by the loss of mosses and lichens (Figs. 3 and 4). Climate manipulations had variable effects on lichens across treatments; nevertheless, warming and warming + watering caused a decrease in lichen cover over time (Fig. 3). Human trampling led to a nearly complete loss of lichen, suggesting that physical disturbances may have more drastic long-term impacts on lichens relative to climate change (Figs. 1 and 4).
Despite the convergence of alternate community states in biocrusts following physical disturbance and climate change treatments, response rates within biocrust communities varied dramatically across warmed and watered treatments. In particular, water addition in the form of frequent, small-volume monsoonal precipitation events led to far more rapid changes in biocrust community structure than did warming. The rapid shift in community structure in response to watering was primarily due to swift moss mortality (35), with a subsequent increase in cyanobacteria cover in watered and warmed + watered treatments (Fig. 3). Quantitative biocrust community assessments in physical disturbance plots were first completed 11 y after the start of trampling; however, visual assessments from early in the experiment indicated rapid effects on biocrust physical and biotic structure, and many other studies verify rapid changes in biocrusts following physical disturbances (32, 45). Thus, we assume that disturbance from human trampling led to a shift toward an alternate state at a rate similar to or faster than that seen for watering treatments.
Although previous reports detailed changes in community structure in response to the watering treatments used here, these studies singularly found shifts linked to rapid moss mortality, likely due to carbon starvation, in response to augmented precipitation (35, 39). However, these reports were completed before the longer term effects of warming on other components of the biocrust communities had manifest. The effects of warming described here are a great cause for concern, as increasing annual temperatures are a near certainty across dryland ecosystems, whereas predictions of future precipitation patterns and regional hydrology remain highly uncertain (28, 29). Indeed, the warming simulated in the climate manipulation treatments (4 °C above ambient) is within the midrange of temperature increases predicted for the US Southwest by 2100 (46). At the same time, moss mortality in response to water additions stands in contrast with the lichen mortality described here, which primarily occurred in response to warming treatments. Variable rates and directional responses of different fractions of the biocrust community to climate manipulations likely stem from important influences of evolutionary history on species’ physiological responses to climate change. Variation in responses to climate change across different fractions of the biocrust community could alter the strength and direction of biotic interactions within communities with different species composition, with possible consequences for local to regional feedbacks among biocrust taxa and novel environmental conditions (47–49). A larger understanding of variation in responses to climate change lies in uncovering the mechanisms behind species’ response patterns. For example, carbon starvation was identified as a probable mechanism underlying rapid moss mortality in watered treatments of our study and as a possible mechanism of both moss and lichen mortality during drought in other systems (50). These results raise the question of whether carbon starvation is also the source of temperature-induced changes in biocrust community composition (Fig. 3) or if a separate set of drivers is at play. Regardless of the cause, differential responses across fractions of the biocrust community to climate factors and even relatively subtle variability among climatic versus physical drivers of change could result in substantial community and functional differences over time.
We found evidence of substantial block (landscape) effects on both community composition and the responses of specific biotic groups to treatments across experiments. These block effects suggest the existence of small-scale gradients in climate and disturbance impacts on biocrust communities. These gradients, possibly in soil nutrients or microclimate, may interact with interannual climatic variation such that areas prone to fluctuations in soil moisture or temperature may become more or less harsh for biocrust organisms over time (51–53). Environmental gradients can also affect the strength and direction of biotic interactions within these communities (47, 54), highlighting the need to consider spatial and temporal influences on species responses in environmental change studies.
The increase in cyanobacteria-dominated soil surfaces, coupled with reduced abundances of mosses and lichens in response to climate manipulations and physical disturbance, is commonly associated with a shift toward early successional biocrust community states (11, 24, 32, 45). This shift toward an early successional state has critical implications for ecosystem processes and functioning, as early successional biocrusts fix less carbon and nitrogen (11, 24, 55, 56) and lose more carbon and nitrogen via leaching (25). Rapid moss mortality in response to watering treatments was associated with decreased concentrations of ammonium (NH4+) and increased concentrations of nitrate (NO3−), suggesting a strong influence of biocrust composition on local nitrogen processes (35). This switch in nitrogen forms can translate to nitrogen being more easily lost through leaching and directly to the atmosphere (57) and can have strong influences on multiple process rates in dryland ecosystems (58, 59). Early successional biocrusts are also more prone to erosion, dust production, and reduced water infiltration, which can have complex, long-lasting effects on local ecosystem processes (25, 32, 60, 61). Thus, drivers that push biocrust communities to earlier successional states—here shown to be both physical and climate disturbance factors—have important implications for soil stability, fertility, and carbon storage, each of which is globally relevant for the extensive dryland biome.
Conclusions
We examined the effects of experimental warming and augmented precipitation on biocrust communities and then compared the impacts of climate manipulations to physical disturbance (replicated human trampling) on biocrust community structure. We found that warming, watering, warming + watering, and physical disturbance had similar effects on biocrust communities, collectively shifting communities toward early successional states. Although physical disturbances can be mitigated (41) and future precipitation patterns are uncertain (28, 42), increasing temperatures (meeting or exceeding those imposed by warming treatments) are considered a near certainty across many dryland ecosystems (29). Thus, our results suggest that climate change will affect biocrusts to an extent similar to changes observed with physical disturbance, leading to a landscape-level shift to early successional states with seemingly limited potential for a return to late successional states. However, given the different rates at which these pressures alter biocrust community structure and the potential for longer term adaptation and species’ shifts, variation in the ultimate effects of these disturbances on ecosystem processes are likely and require continued study.
Ecological state changes in response to global change factors are a growing concern. Transitions to alternate community states in drylands are a particularly poignant issue, as these ecosystems cover roughly 40% of the Earth’s terrestrial surfaces and may hold up to 25% of global soil organic carbon (31). Biocrust communities can represent a substantial portion of primary production in drylands and can locally stabilize and regulate ecosystem processes including carbon and nitrogen cycles as well as short-term hydrologic cycles and surface energy balance (8, 9, 45, 62–64). The role biocrusts play in soil stability is also a topic of significant public interest, due to the human health and safety issues associated with increasing dust storms. Because different components of the biocrust community (i.e., mosses versus lichens) play different roles in the functioning of drylands (8, 10, 56, 65, 66), understanding the factors that control the composition of biocrust communities will be essential to predictions of future dryland function. In addition, because drylands may dominate the interannual variability in global atmospheric CO2 concentrations (67, 68), state transitions in dryland structure could have effects beyond arid and semiarid systems.
Methods
Climate manipulation and physical disturbance experiments were located on the Upper Colorado Plateau, Utah. The region is classified as cool desert with a majority of precipitation occurring in winter and spring. The sites have extensive cover of biocrusts held together primarily by filaments of the cyanobacteria Microcoleus vaginatus and covered by a mosaic of the mosses Syntrichia caninervis and Syntrichia ruralis and various lichens (mostly Collema tenax and Collema coccophorum). Climate manipulations representing model predictions of both increasing temperature and frequency of small (<2 mm) rainfall events for the study region (28, 29, 42) were completed near Castle Valley, Utah (38.6748 N, –109.4163 W, 1,310 m above sea level). The soil of this area is Lithic Torriorthent, a sandy-loam ranging in depth from 17 to 122 cm. Human trampling plots, mimicking disturbances from humans and livestock, were located in Arches National Park, Utah, on soil of the Mido-Sazi complex, a loamy sand with depths to 100 cm.
Climate manipulations began in autumn 2005 in a randomized, complete block design consisting of twenty 2 m × 2.5 m plots divided among four treatments: control (no manipulations), warmed, watered, and warmed + watered. Warming was completed with infrared lamps heating the topsoil (temperature at 1–2 cm depth) by +2 °C above ambient for the first 3 y of the experiment and then by +4 °C above ambient from June 30, 2008 until present. In addition to ambient precipitation, watering treatments consisted of frequent, 1.2 mm “rainfall events” applied by hand sprayers. Watered plots received on average 35 such events (roughly four times the average natural frequency) throughout the summer months starting in 2006 (35–37, 39). Physical disturbance by human trampling started in May 1996 in ten 2 m × 5 m plots, divided as five trampled and five undisturbed controls. Plots were trampled annually in May of successive years until 2011 (69). Biocrust communities were assessed as the relative cover of all visible species present within standardized 40 cm × 40 cm frames. Climate manipulation plots were surveyed twice per year (May and September) from Autumn 2005 to present, with survey frames placed in four repeated locations per plot (6,400 cm2 surveyed in each plot in a repeated-measures design). Trampled–control plot pairs were surveyed in 2007 and 2011, with survey frames placed in 10 random locations within each plot (16,000 cm2 surveyed in each plot).
Biocrust community responses to climate manipulations and trampling were assessed via permutational multivariate analysis of variance (PERMANOVA). PERMANOVAs were performed on Bray–Curtis distance matrices of log-transformed species’ abundances derived from cover measures. PERMANOVAs on climate manipulation data included treatment, block, and time since the start of the experiment as factors, and PERMANOVAs on physical disturbance data included treatment and biocrust survey year (2007 and 2011) as factors, with block effects ruled out by stepwise fitting procedures. Treatment effects of climate manipulations were visualized via PRCs, which measure the differences between the species composition and cover in each treatment compared with controls over time (43, 70). To determine the date when biocrust communities in climate manipulation treatments first differed from controls, the community structure of treatment and the control plots were compared via Dunnett’s tests on PCA axis scores. PRC and PCA were completed on log-transformed species abundance data. To determine effects of climate and disturbance treatments on different fractions of the biocrust community, we calculated relative abundances for three biotic groups common to biocrusts of our sites (mosses, lichens, and cyanobacteria). To account for the repeated-measures design of biocrust surveys, relative cover of the three biotic groups were analyzed with linear mixed-effect models with restricted maximum likelihood estimators. Treatment and time (or sample years in the case of the trampling experiment) were considered fixed effects, and field experimental blocks were considered as random effects. Finally, we compared the long-term effects of climate manipulations (data from the 10th year of the study) and physical disturbance (data from the 11th and 15th year of the study when biocrusts were surveyed) on the relative cover of mosses, lichens, and cyanobacteria by relativizing treatment responses to controls in a manner accounting for cover values in both treatments and controls: Relative Cover Change Index, or RCCI = cover(treatment) – cover(control)/cover(treatment) + cover(control) (71). RCCI values within biotic groups were then compared via Kruskal–Wallis one-way analysis of variance followed by Steel–Dwass nonparametric pairwise comparisons (72). Analyses were completed in R (72).
Acknowledgments
We thank Hilda Smith, Ed Grote, and many technicians for data collection and maintaining experiments. We are grateful to the National Park Service Southeast Utah Group and the Bureau of Land Management Moab Field Office for help with permits and logistics. Our manuscript was improved by comments from Peter Vitousek, Jeffry Mitton, and three anonymous reviewers. This work was supported by the US Department of Energy Office of Science, Office of Biological and Environmental Research Terrestrial Ecosystem Sciences Program, under Award DE-SC-0008168, and the USGS Climate and Land Use, and Ecosystems programs. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1509150112/-/DCSupplemental.
References
- 1.Friedlingstein P, et al. Positive feedback between future climate change and the carbon cycle. Geophys Res Lett. 2001;28(8):1543–1546. [Google Scholar]
- 2.Scheffer M, Carpenter S, Foley JA, Folke C, Walker B. Catastrophic shifts in ecosystems. Nature. 2001;413(6856):591–596. doi: 10.1038/35098000. [DOI] [PubMed] [Google Scholar]
- 3.Field CB, Lobell DB, Peters HA, Chiariello NR. Feedbacks of terrestrial ecosystems to climate change. Annu Rev Environ Resour. 2007;32:1–29. [Google Scholar]
- 4.Luo Y. Terrestrial carbon-cycle feedback to climate warming. Annu Rev Ecol Evol Syst. 2007;38:683–712. [Google Scholar]
- 5.Turner MG. Disturbance and landscape dynamics in a changing world. Ecology. 2010;91(10):2833–2849. doi: 10.1890/10-0097.1. [DOI] [PubMed] [Google Scholar]
- 6.Zhou J, et al. Microbial mediation of carbon-cycle feedbacks to climate warming. Nat Clim Chang. 2012;2(2):106–110. [Google Scholar]
- 7.Lindenmayer DB, Likens GE, Krebs CJ, Hobbs RJ. Improved probability of detection of ecological “surprises”. Proc Natl Acad Sci USA. 2010;107(51):21957–21962. doi: 10.1073/pnas.1015696107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshitake S, Uchida M, Koizumi H, Kanda H, Nakatsubo T. Production of biological soil crusts in the early stage of primary succession on a high Arctic glacier foreland. New Phytol. 2010;186(2):451–460. doi: 10.1111/j.1469-8137.2010.03180.x. [DOI] [PubMed] [Google Scholar]
- 9.Elbert W, et al. Contribution of cryptogamic covers to the global cycles of carbon and nitrogen. Nat Geosci. 2012;5(7):459–462. [Google Scholar]
- 10.Belnap J, Büdel B, Lange OL. Biological soil crusts: Characteristics and distribution. In: Belnap J, Lange OL, editors. Biological Soil Crusts: Structure, Function, and Management. Springer-Verlag; Berlin: 2003a. pp. 3–30. [Google Scholar]
- 11.Housman DC, Powers HH, Collins AD, Belnap J. Carbon and nitrogen fixation differ between successional stages of biological soil crusts in the Colorado Plateau and Chihuahuan Desert. J Arid Environ. 2006;66(4):620–634. [Google Scholar]
- 12.Maestre FT, et al. Changes in biocrust cover drive carbon cycle responses to climate change in drylands. Glob Change Biol. 2013;19(12):3835–3847. doi: 10.1111/gcb.12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Austin AT, et al. Water pulses and biogeochemical cycles in arid and semiarid ecosystems. Oecologia. 2004;141(2):221–235. doi: 10.1007/s00442-004-1519-1. [DOI] [PubMed] [Google Scholar]
- 14.Cable JM, Huxman TE. Precipitation pulse size effects on Sonoran Desert soil microbial crusts. Oecologia. 2004;141(2):317–324. doi: 10.1007/s00442-003-1461-7. [DOI] [PubMed] [Google Scholar]
- 15.Huxman TE, et al. Precipitation pulses and carbon fluxes in semiarid and arid ecosystems. Oecologia. 2004;141(2):254–268. doi: 10.1007/s00442-004-1682-4. [DOI] [PubMed] [Google Scholar]
- 16.Schwinning S, Sala OE. Hierarchy of responses to resource pulses in arid and semi-arid ecosystems. Oecologia. 2004;141(2):211–220. doi: 10.1007/s00442-004-1520-8. [DOI] [PubMed] [Google Scholar]
- 17.Wilske B, et al. The CO2 exchange of biological soil crusts in a semiarid grass-shrubland at the northern transition zone of the Negev desert, Israel. Biogeosciences. 2008;5(3):1411–1423. [Google Scholar]
- 18.Wilske B, et al. Modeling the variability in annual carbon fluxes related to biological soil crusts in a Mediterranean shrubland. Biogeosciences Discuss. 2009;6(4):7295–7324. [Google Scholar]
- 19.Bowker MA, Mau RL, Maestre FT, Escolar C, Castillo-Monroy AP. Functional profiles reveal unique ecological roles of various biological soil crust organisms. Funct Ecol. 2011;25(4):787–795. [Google Scholar]
- 20.Castillo-Monroy AP, et al. Relationships between biological soil crusts, bacterial diversity and abundance, and ecosystem functioning: Insights from a semi-arid Mediterranean environment. J Veg Sci. 2011;22(1):165–174. [Google Scholar]
- 21.Miralles I, Trasar-Cepeda C, Leirós MC, Gil-Sotres F. Labile carbon in biological soil crusts in the Tabernas desert, SE Spain. Soil Biol Biochem. 2013;58:1–8. [Google Scholar]
- 22.Belnap J. Impacts of off-road vehicles on nitrogen cycles in biological soil crusts: Resistance in different U.S. deserts. J Arid Environ. 2002;52(2):155–165. [Google Scholar]
- 23.Hawkes CV. Nitrogen cycling mediated by biological soil crusts and arbuscular mycorrhizal fungi. Ecology. 2003;84(6):1553–1562. [Google Scholar]
- 24.Barger NN, Belnap J, Ojima DS, Mosier A. NO gas loss from biologically crusted soils in Canyonlands National Park, Utah. Biogeochemistry. 2005;75(3):373–391. [Google Scholar]
- 25.Barger NN, Herrick JE, Van Zee JW, Belnap J. Impacts of biological soil crust disturbance and composition on C and N loss from water erosion. Biogeochemistry. 2006;77(2):247–263. [Google Scholar]
- 26.Castillo-Monroy AP, Maestre FT, Delgado-Baquerizo M, Gallardo A. Biological soil crusts modulate nitrogen availability in semi-arid ecosystems: Insights from a Mediterranean grassland. Plant Soil. 2010;333(1-2):21–34. [Google Scholar]
- 27.Delgado-Baquerizo M, Castillo-Monroy AP, Maestre FT, Gallardo A. Plants and biological soil crusts modulate the dominance of N forms in a semi-arid grassland. Soil Biol Biochem. 2010;42(2):376–378. [Google Scholar]
- 28.Christensen NS, Wood AW, Voisin N, Lettenmaier DP, Palmer RN. The effects of climate change on the hydrology and water resources of the Colorado River basin. Clim Change. 2004;62(1-3):337–363. [Google Scholar]
- 29.Solomon S, et al., editors. Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge Univ Press; New York: 2007. [Google Scholar]
- 30.Cayan DR, et al. Future dryness in the southwest US and the hydrology of the early 21st century drought. Proc Natl Acad Sci USA. 2010;107(50):21271–21276. doi: 10.1073/pnas.0912391107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Safriel U, Adeel Z. Dryland systems. In: Hassan R, Scholes R, Ash N, editors. Ecosystems and Human Well-Being, Current State and Trends. Vol 1. Island Press; Washington, DC: 2005. pp. 625–658. [Google Scholar]
- 32.Belnap J, Eldridge D. Disturbance and recovery of biological soil crusts. In: Belnap J, Lange OL, editors. Biological Soil Crusts: Structure, Function, and Management. Springer-Verlag; Berlin: 2003. pp. 363–383. [Google Scholar]
- 33.Zaady E, Kuhn U, Wilske B, Sandoval-Soto L, Kesselmeier J. Patterns of CO2 exchange in biological soil crusts of successional age. Soil Biol Biochem. 2000;32(7):959–966. [Google Scholar]
- 34.Escolar C, Martínez I, Bowker MA, Maestre FT. Warming reduces the growth and diversity of biological soil crusts in a semi-arid environment: Implications for ecosystem structure and functioning. Philos Trans R Soc Lond B Biol Sci. 2012;367(1606):3087–3099. doi: 10.1098/rstb.2011.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reed SC, et al. Changes to dryland rainfall result in rapid moss mortality and altered soil fertility. Nat Clim Chang. 2012;2(10):752–755. [Google Scholar]
- 36.Johnson SL, et al. Increased temperature and altered summer precipitation have differential effects on biological soil crusts in a dryland ecosystem. Glob Change Biol. 2012;18(8):2583–2593. [Google Scholar]
- 37.Yeager CM, et al. Response of biological soil crust diazotrophs to season, altered summer precipitation, and year-round increased temperature in an arid grassland of the colorado plateau, USA. Front Microbiol. 2012;3:358. doi: 10.3389/fmicb.2012.00358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Escolar C, Maestre FT, Rey A. Biocrusts modulate warming and rainfall exclusion effects on soil respiration in a semi-arid grassland. Soil Biol Biochem. 2015;80:9–17. doi: 10.1016/j.soilbio.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zelikova TJ, Housman DC, Grote EE, Neher DA, Belnap J. Warming and increased precipitation frequency on the Colorado Plateau: Implications for biological soil crusts and soil processes. Plant Soil. 2012;355(1-2):265–282. [Google Scholar]
- 40.Reynolds JF, et al. Global desertification: Building a science for dryland development. Science. 2007;316(5826):847–851. doi: 10.1126/science.1131634. [DOI] [PubMed] [Google Scholar]
- 41.Bestelmeyer BT, et al. Desertification, land use, and the transformation of global drylands. Front Ecol Environ. 2015;13(1):28–36. [Google Scholar]
- 42.Weltzin JF, et al. Assessing the response of terrestrial ecosystems to potential changes in precipitation. Bioscience. 2003;53(10):941–952. [Google Scholar]
- 43.Van den Brink PJ, Ter Braak CJF. Principal response curves: Analysis of time-dependent multivariate responses of biological community to stress. Environ Toxicol Chem. 1999;18(2):138–148. [Google Scholar]
- 44.Legendre P, Legendre L. Numerical Ecology. 2nd English Ed Elsevier; Amsterdam: 1998. [Google Scholar]
- 45.Warren SD, Eldridge DJ. Biological soil crusts and livestock in arid ecosystems: Are they compatible? In: Belnap J, Lange OL, editors. Biological Soil Crusts: Structure, Function, and Management. Springer-Verlag; Berlin: 2003. pp. 401–415. [Google Scholar]
- 46.Garfin G, Jardine A, Merideth R, Black M, LeRoy S. Assessment of Climate Change in the Southwest United States: A Report Prepared for the National Climate Assessment. A Report by the Southwest Climate Alliance. Island Press; Washington, DC: 2013. [Google Scholar]
- 47.Bowker MA, Soliveres S, Maestre FT. Competition increases with abiotic stress and regulates the diversity of biological soil crusts. J Ecol. 2010;98(3):551–560. [Google Scholar]
- 48.de Vries FT, Shade A. Controls on soil microbial community stability under climate change. Front Microbiol. 2013;4:265. doi: 10.3389/fmicb.2013.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nemergut DR, et al. Patterns and processes of microbial community assembly. Microbiol Mol Biol Rev. 2013;77(3):342–356. doi: 10.1128/MMBR.00051-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wertin TM, et al. Elevated CO2 did not mitigate the effect of a short-term drought on biological soil crusts. Biol Fertil Soils. 2012;48(7):797–805. [Google Scholar]
- 51.Bowker MA, Belnap J. A simple classification of soil types as habitats of biological soil crusts on the Colorado Plateau, USA. J Veg Sci. 2008;19(6):831–840. [Google Scholar]
- 52.Bowker MA, Belnap J, Davidson DW, Phillips SL. Evidence for micronutrient limitation of biological soil crusts: Importance to arid-lands restoration. Ecol Appl. 2005;15(6):1941–1951. [Google Scholar]
- 53.Cable JM, Ogle K, Williams DG, Weltzin JF, Huxman TE. Soil texture drives responses of soil respiration to precipitation pulses in the Sonoran Desert: Implications for climate change. Ecosystems (N Y) 2008;11(6):961–979. [Google Scholar]
- 54.Maestre FT, et al. Do biotic interactions modulate ecosystem functioning along stress gradients? Insights from semi-arid plant and biological soil crust communities. Philos Trans R Soc Lond B Biol Sci. 2010;365(1549):2057–2070. doi: 10.1098/rstb.2010.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Evans RD, Belnap J. Long-term consequences of disturbance on nitrogen dynamics in an arid ecosystem. Ecology. 1999;80(1):150–160. [Google Scholar]
- 56.Belnap J, Hawkes CV, Firestone MK. Boundaries in miniature: Two examples from soil. Bioscience. 2003b;53(8):739–749. [Google Scholar]
- 57.McCalley CK, Sparks JP. Abiotic gas formation drives nitrogen loss from a desert ecosystem. Science. 2009;326(5954):837–840. doi: 10.1126/science.1178984. [DOI] [PubMed] [Google Scholar]
- 58.Jackson LE, Schimel JP, Firestone MK. Short-term partitioning of ammonium and nitrate between plants and microbes in an annual grassland. Soil Biol Biochem. 1989;21(3):409–415. [Google Scholar]
- 59.Austin AT, Sala OE, Jackson RB. Inhibition of nitrification alters carbon turnover in the Patagonian steppe. Ecosystems. 2006;9(8):1257–1265. [Google Scholar]
- 60.Maestre FT, Huesca M, Zaady E, Bautista S, Cortina J. Infiltration, penetration resistance and microphytic crust composition in contrasted microsites within a Mediterranean semi-arid steppe. Soil Biol Biochem. 2002;34(6):895–898. [Google Scholar]
- 61.Chamizo S, Canton Y, Lázaro R, Solé-Benet A, Domingo F. Crust composition and disturbance drive infiltration through biological soil crusts in semiarid ecosystems. Ecosystems. 2012;15(1):148–161. [Google Scholar]
- 62.Evans RD, Lange OL. Biological soil crusts and ecosystem nitrogen and carbon dynamics. In: Belnap J, Lange OL, editors. Biological Soil Crusts: Structure, Function, and Management. Springer-Verlag; Berlin: 2003. pp. 401–415. [Google Scholar]
- 63.Delgado-Baquerizo M, Maestre FT, Gallardo A. Biological soil crusts increase the resistance of soil nitrogen dynamics to changes in temperatures in a semi-arid ecosystem. Plant Soil. 2013;366(1-2):35–47. [Google Scholar]
- 64.Wei W, Yu Y, Chen L. Response of surface soil hydrology to the micro-pattern of bio-crust in a dry-land Loess environment, China. PLoS One. 2015;10(7):e0133565. doi: 10.1371/journal.pone.0133565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Holst J, et al. Dinitrogen fixation by biological soil crusts in an Inner Mongolian steppe. Biol Fertil Soils. 2009;45(7):679–690. [Google Scholar]
- 66.Zhang P, et al. [Nitrogen fixation potential of biological soil crusts in southeast edge of Tengger Desert, Northwest China] Ying Yong Sheng Tai Xue Bao. 2012;23(8):2157–2164. [PubMed] [Google Scholar]
- 67.Poulter B, et al. Contribution of semi-arid ecosystems to interannual variability of the global carbon cycle. Nature. 2014;509(7502):600–603. doi: 10.1038/nature13376. [DOI] [PubMed] [Google Scholar]
- 68.Ahlström A, et al. Carbon cycle. The dominant role of semi-arid ecosystems in the trend and variability of the land CO2 sink. Science. 2015;348(6237):895–899. doi: 10.1126/science.aaa1668. [DOI] [PubMed] [Google Scholar]
- 69.Kuske CR, Yeager CM, Johnson S, Ticknor LO, Belnap J. Response and resilience of soil biocrust bacterial communities to chronic physical disturbance in arid shrublands. ISME J. 2012;6(4):886–897. doi: 10.1038/ismej.2011.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Frampton GK, Van den Brink PJ, Gould PJL. Effects of spring precipitation on a temperate arable collembolan community analysed using Principal Response Curves. Appl Soil Ecol. 2000;14(3):231–248. [Google Scholar]
- 71.Armas C, Ordiales R, Pugnaire FI. Measuring plant interactions: A new comparative index. Ecology. 2004;85(10):2682–2686. [Google Scholar]
- 72.R Development Core Team 2011 R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna, Austria), www.r-project.org.