Significance
Pathologic vascular growth causes vision impairment in several vascular eye diseases. Specifically targeting molecular signatures distinguishing pathologic neovascularization from normal vessels will allow targeted treatment options. This study demonstrates that miR-150 was specifically enriched in normal retinal vessels and down-regulated in pathologic neovessels in a mouse model of proliferative retinopathy. MiR-150 suppressed pathologic ocular neovascularization in mice with decreased expression of angiogenic target genes and inhibited endothelial cell function in vitro. Loss of miR-150 also promoted vascular sprouting in ex vivo aortic and choroidal assays and laser-induced choroidal neovascularization in mice. These data suggest that endothelial miR-150 is an endogenous suppressor of ocular neovascularization and a drug target for vascular eye diseases.
Keywords: neovascularization, endothelial cells, microRNA, miR-150, retinopathy
Abstract
Pathologic ocular neovascularization commonly causes blindness. It is critical to identify the factors altered in pathologically proliferating versus normally quiescent vessels to develop effective targeted therapeutics. MicroRNAs regulate both physiological and pathological angiogenesis through modulating expression of gene targets at the posttranscriptional level. However, it is not completely understood if specific microRNAs are altered in pathologic ocular blood vessels, influencing vascular eye diseases. Here we investigated the potential role of a specific microRNA, miR-150, in regulating ocular neovascularization. We found that miR-150 was highly expressed in normal quiescent retinal blood vessels and significantly suppressed in pathologic neovessels in a mouse model of oxygen-induced proliferative retinopathy. MiR-150 substantially decreased endothelial cell function including cell proliferation, migration, and tubular formation and specifically suppressed the expression of multiple angiogenic regulators, CXCR4, DLL4, and FZD4, in endothelial cells. Intravitreal injection of miR-150 mimic significantly decreased pathologic retinal neovascularization in vivo in both wild-type and miR-150 knockout mice. Loss of miR-150 significantly promoted angiogenesis in aortic rings and choroidal explants ex vivo and laser-induced choroidal neovascularization in vivo. In conclusion, miR-150 is specifically enriched in quiescent normal vessels and functions as an endothelium-specific endogenous inhibitor of pathologic ocular neovascularization.
Angiogenesis plays important roles in both physiological development and pathological events. Dysregulated angiogenesis is associated with many diseases including cardiovascular diseases, tumorigenesis, proliferative retinopathies, and neurodegeneration (1). In the eye, pathologic retinal neovascularization is characterized by abnormally proliferating tuft-liked structures, which may lead to vision loss (2). During angiogenesis, endothelial cells (ECs) proliferate, sprout, and form new vessels following dual guidance cues from both angiogenic stimulators and inhibitors (3). Vascular endothelial growth factor (VEGF) is targeted in current antiangiogenic therapies for cancers and neovascular eye diseases (4). However, anti-VEGF therapies target the end neovascular stage of vascular eye diseases and therefore do not address the incipient cause of proliferation and ischemia and in fact may affect normal vessel homeostasis (5, 6). It is therefore critical to identify additional intrinsic factors that maintain normal vessel quiescence and control the switch to proliferative neovessels to design improved targeted therapies.
MicroRNAs (miRNAs) are a group of small endogenous noncoding RNA molecules that function by base paring to the complementary sequence in the 3′ untranslated region (3′ UTR) of target mRNAs, inducing their cleavage and translational repression (7). MiRNAs are recognized as key fine-tuning mediators of posttranscriptional regulation. MiRNAs play critical roles in many biological processes, such as cell proliferation, cell death, neuronal differentiation and development, and angiogenesis (8–11). Moreover, dysregulation of miRNA is associated with many diseases, such as cancer and heart diseases, and are being developed as important biomarkers (12, 13). In neovascular diseases, both pro- and antiangiogenic miRNAs were identified in vascular ECs and perivascular cells (8, 13, 14). However, it is not well understood whether miRNAs are intrinsically regulated in proliferative blood vessels to influence ocular vascular diseases.
In this study, we used mouse retinal and choroidal neovascularization (CNV) models to study the role of endogenous miRNAs in regulating pathologic ocular neovascularization, which may also be relevant for angiogenesis in other organs. We demonstrated that expression of miR-150 was highly enriched in quiescent blood vessels isolated from normal retinas and significantly suppressed in pathologic neovessels isolated from retinas under an oxygen-induced proliferative retinopathy model. In addition, we found miR-150 decreases the proliferative function and tube formation of ECs in vitro and decreased the expression of multiple angiogenic target genes: C-X-C chemokine receptor type 4 (CXCR4), Delta like ligand 4 (DLL4), and Frizzled-4 (FZD4). Treatment with miR-150 mimics significantly suppressed neovascularization in vivo in retinopathy. On the other hand, loss of miR-150 in mice promoted angiogenesis in aortic and choroidal explants ex vivo as well as CNV in vivo in a laser-induced model. Together our findings suggest that the vascular-enriched miR-150 is an intrinsic suppressor of EC proliferation and pathologic ocular neovascularization. MiR-150 may represent a potential therapeutic target for diseases with abnormal angiogenesis.
Results
Vascular-Enriched miR-150 Was Significantly Suppressed in Pathologic Neovessels in OIR.
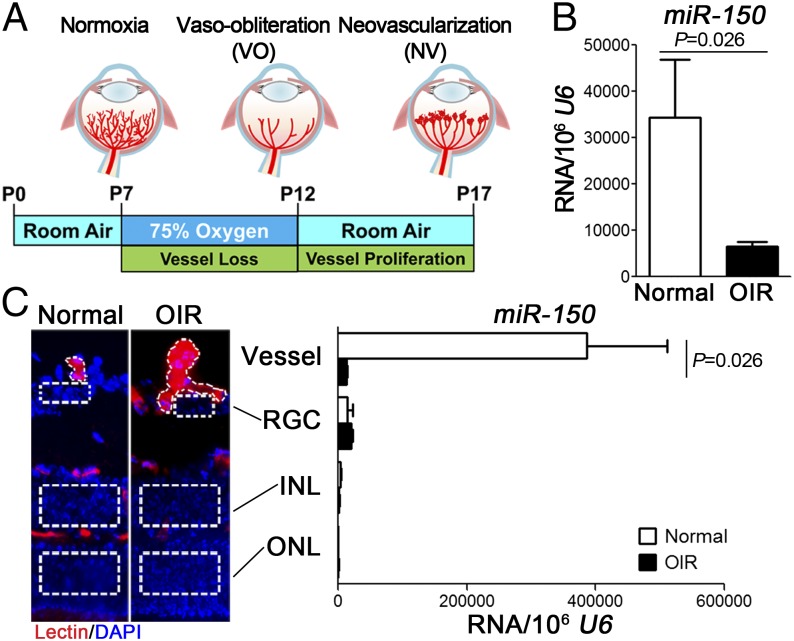
To identify miRNAs specifically affected in pathological vessel growth, we analyzed retinal RNAs isolated from oxygen-induced retinopathy (OIR) and age-matched normoxic mice at postnatal day (P) 17 by miRNA array (Fig. 1A). MiR-150 levels were suppressed about fivefold in OIR retinas compared with normoxic controls as validated by quantitative RT-PCR (qPCR) (Fig. 1B). We next explored the retinal localization of miR-150 in retinal vascular and neuronal layers using laser capture microdissection (LCM) from P17 OIR and control normoxic retinas. The expression level of an EC marker vascular endothelial (VE)-cadherin was comparable between pathologic OIR vessels and normal vessels (SI Appendix, Fig. S1), confirming similar amounts of ECs in LCM isolated vessel samples. MiR-150 was highly enriched in normal blood vessels compared with retinal neurons in normoxic retinas (Fig. 1C), suggesting a potential role of miR-150 in regulating EC function. Moreover, miR-150 expression was drastically reduced (>95%) in pathological neovessels from OIR retinas compared with normal retinal vessels (Fig. 1C), indicating a potential pathogenic role of miR-150 deficiency in regulating ocular neovascularization.
Fig. 1.
MiR-150 was enriched in normal retinal blood vessels and specifically suppressed in pathologic neovessels isolated from OIR retinas. (A) Schematic diagram of OIR. Neonatal mice were exposed to 75% oxygen from P7 to P12 to induce vessel loss and returned to room air from P12 to P17 to induce maximum pathologic neovascularization at P17. (B) Expression levels of miR-150 were significantly suppressed in OIR whole retinas compared with normoxia controls, analyzed by qPCR normalized to U6 snRNA as control (n = 6 per group). (C) Images on the left show representative retinal cross-sections from normoxic and OIR retinas stained with isolectin B4 (red) for ECs and DAPI (blue) for nucleus, with dotted lines highlighting the area for LCM. MiR-150 was found almost exclusively in LCM-isolated normal retinal blood vessels with minimal expression in retinal neurons. In addition, miR-150 levels were significantly suppressed in pathologic vessels isolated from OIR retinas compared with normal vessels (n = 4–6 per group). INL, inner nuclear layer; ONL, outer nuclear layer; RGC, retinal ganglion cells.
MiR-150 Regulated EC Migration, Proliferation, and Tubular Formation.
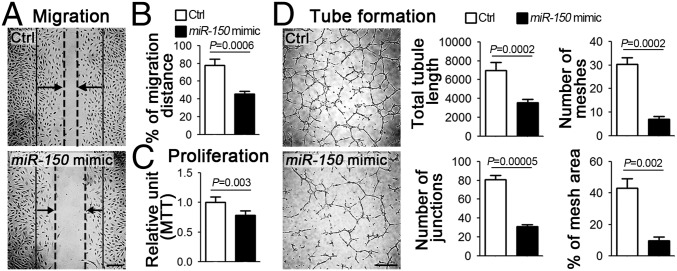
We next examined the angiogenic effects of miR-150 in human retinal microvascular endothelial cells (HRMECs). Cells treated with miR-150 mimic showed ∼42% reduction in migration (Fig. 2 A and B) and ∼22% reduction in proliferation (Fig. 2C) compared with nontargeting miRNA mimic control. In addition, miR-150 substantially suppressed tube formation of HRMECs, resulting in reductions of ∼48% in tubule length, ∼60% in number of junctions, ∼77% in mesh numbers, and ∼78% in mesh area (Fig. 2D). In addition, in the presence of cotreatment with VEGF, miR-150 mimic showed vascular-inhibitory effects independent of VEGF-induced HRMEC proliferation, migration, and tube formation (SI Appendix, Fig. S2). Together these results indicate that miR-150 is a potent suppressor of EC function that may act through VEGF-independent pathways.
Fig. 2.
MiR-150 suppressed EC proliferation, migration, and tube formation in vitro. (A and B) Representative images and quantitative analysis of HRMEC wound healing assay. The solid lines indicate the start of cell migration, the dash lines indicate the front of the migration, and the arrows present the direction of the migration. HRMECs treated with miR-150 mimic had significantly lower migration ability compared with cells treated with nontargeting mimic control (Ctrl) (n = 4–5 per group). (C) MTT assay results showed that miR-150 mimic-treated HRMECs had lower levels of proliferative activity (n = 3 per condition). (D) Representative images and quantitative analysis of tube formation assay characterizing tubule length, junction numbers, mesh numbers, and percentage of mesh area. MiR-150 mimic treatment significantly reduced the capacity of HRMECs to form tubes when cultured on Matrigel (n = 3–5 per group). [Scale bars, 250 μm (A) and 500 μm (D).]
MiR-150 Targeted Multiple Angiogenic Genes CXCR4, DLL4, and FZD4 in EC.
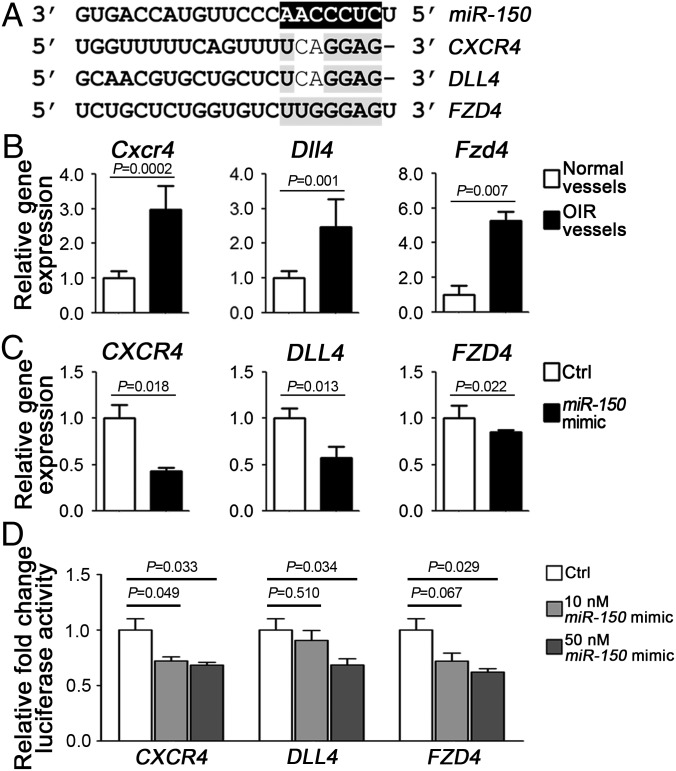
To identify potential target genes of miR-150, we analyzed the seed sequence of miR-150 (CUCCCAA), conserved in both human and murine for complementarity with predicted target mRNAs. Three candidates were identified, which are associated with angiogenesis and retinopathy (15–17): CXCR4, DLL4, and FZD4. All contained potential miR-150 seed targeting sequences in their 3′ UTRs (Fig. 3A). In LCM-isolated pathologic OIR vessels, all three putative targets were significantly up-regulated by ∼threefold (Cxcr4), 2.5-fold (Dll4), or fivefold (Fzd4), respectively, compared with the normoxic control vessels (Fig. 3B), consistent with decreased miR-150 levels in OIR vessels (Fig. 1C). Moreover, miR-150 significantly inhibited expression of CXCR4, DLL4, and FZD4 in HRMECs, compared with nontargeting mimic control (Fig. 3C). Expression levels of other angiogenic factors, VEGF, fibroblast growth factor 2 (FGF2), and angiogenic receptors including vascular endothelial growth factor receptor 1 (VEGFR1), VEGFR2, NOTCH1, low-density lipoprotein receptor-related protein 5 (LRP5), and LRP6, were not significantly affected by miR-150 (SI Appendix, Fig. S3). The direct regulation of miR-150 on its angiogenic targets was further supported by reporter assays. When cotransfected with miR-150 mimic in HEK293T cells, reporter constructs containing target sequences of CXCR4, DLL4, or FZD4 demonstrated significant repression of their luciferase activities in a dose-dependent manner (Fig. 3D). Together these results illustrate that miR-150 is a direct regulator of these angiogenic genes in ECs through targeting their 3′ UTR seed sequences.
Fig. 3.
MiR-150 directly regulated expression levels of angiogenic target genes CXCR4, DLL4, and FZD4 in ECs. (A) Alignment of seed sequences from 3′ UTR regions of candidate target genes (CXCR4, DLL4, and FZD4) with mature miR-150. (B) Expression levels of Cxcr4, Dll4, and Fzd4 were significantly up-regulated in pathologic vessels from OIR retinas compared with normal blood vessels isolated from normoxia control retinas (n = 6 per group). Expression levels were normalized to cyclophlin A (CypA) and relative to normal vessel groups. (C) MiR-150 mimic treatment significantly suppressed expression of CXCR4, DLL4, and FZD4 in HRMECs (n = 3 per group). Expression levels were normalized to β-actin (ACTB) and relative to mimic control (Ctrl). (D) Luciferase reporter assays of potential miR-150 target genes (CXCR4, DLL4, and FZD4) were performed in HEK293T cells. Cells were cotransfected with miR-150 mimic (or mimic control) and constructs of luciferase reporter containing the miR-150 target sequence of CXCR4, DLL4, or FZD4 (as shown in A). Transfection with miR-150 mimic resulted in significant repression of luciferase activities reflecting complementary binding of seed sequence and suppression of target genes expression (n = 3–6 per group). Firefly luciferase activities were normalized to the Renilla luciferase and to the levels in the mimic control (Ctrl).
Deficiency of miR-150 Increased Angiogenesis in Aortic Rings and Choroidal Explants ex Vivo.
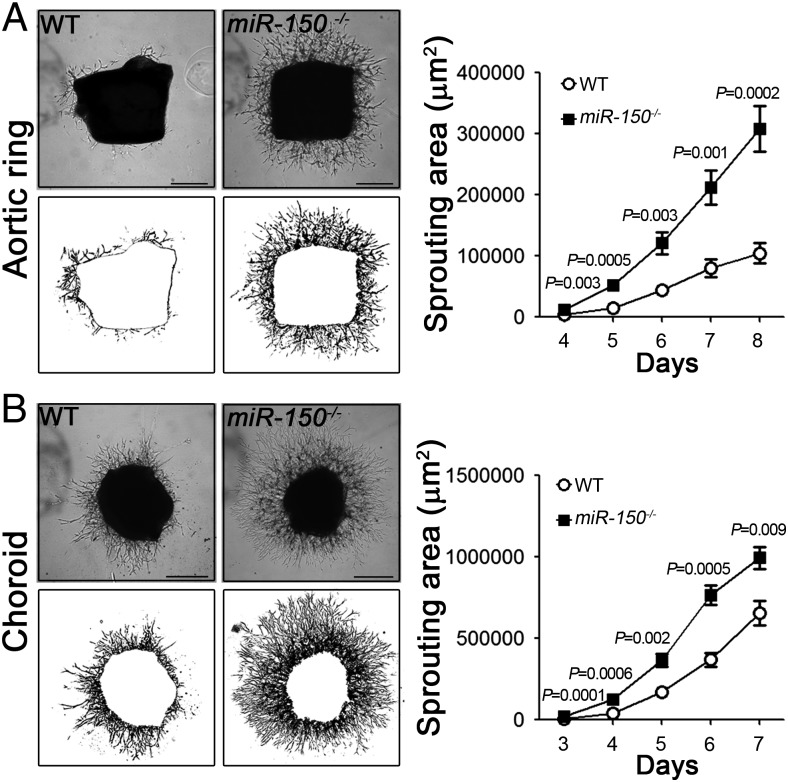
We next investigated the effects of miR-150 on angiogenesis in ex vivo tissue explants isolated from miR-150−/− mice. In an aortic ring angiogenic assay (18), from day 4–8 after aortic ring explanting, there was a significantly larger sprouting area in miR-150−/− aortic rings compared with WT controls (Fig. 4A, ∼threefold at day 8). In the choroid sprouting assay (19), miR-150−/− choroid explants showed extensive outgrowth starting from 3 d after culturing and significantly and consistently larger sprouting areas (1.5-fold at day 7) compared with WT controls (Fig. 4B). In the presence of VEGF treatment, both miR-150−/− aortic ring and choroid explants showed exacerbated vascular outgrowth in an additive manner (SI Appendix, Fig. S4). These data suggest that lack of endothelial miR-150 intrinsically promotes angiogenesis ex vivo and the vascular effects of endothelial miR-150 are additive to external VEGF stimulus.
Fig. 4.
MiR-150 deficiency increased vascular growth in aortic ring and choroidal explant ex vivo. (A) Aortic rings isolated from 4-wk-old miR-150−/− mice showed significantly increased sprouting ability ex vivo compared with those from wild-type (WT) mice (n = 3 per group). (Left panels) Representative images from day 5 of explants (Top) and areas of vascular sprouts quantified (Bottom). (B) Representative images (Top panels) and quantification of microvascular sprouting area (Bottom panels) from miR-150−/− and WT adult mouse choroid explants. Knockout of miR-150 results in significantly increased sprouting area (n = 3 per group). (Scale bar, 500 μm.)
MiR-150 Suppressed Pathologic Retinal Angiogenesis in OIR.
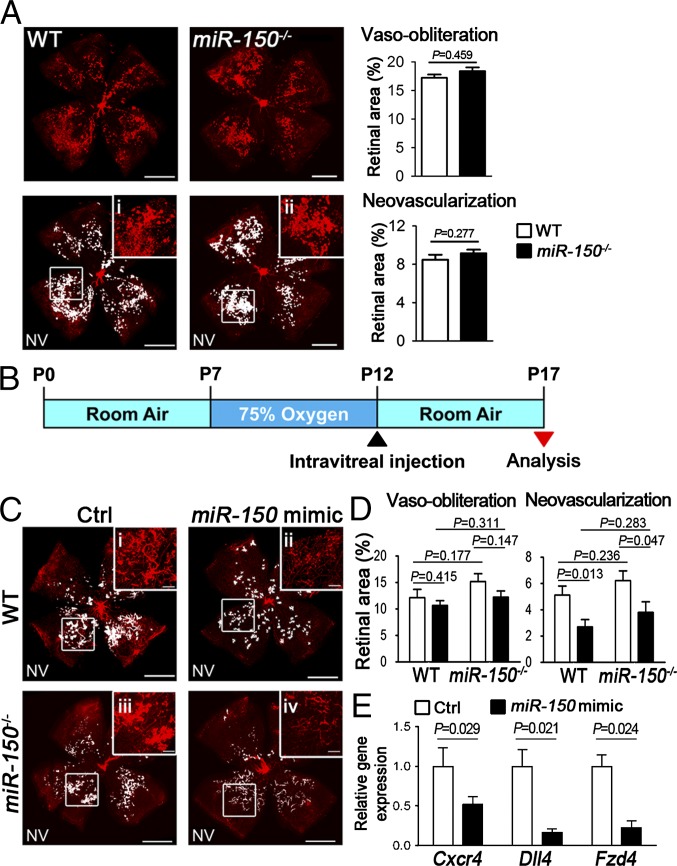
Next we examined retinal vasculature in miR-150−/− and WT mice. At P7, there is no detectable developmental difference in retinal vascular growth areas between miR-150−/− and WT retinas (SI Appendix, Fig. S5), suggesting that miR-150 is likely dispensable for developmental retinal angiogenesis. Because the levels of endogenous miR-150 in WT OIR retinas were already drastically suppressed (Fig. 1 B and C), miR-150−/− mice showed no significant changes in vaso-obliteration and retinal neovascularization compared with WT mice at P17 (Fig. 5A). To determine whether modulation of miR-150 may suppress pathologic retinal vessel growth, OIR mice were treated with intravitreal injection of miR-150 mimic at P12 (Fig. 5B). At P17, miR-150 suppressed retinal neovascularization in OIR by ∼50% in WT retinas and by ∼40% in miR-150−/− retinas compared with their relative contralateral eyes injected with mimic control (Fig. 5 C and D). Moreover, expression levels of Cxcr4, Dll4, and Fzd4 were significantly suppressed in miR-150 mimic-treated P17 WT retinas compared with contralateral retinas treated with mimic control (Fig. 5E), yet retinal Vegfa, Vegfr1, and Vegfr2 levels were not significantly altered (SI Appendix, Fig. S6). Together these data indicate that miR-150 suppressed pathologic neovascularization and the expression of target angiogenic factors in experimental retinopathy.
Fig. 5.
MiR-150 suppressed pathologic neovascularization in OIR. (A) Retinal vaso-obliteration and neovascularization of WT and miR-150−/− retinas in OIR. Retinal flat mounts from P17 WT and miR-150−/− mice were stained with isolectin B4 (red). Pathologic neovascular tufts (NV) were labeled (white highlight), and selected areas were enlarged at high magnification (i and ii). Quantification of vaso-obliteration and neovascularization showed no significant difference between WT and miR-150−/− OIR retinas at P17 (n = 10–18 per group). (B) Schematic diagram of intravitreal injection of miR-150 mimic or mimic control in WT and miR-150−/− OIR eyes at P12, with miR-150 mimic injected in one eye and negative mimic control in the contralateral eye. At P17 in OIR, retinal vasculature was analyzed. (C) Representative images of P17 WT and miR-150−/− OIR retinas with injection of miR-150 mimic or mimic control. Pathologic NVs were labeled as white highlight, and Insets were the selected areas at high magnification (i–iv). (D) Quantitative analysis of vaso-obliteration and pathologic neovascularization at P17 in OIR. MiR-150 mimic treatment significantly reduced pathologic neovascularization in both WT and miR-150−/− mice compared with contralateral retinas treated with mimic control, with no significant difference in vaso-obliteration (n = 12 or 13 per group). (E) Substantial decrease of Cxcr4, Dll4, and Fzd4 expression levels was observed in miR-150 mimic-treated P17 WT OIR retinas (n = 3 per group). Expression levels were normalized to CypA and relative to contralateral eyes injected with negative mimic control. [Scale bars, 1 mm (A and C) and 200 μm (C, i–iv).]
Deficiency of miR-150 Increased Laser-Induced CNV.
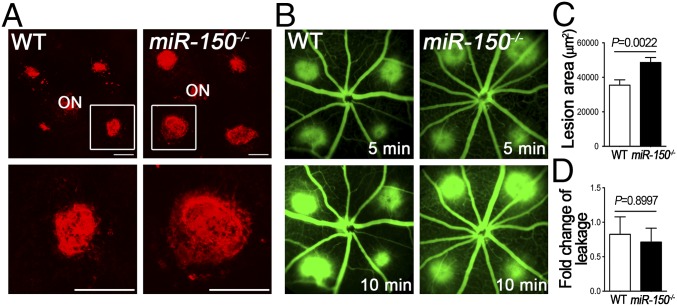
We next evaluated miR-150−/− mice in another angiogenic model of laser-induced CNV (20). At day 7 after laser photocoagulation, miR-150−/− mice had ∼1.4-fold larger CNV lesions than WT mice (Fig. 6A), without significantly impacting the levels of vascular leakage evaluated by fluorescent fundus angiography (Fig. 6B). Together, these findings suggested that miR-150 deficiency resulted in increased pathologic CNV, further supporting an endogenous vaso-inhibitory role of miR-150.
Fig. 6.
MiR-150 deficiency promoted laser-induced CNV. (A) Choroidal flat mounts at 7 d after photocoagulation were stained with isolectin B4 (red) to visualize CNV lesions. (B) Fundus fluorescein angiography images from WT and miR-150−/− mice taken at 1 wk after laser photocoagulation showed the subretinal CNV lesions and associated vascular leakage at 5 and 10 min. (C) Quantitative analysis of lesion size showed that miR-150 deficiency resulted in significantly increased CNV lesion size 7 d after laser exposure (n = 8–14 per group). (D) Quantification analysis revealed no significant difference of the vascular leakage between WT and miR-150−/− mice (n = 7–9 per group). ON, optic nerve. (Scale bar, 500 μm.)
Discussion
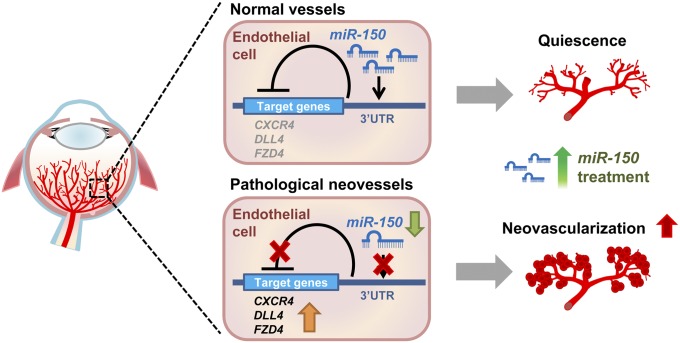
In this study, we identified an endothelium-specific function of miR-150 as a key endogenous suppressor of pathological ocular neovascularization, which is supported by observations in several models of angiogenesis. First, in a mouse OIR model, miR-150 transcript was specifically enriched in normal retinal blood vessels and decreased in pathologic neovessels. Administration of exogenous miR-150 suppressed pathological neovascularization and its target gene expression in OIR, highlighting a vaso-inhibitory role of vascular-enriched miR-150. Second, ex vivo tissue explants from miR-150−/− mice showed notably increased sprouting ability compared with WT explants. In the laser-induced CNV model, miR-150−/− mice showed significantly larger neovascular lesion sizes. All of these findings support a potent antiangiogenic role of endogenous miR-150. Finally, exogenous miR-150 treatment inhibited angiogenic functions specifically in HRMECs and down-regulated multiple angiogenic target genes through direct posttranscriptional control. Collectively, our data suggest that in normal retinal vessels, expression of miR-150 is abundant and may maintain vascular quiescence through repressing its downstream angiogenic target genes—CXCR4, DLL4, and FZD4. In pathological neovessels, however, substantial down-regulation of the angiogenic suppressor miR-150 in ECs may lead to up-regulation of downstream angiogenic targets, contributing to formation of pathologic neovascularization. MiR-150 mimics may compensate for the loss of endogenous miR-150 in pathologic neovessels and thereby alleviate abnormal angiogenesis in neovascular ocular diseases (Fig. 7). This antiangiogenic role of miR-150 is potentially relevant for other vascular diseases as well.
Fig. 7.
Schematic illustration of an endothelium-specific inhibitory role of miR-150 in pathological ocular neovascularization. In normal retinal vessels, endothelial-enriched miR-150 represses the expression of its downstream angiogenic target genes by binding to their 3′ UTRs to maintain quiescence of retinal vessels. In pathological neovessels, expression of endothelial miR-150 is decreased, releasing the repression of downstream angiogenic targets (CXCR4, DLL4, and FZD4) and leading to their up-regulation, thereby contributing to formation of pathologic retinal neovascularization. Supplementing miR-150 may compensate for the loss of miR-150 in pathologic neovessels and alleviate pathologic neovascularization in retinopathy.
Our data localizing miR-150 in normal blood vessels identified a direct cell-specific role of miR-150 in suppressing EC functions and expanded the current knowledge of its cellular localization, previously reported in monocytes, and mature lymphocytes (21, 22). Our findings of miR-150 suppression of vascular EC function is consistent with previous reports of miR-150 in regulating B-cell proliferation, development, and differentiation (22–24) and EC differentiation and vasculogenesis by targeting ZEB1 in human embryonic stem cells (25). A previous study identified multiple miRNAs substantially regulated in ischemic mouse retinas (26), yet the cell-specific roles of these miRNAs in the pathogenesis of neovascular eye diseases were not clear. In addition, several highly expressed miRNAs were identified in human ECs, including miR-126, miR-221/222, miR-21, the let-7 family, the miR-17∼92 cluster, and the miR-23∼24 cluster (27, 28). Our work suggests that miR-150 is an additional EC-enriched miRNA functioning as a molecular regulatory switch controlling EC quiescence and pathologic proliferation.
The intrinsic vaso-inhibitory role of miR-150 was highlighted in two in vivo models of pathologic ocular angiogenesis. Although no significant difference was found in neovascularization between WT and miR-150−/− OIR mice, this observation is likely attributable to very low levels of miR-150 levels in WT OIR retinas, resulting in lack of a significant difference between WT and miR-150−/− OIR retinas. Importantly, intravitreal injection of miR-150 significantly suppressed OIR, suggesting a protective role of exogenously applied miR-150 in inhibiting pathologic retinal angiogenesis. In addition, lack of miR-150 significantly exacerbated CNV lesions in the laser-induced CNV model and increased sprouting in aortic ring and choroidal explants, further supporting an antiangiogenic effect of miR-150. These findings are consistent with another study showing increased lung angiogenesis in miR-150−/− mice in a model of oxygen-induced neonatal lung injury (29). Although these data strongly support a primarily direct effect of vascular-enriched miR-150 on endothelial function and ocular angiogenesis, we cannot rule out potential contributions from circulating miR-150, which was found in monocyte-derived microvesicles to regulate target genes in the recipient ECs and affect pathological angiogenesis in tumor and diabetic models (30, 31). In addition, genetic variation in research mouse strains may also contribute to their observed eye phenotype, as was discovered for rd8 mutation (32), and potentially vascular phenotype. The miR-150−/− mice we used do not harbor rd8 mutation, as validated by genetic testing using PCR and sequencing (SI Appendix, Fig. S7), yet minor unknown background strain differences may exist between the WT (C57BL/6J) and miR-150−/− colonies we used in this study.
The deficiency of miR-150 in pathological OIR neovessels is associated with reciprocal up-regulation of multiple target angiogenic genes that are usually suppressed in quiescent mature vasculature. Indeed, blood vessels isolated from normoxic retinas had high levels of miR-150 and low expression levels of target angiogenic genes, including Cxcr4, Dll4, and Fzd4, that may act separately through stromal cell-derived factor 1 (SDF-1)/CXCR4, Dll4/Notch, or a Wnt signaling pathway to regulate sprouting angiogenesis. The antiangiogenic effect of miR-150 in the eye may be explained by its direct suppression of CXCR4, DLL4, or FZD4, as confirmed in EC culture and in OIR retinas. All of these genes are potent proangiogenic genes associated with ocular angiogenesis. FZD4 is a receptor for Wnt signaling, which is a highly conserved angiogenic pathway activated in both OIR and laser-induced CNV models (16, 33, 34). On the other hand, Dll4/Notch signaling controls vessel sprouting and branching, and inhibition of DLL4 showed reduced pathological neovessels in OIR (35). Moreover, CXCR4, a receptor for SDF-1 signaling, showed increased expression in diabetic epiretinal membranes (36), and inhibition of CXCR4 reduces the laser-induced CNV lesion sizes and leakage in rats (37). Together these findings are consistent with our data of miR-150, a suppressor of Fzd4, Dll4, and Cxcr4, protecting against OIR. In addition, VEGF, a prominent angiogenic growth factor, was reported as a downstream gene regulated by miR-150 (38), yet in our study we did not find significant regulation of VEGF and its receptors’ (VEGFR1 and VEGFR2) expression in miR-150 mimic-treated HRMECs (SI Appendix, Fig. S3) or miR-150 mimic-treated OIR retinas (SI Appendix, Fig. S6). Vegfa expression from isolated retinal pigment epithelium (RPE) was also not significantly affected in miR-150−/− mice compared with WT (SI Appendix, Fig. S8). Moreover, the vascular effects of miR-150 appear to be additive to the effects of VEGF treatment on HRMEC proliferation, migration, tube formation (SI Appendix, Fig. S2), as well as aortic ring and choroidal explant sprouting (SI Appendix, Fig. S4). Together these data suggest that endothelial miR-150 acts in a VEGF-independent manner in our study, although miR-150 may suppress Vegfa expression in other VEGF-enriched cell types in a tissue- and cell-specific context. Although the upstream regulators of miR-150 deficiency in OIR have yet to be identified, one possible regulator is local tissue hypoxia, which may regulate a subset of miRNAs—namely, hypoxamiRs—to influence the posttranscriptional control in hypoxia-controlled cell proliferation, apoptosis, differentiation, and angiogenesis (39–41). Negative regulation of miR-150 by hypoxia-inducible factor (HIF) was reported during hypoxia in liver regeneration (42).
Recent advances in miRNA research have identified many miRNAs expressed in the eye and during retinal angiogenesis as well as regulated in pathologic neovascularization (43). Targeting multiple angiogenic genes using miRNAs may offer a complementary approach for vascular eye disease management and treatment, in addition to current ablative surgeries and pharmacologic interventions, and may be additive to anti-VEGF therapies. As current ongoing trials testing the miRNA approach in other diseases such as cancers are promising (44), the translational prospects of modulation miRNAs including miR-150 in vascular eye diseases are encouraging.
In summary, our study presents direct evidence suggesting that miR-150 is an intrinsic suppressor of pathologic ocular angiogenesis in ECs. MiR-150 was expressed abundantly in quiescent ECs and can suppress abnormal endothelial activation through targeting multiple angiogenic signaling pathways specifically in the endothelium. On the other hand, suppression of endogenous miR-150 in pathologic neovessels may induce endothelial activation to trigger pathological angiogenesis. Our findings of miR-150 as an endothelium-specific intrinsic inhibitor of pathologic ocular angiogenesis suggest the potential of modulating miR-150 as a therapeutic intervention for the treatment of neovascular eye diseases and potentially other vascular diseases.
Materials and Methods
Animals.
Animal studies were approved by the Boston Children’s Hospital Animal Care and Use Committee and adhered to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research (45). MiR-150 knockout mice [B6(C)-Mir150tm1Rsky/J, stock no. 007750] and C57BL/6J mice (stock no. 000664) were obtained from Jackson Laboratory. Age-matched C57BL/6J mice were used as wild-type controls for comparison with the miR-150 knockout mice according to vendor instruction.
OIR Model and Vessel Quantification.
OIR was generated as previously described (46, 47). Neonatal mice with their nursing mother were exposed to 75% oxygen from P7–12 to induce retinopathy and killed at P17, followed by retinal dissection and staining overnight with fluorescent Griffonia simplicifolia isolectin B4 (Invitrogen). Vaso-obliteration and neovascularization in OIR were quantified by using Adobe Photoshop and ImageJ (48). Quantification was performed with the identity of the samples masked, with n being the number of mice quantified.
LCM.
LCM was performed on OIR or normoxia P17 wide-type frozen retinal sections that were dehydrated and stained with isolectin B4 (33). Retinal layers were laser-capture microdissected with the Leica LMD 6000 system (Leica Microsystems) and collected directly into lysis buffer from the RNeasy Micro kit.
Intravitreal Injection.
Intravitreal injections were performed following established protocols (49, 50). We injected 1 µg of miR-150 mimics (Ambion) using a 33-gauge needle behind the limbus of the eye, and the contralateral eye of the same animal was injected with an equal amount of negative control mimics.
Laser-Induced CNV.
Laser photocoagulation was carried out as described previously (18, 51) in 6–8-wk-old miR-150−/− and WT mice with laser-induced CNV analyzed 1 wk postlaser. Additional information regarding laser coagulation and lesion analysis is provided in SI Appendix.
Cell Culture and Assays.
HRMECs and human embryonic kidney 293T cells (HEK293T; ATCC) were cultured for endothelial functional assays (migration, proliferation, and tube formation) and luciferase assays, respectively. FAM-labeled premiRNA (Ambion) was used to analyze transfection efficiency of miRNAs (52) and showed around 90% transfection efficiency in HRMECs (SI Appendix, Fig. S9). Detailed culture and assay conditions are detailed in SI Appendix.
Aortic Ring and Chroidal Sprouting Assays.
Aortic ring and choroidal sprouting assays were performed as previously described (18). Fragments of aortae or RPE/choroid were isolated from 4-wk-old miR-150−/− and WT mice and cultured in Matrigel, imaged daily, and analyzed for vascular growth.
RNA Isolation and qPCR.
Total RNA was extracted from mouse retinas or from HRMEC culture with RNeasy Kit (Qiagen). MiRNA was isolated from retinas or LCM isolated vessels with the miRNeasy Micro Kit (Qiagen). Synthesis of cDNA and qPCR were performed using established protocols as detailed in SI Appendix.
Statistical Analysis.
Results were presented as mean ± SEM for animal studies and nonanimal studies. P values less than 0.05 were considered significant, according to two-tailed Student’s t test (two groups) or ANOVA (more than two groups).
Additional detailed methods are available in SI Appendix.
Supplementary Material
Acknowledgments
We thank Drs. Lois E. H. Smith and Harvey F. Lodish for critical reading of the manuscript, Dr. John Paul SanGiovanni for helpful discussion, and Aimee M. Juan and Ricky Z. Cui for their excellent technical assistance. This work was supported by NIH/National Eye Institute Grant R01 EY024963, Blind Childrens Center, BrightFocus Foundation, Boston Children’s Hospital (BCH) Ophthalmology Foundation, a BCH career development award, Massachusetts Lions Eye Research Fund Inc., and Alcon Research Institute (to J.C.) and Taiwan Ministry of Science and Technology Postdoctoral Research Abroad Fellowship 104-2917-I-564-026 (to C.-H.L.). J.L. was supported by the Joint PhD Program of the Chinese Scholarship Council.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1508426112/-/DCSupplemental.
References
- 1.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1(1):27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 2.Afzal A, et al. Retinal and choroidal microangiopathies: Therapeutic opportunities. Microvasc Res. 2007;74(2-3):131–144. doi: 10.1016/j.mvr.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 3.Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146(6):873–887. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 4.Crawford Y, Ferrara N. VEGF inhibition: Insights from preclinical and clinical studies. Cell Tissue Res. 2009;335(1):261–269. doi: 10.1007/s00441-008-0675-8. [DOI] [PubMed] [Google Scholar]
- 5.Darlow BA, Ells AL, Gilbert CE, Gole GA, Quinn GE. Are we there yet? Bevacizumab therapy for retinopathy of prematurity. Arch Dis Child Fetal Neonatal Ed. 2013;98(2):F170–F174. doi: 10.1136/archdischild-2011-301148. [DOI] [PubMed] [Google Scholar]
- 6.Wallace DK, Wu KY. Current and future trends in treatment of severe retinopathy of prematurity. Clin Perinatol. 2013;40(2):297–310. doi: 10.1016/j.clp.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 7.He L, Hannon GJ. MicroRNAs: Small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5(7):522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 8.Anand S, Cheresh DA. Emerging role of micro-RNAs in the regulation of angiogenesis. Genes Cancer. 2011;2(12):1134–1138. doi: 10.1177/1947601911423032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang TC, Mendell JT. microRNAs in vertebrate physiology and human disease. Annu Rev Genomics Hum Genet. 2007;8:215–239. doi: 10.1146/annurev.genom.8.080706.092351. [DOI] [PubMed] [Google Scholar]
- 10.Krek A, et al. Combinatorial microRNA target predictions. Nat Genet. 2005;37(5):495–500. doi: 10.1038/ng1536. [DOI] [PubMed] [Google Scholar]
- 11.Lim LP, et al. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433(7027):769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- 12.Du L, Pertsemlidis A. Cancer and neurodegenerative disorders: Pathogenic convergence through microRNA regulation. J Mol Cell Biol. 2011;3(3):176–180. doi: 10.1093/jmcb/mjq058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nicoli S, et al. MicroRNA-mediated integration of haemodynamics and Vegf signalling during angiogenesis. Nature. 2010;464(7292):1196–1200. doi: 10.1038/nature08889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Westenskow PD, et al. Ras pathway inhibition prevents neovascularization by repressing endothelial cell sprouting. J Clin Invest. 2013;123(11):4900–4908. doi: 10.1172/JCI70230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wen J, Zhang JQ, Huang W, Wang Y. SDF-1α and CXCR4 as therapeutic targets in cardiovascular disease. Am J Cardiovasc Dis. 2012;2(1):20–28. [PMC free article] [PubMed] [Google Scholar]
- 16.Ye X, et al. Norrin, frizzled-4, and Lrp5 signaling in endothelial cells controls a genetic program for retinal vascularization. Cell. 2009;139(2):285–298. doi: 10.1016/j.cell.2009.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gale NW, et al. Haploinsufficiency of delta-like 4 ligand results in embryonic lethality due to major defects in arterial and vascular development. Proc Natl Acad Sci USA. 2004;101(45):15949–15954. doi: 10.1073/pnas.0407290101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J, et al. Endothelial TWIST1 promotes pathological ocular angiogenesis. Invest Ophthalmol Vis Sci. 2014;55(12):8267–8277. doi: 10.1167/iovs.14-15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shao Z, et al. Choroid sprouting assay: An ex vivo model of microvascular angiogenesis. PLoS One. 2013;8(7):e69552. doi: 10.1371/journal.pone.0069552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lambert V, et al. Laser-induced choroidal neovascularization model to study age-related macular degeneration in mice. Nat Protoc. 2013;8(11):2197–2211. doi: 10.1038/nprot.2013.135. [DOI] [PubMed] [Google Scholar]
- 21.Monticelli S, et al. MicroRNA profiling of the murine hematopoietic system. Genome Biol. 2005;6(8):R71. doi: 10.1186/gb-2005-6-8-r71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao C, et al. MiR-150 controls B cell differentiation by targeting the transcription factor c-Myb. Cell. 2007;131(1):146–159. doi: 10.1016/j.cell.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 23.Zhou B, Wang S, Mayr C, Bartel DP, Lodish HF. miR-150, a microRNA expressed in mature B and T cells, blocks early B cell development when expressed prematurely. Proc Natl Acad Sci USA. 2007;104(17):7080–7085. doi: 10.1073/pnas.0702409104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas MD, Kremer CS, Ravichandran KS, Rajewsky K, Bender TP. c-Myb is critical for B cell development and maintenance of follicular B cells. Immunity. 2005;23(3):275–286. doi: 10.1016/j.immuni.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 25.Luo Z, et al. MicroRNA-200C and -150 play an important role in endothelial cell differentiation and vasculogenesis by targeting transcription repressor ZEB1. Stem Cells. 2013;31(9):1749–1762. doi: 10.1002/stem.1448. [DOI] [PubMed] [Google Scholar]
- 26.Shen J, et al. MicroRNAs regulate ocular neovascularization. Mol Ther. 2008;16(7):1208–1216. doi: 10.1038/mt.2008.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poliseno L, et al. MicroRNAs modulate the angiogenic properties of HUVECs. Blood. 2006;108(9):3068–3071. doi: 10.1182/blood-2006-01-012369. [DOI] [PubMed] [Google Scholar]
- 28.Wang S, Olson EN. AngiomiRs--Key regulators of angiogenesis. Curr Opin Genet Dev. 2009;19(3):205–211. doi: 10.1016/j.gde.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Narasaraju T, et al. Role of microRNA-150 and glycoprotein nonmetastatic melanoma protein B in angiogenesis during hyperoxia-induced neonatal lung injury. Am J Respir Cell Mol Biol. 2015;52(2):253–261. doi: 10.1165/rcmb.2013-0021OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J, et al. Microvesicle-mediated transfer of microRNA-150 from monocytes to endothelial cells promotes angiogenesis. J Biol Chem. 2013;288(32):23586–23596. doi: 10.1074/jbc.M113.489302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y, et al. Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol Cell. 2010;39(1):133–144. doi: 10.1016/j.molcel.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 32.Mattapallil MJ, et al. The Rd8 mutation of the Crb1 gene is present in vendor lines of C57BL/6N mice and embryonic stem cells, and confounds ocular induced mutant phenotypes. Invest Ophthalmol Vis Sci. 2012;53(6):2921–2927. doi: 10.1167/iovs.12-9662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen J, et al. Wnt signaling mediates pathological vascular growth in proliferative retinopathy. Circulation. 2011;124(17):1871–1881. doi: 10.1161/CIRCULATIONAHA.111.040337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu Y, et al. Pathogenic role of the Wnt signaling pathway activation in laser-induced choroidal neovascularization. Invest Ophthalmol Vis Sci. 2013;54(1):141–154. doi: 10.1167/iovs.12-10281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lobov IB, et al. Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc Natl Acad Sci USA. 2007;104(9):3219–3224. doi: 10.1073/pnas.0611206104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abu El-Asrar AM, Struyf S, Verbeke H, Van Damme J, Geboes K. Circulating bone-marrow-derived endothelial precursor cells contribute to neovascularization in diabetic epiretinal membranes. Acta Ophthalmol. 2011;89(3):222–228. doi: 10.1111/j.1755-3768.2009.01700.x. [DOI] [PubMed] [Google Scholar]
- 37.Lee E, Rewolinski D. Evaluation of CXCR4 inhibition in the prevention and intervention model of laser-induced choroidal neovascularization. Invest Ophthalmol Vis Sci. 2010;51(7):3666–3672. doi: 10.1167/iovs.09-3802. [DOI] [PubMed] [Google Scholar]
- 38.Yan B, et al. lncRNA-MIAT regulates microvascular dysfunction by functioning as a competing endogenous RNA. Circ Res. 2015;116(7):1143–1156. doi: 10.1161/CIRCRESAHA.116.305510. [DOI] [PubMed] [Google Scholar]
- 39.Loscalzo J. The cellular response to hypoxia: Tuning the system with microRNAs. J Clin Invest. 2010;120(11):3815–3817. doi: 10.1172/JCI45105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nallamshetty S, Chan SY, Loscalzo J. Hypoxia: A master regulator of microRNA biogenesis and activity. Free Radic Biol Med. 2013;64:20–30. doi: 10.1016/j.freeradbiomed.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bertero T, et al. MicroRNA target identification: Lessons from hypoxamiRs. Antioxid Redox Signal. 2014;21(8):1249–1268. doi: 10.1089/ars.2013.5648. [DOI] [PubMed] [Google Scholar]
- 42.Yu ZY, Bai YN, Luo LX, Wu H, Zeng Y. Expression of microRNA-150 targeting vascular endothelial growth factor-A is downregulated under hypoxia during liver regeneration. Mol Med Rep. 2013;8(1):287–293. doi: 10.3892/mmr.2013.1493. [DOI] [PubMed] [Google Scholar]
- 43.Agrawal S, Chaqour B. MicroRNA signature and function in retinal neovascularization. World J Biol Chem. 2014;5(1):1–11. doi: 10.4331/wjbc.v5.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ling H, Fabbri M, Calin GA. MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat Rev Drug Discov. 2013;12(11):847–865. doi: 10.1038/nrd4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. The Association for Research in Vision and Ophthalmology (2015) Statement for the Use of Animals in Ophthalmic and Visual Research. Available at www.arvo.org/About_ARVO/Policies/Statement_for_the_Use_of_Animals_in_Ophthalmic_and_Visual_Research/
- 46.Connor KM, et al. Quantification of oxygen-induced retinopathy in the mouse: A model of vessel loss, vessel regrowth and pathological angiogenesis. Nat Protoc. 2009;4(11):1565–1573. doi: 10.1038/nprot.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith LE, et al. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35(1):101–111. [PubMed] [Google Scholar]
- 48.Stahl A, et al. Computer-aided quantification of retinal neovascularization. Angiogenesis. 2009;12(3):297–301. doi: 10.1007/s10456-009-9155-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen J, et al. Suppression of retinal neovascularization by erythropoietin siRNA in a mouse model of proliferative retinopathy. Invest Ophthalmol Vis Sci. 2009;50(3):1329–1335. doi: 10.1167/iovs.08-2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun Y, et al. Nuclear receptor RORalpha regulates pathologic retinal angiogenesis by modulating SOCS3-dependent inflammation. Proc Natl Acad Sci USA. 2015;112(33):10401–10406. doi: 10.1073/pnas.1504387112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gong Y, et al. Optimization of an image-guided laser-induced choroidal neovascularization model in mice. PLoS One. 2015;10(7):e0132643. doi: 10.1371/journal.pone.0132643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu D, et al. Brain endothelial miR-146a negatively modulates T-cell adhesion through repressing multiple targets to inhibit NF-κB activation. J Cereb Blood Flow Metab. 2015;35(3):412–423. doi: 10.1038/jcbfm.2014.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.