Significance
Maternal care plays an important role in the development of the offspring’s social behaviors through the programming of relevant neural and hormonal systems. However, it is unclear how specific maternal behaviors, such as breastfeeding and genetic variation related to the oxytocin system, contribute to emerging social behaviors in human infants. We therefore examined infants’ attention to emotional eyes. Our results revealed that infants with the genotype previously associated with decreased availability of oxytocin and an increased rate of autism were most affected by extended durations of exclusive breastfeeding. Namely, these infants showed increased attention to happy eyes and decreased attention to angry eyes. This finding suggests that breastfeeding experience enhances prosocial tendencies in infants that are genetically at risk for autism.
Keywords: CD38, oxytocin, breastfeeding, emotion perception, infancy
Abstract
Attending to emotional information conveyed by the eyes is an important social skill in humans. The current study examined this skill in early development by measuring attention to eyes while viewing emotional faces in 7-mo-old infants. In particular, we investigated individual differences in infant attention to eyes in the context of genetic variation (CD38 rs3796863 polymorphism) and experiential variation (exclusive breastfeeding duration) related to the oxytocin system. Our results revealed that, whereas infants at this age show a robust fear bias (increased attention to fearful eyes), their attention to angry and happy eyes varies as a function of exclusive breastfeeding experience and genetic variation in CD38. Specifically, extended exclusive breastfeeding duration selectively enhanced looking preference to happy eyes and decreased looking to angry eyes. Importantly, however, this interaction was impacted by CD38 variation, such that only the looking preferences of infants homozygous for the C allele of rs3796863 were affected by breastfeeding experience. This genotype has been associated with reduced release of oxytocin and higher rates of autism. In contrast, infants with the CA/AA genotype showed similar looking preferences regardless of breastfeeding exposure. Thus, differences in the sensitivity to emotional eyes may be linked to an interaction between the endogenous (CD38) and exogenous (breastfeeding) availability of oxytocin. These findings underline the importance of maternal care and the oxytocin system in contributing to the early development of responding to social eye cues.
Sensitive responding to emotions in others is a vital social skill that helps us relate to others, predict their actions, and coordinate our own behavior during social interactions. Given its critical importance for effective social functioning, it is not surprising that the ability to detect and distinguish between emotional expressions emerges early in human development. Findings from behavioral and neuroscience studies indicate that infants’ ability to discriminate between positive and negative emotional expressions emerges in the first year of life (see refs. 1 and 2 for reviews). For example, by around 7 mo of age, infants distinguish between fearful and happy facial expressions and show increased allocation of attention to fear (3). This enhanced attention to fear in others marks the emergence of a “fear bias” in infancy, which is thought to orient and alert the infant to potentially threatening situations (2, 4). Prior work with adults demonstrates that emotion perception and, in particular, fear detection (eyes wide open) rely heavily on information from the eye region and occur even in the absence of conscious perception (5–8).The importance of eye cues for emotion perception has recently been studied in infants. In an event-related brain potential (ERP) study, 7-mo-old infants were found to discriminate between fear and happiness on the basis of eye cues alone (9). Similar to adults, 7-mo-old infants discriminated between emotional eyes without consciously perceiving them (see ref. 10 for more information regarding conscious and unconscious processing in infants).
Studies on autism spectrum disorder (ASD), a neurodevelopmental disorder characterized by severe social impairments, provide more evidence to suggest that attending to eyes plays an important role in early social development. In particular, there is evidence to suggest abnormalities in looking to the eyes in toddlers, adolescents, and adults with ASD (refs. 11–13, respectively). However, it should also be mentioned that some studies do not find impaired looking to the eyes among individuals with ASD, which may be explained by differences in the stimuli used, in the age ranges tested, and in the symptom severity considered across studies (see ref. 14 for a review). A recent study by Jones and Klin (11) investigated attention to eyes from 2 to 24 mo in infants at low or high risk for autism (high risk: infants with ASD siblings). Among infants later diagnosed with autism, attention to the eyes while viewing videos of naturalistic caregiver interactions was initially present but began to decline between 2 and 6 mo of age. Furthermore, attention to eyes measured over the course of 24 mo negatively correlated with symptom severity. Thus, children later diagnosed with autism initially attend to eyes as much as typically developing infants but later in infancy exhibit a steady decline in attending to the eyes whereas typically developing infants maintain a constant level of attention to the eyes (11). This finding points to an important period in infancy during which attention to eyes may develop along different trajectories. Furthermore, the results from Jones and Klin (11) support the notion that the development of reduced attention to eyes and eye cues during infancy may be one of the earliest detectable warning signs of autism (see also ref. 15).
In both autistic and healthy populations, oxytocin has been related to orientation to eyes and to the detection of emotional states from the eye region (16, 17). Oxytocin is a neurohormone synthesized in the paraventricular and supraoptic nuclei of the hypothalamus, released both peripherally into the bloodstream and centrally within the brain (18). It is best known for its role in mammalian reproductive and parental behaviors (19, 20). However, it serves much broader functions and has been implicated in the modulation of a host of social behaviors, with a special emphasis on cooperative behaviors based on empathy and trust (21, 22). With respect to the current context, a recent study by Auyeung et al. (17) reported a facilitation of eye contact with oxytocin administration in a real-time social interaction. This increase in eye contact was exhibited in both autistic and healthy adults. Oxytocin administration has also been shown to increase eye gaze to static faces in adults with autism (23) and has further been linked to improved emotion recognition from the eye region of static images in adolescents with autism (24). Moreover, the administration of oxytocin to children (25) and adults (26) diagnosed with autism has been shown to selectively enhance brain responses to social stimuli compared with physical control stimuli. In healthy adults, oxytocin administration increases the duration and frequency of gaze to the eye region while viewing neutral faces (27) and increases orienting of attention to emotional gaze cues (28). It also facilitates recognition of mental states of others when provided with cues from the eye region (29). Moreover, there is considerable evidence suggesting that oxytocin plays a specific role in facilitating the processing of positive emotions and events (27, 30–32). This increase in the salience of positive emotions might also be linked to, or explained by, a reduction of the salience of negative social stimuli (33–36). Taken together, oxytocin seems to increase the salience of prosocial positive stimuli, which may be facilitated by increased attention to the eye region during social interactions.
Genetic variation that affects oxytocin neurotransmission has been linked to individual differences in social behavior (see refs. 37 and 38 for reviews). Specifically, the ectoenzyme CD38 (Cluster of Differentiation 38) is considered to play a major role in regulating social behavior due to its effect on the release of oxytocin (39). Knockout mice that lack the Cd38 gene show severe social deficits (i.e., amnesia of conspecifics) and have been discussed as a rodent model of autism (40, 41). Indeed, research points to a reduced expression of CD38 in the lymphoblastoid cell lines of autistic patients (42). With respect to naturally occurring variation at this gene, a common single-nucleotide polymorphism (SNP) has been described that occurs on an intron of this gene in humans, CD38 rs3796863. Genetic variation at this locus has been associated with autism (40, 41). Specifically, the C allele is reportedly overtransmitted in populations with autism and also associated with lower CD38 expression (42, 43). Furthermore, ASD in individuals with the CC genotype is characterized by more severe symptoms, such as restricted, repetitive, and stereotyped patterns of behavior, than ASD in individuals carrying the A allele (43). In healthy populations, individuals who are homozygous for the “risk allele” (CC) have lower plasma levels of oxytocin than CA/AA carriers (44, 45). When presented with social stimuli (both faces and scenes), adults with the CC genotype exhibit slower reaction times, increased fusiform gyrus activation (46), and increased amygdala activation (47) compared with adults with the CA/AA genotypes. At the behavioral level, parents homozygous for the C allele have been shown to touch their infants less during a free play session than A allele carriers (45). In summary, this literature suggests that genetic variation in CD38 is associated with systematic neural and behavioral differences in social functioning.
Apart from genetic variation within the oxytocin system itself, maternal behaviors that influence the exogenous availability of oxytocin might also contribute to individual differences in emotion processing, namely breastfeeding. Plasma oxytocin rises in mothers in response to suckling (48), and a similar rise in oxytocin occurs within the mothers’ milk itself (49). Although not directly studied in human infants, breastfeeding has been suggested to increase oxytocin levels in infants directly through breast milk, as well as indirectly through caring touch and warmth (50). Research with dairy calves and rat pups provides evidence that plasma oxytocin levels rise as a direct result of suckling (49, 51). For example, Takeda et al. (49) demonstrated that concentrations of radioactively marked oxytocin injected into rat dams were found in both the plasma and gastric contents of neonates after suckling. The duration in which human infants are fed with breast milk exclusively (exclusive breastfeeding; EBF) is recognized to facilitate cognitive (52–56) and neural development (57–59). However, only recently, EBF has been linked to differences in emotion processing in both mothers (60) and infants (61). In both studies, an extended duration of EBF was associated with increased sensitivity to happiness in others. These breastfeeding studies are in line with oxytocin administration studies, in which oxytocin has been found to increase sensitivity to positive expressions in others (i.e., ref. 30). It is thus likely that the influence of EBF on positive emotion perception is related to increased oxytocin levels in both mothers and infants.
The Current Study
The goal of the current study was to systematically investigate infants’ responses to emotional eyes and to examine how genetic variation in CD38 and variation in breastfeeding behavior impact individual differences in emotional eye processing. The specific aim of the current study was threefold: (i) to find out whether infants at 7 mo of age are able to differentially attend to emotional eye cues (and show a fear bias as seen for emotional faces), (ii) to examine whether exclusive breastfeeding duration, as a proxy for exogenously available oxytocin, impacts infants’ attention toward the eye region of specific emotions, and (iii) to further investigate whether genetic differences related to central oxytocin release (endogenously available oxytocin) modulate attention to emotional eyes and whether and how this possible influence might interact with exclusive breastfeeding duration (see Figs. 1 and 2 for presentation paradigm and stimuli examples). Based on prior work on emotional face perception in infancy, we hypothesized that 7-mo-old infants are able to discriminate between emotions (1). More specifically, we predicted that infants would show increased attention to fear compared with other emotions (3, 62–65). We further hypothesized that duration of EBF is associated with differences in infants’ attention to emotional information. As shown in prior work with infants and mothers (60, 61), we hypothesized that breastfeeding exposure would increase looking to happy eyes. Critically, we also hypothesized that the effect of EBF duration interacts with genetic variation within the oxytocin system. Specifically, based on prior work with rodents (41) and both autistic and healthy humans (43, 45), we predicted that the impact of breastfeeding might be greater for infants with the CC genotype (homozygous for the risk allele) of CD38 rs3796863. Because breastfeeding is thought to be an exogenous source of oxytocin (41, 49, 51), it might help up-regulate oxytocin levels (and function) in infants with the “risk” CC genotype presumably characterized by low oxytocin levels. It is also important to mention that the choice and the extent of breastfeeding may be impacted by potentially confounding maternal factors such as maternal age and education (66, 67). The current study therefore obtained information regarding these attributes and other variables through questionnaires.
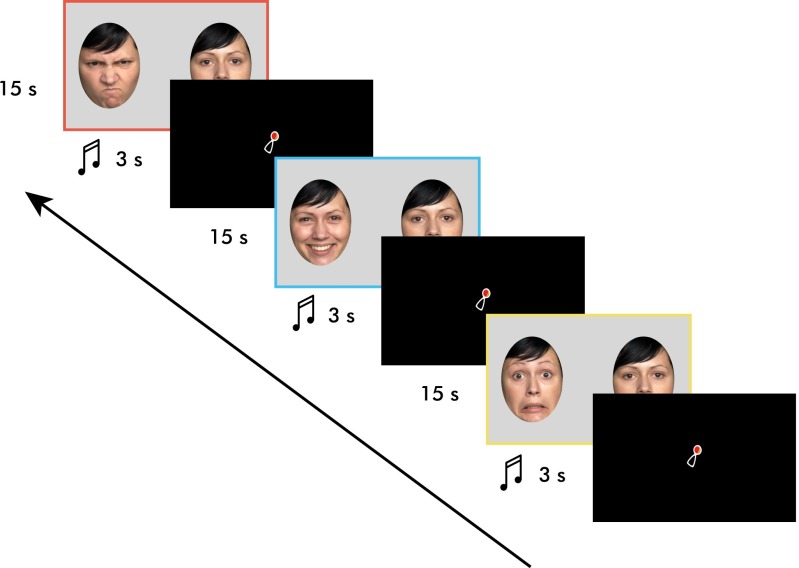
Fig. 1.
Presentation paradigm. Please note that the side in which the emotional face was presented was counterbalanced. Colored borders are shown to aid the reader in assessing the graphs below but were not presented to the infants. Stimulus duration is represented in seconds (s).
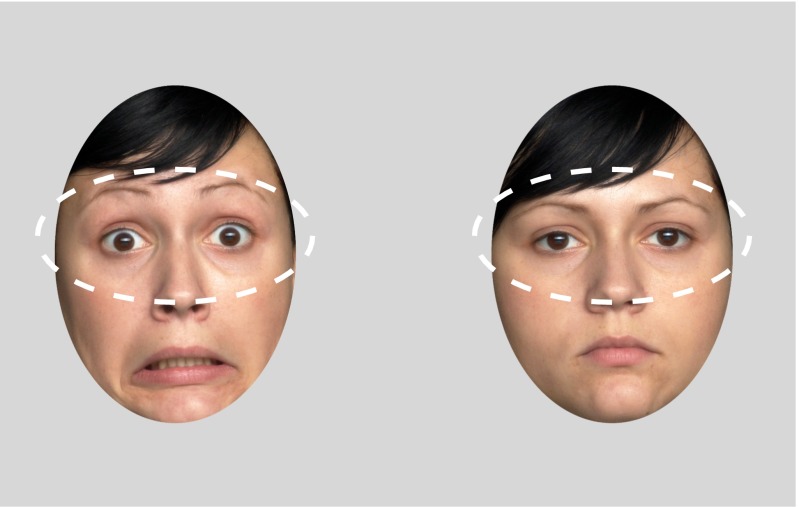
Fig. 2.
Regions of interest (ROIs). Dashed lines represent the ROIs analyzed for each emotion and actress.
Results
CD38 rs3796863 genotype frequencies in the infants are displayed in Table 1 and are similar to those observed in other studies (46, 47). No Mendelian inconsistencies were detected in inheritance of alleles from mothers to offspring (see Table S1 for maternal genotype frequencies). Hardy–Weinberg equilibrium was tested separately in mothers and in offspring. No deviations from Hardy–Weinberg equilibrium were detected (all P values > 0.05). Sex distribution and age differed significantly neither between genotype groups (CC versus CA/AA) nor between EBF groups (low versus high) (all P values > 0.05). The majority of studies relating the polymorphism rs3796863 (CD38) with aspects of social behavior and plasma oxytocin find differences between homozygous risk allele carriers (CC) and A carriers (CA/AA) (44–47, 68). In keeping with prior studies, we conducted our analyses by grouping genotypes in the same manner (CC versus CA/AA).
Table 1.
Infant CD38 rs3796863 genotype frequencies
| Genotype | Frequency | % |
| CC | 44 (18 F, 26 M) | 44.9 |
| CA | 49 (27 F, 22 M) | 50 |
| AA | 5 (4 F, 1 M) | 5.1 |
F, females; M, males.
Table S1.
Maternal CD38 rs3796863 genotype frequencies
| Genotype | Frequency | % |
| CC | 60 | 61.2 |
| CA | 29 | 29.6 |
| AA | 9 | 9.2 |
Note that the maternal genotypes were used to conduct Mendelian inheritance analyses and are shown only for the sake of completeness. They were not used in any of the main analyses of this study.
As our main analysis, we conducted a three (emotion: anger, happiness, fear) by two (EBF: low, high) by two (CD38: CC, CA/AA) repeated-measures analysis of covariance (ANCOVA) with looking preference to the eye region as the dependent variable. Covariates included in our analysis were maternal age, maternal education, and the percentage of currently breastfed meals (Table S2). Maternal age and education were included because prior work indicates that these factors are associated with breastfeeding behavior and should therefore be controlled as covariates (52–55, 57–59). Based on prior work with infants who are still breastfed (60, 61), we also included currently breastfed meals as a covariate because it allowed us to ascertain that breastfeeding effects can be attributed to exclusive breastfeeding duration (EBF): that is, the prolonged breastfeeding experience, rather than current breastfeeding behavior as reflected in currently breastfed meals. The main and interaction effects from this omnibus ANCOVA analysis and the subsequent follow-up tests are reported in the following paragraphs.
Table S2.
Descriptive statistics for main breastfeeding variables and covariates maternal age and education split by genotype and breastfeeding groups
| Variable | CC | CA/AA | ||
| EBF low | EBF high | EBF low | EBF high | |
| EBF duration, d | 115.52 (12.77) | 193.55 (3.69) | 120.77 (9.31) | 188.42 (2.31) |
| Currently breastfed meals, % | 38.32 (8.16) | 71.64 (4.02) | 35.36 (5.79) | 63.61 (5.53) |
| Maternal age, y | 31.45 (1.03) | 30.86 (0.86) | 31.00 (0.64) | 32.78 (1.13) |
| Maternal education, y | 16.55 (0.83) | 16.82 (0.54) | 15.87 (0.59) | 17.39 (3.81) |
Values are presented as mean (SEM).
Emotion.
Our analysis revealed a main effect of emotion [F(2,178) = 10.530, P < 0.001, η2 = 0.106]. Post hoc pairwise comparisons revealed that infants showed an increased looking preference to fearful eyes (mean = 0.59, SEM = 0.020) compared with happy eyes (M = 0.49, SEM = 0.021) and compared with angry eyes (mean = 0.47, SEM = 0.020), Bonferroni-adjusted P < 0.001 and P = 0.001, respectively. Looking to happy eyes and angry eyes did not differ significantly (Bonferroni-adjusted P = 1.00).
Emotion and Breastfeeding.
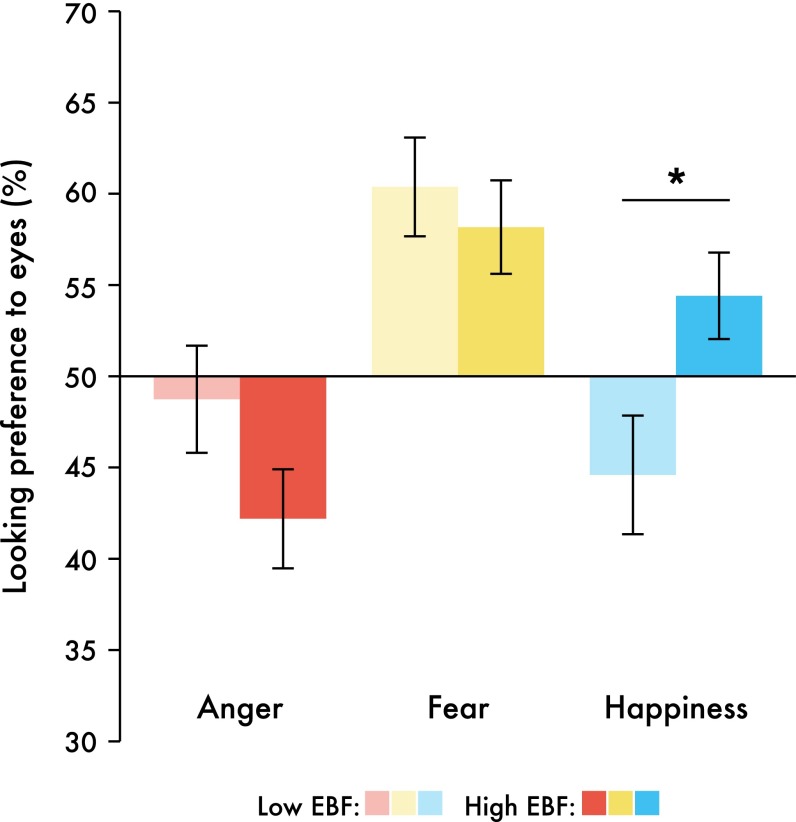
Our analysis further revealed a significant interaction between the factors emotion and EBF [F(2,178) = 3.6, P = 0.029, η2 = 0.039]. One-way ANOVAs conducted for the three emotions separately revealed that this interaction was driven by happiness. Specifically, infants in the high exclusive breastfeeding group showed higher looking preferences to happy eyes than infants in the low exclusive breastfeeding group [F(1,96) = 5.591, P = 0.02]. Anger and fear looking preferences did not differ by breastfeeding experience [F(1,96) = 2.613, P = 0.109 and F(1,96) = 0.343, P = 0.56, respectively] (Fig. 3).
Fig. 3.
Bar graph illustrating the interaction between EBF and emotion. Infants with a high EBF duration displayed significantly longer looking preferences to happy eyes than infants with low EBF. *P < 0.05; n = 98; data are presented in raw form and bars represent ±1 SEM.
As seen in Fig. 3, it seems that EBF might impact attention to angry eyes in the opposite direction of that to happy eyes, such that infants in the high EBF group have a higher looking preference to happiness and a lower looking preference to anger than infants in the low EBF group. Therefore, we explored a potential linear relationship between EBF duration and the bias toward either happy eyes or angry eyes. A difference score was created [(looking preference to happy eyes) − (looking preference to angry eyes)] on which we could conduct a forced-entry regression. With EBF, maternal education, and maternal age as predictors, only EBF significantly predicted this difference score (Table 2). As the duration of EBF linearly increases, a looking preference toward angry eyes shifts toward one for happy eyes (Fig. S1). This pattern might also reflect an avoidance of angry eyes with increased EBF duration. For results of one-sample t tests comparing each preference score to chance (50%), see Fig. S2.
Table 2.
Regression analysis indicating a positive prediction of EBF duration on looking preference to happy eyes minus looking preference to angry eyes
| Model | B | SEM | β | t value | P value |
| (Constant) | 0.025 | 0.031 | 0.826 | 0.411 | |
| EBF | 0.089 | 0.032 | 0.282 | 2.821 | 0.006 |
| Maternal education | −0.004 | 0.033 | −0.013 | −0.120 | 0.905 |
| Maternal age | 0.050 | 0.033 | 0.159 | 1.524 | 0.131 |
Model summary: F(3,92) = 3.736, P = 0.014, R2 = 0.109.
Fig. S1.
Partial regression plot illustrating the prediction of EBF on infant looking preference to happy eyes minus angry eyes. Please note that the residuals from the regression are plotted on the x and y axes. Points above zero represent a bias toward happy stimuli whereas points below zero represent a bias toward anger. P = 0.006, n = 98.
Fig. S2.
Bar graphs illustrating the results of one-sample t tests in which we compared each looking preference score to chance (50%) when (A) split by EBF group and (B) split by EBF and genotype groups. †P < 0.05, ††P < 0.01, and †††P < 0.001.
Emotion, Breastfeeding, and CD38.
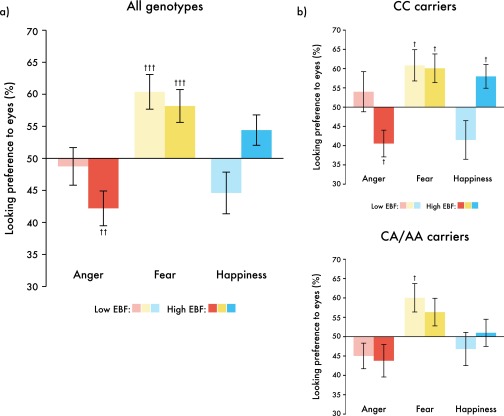
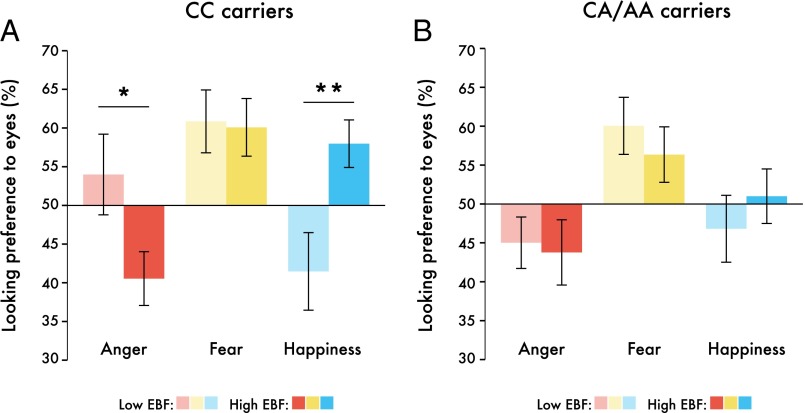
Critically, our analysis also revealed a three-way interaction between the factors emotion, EBF, and CD38 genotype [F(2,178) = 3.287, P = 0.04, η2 = 0.036]. To explore this interaction further, three (emotion: anger, happiness, fear) by two (EBF: high, low) repeated-measures ANCOVAs were conducted separately for each of the two CD38 genotype groups. We found a main effect of emotion for both genotype groups [CC, F(2,74) = 3.514, P = 0.035, η2 = 0.087; CA/AA, F(2,98) = 6.378, P = 0.002, η2 = 0.115]. However, the interaction between the factors EBF and emotion was only significant within CC carriers [F(2,74) = 5.151, P = 0.008, η2 = 0.112] (Fig. 4). Within the CC genotype, infants in the high exclusive breastfeeding group showed a greater preference for happy eyes (increased looking) and a greater avoidance of angry eyes (reduced looking) than infants in the low exclusive breastfeeding group [happiness, F(1,42) = 7.886, P = 0.008; anger, F(1,42) = 4.613, P = 0.038]. In the CA/AA genotype group, no such effects were observed. The looking preference averages for each breastfeeding group and genotype are displayed in Table 3.
Fig. 4.
Infant looking preferences were modulated by an interaction between EBF and CD38 genotype. Bar graphs represent emotion and EBF interactions for (A) CC carriers and (B) CA/AA carriers separately. *P < 0.05, **P < 0.01; n = 98; data are presented in raw form and bars represent ±1 SEM.
Table 3.
Looking preferences (%) for each emotion, split by genotype and breastfeeding groups. Values are presented as mean (SEM)
| Emotion | CC | CA/AA | ||
| EBF low | EBF high | EBF low | EBF high | |
| Angry eyes | 53.30 (4.21) | 44.12 (4.40) | 41.61 (3.58) | 47.02 (4.08) |
| Fearful eyes | 60.46 (4.24) | 60.12 (4.42) | 59.30 (3.60) | 57.21 (4.10) |
| Happy eyes | 38.79 (4.45) | 60.40 (4.64) | 45.69 (3.78) | 51.51 (4.30) |
Values are presented as mean (SEM).
Additional Effects.
Our analysis also revealed main effects of the covariates maternal education and currently breastfed meals [F(1,89) = 6.494, P = 0.013, η2 = 0.068, and F(1,89) = 4.502, P = 0.037, η2 = 0.048, respectively]. Note that, despite the observed main effects of these covariates, removing them as covariates from the statistical model leaves the main findings reported from the omnibus repeated measures ANOVA above unchanged. To further explore the main effects of the two covariates maternal education and currently breastfed meals, we computed correlations with looking preferences to emotional eyes for both maternal education and currently breastfed meals. The correlation analysis showed that only looking preference to angry eyes, but not happy or fearful eyes, correlated negatively with percentage of breastfed meals [r(97) = −0.289, P = 0.004] and with maternal education [r(97) = −0.241, P = 0.017]. Specifically, the greater the maternal education and percentage of breastfed meals, the greater was infants’ preference for neutral eyes compared with angry eyes, which might reflect an avoidance of angry eyes. With respect to percentage of breastfed meals, this result is similar to a study with mothers in which the percentage of breastfed meals slowed down reaction times when recognizing angry faces (60). Maternal education and percentage of breastfed meals showed a positive relation but were not significantly correlated with each other [r(97) = 0.162, P = 0.113]. There were no interactions between covariates, and the covariates did not differ across genotype groups. Relevant data files have been deposited in the Center for Open Science digital repository (https://osf.io/6pnaj).
Discussion
In the current study, we examined infants’ responses to emotional eyes in the context of common genetic variation within the CD38 gene and variation in exclusive breastfeeding experience. Our analysis of infants’ looking patterns yielded three main findings. First, as a group, 7-mo-old infants showed a fear bias: increased looking toward fearful eyes compared with happy and angry eyes. Second, infants’ increased breastfeeding experience was associated with enhanced looking to happy eyes and aversion of angry eyes. Third, these effects of breastfeeding on emotional eye processing were observed only in infants with the CC genotype of the CD38 gene (rs3796863). Our findings suggest that differences in the sensitivity to emotional information conveyed through the eyes are linked to the oxytocin system. Specifically, the pattern of results points to an interaction between factors impacting the endogenous (CD38) and exogenous (breastfeeding) availability of oxytocin. This finding underlines the importance of the oxytocin system in contributing to sensitivity to emotional cues from the eyes. We will now discuss the three main findings in turn.
Emotion.
Our results show that, at the age of 7 mo, infants’ looking to fearful eyes is significantly increased compared with happy and angry eyes. This finding demonstrates that, as a group, infants show what has been termed a fear bias (4). This increased attention to fear in others is in line with prior behavioral and neuroscience work with infants of that age (3, 62–65, 69). Enhanced attention to fear has been argued to serve important adaptive functions because it is thought to alert the individual to potential dangers and threats in the environment (69). Interestingly, although fearful and angry faces both represent negative expressions that might be associated with threat, our results show that infants dedicate more attention to fearful eyes than angry eyes. This outcome indicates that, rather than indiscriminately heightening attention to negative cues, infants selectively increase looking at the eyes of someone experiencing fear. This finding is in agreement with work in adults showing that fearful expressions elicit approach behavior and are linked to helping whereas angry expressions elicit avoidance behavior (70–72). Therefore, selective enhanced attention to fearful eyes as seen in our infant data may reflect infants’ approach and empathic concern for others in distress (experiencing fear) (73). Alternatively, infants may also look longer at fearful eyes because they expect gaze cues that help them identify the source of threat (74) or because they exhibit a heightened perceptual sensitivity to the large eye whites characteristic of fearful eyes (9). Regardless of the exact nature of this effect, the current findings provide evidence for the notion that infants of this age show a robust fear bias.
Emotion and Breastfeeding.
Our analysis further revealed an interaction between the factors emotion and exclusive breastfeeding duration (EBF). Importantly, however, the two-way interaction between emotion and EBF was further qualified by a three-way interaction including CD38 variation and can therefore be fully interpreted only when taking into account infant CD38 genotype. Before a detailed discussion of the role of CD38 genotype, we nonetheless briefly summarize and discuss our findings with respect to variation in EBF because they replicate and critically extend prior work on that topic with infants and mothers. More specifically, our results show that infants who were exclusively breastfed for a longer duration exhibit increased looking to happy eyes than those infants exclusively breastfed for a shorter duration. This finding confirms our prediction based on previous work in which longer exclusive breastfeeding durations were associated with greater attention allocation in infants to happy body expressions as indexed by ERP measures (61). This increased sensitivity to happiness in others as a function of exclusive breastfeeding duration is also seen in mothers (60), suggesting that infants’ and mothers’ responses to happiness are similarly affected by breastfeeding experience. Moreover, our regression analyses showed an association between currently breastfed meals and infants’ responses to angry eyes. Specifically, as the percentage of breastfed meals increased, the looking toward angry eyes decreased. This effect of breastfeeding on looking away from angry eyes, although not statistically significant, seems to be in a similar direction also when considering duration of EBF (Fig. 3). This aversion of angry eyes is similar to what has been found in mothers (60), in which the percentage of breastfed meals slowed down reaction time when recognizing angry faces. Such an increased sensitivity to positive emotional expressions in both mothers and infants might be important in fostering positive social interactions and thereby might facilitate affiliation and bonding in human development (75). It is also in general agreement with accounts that assign an important role to maternal behaviors in the early development of social functioning (76, 77). It is also important to emphasize that the effects of the breastfeeding variables obtained in the current infant study resemble the effects of oxytocin administration in prior work on emotional face perception with adults (30, 32). Taken together, our findings indicate that increased breastfeeding experience, presumably through its effects on the oxytocin system, is associated with enhanced sensitivity to happy eyes and a decreased sensitivity to angry eyes. This interpretation is in line with accounts that view oxytocin’s role in social functioning as facilitating approach while simultaneously reducing withdrawal tendencies (36).
Emotion, Breastfeeding, and CD38.
Whether and how the effects of breastfeeding are moderated by genetic variation related to the central release of oxytocin (CD38) will be discussed in this section. Addressing this question can shed light on how endogenous and exogenous variables affecting the oxytocin system interact in contributing to infants’ sensitivity to emotional eyes. Our results show that the association between EBF duration and specific differences in emotional eye processing is present only in individuals homozygous for the C allele of CD38 rs3796863. Specifically, among infants with the CC genotype (lower endogenous oxytocin), those with longer durations of EBF (higher exogenous oxytocin) experience exhibited enhanced looking to happy eyes and decreased looking (even looking away, avoidance) of angry eyes, compared with infants of the same genotype but with shorter durations of EBF. This finding was in line with our prediction based on prior work with rodents (41) and both autistic and healthy humans (43–45, 68), implicating the CC genotype in impaired social functioning associated with reduced oxytocin levels. Critically, our results suggest that breastfeeding, probably through its role as an exogenous source of oxytocin (49, 51), may help regulate oxytocin levels (and function) specifically in infants with the CC genotype who may have a greater risk for social dysfunction. It seems that low endogenous oxytocin, albeit genetically determined, can be corrected for in a sense by an environmental manipulation, namely, extended breastfeeding. This finding may have important implications regarding genetic hard wiring, and we suggest the notion that other instances of perceived genetic determinism can be minimized or neutralized by appropriate behavioral interventions, which in our study are represented by breastfeeding. This paper adds to the growing consensus that, although genes are important in human behavior, they are not deterministic.
More generally, the obtained results indicate that infants in the CC genotype group are more susceptible to the effects of their maternal environment (breastfeeding experience). To find such a pattern of differential susceptibility to breastfeeding experience on the basis of the genotype concurs with accounts stipulating that putative “risk genes” might in some instances be more appropriately conceptualized as “plasticity genes” (78). Rather than reflecting a predisposed risk factor, some genes may essentially make the individual infant more susceptible to context, whereby the direction of the effect depends on the context. Namely, in the current study, in the CC genotype group, shorter durations of breastfeeding are associated with reduced attention to happy eyes and increased attention to angry eyes whereas longer durations of breastfeeding had opposite effects (increased attention to happy eyes and decreased attention to angry eyes). According to this view, the current results point to a potential plasticity gene (CC genotype) involved in regulating oxytocin effects on social functioning during early development related to responding to emotional eyes. A critical question that arises from this investigation is whether infancy represents a sensitive period in human development during which such plasticity is observed. It should also be considered that this period of plasticity may well extend into adolescence (79). Relatedly, it will be important to find out whether the biases seen in 7-mo-old infants’ attention to emotional eyes based on the interaction between breastfeeding and CD38 genotype endure beyond infancy and, if so, how they impact social behavior in children more globally. Ultimately, longitudinal work is needed to elucidate these critical questions.
Limitations and Future Directions.
Apart from the need for longitudinal data to examine potential long-term effects, in future work, it will also be critical to investigate the neural processes that are involved in the interaction between breastfeeding and CD38 genotype when accounting for individual differences in the attention to social eye cues. Prior work has shown that oxytocin exerts neurophysiological effects in the brain by directly targeting fast-spiking interneurons. Specifically, there is work demonstrating that the administration of oxytocin in rats increases throughput of output spikes, sharpens spike timing, and suppresses background firing, which improves circuit-level signal-to-noise ratios and can thereby increase the salience of certain stimuli (80). This signal-to-noise enhancement can be impacted by certain developmental events and experiences. For example, when female rats become dams, responses to pup distress calls become enhanced by balancing cortical inhibition, and the same effect can be mimicked in virgin female rats by administering oxytocin (81). This literature supports the notion that oxytocin plays a role in enhancing social responsiveness. Moreover, as previously mentioned, the administration of oxytocin to children (25) and adults (26) diagnosed with autism has been shown to selectively enhance brain responses to social stimuli compared with physical control stimuli. Interestingly, it has also been observed that, for some individuals with ASD, oxytocin administration is more effective in enhancing brain responses to social cues than in others (25). Our infant data suggest that there are genetic factors, namely CD38 genotype, that may play a role in accounting for how effective exogenously stimulated oxytocin levels enhance responding to social cues. It may thus be important to examine whether CD38 variation can also account for the effectiveness of oxytocin administration in ASD.
As a further consideration and limitation, it must be stressed that breastfeeding is a dynamic and complex process that involves and impacts several hormonal, physiological, and psychological systems (56). It is therefore difficult to unpack and to determine the exact mechanisms by which breastfeeding exerts the effects seen in the current study. For example, breastfeeding involves other behaviors such as physical contact associated with warmth and pleasant touch (50), both of which have been shown to, on the one hand, reduce heart rate in infants (82) and, on the other hand, enhance emotion regulation, social engagement, and pain analgesia (83, 84). Furthermore, factors related to attachment quality, especially maternal sensitivity, have been shown to impact breastfeeding behavior, with increased maternal sensitivity related to prolonged breastfeeding rates (85). These physiological, behavioral, and psychological variations are all important factors to take into account in future studies on the effects of breastfeeding on social functioning in infancy.
Conclusion
All in all, the current study demonstrates that differential attention to emotional eye cues, as a vital social skill, develops during infancy. Importantly, differences in this sensitivity to emotional eye cues emerge in the context of an interaction of factors associated with genetic variation in CD38 and breastfeeding experience. This finding provides evidence that, early in human ontogeny, endogenous and exogenous factors involved in regulating oxytocin levels contribute to individual differences in emotional attention. This research points to early emerging biases in emotion processing that may plastically shape pathways of socio-emotional development.
Materials and Methods
Participants.
Ninety-eight 7-mo-old infants (49 females, 49 males) participated with their mothers in this study. Infants’ age ranged from 204 to 232 d (mean = 214 d, SEM = 0.56), and mothers’ age ranged from 22 to 45 y (mean = 31.49, SEM = 0.45). All infants were typically developing, had a normal birth weight (>2,500 g), were born full-term (37–41 wk), and were of European descent. There was no known history of autism spectrum disorder either in any of the participating mothers or in any of the older siblings. Seventy-eight infants had standard vaginal deliveries, and 20 were delivered via caesarean section. All but one infant had mothers that were still on maternity leave up to the time of testing. Informed consent was provided by the infants’ parents before participation in the study. All procedures were approved by the Leipzig University Medical School Ethics Committee and were performed in accordance with the Declaration of Helsinki. Parents were reimbursed for travel, and infants received a toy and a T-shirt.
Breastfeeding.
A questionnaire developed in house was completed by mothers to obtain detailed information on breastfeeding experience (60, 61). Mothers filled out a table that detailed a feeding plan for a typical day. The percentage of breastfed meals was calculated by dividing the number of breastfed meals by the number of total meals. Mothers also indicated whether they were still exclusively breastfeeding and, if not, at what age (infant) they ceased. Exclusive breastfeeding was defined as providing breast milk as the only source of nutrition for the infant. Additional demographic details were provided, including the mothers’ education and job status, immigration history of both parents, number of caretakers, parity, and current stress level.
Stimuli.
Photographs of three females expressing happiness, fear, anger, and neutrality were chosen from a published and validated stimulus set (FACES Collection) (86). The selection was based on an average recognition rate of at least 90% from adult raters across all emotional expressions. Furthermore, we excluded actresses whose hair obstructed the face in any way. Stimuli were created such that each emotional face was presented side-by-side with the neutral face of the same actress. Using Adobe Photoshop (Version CS5), facial expressions were placed within two predetermined ovals within a light gray frame (always located in the same positions, at the same distance and aligned with each other) (Fig. 2) that removed potentially distracting outer features of the faces such as the ears and parts of the hair. The facial expressions were placed within (below) this frame and were moved and resized to align with geometrically fixed markers for the position of the two eyes, the nose, and the mouth. This editing procedure ensured that all facial stimuli were presented in the same position, all facial features were aligned, and the eyes were within the designated region of interest (ROI) created in Tobii Studio. Facial ovals were 23 cm high and 16.5 cm wide and presented on a 32 × 52-cm (24-inch diagonal) computer monitor placed about 60 cm in front of the infant. Every trial consisted of the presentation of two faces shown side-by-side: one neutral face with no emotional content, and one expression displaying either happiness, fear, or anger. The two faces displayed simultaneously during each trial were aligned and presented 25 cm apart from each other (nose-to-nose distance). The presentation of an emotional face and a neutral face simultaneously allowed for the direct comparison of infants’ looking preference for emotional faces compared with neutral faces (Figs. 1 and 2). Information on the calculation of a preference score is located in Data Analysis.
A three-second video clip of a shaking rattle was used as an attention-getter to draw infants’ attention to the center of the screen before each experimental trial. The rattle measured 3 cm × 1.5 cm and was presented in the center of the screen. It was accompanied by three tones of alternating frequencies (ranging from 109 Hz to 262 Hz).
Procedure.
Infants were seated on a parent’s lap, ∼60 cm away from the presentation monitor. The experimental area was separated from the experimenter’s control desk with a room divider. The region behind the computer monitor was covered with a black curtain to prevent any possible distractions for the infant. A small plastic ring was provided for each infant to hold during stimulus presentation. A Tobii ×120 eye tracker was set up at the bottom of the computer monitor to record infant looking behavior. Stimuli were presented through Tobii Studio (Version 3.2). Before stimulus presentation, a five-point calibration procedure was administered. At the five calibration locations (four corners and the center of the screen), a moving rattle of the length of 3 cm was shown. The designated area within the rattle stimulus comprised a visual angle of ∼4.6°. The rattle was combined with a beeping sound. For successful calibration, infants needed to fixate within this designated fixation area at all five locations. The calibration procedure was repeated for infants that did not successfully fixate. Additional information regarding the infant calibration procedure is available in the Tobii user manual (available for free download at www.tobii.com).
Every infant viewed nine trials, such that each emotion was presented three times and by each actress. Stimulus presentation was pseudorandomized such that no emotion and no actress were displayed twice in a row. Presentation of the emotion on the left or right side was counterbalanced for each infant. Because there were nine trials, half of the infants had one extra trial where the emotional face was presented on the left whereas the other half had one extra trial where the emotional face was presented on the right. Infant behavior was recorded online such that the experimenter could assess attention and had full control over the presentation of each trial. Each trial began with a 3-s attention-getter in the center of the screen (the same moving rattle plus beeping combination used for calibration). Experimental trials were presented for 15 s. During that period, infants were able to freely explore the faces and look toward and away from the screen as often as they pleased. The entire eye-tracking session lasted approximately 3 min.
Genotyping.
Saliva samples were collected from infants and their mothers at a previous visit (at 5 mo of age). Sponges were used to collect saliva from infants (OG-250 kit; DNA Genotek), and collection tubes were used for mothers (OG-500 kit; DNA Genotek). Infants and mothers had no food or drink within the 30 min before collection. Samples were stored at room temperature, and DNA was extracted using the DNA Genotek manual purification protocol. Genotyping of CD38 rs3796863 was performed with a 5′-nuclease assay. Primers and probes were from Applied Biosystems (TaqMan SNP Genotyping Assay). PCR was conducted with HotStarTaq Plus DNA polymerase and Q-solution (Qiagen) in a Bio-Rad C1000 instrument with a CFX96 fluorescence reading module, with the following thermal protocol: enzyme activation, −95 °C for 5 min; cycling, −95 °C for 15 s, 60 °C for 1 min, 45 times.
Data Analysis.
The duration of exclusive breastfeeding (EBF) was negatively skewed in our sample: Zskewness = −5.84, P < 0.001; Zkurtosis = 3.12, P < 0.01 (mean = 151.38 d, SEM = 5.59; median = 167.40 d). We therefore used a median split to create categorical groups of low and high EBF for further analysis and visualization (low EBF, mean = 118.59 d, SEM = 7.53; high EBF, mean = 190.87 d, SEM = 2.15) (87). Regions of interest (ROIs) were created within Tobii Studio. ROIs comprised the eye regions of the face stimuli used (Fig. 2). The total looking times for each emotional eye region were extracted for each infant and trial (see Table S3 for raw values). We then computed average looking preference scores by calculating the proportion of looking time to each emotion [anger (A), fear (F), happiness (H)] and its corresponding neutral face [neutral (A), neutral (F), neutral (H)]. The looking preference for any given emotion (X) was computed as follows: looking preference (X) = looking time (emotion X)/[looking time (emotion X) + looking time (neutral X)].
Table S3.
Raw looking durations (seconds) for each emotion, split by genotype and breastfeeding groups
| Emotion | CC | CA/AA | ||
| EBF low | EBF high | EBF low | EBF high | |
| Angry eyes | 3.93 (0.54) | 4.00 (0.57) | 4.61 (0.62) | 5.08 (0.79) |
| Fearful eyes | 5.56 (0.94) | 5.72 (0.75) | 6.89 (0.97) | 8.00 (1.06) |
| Happy eyes | 3.45 (0.60) | 4.85 (0.60) | 4.21 (0.62) | 6.10 (0.92) |
Values are presented as mean (SEM). Total looking durations did not differ significantly between EBF or CD38 groups.
We were therefore able to compare the percentages of looking time toward anger, fear, and happiness, while taking into account the time the infant spent looking at neutral stimuli (see Fig. 2 for stimulus example). Critically, looking preferences were calculated only on the basis of trials during which the infant had fixated (looked at) both the emotional face and the neutral face at least once. This guideline served as a minimum criterion for a trial to be included in the analysis. Note that infants had 15 s to freely explore, scan, and compare between both faces presented simultaneously on the screen. To present two faces side-by-side for this duration is an experimental procedure commonly used to determine visual preferences for facial expressions in infants of this age (see refs. 3 and 88). Of the maximum number of nine trials, infants contributed an average of mean = 8.85 trials (SEM = 0.07; range = 4–9). All infants contributed at least one trial per emotion to the analysis, and the great majority of infants (n = 92) contributed all nine trials to the analysis. The number of trials included in the analysis did not differ between EBF groups or genotype groups (all P values > 0.05).
The heat maps of absolute looking durations for each infant during each attention-getter/centering stimulus were visually inspected throughout the session. The visual inspection of these heat maps revealed that the infants fixated on the moving rattle during all nine attention-getters, indicating first that there was no drift across the experimental session, and second that infants maintained central looking before the presentation of the facial stimuli.
Questionnaires.
Interpersonal Reactivity Index (89).
The Interpersonal Reactivity Index (IRI) evaluates dispositional empathy. This questionnaire has four subscales and was given to mothers to assess the tendency one has to take the point of view of others (PT), the tendency to experience feelings of sympathy and compassion for those less fortunate (EC), the tendency to experience feelings of distress in response to discomfort in others (PD), and the tendency to transpose oneself into fictional situations such as novels or movies (FS).
The Positive and Negative Affect Schedule (90).
The Positive and Negative Affect Schedule (PANAS) assessed the general mood of the mother over the last 12 mo and calculated both a positive (PA) and negative affect (NA) score.
Additional questionnaires.
Additional questionnaires were administered to mothers when infants were 5 mo of age (about 2 mo before the current study) and were explored in analyses reported here. A short form of the Social Support Questionnaire (SSQ6) was filled out by mothers (91). The SSQ6 comprises two subscales that measure the amount of social support one experiences (SSQ Number), as well as the satisfaction one experiences with the support (SSQ Satisfaction). The Parental Sense of Competence (PSOC) questionnaire assessed how knowledgeable and capable each mother felt with parenting (92). Lastly, the Edinburgh Postnatal Depression Questionnaire (EPDS) was administered to each mother to detect potential signs of postpartum depression (93).
Analysis of Questionnaire and Demographic Data.
To identify and rule out potentially confounding factors, analyses (one-way ANOVAs) were performed to compare between EBF and genotype groups for the questionnaire and demographic data. These variables included demographic factors such as maternal age and infant age, parity, years of maternal education, number of caretakers, and current subjective stress level of the mother. Additional maternal self-report measures were analyzed, including amount of, and satisfaction with, social support, parental sense of competence, Edinburgh postnatal depression score, positive and negative affect score, and all subscores on the interpersonal reactivity index (empathic concern, fantasy seeking, personal distress, perspective taking). None of these variables differed significantly between EBF groups or genotype groups (all P values > 0.1). Moreover, there was no difference between EBF groups or genotype groups with respect to the delivery method (vaginal birth versus caesarean section) [χ2(1) = 1.206, P = 0.272; χ2(1) = 1.037, P = 0.309, respectively]. The absence of differences across EBF and genotype groups with respect to these variables helps to rule out that any of those variables account for the effects reported in the current study.
Acknowledgments
We thank Jenny Tippmann and Caterina Boettcher for invaluable help with data collection, and Aileen Pang Yu Wen for assistance with DNA analysis. We also thank all families who participated in this study. This work was supported by funding awarded by the Max Planck Society (to T.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. K.A.P. is a guest editor invited by the Editorial Board.
Data deposition: Relevant data files have been deposited in the Center for Open Science digital repository, https://osf.io/6pnaj.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1506352112/-/DCSupplemental.
References
- 1.Grossmann T. The early development of processing emotions in face and voice. In: Belin P, Campanella S, Ethofer T, editors. Integrating Face and Voice in Person Perception. Springer; New York: 2013. pp. 95–116. [Google Scholar]
- 2.Vaish A, Grossmann T, Woodward A. Not all emotions are created equal: The negativity bias in social-emotional development. Psychol Bull. 2008;134(3):383–403. doi: 10.1037/0033-2909.134.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peltola MJ, Leppänen JM, Mäki S, Hietanen JK. Emergence of enhanced attention to fearful faces between 5 and 7 months of age. Soc Cogn Affect Neurosci. 2009;4(2):134–142. doi: 10.1093/scan/nsn046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leppänen JM, Nelson CA. Early development of fear processing. Curr Dir Psychol Sci. 2012;21(3):200–204. [Google Scholar]
- 5.Adolphs R, et al. A mechanism for impaired fear recognition after amygdala damage. Nature. 2005;433(7021):68–72. doi: 10.1038/nature03086. [DOI] [PubMed] [Google Scholar]
- 6.Baron-Cohen S. Mindblindness: An Essay on Autism and Theory of Mind. MIT Press; Cambridge: 1995. [Google Scholar]
- 7.Lewis MB, Edmonds AJ. Face detection: Mapping human performance. Perception. 2003;32(8):903–920. doi: 10.1068/p5007. [DOI] [PubMed] [Google Scholar]
- 8.Whalen PJ, et al. Human amygdala responsivity to masked fearful eye whites. Science. 2004;306(5704):2061. doi: 10.1126/science.1103617. [DOI] [PubMed] [Google Scholar]
- 9.Jessen S, Grossmann T. Unconscious discrimination of social cues from eye whites in infants. Proc Natl Acad Sci USA. 2014;111(45):16208–16213. doi: 10.1073/pnas.1411333111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kouider S, et al. A neural marker of perceptual consciousness in infants. Science. 2013;340(6130):376–380. doi: 10.1126/science.1232509. [DOI] [PubMed] [Google Scholar]
- 11.Jones W, Klin A. Attention to eyes is present but in decline in 2-6-month-old infants later diagnosed with autism. Nature. 2013;504(7480):427–431. doi: 10.1038/nature12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Arch Gen Psychiatry. 2002;59(9):809–816. doi: 10.1001/archpsyc.59.9.809. [DOI] [PubMed] [Google Scholar]
- 13.Spezio ML, Adolphs R, Hurley RS, Piven J. Abnormal use of facial information in high-functioning autism. J Autism Dev Disord. 2007;37(5):929–939. doi: 10.1007/s10803-006-0232-9. [DOI] [PubMed] [Google Scholar]
- 14.Guillon Q, Hadjikhani N, Baduel S, Rogé B. Visual social attention in autism spectrum disorder: Insights from eye tracking studies. Neurosci Biobehav Rev. 2014;42:279–297. doi: 10.1016/j.neubiorev.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 15.Senju A, Johnson MH. Atypical eye contact in autism: Models, mechanisms and development. Neurosci Biobehav Rev. 2009;33(8):1204–1214. doi: 10.1016/j.neubiorev.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Guastella AJ, Mitchell PB, Dadds MR. Oxytocin increases gaze to the eye region of human faces. Biol Psychiatry. 2008;63(1):3–5. doi: 10.1016/j.biopsych.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 17.Auyeung B, et al. Oxytocin increases eye contact during a real-time, naturalistic social interaction in males with and without autism. Transl Psychiatry. 2015;5:e507. doi: 10.1038/tp.2014.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: A dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol. 2004;25(3-4):150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Pedersen CA, Ascher JA, Monroe YL, Prange AJJ., Jr Oxytocin induces maternal behavior in virgin female rats. Science. 1982;216(4546):648–650. doi: 10.1126/science.7071605. [DOI] [PubMed] [Google Scholar]
- 20.Lincoln DW, Paisley AC. Neuroendocrine control of milk ejection. J Reprod Fertil. 1982;65(2):571–586. doi: 10.1530/jrf.0.0650571. [DOI] [PubMed] [Google Scholar]
- 21.Carter CS. Oxytocin pathways and the evolution of human behavior. Annu Rev Psychol. 2014;65:17–39. doi: 10.1146/annurev-psych-010213-115110. [DOI] [PubMed] [Google Scholar]
- 22.Rilling JK, Young LJ. The biology of mammalian parenting and its effect on offspring social development. Science. 2014;345(6198):771–776. doi: 10.1126/science.1252723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andari E, et al. Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proc Natl Acad Sci USA. 2010;107(9):4389–4394. doi: 10.1073/pnas.0910249107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guastella AJ, et al. Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biol Psychiatry. 2010;67(7):692–694. doi: 10.1016/j.biopsych.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 25.Gordon I, et al. Oxytocin enhances brain function in children with autism. Proc Natl Acad Sci USA. 2013;110(52):20953–20958. doi: 10.1073/pnas.1312857110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Domes G, et al. Effects of intranasal oxytocin on the neural basis of face processing in autism spectrum disorder. Biol Psychiatry. 2013;74(3):164–171. doi: 10.1016/j.biopsych.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 27.Guastella AJ, Mitchell PB, Mathews F. Oxytocin enhances the encoding of positive social memories in humans. Biol Psychiatry. 2008;64(3):256–258. doi: 10.1016/j.biopsych.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 28.Tollenaar MS, Chatzimanoli M, van der Wee NJA, Putman P. Enhanced orienting of attention in response to emotional gaze cues after oxytocin administration in healthy young men. Psychoneuroendocrinology. 2013;38(9):1797–1802. doi: 10.1016/j.psyneuen.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 29.Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin improves “mind-reading” in humans. Biol Psychiatry. 2007;61(6):731–733. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 30.Marsh AA, Yu HH, Pine DS, Blair RJ. Oxytocin improves specific recognition of positive facial expressions. Psychopharmacology (Berl) 2010;209(3):225–232. doi: 10.1007/s00213-010-1780-4. [DOI] [PubMed] [Google Scholar]
- 31.Gamer M, Büchel C. Oxytocin specifically enhances valence-dependent parasympathetic responses. Psychoneuroendocrinology. 2012;37(1):87–93. doi: 10.1016/j.psyneuen.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 32.Domes G, Steiner A, Porges SW, Heinrichs M. Oxytocin differentially modulates eye gaze to naturalistic social signals of happiness and anger. Psychoneuroendocrinology. 2013;38(7):1198–1202. doi: 10.1016/j.psyneuen.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Parr LA, Modi M, Siebert E, Young LJ. Intranasal oxytocin selectively attenuates rhesus monkeys’ attention to negative facial expressions. Psychoneuroendocrinology. 2013;38(9):1748–1756. doi: 10.1016/j.psyneuen.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kirsch P, et al. Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci. 2005;25(49):11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Simplicio M, Massey-Chase R, Cowen PJ, Harmer CJ. Oxytocin enhances processing of positive versus negative emotional information in healthy male volunteers. J Psychopharmacol. 2009;23(3):241–248. doi: 10.1177/0269881108095705. [DOI] [PubMed] [Google Scholar]
- 36.Kemp AH, Guastella AJ. The role of oxytocin in human affect: A novel hypothesis. Curr Dir Psychol Sci. 2011;20(4):222–231. [Google Scholar]
- 37.Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: Social neuropeptides for translational medicine. Nat Rev Neurosci. 2011;12(9):524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- 38.Kumsta R, Heinrichs M. Oxytocin, stress and social behavior: Neurogenetics of the human oxytocin system. Curr Opin Neurobiol. 2013;23(1):11–16. doi: 10.1016/j.conb.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 39.Jin D, et al. CD38 is critical for social behaviour by regulating oxytocin secretion. Nature. 2007;446(7131):41–45. doi: 10.1038/nature05526. [DOI] [PubMed] [Google Scholar]
- 40.Higashida H, Yokoyama S, Kikuchi M, Munesue T. CD38 and its role in oxytocin secretion and social behavior. Horm Behav. 2012;61(3):351–358. doi: 10.1016/j.yhbeh.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 41.Higashida H, et al. CD38 gene knockout juvenile mice: A model of oxytocin signal defects in autism. Biol Pharm Bull. 2011;34(9):1369–1372. doi: 10.1248/bpb.34.1369. [DOI] [PubMed] [Google Scholar]
- 42.Lerer E, et al. Low CD38 expression in lymphoblastoid cells and haplotypes are both associated with autism in a family-based study. Autism Res. 2010;3(6):293–302. doi: 10.1002/aur.156. [DOI] [PubMed] [Google Scholar]
- 43.Munesue T, et al. Two genetic variants of CD38 in subjects with autism spectrum disorder and controls. Neurosci Res. 2010;67(2):181–191. doi: 10.1016/j.neures.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 44.Feldman R, Gordon I, Influs M, Gutbir T, Ebstein RP. Parental oxytocin and early caregiving jointly shape children’s oxytocin response and social reciprocity. Neuropsychopharmacology. 2013;38(7):1154–1162. doi: 10.1038/npp.2013.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feldman R, et al. Sensitive parenting is associated with plasma oxytocin and polymorphisms in the OXTR and CD38 genes. Biol Psychiatry. 2012;72(3):175–181. doi: 10.1016/j.biopsych.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 46.Sauer C, Montag C, Wörner C, Kirsch P, Reuter M. Effects of a common variant in the CD38 gene on social processing in an oxytocin challenge study: Possible links to autism. Neuropsychopharmacology. 2012;37(6):1474–1482. doi: 10.1038/npp.2011.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sauer C, Montag C, Reuter M, Kirsch P. Imaging oxytocin × dopamine interactions: An epistasis effect of CD38 and COMT gene variants influences the impact of oxytocin on amygdala activation to social stimuli. Front Neurosci. 2013;7:45. doi: 10.3389/fnins.2013.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dawood MY, Khan-Dawood FS, Wahi RS, Fuchs F. Oxytocin release and plasma anterior pituitary and gonadal hormones in women during lactation. J Clin Endocrinol Metab. 1981;52(4):678–683. doi: 10.1210/jcem-52-4-678. [DOI] [PubMed] [Google Scholar]
- 49.Takeda S, Kuwabara Y, Mizuno M. Concentrations and origin of oxytocin in breast milk. Endocrinol Jpn. 1986;33(6):821–826. doi: 10.1507/endocrj1954.33.821. [DOI] [PubMed] [Google Scholar]
- 50.Uvnäs-Moberg K. Oxytocin may mediate the benefits of positive social interaction and emotions. Psychoneuroendocrinology. 1998;23(8):819–835. doi: 10.1016/s0306-4530(98)00056-0. [DOI] [PubMed] [Google Scholar]
- 51.Lupoli B, Johansson B, Uvnäs-Moberg K, Svennersten-Sjaunja K. Effect of suckling on the release of oxytocin, prolactin, cortisol, gastrin, cholecystokinin, somatostatin and insulin in dairy cows and their calves. J Dairy Res. 2001;68(2):175–187. doi: 10.1017/s0022029901004721. [DOI] [PubMed] [Google Scholar]
- 52.Mortensen EL, Michaelsen KF, Sanders SA, Reinisch JM. The association between duration of breastfeeding and adult intelligence. JAMA. 2002;287(18):2365–2371. doi: 10.1001/jama.287.18.2365. [DOI] [PubMed] [Google Scholar]
- 53.Oddy WH. Long-term health outcomes and mechanisms associated with breastfeeding. Expert Rev Pharmacoecon Outcomes Res. 2002;2(2):161–177. doi: 10.1586/14737167.2.2.161. [DOI] [PubMed] [Google Scholar]
- 54.Kramer MS, et al. Infant growth and health outcomes associated with 3 compared with 6 mo of exclusive breastfeeding. Am J Clin Nutr. 2003;78(2):291–295. doi: 10.1093/ajcn/78.2.291. [DOI] [PubMed] [Google Scholar]
- 55.Daniels MC, Adair LS. Breast-feeding influences cognitive development in Filipino children. J Nutr. 2005;135(11):2589–2595. doi: 10.1093/jn/135.11.2589. [DOI] [PubMed] [Google Scholar]
- 56.Raju TN. Breastfeeding is a dynamic biological process--not simply a meal at the breast. Breastfeed Med. 2011;6:257–259. doi: 10.1089/bfm.2011.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Isaacs EB, et al. Impact of breast milk on intelligence quotient, brain size, and white matter development. Pediatr Res. 2010;67(4):357–362. doi: 10.1203/PDR.0b013e3181d026da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kafouri S, et al. Breastfeeding and brain structure in adolescence. Int J Epidemiol. 2013;42(1):150–159. doi: 10.1093/ije/dys172. [DOI] [PubMed] [Google Scholar]
- 59.Deoni SC, et al. Breastfeeding and early white matter development: A cross-sectional study. Neuroimage. 2013;82:77–86. doi: 10.1016/j.neuroimage.2013.05.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Krol KM, Kamboj SK, Curran HV, Grossmann T. Breastfeeding experience differentially impacts recognition of happiness and anger in mothers. Sci Rep. 2014;4:7006. doi: 10.1038/srep07006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Krol KM, Rajhans P, Missana M, Grossmann T. Duration of exclusive breastfeeding is associated with differences in infants’ brain responses to emotional body expressions. Front Behav Neurosci. 2014;8:459. doi: 10.3389/fnbeh.2014.00459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nelson CA, De Haan M. Neural correlates of infants’ visual responsiveness to facial expressions of emotion. Dev Psychobiol. 1996;29(7):577–595. doi: 10.1002/(SICI)1098-2302(199611)29:7<577::AID-DEV3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 63.Kotsoni E, de Haan M, Johnson MH. Categorical perception of facial expressions by 7-month-old infants. Perception. 2001;30(9):1115–1125. doi: 10.1068/p3155. [DOI] [PubMed] [Google Scholar]
- 64.de Haan M, Johnson MH, Halit H. Development of face-sensitive event-related potentials during infancy: A review. Int J Psychophysiol. 2003;51(1):45–58. doi: 10.1016/s0167-8760(03)00152-1. [DOI] [PubMed] [Google Scholar]
- 65.Reynolds GD, Richards JE. Familiarization, attention, and recognition memory in infancy: An event-related potential and cortical source localization study. Dev Psychol. 2005;41(4):598–615. doi: 10.1037/0012-1649.41.4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bertini G, et al. Maternal education and the incidence and duration of breast feeding: A prospective study. J Pediatr Gastroenterol Nutr. 2003;37(4):447–452. doi: 10.1097/00005176-200310000-00009. [DOI] [PubMed] [Google Scholar]
- 67.Scott JA, Binns CW. Factors associated with the initiation and duration of breastfeeding: A review of the literature. Breastfeed Rev. 1999;7(1):5–16. [PubMed] [Google Scholar]
- 68.Algoe SB, Way BM. Evidence for a role of the oxytocin system, indexed by genetic variation in CD38, in the social bonding effects of expressed gratitude. Soc Cogn Affect Neurosci. 2014;9(12):1855–1861. doi: 10.1093/scan/nst182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peltola MJ, Hietanen JK, Forssman L, Leppänen JM. The emergence and stability of the attentional bias to fearful faces in infancy. Infancy. 2013;18(6):905–926. [Google Scholar]
- 70.Marsh AA, Ambady N, Kleck RE. The effects of fear and anger facial expressions on approach- and avoidance-related behaviors. Emotion. 2005;5(1):119–124. doi: 10.1037/1528-3542.5.1.119. [DOI] [PubMed] [Google Scholar]
- 71.Marsh AA, Kozak MN, Ambady N. Accurate identification of fear facial expressions predicts prosocial behavior. Emotion. 2007;7(2):239–251. doi: 10.1037/1528-3542.7.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marsh AA, et al. The influence of oxytocin administration on responses to infant faces and potential moderation by OXTR genotype. Psychopharmacology (Berl) 2012;224(4):469–476. doi: 10.1007/s00213-012-2775-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Davidov M, Zahn-Waxler C, Roth-Hanania R, Knafo A. Concern for others in the first year of life: Theory, evidence, and avenues for research. Child Dev Perspect. 2013;7(2):126–131. [Google Scholar]
- 74.Hoehl S, Wiese L, Striano T. Young infants’ neural processing of objects is affected by eye gaze direction and emotional expression. PLoS One. 2008;3(6):e2389. doi: 10.1371/journal.pone.0002389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Feldman R. Bio-behavioral synchrony: A model for integrating biological and microsocial behavioral processes in the study of parenting. Parent Sci Pract. 2012;12(2-3):154–164. [Google Scholar]
- 76.Kappeler L, Meaney MJ. Epigenetics and parental effects. BioEssays. 2010;32(9):818–827. doi: 10.1002/bies.201000015. [DOI] [PubMed] [Google Scholar]
- 77.Hrdy SB. Mothers and Others: The Evolutionary Origins of Mutual Understanding. Harvard Univ Press; Cambridge, MA: 2009. [Google Scholar]
- 78.Belsky J, et al. Vulnerability genes or plasticity genes? Mol Psychiatry. 2009;14(8):746–754. doi: 10.1038/mp.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Galván A. Insights about adolescent behavior, plasticity, and policy from neuroscience research. Neuron. 2014;83(2):262–265. doi: 10.1016/j.neuron.2014.06.027. [DOI] [PubMed] [Google Scholar]
- 80.Owen SF, et al. Oxytocin enhances hippocampal spike transmission by modulating fast-spiking interneurons. Nature. 2013;500(7463):458–462. doi: 10.1038/nature12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Marlin BJ, Mitre M, D’amour JA, Chao MV, Froemke RC. Oxytocin enables maternal behaviour by balancing cortical inhibition. Nature. 2015;520(7548):499–504. doi: 10.1038/nature14402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fairhurst MT, Löken L, Grossmann T. Physiological and behavioral responses reveal 9-month-old infants’ sensitivity to pleasant touch. Psychol Sci. 2014;25(5):1124–1131. doi: 10.1177/0956797614527114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Feldman R, Rosenthal Z, Eidelman AI. Maternal-preterm skin-to-skin contact enhances child physiologic organization and cognitive control across the first 10 years of life. Biol Psychiatry. 2014;75(1):56–64. doi: 10.1016/j.biopsych.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 84.Gray L, Watt L, Blass EM. Skin-to-skin contact is analgesic in healthy newborns. Pediatrics. 2000;105(1):e14. doi: 10.1542/peds.105.1.e14. [DOI] [PubMed] [Google Scholar]
- 85.Britton JR, Britton HL, Gronwaldt V. Breastfeeding, sensitivity, and attachment. Pediatrics. 2006;118(5):e1436–e1443. doi: 10.1542/peds.2005-2916. [DOI] [PubMed] [Google Scholar]
- 86.Ebner NC, Riediger M, Lindenberger U. FACES—a database of facial expressions in young, middle-aged, and older women and men: Development and validation. Behav Res Methods. 2010;42(1):351–362. doi: 10.3758/BRM.42.1.351. [DOI] [PubMed] [Google Scholar]
- 87.Katz MH. Study Design and Statistical Analysis: A Practical Guide for Clinicians. Cambridge Univ Press; Cambridge, MA: 2006. [Google Scholar]
- 88.Grossmann T, Striano T, Friederici AD. Developmental changes in infants’ processing of happy and angry facial expressions: a neurobehavioral study. Brain Cogn. 2007;64(1):30–41. doi: 10.1016/j.bandc.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 89.Davis MH. The effects of dispositional empathy on emotional reactions and helping: A multidimensional approach. J Pers. 1983;51(2):167–184. [Google Scholar]
- 90.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 91.Sarason IG, Sarason BR, Shearin EN, Pierce EN, Pierce GR. A brief measure of social support: Practical and theoretical implications. J Soc Pers Relat. 1987;4(4):497–510. [Google Scholar]
- 92.Johnston C, Mash EJ. A measure of parenting satisfaction and efficacy. J Clin Child Psychol. 1989;18(2):167–175. [Google Scholar]
- 93.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression: Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150(6):782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]