Significance
Using landscape-scale traffic noise playbacks to create a “phantom road,” we find that noise, apart from other factors present near roads, degrades the value of habitat for migrating songbirds. We found that nearly one third of the bird community avoided the phantom road. For some bird species that remained despite noise exposure, body condition and stopover efficiency (ability to gain body condition over time) decreased compared with control conditions. These findings have broad implications for the conservation of migratory birds and perhaps for other wildlife, because factors driving foraging behavior are similar across animals. For wildlife that remains in loud areas, noise pollution represents an invisible source of habitat degradation.
Keywords: traffic noise pollution, songbird migration, habitat degradation, foraging-vigilance trade-off, perceived predation risk
Abstract
Decades of research demonstrate that roads impact wildlife and suggest traffic noise as a primary cause of population declines near roads. We created a “phantom road” using an array of speakers to apply traffic noise to a roadless landscape, directly testing the effect of noise alone on an entire songbird community during autumn migration. Thirty-one percent of the bird community avoided the phantom road. For individuals that stayed despite the noise, overall body condition decreased by a full SD and some species showed a change in ability to gain body condition when exposed to traffic noise during migratory stopover. We conducted complementary laboratory experiments that implicate foraging-vigilance behavior as one mechanism driving this pattern. Our results suggest that noise degrades habitat that is otherwise suitable, and that the presence of a species does not indicate the absence of an impact.
Human infrastructure shapes animal behaviors, distributions, and communities (1, 2). A meta-analysis of 49 datasets from across the globe found that bird populations decline within 1km of human infrastructure, including roads (2). Observational studies of birds near roads implicate traffic noise as a primary driver of these declines (3). Road ecology research has also shown negative correlations between traffic noise levels and songbird reproduction (4, 5). Birds that produce low frequency songs, likely masked by traffic noise, show the strongest avoidance of roads (6).
There is now substantial evidence that anthropogenic noise has detrimental impacts on a variety of species (3, 7–10). For example, work in natural gas extraction fields has demonstrated that compressor station noise alters songbird breeding distribution and species richness (11–13). However, explicit experiments would help to further rule out other characteristics of infrastructure, such as visual disturbance, collisions, chemical pollution, and edge effects, which might be driving these patterns (3). In addition, although these studies implicate noise as a causal factor in population declines, many individuals remain despite noise exposure (3), but at what cost? Proposed causes of decreased fitness for birds in noise include song masking, interference with mate evaluation, nonrandom distribution of territorial individuals, disruption of parent-chick communication, reduced foraging opportunities, and/or alterations in the foraging/vigilance trade-off (3, 4).
Here we parse the independent role of traffic noise from other aspects of roads experimentally by playing traffic sounds in a roadless area, creating a ‘phantom road’. We focus on birds during migratory stopover, because energy budgets are streamlined; foraging, vigilance, and rest dominate activity (14). To meet the amplified physiological needs of sustained nocturnal migratory flights, birds must increase foraging during periods of stopover while maintaining appropriate vigilance levels (14, 15). Any interference with foraging will decrease stopover efficiency and thus reduce migration speed, a likely surrogate for fitness (14), thereby increasing exposure to significant mortality risks during what can be the most perilous stage of a migratory bird’s life cycle (16). Anthropogenic noise might disrupt the foraging-vigilance tradeoff by acting as a form of perceived predation risk (17, 18) or by reducing sensory awareness via distraction or acoustic masking (3, 19). Using the “phantom road” experimental approach, we previously conducted count surveys of bird distributions at this site, finding a decrease in overall bird numbers of more than 25% (20). We hypothesized that the subset of birds choosing to stay at the site would experience other negative effects of traffic noise, and we predicted that the birds that remained would exhibit lower body condition and reduced ability to increase body condition (i.e., reduced stopover efficiency) in noise.
To test these predictions we used an array of speakers to recreate the soundscape of a ∼0.5 km section of highway along a ridge in southwest Idaho. This approach enabled us to turn the traffic noise on and off throughout fall migration at our phantom road site, and compare it with a nearby quiet control site, creating a modified before-after-control-impact design (Fig. 1). Alternating noise on/off every four days, we sampled a different set of migrants during each block as birds arrived and departed from the stopover site (SI Text). We measured sound levels (hourly level-equivalent, or LEQ) continuously during the season using acoustic recording units placed at mist net locations (Fig. 1A). We compared mist-net capture rate (birds/net/hr) across site (control vs. phantom road) and noise treatment (on vs. off) to investigate whether birds were leaving or staying when exposed to traffic noise (SI Text). Similar to our survey work (20), our best-fitting model indicated that capture rate decreased by 31% during phantom traffic noise playback, demonstrating that anthropogenic noise, independent of other road forces, fundamentally shapes bird distributions. However, 69% of birds remained despite the noise (Table S1, Dataset S1, and SI Text).
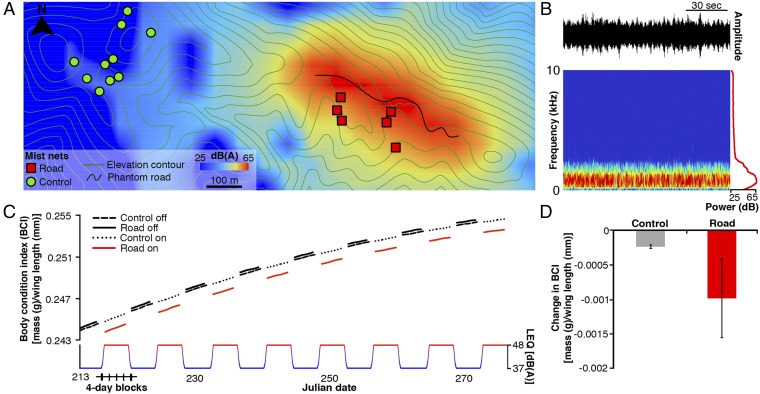
Fig. 1.
Phantom road playback causes songbird body condition decline. (A) Estimated sound levels [dB(A) 1 h LEQ: The level of a constant sound over a specified time period that has the same energy as the actual (unsteady) sound over the same interval] during periods when speakers were on: from August through October 2012–2013 in the Boise Foothills, Idaho. Sound level was modeled using NMSim (Wyle Laboratories) (20). Circles (control) and squares (road) represent capture sites. With the noise on, mean sound levels at the phantom road capture sites increased by 11 dB(A) to 48 dB(A) (SE = 0.3), whereas the control site averaged 2 dB(A) louder with noise on (mean± SE; 41 dB(A) ± 0.2). With noise off, sound levels averaged 39 dB(A) (SE = 0.2) at the control capture sites and 37 dB(A) (SE = 0.3) at the phantom road. Elevation contours are 50 m. (B) A 2-min sample of the phantom road file displayed as an oscillogram, a spectrogram and a power spectrum. (C) Predicted values for body condition index (BCI) as birds add fuel throughout fall migration. Estimates are based on the AIC-best model for BCI for all captures combined, with species as a random intercept. A consistent full SD change in BCI is evident during each noise-on block (pattern of noise on blocks displayed along the x axis) throughout the migratory period. (D) Predicted mean change in BCI at the control and phantom road sites between noise off and noise on periods across the entire study. Error bars represent SE. These differences in BCI (and associated error) are derived from the average of the predictions presented in C.
Table S1.
Sample sizes of the 51 songbird species captured at the Phantom Road and control sites in southwestern Idaho during fall migration in 2012 and 2013
| Species | Latin name | n | Body condition | Stopover efficiency | Capture rate |
| All species | 9,924 | -* | −* | ||
| Ruby-crowned kinglet | Regulus calendula | 2,677 | +* | −* | |
| White-crowned sparrow | Zonotrichia leucophrys | 1,220 | −* | ||
| Dark-eyed junco | Junco hyemalis | 760 | −* | ||
| Yellow-rumped warbler | Setophaga coronata | 582 | |||
| Dusky flycatcher | Empidonax oberholseri | 473 | |||
| Western tanager | Piranga ludoviciana | 418 | − | ||
| Yellow warbler | Setophaga petechia | 409 | |||
| Spotted towhee | Pipilo maculatus | 370 | −* | − | |
| Orange-crowned warbler | Oreothlypis celata | 310 | |||
| MacGillivray's warbler | Geothlypis tolmiei | 305 | −* | − | |
| Warbling vireo | Vireo gilvus | 285 | |||
| Cassin's vireo | Vireo cassinii | 198 | +* | ||
| Hammond's flycatcher | Empidonax hammondii | 174 | |||
| Wilson's warbler | Cardellina pusilla | 166 | |||
| Nashville warbler | Oreothlypis ruficapilla | 165 | |||
| Townsend's solitaire | Myadestes townsendi | 144 | |||
| Townsend's warbler | Setophaga townsendi | 132 | |||
| Chipping sparrow | Spizella passerina | 126 | |||
| American robin | Turdus migratorius | 118 | −* | ||
| Hermit thrush | Catharus guttatus | 113 | |||
| Cassin's finch | Haemorhous cassinii | 112 | −* | +* | −* |
| Swainson’s thrush | Catharus ustulatus | 95 | Species-specific models not built for those with n < 100 | ||
| Mountain chickadee | Poecile gambeli | 89 | |||
| Red-breasted nuthatch | Sitta canadensis | 85 | |||
| Black-headed grosbeak | Pheucticus melanocephalus | 62 | |||
| Lazuli bunting | Passerina amoena | 48 | |||
| Golden-crowned kinglet | Regulus satrapa | 44 | |||
| Brewer’s sparrow | Spizella breweri | 42 | |||
| Pine siskin | Spinus pinus | 37 | |||
| “Western” flycatcher species complex | Empidonax occidentalis/difficilis | 29 | |||
| Fox sparrow | Passerella iliaca | 25 | |||
| House wren | Troglodytes aedon | 22 | |||
| Western wood-pewee | Contopus sordidulus | 19 | |||
| Black-capped chickadee | Poecile atricapillus | 18 | |||
| Golden-crowned sparrow | Zonotrichia atricapilla | 11 | |||
| Brown creeper | Certhia americana | 6 | |||
| Gray flycatcher | Empidonax wrightii | 6 | |||
| Cedar waxwing | Bombycilla cedrorum | 4 | |||
| Willow flycatcher | Empidonax traillii | 4 | |||
| Bullock’s oriole | Icterus bullockii | 3 | |||
| Least flycatcher | Empidonax minimus | 3 | |||
| Lincoln’s sparrow | Melospiza linconii | 3 | |||
| American redstart | Setophaga ruticilla | 2 | |||
| Song sparrow | Melospiza melodia | 2 | |||
| Vesper sparrow | Pooecetes gramineus | 2 | |||
| Cape May warbler | Setophaga tigrina | 1 | |||
| Evening grosbeak | Coccothraustes vespertinus | 1 | |||
| Pacific wren | Troglodytes pacificus | 1 | |||
| Rose-breasted grosbeak | Pheucticus ludovicianus | 1 | |||
| Savannah sparrow | Passerculus sandwichensis | 1 | |||
| Steller’s jay | Cyanocitta stelleri | 1 | |||
Twenty-one species had large enough sample sizes to allow for testing individually (those with n > 100) and showed varying responses to traffic noise at the phantom road study site. Responses to increased noise are indicated as positive (+), negative (−), or no response (blank). We considered a response to noise to exist if a model including either a covariate for dB(A) or the interaction between factors indicating noise on vs. off and control vs. road locations was within ΔAIC < 2 of the best model and 85% confidence intervals excluded zero. An asterisk (*) indicates that 95% confidence intervals excluded zero.
Focusing on birds exposed to a gradient of sound levels, we examined differences in body condition index (BCI) of newly captured birds. BCI is a size-adjusted metric of body mass calculated as mass (g)/natural wing chord (mm). Small changes in BCI represent large differences in condition (21). During migration, high body condition signifies birds with the energy stores needed for long migratory flights (15). The best-fitting model showed that as noise exposure increased, overall BCI of the bird community remaining at the road site decreased (Fig. 1C, Table S1, Datasets S1 and S2, and SI Text). In fact, BCI in noise declined by a full SD compared with the community mean in control conditions. In the absence of noise, BCI of the songbird community at the phantom road site did not differ from the values at the control site, indicating both were suitable stopover locations (Fig. 1C). Models for individual species showed 5 of 21 species significantly decreased BCI in noise. Iterative exposure to noise during the multiple stopovers of saltatory migration may ultimately result in mortality (16) or, in a better case scenario, reduced fitness manifested from slower migration speed (14) which would likely impact fitness and survival in the subsequent life history stage (22).
Because we turned the phantom road off overnight to match typical diel traffic patterns, it is likely that nocturnal migrants (the majority of species in this study; see ref. 23) chose to land at our site when it was quiet, before the phantom road playbacks began in the morning. In effect, diurnally varying traffic noise might function as an ecological trap (24) for migrants. Although staying in traffic noise has a cost, the energetic outlay for individuals to leave a given site might be even greater. Birds with low body condition are less likely to embark on migratory journeys than those in good condition, and depending on the suitability of surrounding habitat, it may not be worth the risk to disperse once landed (25). We cannot differentiate whether the lower BCI we documented in traffic noise is the result of (i) higher body condition birds leaving the population or (ii) birds losing body condition over the duration of noise exposure. We saw both reduced mean body condition and reduced bird numbers, suggesting that at least some birds with the energetic stores to migrate chose to leave the site and escape the costs of remaining in noise (25).
To examine if the birds that remained in noise were suffering reduced ability to add migratory fuel (i.e., increase BCI), we regressed BCI of new captures against time of day to estimate stopover efficiency. Comparing stopover efficiency of individuals between sites provides an essential metric to compare the relative value of stopover habitat (SI Text). The best-fitting model for the entire songbird community included noise intensity level [dB(A)] although the confidence intervals overlapped zero (SI Text). For nine individual species, the best-fitting model included a noise variable, however the confidence intervals overlapped zero for all but 3 of these species (Table S1).
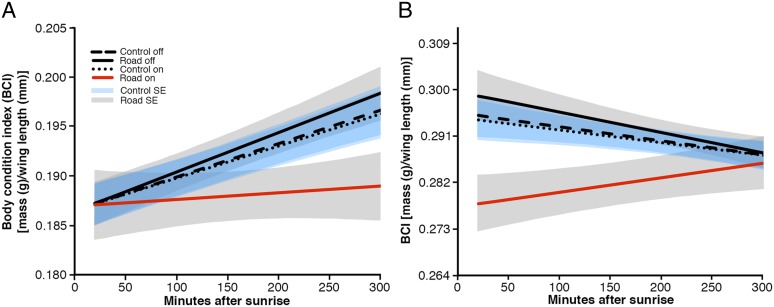
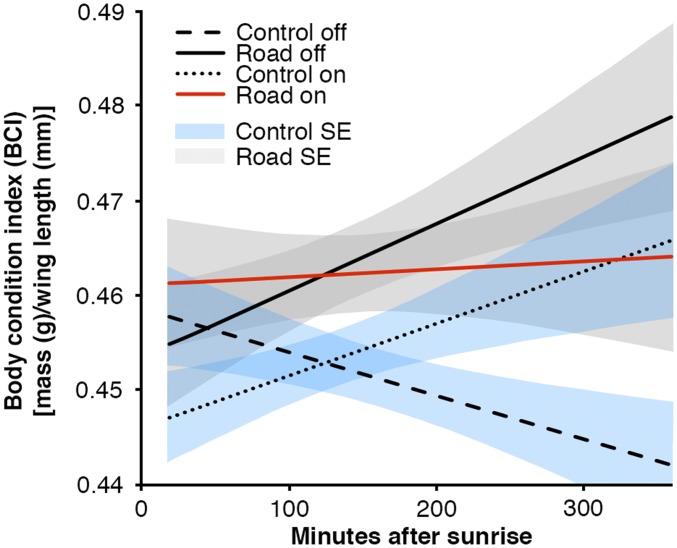
For MacGillivray’s warblers, the best-fitting model showed that stopover efficiency substantially decreased with increasing decibel levels. MacGillivray’s warblers did not show reduced capture rates in noise, and were the species that showed the strongest negative responses for both BCI and stopover efficiency, indicating that individuals stayed but did poorly in noise (Fig. 2A and Tables S1 and S2). In contrast, Cassin’s finches had significantly increased stopover efficiency in noise and a decreased capture rate (Fig. 2B and Tables S1 and S2). This increase in stopover efficiency might reflect decreased competition for food resources in noise. Although stopover efficiency was increased in noise (Fig. 2B), Cassin’s finches showed lower initial BCI in traffic noise (Fig. 2B), perhaps indicating individuals with higher BCI left the site during noise exposure. The best models for spotted towhees showed a reduced capture rate and also indicated different stopover efficiencies between on-off periods at the control and road sites with efficiency being negatively affected by noise along the phantom road (Fig. S1 and Tables S1 and S2).
Fig. 2.
Stopover efficiency is altered in noise. Predicted values for stopover efficiency for MacGillivray’s warblers (A) and Cassin’s finches (B). Estimates were made using average day of season using the AIC-best model for BCI for all captures combined. Values were predicted by inputting average dB(A) levels for each site. Values are shown for the control site noise off [avg. 42 dB(A)], control site noise on [43 dB(A)], phantom site noise off [40 dB(A)], and phantom site noise on [51 dB(A)]. Blue shading represents SE for the control site whereas gray shading represents SE for the phantom road.
Table S2.
Number of parameters (k), Bias-corrected Akaike’s Information Criterion value (AIC), the difference between a given model and the model with the lowest AIC value (ΔAIC), and the AIC weight of models for white-crowned sparrow laboratory foraging and vigilance experiments
| Model | k | AIC | ∆AIC | wi |
| Foraging: head down duration (s) | ||||
| dB | 2 | −54.134 | 0.00 | 0.51 |
| dB+trial+time | 4 | −52.2161 | 1.92 | 0.20 |
| dB+day | 3 | −52.1663 | 1.97 | 0.19 |
| dB+day+trial+time | 5 | −50.4667 | 3.67 | 0.08 |
| null | 2 | −47.5941 | 6.54 | 0.02 |
| Vigilance: head up rate (head lifts per s) | ||||
| dB+day | 3 | −23.543 | 0.00 | 0.70 |
| null | 2 | −20.4975 | 3.05 | 0.15 |
| dB+day+trial+time | 5 | −19.7983 | 3.74 | 0.11 |
| dB | 2 | −17.2415 | 6.30 | 0.03 |
| dB+trial+time | 4 | −13.7057 | 9.84 | 0.01 |
| Foraging bout | ||||
| dB | 2 | 292.0977 | 0.00 | 0.70 |
| dB+day | 3 | 293.9805 | 1.88 | 0.27 |
| dB+day+trial+time | 5 | 295.577 | 3.48 | 0.12 |
| null | 2 | 296.9985 | 4.90 | 0.06 |
| dB+day+trial+time | 5 | 297.4884 | 5.39 | 0.05 |
Fig. S1.
Predicted values from the AIC-best model of spotted towhee stopover efficiency for the control site when the road was off [dB(A) 42], control site with road on [dB(A) 43], phantom road with noise turned off [dB(A) 40], and the phantom road with the noise on [dB(A) 51]. Blue shading represents SE for the control site, whereas gray shading represents SE for the phantom road.
It seems that for species impacted by noise, different strategies exist for managing the consequences, which might be based on differences in life history traits such as territoriality during stopover, migratory strategy, or flocking behavior. Our species-specific results show that birds may stay and incur a cost of remaining in noise (e.g., MacGillivray’s warblers), or choose to leave (e.g., Cassin’s finches). Leaving the noisy area may allow some species to avoid the costs of noise or a species may still experience the impacts of noise despite some individuals leaving (e.g., spotted towhees). Together, our observations of overall changes in the BCI of the entire bird community and of several individual species, as well as the changes in stopover efficiency of spotted towhee and MacGillivray’s warbler, demonstrate that addition of traffic noise alone, without the other variables associated with actual roadways, can significantly decrease the value of a stopover site.
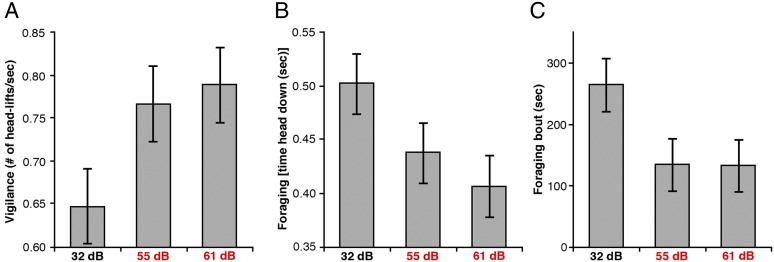
In support of our field results, we conducted a controlled laboratory study to test whether traffic noise alters the foraging-vigilance tradeoff in songbirds and could thus mechanistically underpin our field data (SI Text). We focused on the second most common species from our field study, white-crowned sparrow (Zonotrichia leucophrys), a species that also decreased BCI in noise, to investigate the reduction in foraging and increase in vigilance implied by our community-wide body condition analysis. We quantified head-down duration (i.e., foraging) and head-up rate (i.e., vigilance), because these are known measures of avian visual vigilance that change when auditory surveillance is limited and that correlate with food intake and ability to detect predator attacks (26). We also measured feeding duration (no. seconds per 8-min trial spent feeding) to quantify overall feeding bout duration. Using the same playback file as our field experiment, we played 61 dB(A) and 55 dB(A) traffic noise treatments, plus a silent control track [32 dB(A)] to foraging sparrows (n = 20). White-crowned sparrows decreased foraging by ∼8%, increased vigilance levels by ∼21%, and decreased feeding duration by ∼30% when exposed to traffic noise [61 dB(A); Fig. 3, Movies S1 and S2, and Dataset S3]. Vigilance behavior of individuals did not change based on the number of trials experienced, indicating birds did not habituate to the noise (SI Text and Table S2). During energetically demanding periods in a bird’s life, increasing vigilance can reduce survival because of increased starvation risk (27). In contrast to song masking, which can be partially overcome by frequency shifting (28), release from masking is not possible for auditory cues necessary for aural vigilance (7). With limited auditory information, animals must resort to other methods such as visual scans to compensate for the increase in perceived predation risk, perhaps driven by masking of communication calls and predator-generated sounds (26, 29).
Fig. 3.
The foraging/vigilance trade-off is altered in noise. White-crowned sparrows foraging in traffic noise at 61 and 55 dB(A) had reduced foraging rates (A), increased vigilance (B), and decreased foraging bout duration (C) compared with trials in ambient conditions [32 dB(A)]. Data are means ± SE. [Mean head up rate (head lifts/s) for 61 dB(A) = 0.79 ± 0.06, 55 dB(A) = 0.77 ± 0.05, 32 dB(A) = 0.65 ± 0.05. Mean head down duration (s): 61 dB(A) = 0.41 ± 0.03, 55 dB(A) = 0.44 ± 0.04, 32 dB(A) = 0.50 ± 0.04. Mean foraging bout duration (s): 61 dB(A) = 159.25 ± 28.0 55 dB(A) = 147 ± 32.5 32 dB(A) = 228 ± 33.7]. Birds showed more head lifts/s (β = 0.005 ± 0.002), decreased the amount of time spent with their heads down searching for seeds (β = −0.003 ± 0.001), and decreased total feeding duration (β = −4.589 ± 1.944; Movies S1 and S2) during noise playback compared with ambient conditions.
Our behavioral investigations in the laboratory offer compelling evidence that the body condition changes measured in the field were due at least in part to a change in foraging and vigilance behavior, but our field results could be due to a combination of factors that also deserve consideration. For example, noise might also increase physiological stress levels (ref. 30, but see ref. 31) that could cause additional declines in body condition. However, we view it as unlikely that noise can cause a stress response independent of a change in behavior. In addition, noise might indirectly change foraging rates through alterations in prey search time, sleep, or territoriality. For instance, our phantom road might have disrupted foraging behavior by reducing the acoustic detectability of insect prey (32) or reducing insect numbers. We did not test for changes in insect abundance or distribution, but because we found noise impacts on a mixed community of both frugivorous and insectivorous birds (Table S1 and Dataset S1), it seems unlikely that altered insect numbers explain a significant component of the observed patterns. Effects were consistent between the 4-d noise-on blocks throughout migration, despite documented seasonal variation in fruit and arthropod availability at the site (33), so it is more likely that changes in bird behavior drove these responses. Our experimental design was not able to determine whether noise disrupts territoriality or dominance hierarchies during stopover. However, both territorial and nonterritorial species showed negative effects of noise (23) (Table S1 and Datasets S1 and S4). We expect that a subset of these indirect effects plus the behavioral changes quantified in the laboratory contributed to the body condition declines seen in our field experiment. Because provisioning is a constant requirement for birds throughout the year, other effects of noise that occur outside of migration (e.g., refs. 4 and 5) would be in addition to, rather than instead of, the impacts we document here.
Previous work that failed to find a change in animal distributions near roads or other infrastructure has assumed a lack of negative impacts from loud human activities (2, 3). Our results demonstrate that individuals may remain in an area with high levels of noise yet suffer significant costs. We found that different species chose different strategies: to either leave noisy areas, or stay and perhaps incur the costs of noise (Fig. 1, Fig. S2, and Table S1). We exposed the bird community at our phantom road to sound levels similar to some suburban neighborhoods [∼55 dB(A) hourly LEQ] (34). Many protected areas and high-value habitats are currently exposed to these levels, and would benefit from noise relief measures (35, 36). The impact of noise reaches far beyond the physical footprint of human infrastructure. Unlike other aspects of roads, noise impacts can be minimized without removing the road itself. Substrate alteration and speed limit reduction on existing roads can significantly lower decibel levels (34).
Fig. S2.
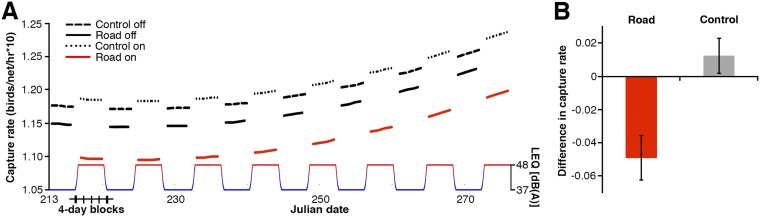
(A) Model output predictions for capture rates of birds mist netted at the control and phantom road sites during 2012 and 2013. Capture rate at the phantom road site was significantly lower when the noise was on, whereas capture rates at the control site did not change significantly in response to noise. (B) Mean change in capture rate at the control and phantom sites with noise on. Error bars represent SE. These differences in capture rate (and associated error) are derived from the average of the predictions presented in A.
Our results reveal the need for attention to noise impacts beyond distributional shifts (3). For individuals that remain in areas disturbed by loud human activities, noise pollution represents an invisible source of habitat degradation that has been largely ignored: Traffic noise degrades habitat value but leaves no physical signs of change. Stopover habitat loss and degradation have been identified as major contributing factors to migratory songbird declines worldwide (37, 38). Migrants are exposed to an unknown risk landscape at stopover sites and must therefore rely heavily on increased vigilance to compensate (39–41). Unlike resident species, successful conservation of migratory species requires protection of habitats in breeding, wintering, and stopover locations (41). In addition, reduction in condition or delay in migration could have carry-over effects into the overwintering or breeding seasons (42). Further understanding of anthropogenic noise’s impact on body condition is key, as it is an important predictor of fitness across taxa and life stage (22). When managing natural systems, we should ensure that the habitat we protect remains of high quality, including the quality of the acoustic environment.
All birds caught during this project were mist-netted and banded under the Intermountain Bird Observatory’s federal permit (22929) and Idaho Department of Fish and Game permit (764–13-000039). All experiments were approved by Boise State University’s Institutional Animal Care and Use Committee (006-AC12-007 and 006-AC13-002).
SI Text
SI Methods
Field Experiment Materials and Methods.
Study site and data collection.
We conducted our study at two adjacent sites in southwestern Idaho (43°36’N, 116°05’W) during the 2012 and 2013 fall bird migration seasons. Both study sites are located on the Idaho Fish and Game’s Boise River Wildlife Management Area along the southernmost edge of the Boise Foothills (Fig. 1). Our control site was located on Lucky Peak and is the banding site for the Intermountain Bird Observatory’s long-term fall migration study; it has operated for the last 17 y. The second site was newly pioneered in 2012 for the purpose of this experimental study and is located 0.95 km east of Lucky Peak. Both the experimental and control sites are characterized by a habitat mosaic of (i) mountain shrubland: dominated by bittercherry (P. emarginata) with a mix of other shrubs including chokecherry (Prunus virginiana) and Scouler’s willow (Salix scouleriana), (ii) conifer forest: dominated by Douglas-fir (Pseudotsuga menziesii) with a mountain ninebark (Physocarpus malvaceus) understory, and (iii) shrub steppe: consisting of mountain big sagebrush (Artemisia tridentata vaseyana), bitterbrush (Purshia tridentata), and rabbitbrush (Chrysothamnus sp.) with an understory of various grasses and forbs (see ref. 43 for more in-depth site information).
Birds were netted at both sites between 19 August to 9 October in 2012, and 2 August to 8 October in 2013. We captured birds using Ecotone brand mist nets (12 × 2.6 m, 32-mm mesh) placed in the mountain shrubland habitat in locations that would maximize capture rates (44). The control site at Lucky Peak had 10 nets, whereas the phantom road site had 6. We placed nets so that habitat and shrub height were similar between sites and net locations remained the same on all days and between years. We began netting at sunrise and continued for 5 h, except occasions with heavy precipitation or high winds. Sunrise ranged from 0635 at the start of the season to 0755 at the end of the season. We cleared nets at 20–30 min intervals, depending on weather. We operated nets on the first, second, and fourth days of every 4-d interval, and netted every day at the control site. For analyses, we included data only from days that nets were operating at both sites (i.e., we only used data from 3 d out of every 4-d block).
Once captured, we returned birds to the banding station where they were fitted with standard, individually numbered aluminum USGS leg bands. We identified each bird to species, and aged and sexed individuals based on Pyle (45). We recorded the date and time of capture to the nearest 10 min and collected additional data on each bird following the standard protocol of the Intermountain Bird Observatory’s long-term study (46). We also recorded the mass in grams, and unflattened wing chord of each bird to the nearest millimeter.
Phantom road.
At the phantom road site we placed 15 pairs of speakers in Douglas-fir trees at a height of 4 m from the crest of the ridge, at the interface between forest and mountain shrubland habitat. We amplified the speakers [Dayton Audio RPH16 Round 16’ PA Horns paired with MCM Electronics 40-W midrange compression drivers; +5 dB(A), 400–3,000 Hz] with Parts Express 2 W × 2 channel, 4-ohm, Class D amplifiers and played back sound files (MP3, 128 kbps) using Olympus LS-7 and Roland R-05 audio players. We powered amplifiers and audio players with arrays of LiFePO4 (Batteryspace) batteries housed in waterproof plastic containers. One speaker of each pair pointed north into the conifer forest while the other faced south into the mountain shrubland. We spaced the speaker pairs at ∼30-m intervals along the ridge to create a “line source” of sound that replicated an actual highway. The geometry of a sound source can have profound impacts on the scale of noise exposure. Point sources (e.g., generators, gas-compressor stations, a single car) lose sound energy at ∼6 dB per doubling of distances, whereas line sources (e.g., a busy roadway or train) fall off at ∼3 dB per doubling of distance.
We played traffic noise recorded within Glacier National Park. To create the playback file, we combined files of 12 individual cars recorded at known distances, decibel levels and speeds. We chose car pass-by events based on clarity of recording, decibel level and speed. We created a 1 min file of 12 car pass-by events and repeated this file without shuffling. Because any possible habituation would have only reduced our ability to detect changes, we see this as a minor concern. Our playback file therefore contained 720 pass-by events per hour of cars traveling at ∼45 miles per hour, traffic levels and speeds found along roads in some of the most visited protected areas globally. Our playback file further simulated the frequency profile of typical traffic noise with most of the energy of the noise between 0 and 3 kHz with a peak around 1 kHz. We elected to not reproduce the very low frequencies in traffic noise (<300 Hz) due to the logistical and resource constraints of creating these long wavelengths (sensu ref. 47). As these frequencies are either poorly or not perceived by songbirds (48) we see this as an acceptable limitation.
We set the speaker levels so that the 1 min LEQ reading was ∼55 dB(A) (±3 dB) at 50 m from the phantom road. LEQ values are the level of a constant sound over a specified time period that has the same energy of the actual, fluctuating energy over that same time period (35). We played MP3 files of traffic noise in four-day blocks, alternating with four days without noise playback. During noise-on days, noise played from 0430 until 2100 local time, with a 30-min fade-on and fade-off period to approximate typical traffic flow patterns and to avoid startling birds. During noise playback, noise levels were 11 dB(A) higher at the phantom road site and 1 dB(A) higher at the control site. The nearest drivable dirt path was 750 m from the phantom road site, and the nearest paved road was 4 km away. The drivable path near our site was a gated, dead end road used to provide access to the study site for the research team.
We chose 4-d long noise-on and noise-off blocks because almost all species that use our site during autumn stopover remain for fewer than 8 d on average (46). Thus, each noise-on/noise-off block was likely independent as individuals left during the course of a block, no individual bird was likely to be present for more than one noise-on or noise-off period. By running models using only newly captured birds, we further ensured the independence of each block in our analyses.
To measure dB(A) at our site, we used 6,744 h of recordings from eight acoustic recording units that ran simultaneously during the 2012 and 2013 fall migration seasons. This amount of continuous recording is, to date, the most thorough quantification of the acoustic environment to be undertaken. Using a custom program (Damon Joyce, NPS, AUDIO2NVSPL) we converted the MP3 recordings into an hourly sound pressure level format. We then converted those values to hourly LEQ values in dB(A) using another custom program (Damon Joyce, NPS, Acoustic Monitoring Toolbox). We averaged the hourly background LEQ during noise-on hours (05.00 through to 21.00) across the noise-on and noise-off blocks, creating separate noise-on and noise-off LEQs.
We placed four recording units at each site, and ensured that each net was within <15 m of a recording unit. Because each recording unit measures dB levels within an ∼50 m radius, we placed one recording unit equidistant between nets that were <20 m apart to measure the dB(A) of the net area. Nets that shared a recording unit were assigned the same dB(A) values for analyses.
We chose to use the hours of 5.00 through 21.00 for two reasons: (i) because we only played traffic noise during those hours, and our goal was to measure the differences between sites during noise on and noise off blocks, and (ii) the design of the wind screens used to protect the recorders provided shelter for nocturnal tree crickets. During night hours, tree crickets sang from perches on top of our MP3 recording units, creating erroneous LEQs. Therefore, our nighttime recordings did not accurately represent the actual background sound levels of our site and could not be used for analysis. The custom programs used only allowed for full hours to be analyzed, therefore we included the full hour of 21.00 although this included the ramp down period.
Analysis.
We analyzed all data using the function lmer (49) in Program R (R Development Core Team 2011). We used data from 51 bird species (9,924 individuals) to build three sets of models for (i) BCI, (ii) stopover efficiency, and (iii) capture rate, including combinations of variables for dB(A), minute after sunrise, noise, site, and linear and quadratic effects of day, plus random intercepts for year. We also built intercept-only models. For all three model sets we ran competing models using noise as either a continuous variable [dB(A)] or a binary (on/off) variable (noise*site interaction models). We compared these noise models because they represent two separate hypotheses. Models including dB(A) test the hypothesis that birds show a functional response to noise and respond in a gradient to increasing noise intensity. Models using an interaction term of noise*site test the hypothesis that the presence of noise at the phantom road site, regardless of intensity, determines the response. In other words, these competing models were used to determine whether birds were responding to noise on a fine or coarse scale. We ranked and compared the models using Akaike’s Information Criterion, (ref. 50; Dataset S1). We considered covariates to be useful for inference if their 85% confidence intervals excluded zero. We used 85% confidence intervals instead of the traditional 95% because they are more appropriate when selecting models using AIC (51). To aid in interpretation, however, we also indicate where 95% confidence intervals exclude zero (Table S1). We used species and the nearest acoustic recording unit as random variables. It was important to use the nearest recording unit as a random variable because some mist nets in our study were paired, and therefore shared one recording unit. Including recording unit as a random variable controlled for repeated sampling of decibel levels at some nets. For this study we were interested in the avian community as a whole, but also analyzed species with large enough sample sizes (those with n > 100; Table S1 and Dataset S2). We visually inspected plots of residuals for the AIC-best model for each analysis and all inspected models appeared to conform to assumptions of normality and heteroskedasticity.
Capture rate analysis.
We calculated the capture rate as the number of birds caught/net/hour. This index is slightly different from an index of birds caught/‘net hour’ that is traditionally used in mist netting literature to measure the capture rate at a netting station. This resulted in a similar, but smaller magnitude value for capture rate than is typically reported in mist netting studies. We used this alternative capture rate calculation because we analyzed our capture data on a per-net basis (rather than per-station) as each net had a different dB(A) value. We considered capture rate an accurate index of migrant relative abundance at each site, based on past research and previous comparisons of netting and count surveys during migration at this and other sites (43, 52). Because we controlled for the habitat around mist nets at the control and phantom road sites in this study, we feel confident that capture rate is a valid comparison between sites. The best-fit model for capture rate included an interaction between noise and site (interaction β = -6.09e-03 ± 1.70e-03).
Body condition index analysis.
We used Body condition Index (hereafter “BCI”: calculated as the birds’ mass/wing chord) of newly captured birds as a proxy for the energetic condition of migrants at our site. BCI and fat scores were highly correlated in the migrating songbird community we studied, however fat scores were measured on a much coarser, categorical scale. The same models for fat (measured on a 5 point scale) showed similar trends compared with our BCI models, however the confidence intervals overlapped zero, likely based on the broad variation of fat stores characterized by each fat score value. Also, the variable for fat score is a factor rather than a continuous variable, which decreased the power of the models. Therefore, we used BCI for our model analysis as it offered a finer index of migrant condition. Additionally, although fat makes up the most substantial proportion of energy stores used by migrants during nocturnal flights, protein and hydration levels also play a role in determining a bird’s potential flight distance (53). Therefore, body condition is a useful measure that incorporates condition indices such as fat and pectoral muscle scores that are easily quantified through external observations, as well as less-visible accumulations of protein elsewhere in the body. Increased mass during migration has potential to be detrimental at high levels, however evidence shows that carrying fuel loads is likely cheaper than previously predicted so that maximum flight range is not necessarily lowered by normal levels of fat storage (54). In trans-Saharan migrants crossing an ecological barrier, Sedge Warblers (Acrocephalus schoenobaenus) were found to have a reduced ability to evade predators at extreme fat loads (>60% lean body mass; ref. 55). In species not facing an extreme ecological barrier (e.g., desert or ocean crossings), such as the community we studied at Lucky Peak, birds’ risk of predation has not been found to increase within normal body mass ranges (56). In fact, Dierschke found that lighter birds are more likely to be captured by predators than heavier individuals (57). For the purposes of our analyses and interpretation, we assume that birds in our study were not carrying above-optimal fat stores, since birds at our site do not accumulate fat scores of such magnitude, and migrant passerines are known to adaptively regulate their fat stores to balance the risks between starvation and predation (58, 59). Our best-fitting model included a continuous covariate for dB(A), showing that as noise exposure increased, overall BCI of birds remaining at the road site decreased [dB(A) = -1.08e-04 ± 4.76e-05; Fig. 1C].
Stopover efficiency analysis.
We calculated the stopover efficiency of species at our site using linear mixed-effects models with Gaussian distributions and identity links. By regressing the body condition index of each newly captured bird against capture time (calculated as minute after sunrise), we quantified migrants’ ability to gain body condition throughout the day, i.e., their stopover efficiency (21, 46, 60, 61). In our study design, the noise off days at the phantom road site acted as an internal control, while the data collected at the control site allowed further control for weather and migration variability. Using regression based on new captures to calculate condition gain is thought to be a less-biased method of calculating gain during stopover (21). In addition to a problem of small sample sizes, the historic method of using the mass of a single individual at multiple captures may be biased because an individual’s condition influences its probability of recapture (21). For community-wide analysis, we included species as both a random intercept and random slope in our models. The best-fitting model for community stopover efficiency, included an interaction between dB(A) and minute after sunrise but the confidence intervals overlapped zero (interaction β = -4.94e-07 ± 4.48e-07).
Species-specific models.
We built linear mixed effect models for all 51 species combined, then tested the same set of models on 21 species individually (those with n > 100; Table S1 and Dataset S2). Seven species analyzed individually showed decreased stopover efficiency or BCI in noise (Table S1).
Laboratory Materials and Methods.
Captive sparrows.
We mist-netted 20 Gambel’s white-crowned sparrows (Zonotrichia leucophrys gambelii) from Deer Flat National Wildlife Refuge in southwestern Idaho under the Idaho Department of Fish and Game permit (764–13-000039), and approved by Boise State University IACUC (006-AC12-007). We brought birds into the laboratory in groups of five, captured between March 16 and April 16, 2013. We individually marked each bird with an aluminum USGS band, under federal banding permit (22929). While birds were in captivity we used strips of plastic tape wrapped around their federal bands to temporarily color mark individuals. The plastic was removed before release. Adjacent individual cages allowed birds to remain within sight and sound of their flock mates while held in the Sensory Ecology Lab animal housing room at Boise State University. Birds had access to water ad libitum at all times, including during experiments, and we provided a seed mix ad libitum in their individual cages when foraging trials or pretrial acclimations were not underway. The temperature-controlled housing room was set to 19 °C and a 12.2:11.8 light:dark cycle with 30 min twilights to match average outdoor conditions at the time of experiments. We kept birds an average of 5 d, and none were held longer than 7 d; they were released at the location of their original capture.
Experimental set-up.
We conducted experiments inside the flight room in the Sensory Ecology Lab at Boise State University. The flight room is a 38-m2 room lined with anechoic foam and has a background sound level of ∼32 dB(A). In the center of the room we constructed a 1.5 × 2.5 m foraging arena covered in 2 cm of medium-grain sand. White Millet (Panicum miliaceum) seeds scattered evenly over the sand provided homogenous foraging conditions during trials. We maintained a high density of millet seeds (∼100 g) in the foraging arena so that the supply available to birds during a given trial was not depletable, allowing them to forage at their maximal rate without influencing search time. White-crowned sparrows are omnivorous and feed on bitter cherries as well as grains at our field study site (33), thus, although we only measured their behavior while feeding on seeds, we in fact measured the vigilance of a partially frugivorous species from our field study. We placed three natural branches as perches at the edge of the foraging arena at a height of ∼0.75 m. After initial capture, we placed birds in the flight room and allowed them to acclimate for a full day with access to seed in the foraging arena. We ensured that birds had learned to feed on the arena, access water dishes, and sit on the perches before we began trials.
During experiments, we allowed birds to feed in their individual cages for 30 min each morning before trials, and then removed access to food 1.5 h before the start of an individual’s first trial of the day. During this period without food, birds spent 50 min in their individual cages, after which we moved a bird from its cage into the flight room and allowed it 40 additional minutes to acclimate to the room. During this time the foraging arena was covered to prevent access to seed. At the beginning of each trial, we entered the room to remove the cover over the area and to start video recording. We then returned to an adjacent room where we could observe the trial on a live video feed and control noise playback.
Speakers (Bird speakers; frequency response 70 Hz - 25 kHz; ± 3 dB) at opposite ends of the flight room broadcast sound evenly [±2 dB(A)] over the foraging arena during noise treatment trials. To allow for comparison between these results and the field experiment, we used the same sound files and matched dB(A) settings for both experiments. We used the phantom road file to create 8-min-long sound files, adjusting the files to match the required dB(A) levels.
Behavioral observations.
Foraging trials lasted for 8 min and all bird activity and foraging behavior was video recorded during the experimental period. We used an HD video camera (Sony HDV 1080i and Canon XA10 models) to record foraging behavior of individuals during trials. During each foraging trial we played one of three randomly-selected noise treatments: 61 dB(A) traffic noise, 55 dB(A) traffic noise or a silent control track, for 8 min. The sound files used a 5 second fade-in at the beginning and end of the traffic noise so that birds were not startled by the onset of the noise treatment. At the end of the 8 min we stopped the video recording and covered the foraging arena.
We randomly selected treatment order and ran a focal bird through the three, 8-min noise trials. We covered food for 40 min between foraging trials to ensure birds were hungry at the start of the next experiment. By covering the foraging arena while allowing birds to remain in the flight room, we eliminated the need to capture and handle birds after each trial and greatly reduced their apparent stress levels.
During preliminary trials, we found that all birds began investigating the covered foraging arena in search of food between 25–35 min after they had last eaten. We therefore chose a 40 min wait time to make sure that all birds were ready to forage at the start of the next trial. We chose an 8-min trial duration because birds did not forage for the entire 8 min during preliminary tests, so we assumed that they were satiated before the end of the trial and would therefore be equally hungry at the start of each subsequent trial (i.e., the birds did not accumulate hunger throughout the day). By observing the sparrows’ naturally-preferred foraging schedule, we were able create an experimental schedule that allowed for the most trials to be conducted in one day without prolonged food deprivation.
Analysis.
We recorded several foraging and vigilance variables based on analysis of the 30-fps HD videos for each trial. By playing back the videos frame by frame we were able to track the timing of each movement during a foraging session. For each trial we analyzed foraging-vigilance behavior during a 30-s foraging session. We defined the start of a foraging session as 5 consecutive pecks separated by less than 10 s (26) and selected the first 30-s foraging session within each trial to ensure equal hunger levels between birds. We recorded duration of head-up and down periods, and head up and down rate for each 30-s foraging session (26). We defined head-up as when the sparrow’s head was above the level of its back and head-down when the head was below the level of the back. We used head up rate and mean duration of head down period during trials to quantify the sparrows’ vigilance during foraging sessions. For each trial we also recorded the total amount of time birds spent feeding (using the same criteria of 5 consecutive pecks separated by less than 10 s) during the 8 min to calculate an overall “feeding duration.”
We built three sets of linear mixed-effects models with Gaussian distributions and identity links for (i) head up rate (head lifts/sec), (ii) mean head down duration (sec), and (iii) feeding duration, including combinations of variables for dB(A), time of day, trial number, and day, where trial number indicated the number of trials an individual sparrow had experienced. We also built intercept-only models. We ranked and compared the models using Akaike’s Information Criterion (ref. 50; Table S2 and Fig. 3). We used individual as a random variable, and considered covariates to be useful for inference if their 85% confidence intervals excluded zero. We used 85% confidence intervals instead of the traditional 95% because they are more appropriate when selecting models using AIC (51). However, note that inference from the laboratory study would have been the same had we used 95% confidence intervals.
SI Discussion: Species-Specific Results
Species-specific models indicate varied strategies in the migrant community in response to noise. Of those with changes in either of the BCI measures or capture rate, the following patterns emerged: (i) Three species had lower BCI in noise, but did not leave the site (i.e., did not exhibit a lower capture rate in noise). (ii) One species decreased in abundance in traffic noise but individuals that remained did not show reduced BCI or stopover efficiency. (iii) Two species showed reduced BCI in noise, and reduced capture rate when noise was on. (iv) One species, Cassin’s vireo (Vireo cassinii), had increased BCI in noise, and did not leave the site. (v) In one species, ruby-crowned kinglet, some individuals left the site, while the ones that remained showed increases in BCI. (vi) Cassin’s finches (Carpodacus cassinii) had lower overall BCI, but compensated by increasing their stopover efficiency. Of the 21 species examined, 9 showed a negative response to noise.
Although, at first glance, it seems difficult to explain these varied responses, a few dynamics might be at play. First, variation in species’ behavior likely affects the “choice” to stay in the noise or leave; i.e., the cost of searching for another stopover area might be perceived as higher (or lower) than remaining in a suboptimal site (15). Second, if some sensitive individuals depart and food availability in the noisy area remains the same, this could make foraging more efficient for the remaining birds. Thus, individuals of some species that remain in the noise might be able to make up for their increased vigilance by easier prey acquisition whereas foraging behavior of other species might not allow for increased efficiencies to offset costs to vigilance. For example, two species showed a positive response in body condition (Cassin’s vireo, ruby-crowned kinglet). This result might be a manifestation of birds with high fat stores deciding to remain in noise while low-condition birds decide to leave, perhaps a consequence of a difference in foraging requirements between lean and fat birds (62). Fat birds with enough energy stores to migrate the next night may not need to forage much or at all during the day, thus negating costs to their foraging-vigilance tradeoff caused by noise. Lean birds conversely rely heavily on foraging through the day to replenish energy stores (15). The cost of remaining in noise and reducing foraging may therefore be too great, causing lean birds to vacate the phantom road site.
It is also possible that some species’ foraging strategy enables them to remain more vigilant while foraging. For example, Cassin’s vireos hunt larger prey using a sit-and-wait strategy (33), meaning that they may be able to more easily remain vigilant between large meals while searching for their next food item. One species showed increased stopover efficiency (Cassin’s finch), which suggests that at least some individuals of a species were able to take advantage of the reduced abundance of other migratory birds in noise.
Spotted towhees present an example of the varied strategies used by the bird community in response to noise. Our control site has historically been a location where towhees lose mass (33), and our analysis supports this, showing that stopover efficiency at the control site is negative (Fig. S1). In contrast, without noise, our phantom road site appears to be a high-value stopover site for towhees, where stopover efficiency is high. Our capture rate and stopover efficiency results seem to indicate that high-quality birds might prefer the phantom road location but when noise is on they move to the control site, leaving lower quality birds in noise. Regardless of the underlying mechanism, noise reduced the stopover efficiency of towhees along the phantom road.
For the entire community, it is likely that sensitive individuals were the first to leave in response to noise disturbance, whereas more tolerant individuals remained (ref. 63, reviewed in ref. 3). Only exploration of food availability, foraging strategies, and predator-prey dynamics with and without noise would allow us to further elucidate the different responses of migratory birds to experimental noise exposure.
Supplementary Material
Acknowledgments
We thank Kurt Fristrup for input on study design and commenting on the manuscript. We thank Jennifer Forbey, Clint Francis, Julie Heath, and Nick Fuzessery for providing comments on the manuscript. Krista Muller of the Idaho Department of Fish and Game Boise River Wildlife Management Area provided support and access to our study site. We thank Brian Leavell, Dan Mennitt, Tate Mason, David Anderson, Alexis Billings, Jarrod Zacher, Adam Keener, Randy Nuxoll, and the Intermountain Bird Observatory. We especially thank Elizeth Cinto Mejía and Mitchell Levenhagen, Andrea Ball, Luke Eberhart-Phillips, Michael Fuss, Callie Gesmundo, Greg Kaltenecker, Lindsey Lockwood, Jesus Lopez Angulo, Garrett MacDonald, Krystie Miner, Zoe Mroz, Zak Pohlen, Jessica Pollock, Eric Ripma, Jeff Roelke, Teague Scott, Micah Scholer, Jacob Shorty, Rose Swift, Elizabeth Urban, Benjamin Wright, C. R. Jepsen, and T. Dillard, who helped to develop, implement, and maintain the Phantom Road. This study was funded by the Natural Sounds and Night Skies Division of the National Park Service (CESUcH8R07060001). Boise State University Office of Research and Department of Biological Sciences, and the National Science Foundation (CNH1414171) provided additional funding. Addison Mohler and the Deer Flat National Wildlife Refuge provided support for our laboratory project.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 11995.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1504710112/-/DCSupplemental.
References
- 1.Fahrig L, Rytwinski T. Effects of roads on animal abundance: An empirical review and synthesis. Ecol Soc. 2009;14(1):21. [Google Scholar]
- 2.Benítez-lópez A, Alkemade R, Verweij PA. The impacts of roads and other infrastructure on mammal and bird populations: A meta-analysis. Biol Conserv. 2010;143(6):1307–1316. [Google Scholar]
- 3.Francis CD, Barber JR. A framework for understanding noise impacts on wildlife: An urgent conservation priority. Front Ecol Environ. 2013;11(6):305–313. [Google Scholar]
- 4.Halfwerk W, Holleman LJM, Lessells CKM, Slabbekoorn H. Negative impact of traffic noise on avian reproductive success. J Appl Ecol. 2011;48:210–219. [Google Scholar]
- 5.Reijnen R, Foppen R. Impact of road traffic on breeding bird populations. In: Davenport J, Davenport JL, editors. The Ecology of Transportation: Managing Mobility for the Environment. Springer; Dordrecht, The Netherlands: 2006. pp. 255–274. [Google Scholar]
- 6.Goodwin SE, Shriver WG. Effects of traffic noise on occupancy patterns of forest birds. Conserv Biol. 2011;25(2):406–411. doi: 10.1111/j.1523-1739.2010.01602.x. [DOI] [PubMed] [Google Scholar]
- 7.Barber JR, Crooks KR, Fristrup KM. The costs of chronic noise exposure for terrestrial organisms. Trends Ecol Evol. 2010;25(3):180–189. doi: 10.1016/j.tree.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Siemers BM, Schaub A. Hunting at the highway: Traffic noise reduces foraging efficiency in acoustic predators. Proc R Soc Lond B Biol Sci. 2011;278(1712):1646–1652. doi: 10.1098/rspb.2010.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kight CR, Swaddle JP. How and why environmental noise impacts animals: An integrative, mechanistic review. Ecol Lett. 2011;14(10):1052–1061. doi: 10.1111/j.1461-0248.2011.01664.x. [DOI] [PubMed] [Google Scholar]
- 10.Bunkley JP, McClure CJW, Kleist NJ, Francis CD, Barber JR. Anthropogenic noise alters bat activity levels and echolocation calls. Glob Ecol Conserv. 2015;3:62–71. [Google Scholar]
- 11.Habib L, Bayne EM, Boutin S. Chronic industrial noise affects pairing success and age structure of ovenbirds Seiurus aurocapilla. J Appl Ecol. 2006;44(1):176–184. [Google Scholar]
- 12.Bayne EM, Habib L, Boutin S. Impacts of chronic anthropogenic noise from energy-sector activity on abundance of songbirds in the boreal forest. Conserv Biol. 2008;22(5):1186–1193. doi: 10.1111/j.1523-1739.2008.00973.x. [DOI] [PubMed] [Google Scholar]
- 13.Francis CD, Ortega CP, Cruz A. Noise pollution changes avian communities and species interactions. Curr Biol. 2009;19(16):1415–1419. doi: 10.1016/j.cub.2009.06.052. [DOI] [PubMed] [Google Scholar]
- 14.Hedenström A. Adaptations to migration in birds: Behavioural strategies, morphology and scaling effects. Philos Trans R Soc Lond B Biol Sci. 2008;363(1490):287–299. doi: 10.1098/rstb.2007.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berthold P. Control of Bird Migration. Chapman and Hall; London: 1996. [Google Scholar]
- 16.Sillett TS, Holmes RT. Variation in survivorship of a migratory songbird throughout its annual cycle. J Anim Ecol. 2002;71:296–308. [Google Scholar]
- 17.Frid A, Dill LM. Human-caused disturbance stimuli as a form of predation risk. Conserv Ecol. 2002;6(1):11. [Google Scholar]
- 18.Shannon G, Angeloni LM, Wittemyer G, Fristrup KM, Crooks KR. Road traffic noise modifies behaviour of a keystone species. Anim Behav. 2014;94:135–141. [Google Scholar]
- 19.Purser J, Radford AN. Acoustic noise induces attention shifts and reduces foraging performance in three-spined sticklebacks (Gasterosteus aculeatus) PLoS One. 2011;6(2):e17478. doi: 10.1371/journal.pone.0017478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McClure CJW, Ware HE, Carlisle J, Kaltenecker G, Barber JR. An experimental investigation into the effects of traffic noise on distributions of birds: Avoiding the phantom road. Proc R Soc Lond B Biol Sci. 2013;280(1773):20132290. doi: 10.1098/rspb.2013.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winker K, Warner DW, Weisbrod AR. Daily mass gains among woodland migrants at an inland stopover site. Auk. 1992;109(4):853–862. [Google Scholar]
- 22.Marra PP, Hobson KA, Holmes RT. Linking winter and summer events in a migratory bird by using stable-carbon isotopes. Science. 1998;282(5395):1884–1886. doi: 10.1126/science.282.5395.1884. [DOI] [PubMed] [Google Scholar]
- 23.Poole A, editor. The Birds of North America Online. Cornell Laboratory of Ornithology; Ithaca: 2005. [Google Scholar]
- 24.Robertson BA, Hutto RL. A framework for understanding ecological traps and an evaluation of existing evidence. Ecology. 2006;87(5):1075–1085. doi: 10.1890/0012-9658(2006)87[1075:affuet]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 25.Smith AD, McWilliams SR. What to do when stopping over: Behavioral decisions of a migrating songbird during stopover are dictated by initial change in their body condition and mediated by key environmental conditions. Behav Ecol. 2014;25(6):1423–1435. [Google Scholar]
- 26.Quinn JL, Whittingham MJ, Butler SJ, Cresswell W. Noise, predation risk compensation and vigilance in the chaffinch Fringilla coelebs. J Avian Biol. 2006;37(6):601–608. [Google Scholar]
- 27.Watson M, Aebischer NJ, Cresswell W. Vigilance and fitness in grey partridges Perdix perdix: The effects of group size and foraging-vigilance trade-offs on predation mortality. J Anim Ecol. 2007;76(2):211–221. doi: 10.1111/j.1365-2656.2006.01194.x. [DOI] [PubMed] [Google Scholar]
- 28.Mockford EJ, Marshall RC. Effects of urban noise on song and response behaviour in great tits. Proc R Soc Lond B Biol Sci. 2009;276(1669):2979–2985. doi: 10.1098/rspb.2009.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gavin SD, Komers PE. Do pronghorn (Antilocapra americana) perceive roads as a predation risk? Can J Zool. 2006;84:1775–1780. [Google Scholar]
- 30.Blickley JL, et al. Experimental chronic noise is related to elevated fecal corticosteroid metabolites in lekking male greater Sage-Grouse (Centrocercus urophasianus) PLoS One. 2012;7(11):e50462. doi: 10.1371/journal.pone.0050462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crino OL, Van Oorschot BK, Johnson EE, Malisch JL, Breuner CW. Proximity to a high traffic road: Glucocorticoid and life history consequences for nestling white-crowned sparrows. Gen Comp Endocrinol. 2011;173(2):323–332. doi: 10.1016/j.ygcen.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 32.Montgomerie R, Weatherhead PJ. How robins find worms. Anim Behav. 1997;54(1):143–151. doi: 10.1006/anbe.1996.0411. [DOI] [PubMed] [Google Scholar]
- 33.Carlisle JD, Olmstead KL, Richart CH, Swanson DL. Food Availability, Foraging Behavior, and Diet of Autumn Migrant Landbirds in the Boise Foothills of Southwestern Idaho. Condor. 2012;114(3):449–461. [Google Scholar]
- 34.Wayson RL. Relationship between Pavement Surface Texture and Highway Traffic Noise. Vol 268 Transportation Research Board; Washington, DC: 1998. [Google Scholar]
- 35.Barber JR, et al. Anthropogenic noise exposure in protected natural areas: Estimating the scale of ecological consequences. Landscape Ecol. 2011;9:1281–1295. [Google Scholar]
- 36.Lynch E, Joyce D, Fristrup K. An assessment of noise audibility and sound levels in U.S. National Parks. Landscape Ecol. 2011;26(9):1297–1309. [Google Scholar]
- 37.Sanderson FJ, Donald PF, Pain DJ, Burfield IJ, van Bommel FPJ. Long-term population declines in Afro-Palearctic migrant birds. Biol Conserv. 2006;131(1):93–105. [Google Scholar]
- 38.Robbins CS, Sauer JR, Greenberg RS, Droege S. Population declines in North American birds that migrate to the neotropics. Proc Natl Acad Sci USA. 1989;86(19):7658–7662. doi: 10.1073/pnas.86.19.7658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomson RL, Forsman JT, Sarda-Palomera F, Monkkonnen M. Fear factor: Prey habitat selection and its consequences in a predation risk landscape. Ecography (Cop) 2006;29(4):507–514. [Google Scholar]
- 40. Schmidt KA, Dall SRX, Gils JA Van (2010) The ecology of information: An overview on the ecological significance of making informed decisions. Oikos 119(May 2009):304–316.
- 41.Faaborg J, et al. Conserving migratory land birds in the New World Do we know enough? Ecol Applications. 2010;20(2):398–418. doi: 10.1890/09-0397.1. [DOI] [PubMed] [Google Scholar]
- 42.Reudink MW, et al. Non-breeding season events influence sexual selection in a long-distance migratory bird. Proc R Soc Lond B Biol Sci. 2009;276(1662):1619–1626. doi: 10.1098/rspb.2008.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carlisle JD, Stock SL, Kaltenecker GS, Swanson DL. Habitat associations, relative abundance, and species richness of autumn landbird migrants in southwestern Idaho. Condor. 2004;106:549–566. [Google Scholar]
- 44.Ralph CJ, Geupel GR, Pyle P, Martin TE, DeSante DF. 1993. Handbook of Field Methods for Monitoring Landbirds (USDA Forest Service General Technical Report PSW-GTR-144)
- 45.Pyle P. Identification Guide to North American birds. Part 1. Slate Creek Press; Bolinas, CA: 1997. [Google Scholar]
- 46.Carlisle JD, Kaltenecker GS, Swanson DL. Stopover Ecology of Autumn Landbird Migrants in the Boise Foothills of Southwestern Idaho. Condor. 2005;107(2):244–258. [Google Scholar]
- 47.Halfwerk W, Slabbekoorn H. A behavioural mechanism explaining noise-dependent frequency use in urban birdsong. Anim Behav. 2009;78(6):1301–1307. [Google Scholar]
- 48.Dooling RJ, Popper AN. 2007. The effects of highway noise on birds (The California Department of Transportation Division of Environmental Analysis, Sacramento, CA), Vol 74.
- 49.Bates D, Maechler M, Bolker B. 2012. lme4: Linear mixed-effects models using S4 classes. R version 3.1-102. See https://cran.r-project.org/web/packages/lme4/index.html.
- 50.Akaike HAI. A new look at the statistical model identification. IEEE Trans Automat Contr. 1974;19:716–723. [Google Scholar]
- 51.Arnold TW. Uninformative parameters and model aelection using Akaike’s information criterion. J Wildl Manage. 2010;74(6):1175–1178. [Google Scholar]
- 52.Wang Y, Finch DM. Consistency of mist netting and point counts in assessing landbird species richness and relative abundance during migration. Condor. 2002;104:59–72. [Google Scholar]
- 53.Klaassen M, Hoye BJ, Nolet BA, Buttemer WA. Ecophysiology of avian migration in the face of current global hazards. Philos Trans R Soc Lond B Biol Sci. 2012;367(1596):1719–1732. doi: 10.1098/rstb.2012.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kvist A, Lindström A, Green M, Piersma T, Visser GH. Carrying large fuel loads during sustained bird flight is cheaper than expected. Nature. 2001;413(6857):730–732. doi: 10.1038/35099556. [DOI] [PubMed] [Google Scholar]
- 55.Kullberg C, Jakobsson S, Fransson T. High migratory fuel loads impair predator evasion in Sedge Warblers. Auk. 2000;117(4):1034–1038. [Google Scholar]
- 56.Van der Veen IT. Effects of predation risk on diurnal mass dynamics and foraging routines of yellowhammers (Emberiza citrinella) Behavioral Ecol. 1999;10(5):545–551. [Google Scholar]
- 57.Dierschke V. Predation hazard during migratory stopover: Are light or heavy birds under risk? J Avian Biol. 2003;34(1):24–29. [Google Scholar]
- 58.McNamara JM, Houston AI. The value of fat reserves and the tradeoff between starvation and predation. Acta Biotheor. 1990;38(1):37–61. doi: 10.1007/BF00047272. [DOI] [PubMed] [Google Scholar]
- 59.Witter MS, Cuthill IC. The ecological costs of avian fat storage. Philos Trans R Soc Lond B Biol Sci. 1993;340(1291):73–92. doi: 10.1098/rstb.1993.0050. [DOI] [PubMed] [Google Scholar]
- 60.Dunn EH. Mass change during migration stopover: A comparison of species groups and sites. J Ornithol. 2001;72:419–432. [Google Scholar]
- 61.Bonter DN, Donovan TM, Brooks EW, Hobson KA. Daily mass changes in landbirds during migration stopover on the south shore of Lake Ontario. Auk. 2007;124:122–133. [Google Scholar]
- 62.Graber JW, Graber RR. Feeding rates of warblers in spring. Condor. 1983;85(2):139–150. [Google Scholar]
- 63.Bejder L, Samuels A, Whitehead H, Finn H, Allen S. Impact assessment research: Use and misuse of habituation, sensitisation and tolerance in describing wildlife responses to anthropogenic stimuli. Mar Ecol Prog Ser. 2009;395:177–185. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.