Significance
The cerebral cortex is the most highly evolved structure in the human brain. Generating the correct number and types of neurons is crucial for brain function. We show a central role of the Lhx2 homeoprotein in this task: deleting Lhx2 in cortical progenitors leads to a temporal shift of neurogenesis initiation, resulting in a much smaller cortex with decreased numbers of neurons in all cortical layers. Further, we found that Lhx2 is required for the Wnt/β-catenin pathway to maintain progenitor proliferation. Using a parsimonious mathematical model, we demonstrated that such disruptions of neurogenesis timing are enough to explain the cortical size and thickness modifications observed. Our findings enlighten how neurogenesis timing is regulated molecularly and how it affects cortical size and organization.
Keywords: cortical neurogenesis, Lhx2, β-catenin
Abstract
The timing of cortical neurogenesis has a major effect on the size and organization of the mature cortex. The deletion of the LIM-homeodomain transcription factor Lhx2 in cortical progenitors by Nestin-cre leads to a dramatically smaller cortex. Here we report that Lhx2 regulates the cortex size by maintaining the cortical progenitor proliferation and delaying the initiation of neurogenesis. The loss of Lhx2 in cortical progenitors results in precocious radial glia differentiation and a temporal shift of cortical neurogenesis. We further investigated the underlying mechanisms at play and demonstrated that in the absence of Lhx2, the Wnt/β-catenin pathway failed to maintain progenitor proliferation. We developed and applied a mathematical model that reveals how precocious neurogenesis affected cortical surface and thickness. Thus, we concluded that Lhx2 is required for β-catenin function in maintaining cortical progenitor proliferation and controls the timing of cortical neurogenesis.
Understanding how genetic mechanisms interact to set up a precise developmental timing is a fundamental issue in biology. In the cerebral cortex, excitatory neurons are generated by progenitor cells in the dorsal telencephalon (dTel) lining the lateral ventricle. During the early developmental stages, cortical progenitors undergo symmetric divisions, resulting in the proliferation of progenitors and thereby allowing expansion of the developing cortex. Soon after, cortical progenitors start generating distinct types of neurons through asymmetric differentiative divisions (1–5). The precise timing of the switch from proliferative division to differentiative division is crucial to determining the number of cortical neurons, and thus the cortical size.
The switch from proliferation to differentiation is reportedly regulated by the canonical Wnt signaling pathway, in which β-catenin (β-Cat) is the major downstream effector. In the absence of Wnt signaling, β-Cat is phosphorylated by glycogen synthase kinase 3 and targeted for proteosome degradation. Once Wnt ligands bind to the Frizzled-Lrp5/6 receptors, the activity of glycogen synthase kinase 3-Axin-APC (adenomatous polyposis coli) destruction complex is inhibited. As a consequence, β-Cat accumulates in the cytoplasm, translocates to the nucleus, and activates downstream gene transcription together with the lymphoid enhancer-binding factor (LEF)/T-cell factor (TCF) transcription factors (6). Overexpression of the stabilized, N-terminally truncated form of β-Cat in cortical progenitors during early neurogenesis promotes their overproliferation (7, 8), whereas inactivation of β-Cat in the cortex promotes neurogenesis (9, 10). However, stabilized β-Cat was also shown to promote cortical progenitor differentiation (11). Thus, it has been proposed that Wnt/β-Cat signaling promotes proliferation and differentiation of cortical progenitors at early and late developmental stages, respectively (12). This raises the essential and largely open question of how Wnt/β-Cat regulates cortical progenitor proliferation and differentiation.
The LIM-homeodomain transcription factor Lhx2 plays an important role in cortical development. In the neocortex, Lhx2 is expressed by neocortical progenitors within the ventricular zone (VZ) of the dTel throughout cortical neurogenesis. Lhx2 was shown to play stage-specific roles determining the fate of cortical progenitors during early stages of corticogenesis (13–15). Further, the mutant mice with Lhx2 deleted in the neural progenitors at embryonic day 11.5 (E11.5) by Nestin-cre exhibited a significantly smaller neocortex than WT mice (16). Overall, these previous studies showed that Lhx2 is important for the determination and maintenance of neocortical progenitors, although how Lhx2 regulates the proliferation and differentiation of cortical progenitors is unclear.
In this study, we identified a role for Lhx2 in regulating the function of the Wnt/β-Cat signaling pathway in maintaining progenitor proliferation. The deletion of Lhx2 in cortical progenitors leads to a temporal shift of neurogenesis. We found that by regulating how cortical progenitors respond to Wnt/β-Cat signaling, Lhx2 regulates cortex size. By delaying neuronal differentiation and maintaining progenitor symmetric division, Lhx2 allows a suitable increase of the numbers of cortical progenitors needed to develop a proper cortex.
Results
Lhx2 Regulates the Timing of Sequential Cortical Neurogenesis.
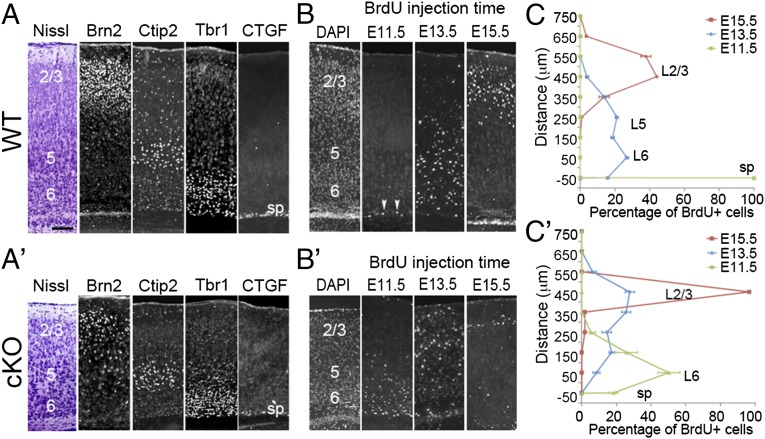
The deletion of Lhx2 in the cortical progenitors at E11.5 by Nestin-cre in Lhx2 conditional knockout (cKO, Lhx2f/f:Nestin-cre) leads to a significantly smaller and thinner cortex, although all six cortical layers were generated in the cKO, and the relative positions of cortical neurons in each were generally normal (16). To investigate the mechanisms for Lhx2 to regulate cortical neurogenesis, we first examined the timing of neurogenesis by injecting BrdU into pregnant mothers when embryos were at different developmental stages and analyzing the distribution of BrdU-labeled neurons at postnatal day 7 (P7), when the six cortical layers are easily distinguishable. To define each cortical layer in WT (Lhx2f/+ or Lhx2f/f) and Lhx2 cKO cortex, we analyzed the expression of cortical layer markers in P7 brains (17, 18). We found that in both WT and cKO cortex, the CTGF (connective tissue growth factor)-expressing subplate neurons were distributed in a single layer. We used Brn2, Ctip2, and the Tbr1 expression domain to determine layers (L) 2/3, 5, and 6, respectively (Fig. 1 A and A′). We found that the number of cells in all layers all significantly decreased in cKO cortex (SI Appendix, Fig. S1). In WT cortices, almost all neurons generated at E11.5 contributed to the subplate, whereas most neurons generated at E13.5 and E15.5 contributed to the deep (L6, L5) and superficial (L2/3) layers, respectively (Fig. 1 B and C) (17). In cKO cortices, although the inside-out organization was maintained, we found that neuronal birth dates shifted to earlier points. For example, in the cKO brain, most neurons born at E11.5 contributed to layer 6, and most neurons born at E13.5 contributed to superficial layers. In cKO cortices, the number of neurons generated at E15.5 dramatically decreased relative to WT, and these neurons were located superficially in layer 2/3 (Fig. 1 B′ and C′). BrdU birth dating analyses suggested that Lhx2 deletion in cortical progenitors altered the timing of neurogenesis. To confirm this, we examined expression of neuronal markers at E13.5. We observed comparable numbers of Reelin-positive Cajal-Retzius cells in WT and cKO cortices, whereas the cortical plate, which is labeled by Tbr1, Ctip2, and Satb2, was relatively thicker in the cKO cortex (SI Appendix, Fig. S2). Overall, these findings confirmed that cortical neurons are produced earlier in Lhx2 cKO.
Fig. 1.
Lhx2 regulates the timing of sequential neurogenesis. (A and A′) Immunostaining for markers of specific cortical layers on coronal sections of P7 WT (A) and cKO (A′) cortices. In both WT and cKO samples, six neuronal layers are present, including the Brn2-expressing L2/3 (2/3), Ctip2-expressing L5 (5), Tbr1-expressing L6 (6), and CTGF-expressing subplate (sp). (B and B′) Immunostaining for BrdU on coronal sections of P7 WT (B) and cKO (B′) cortices. BrdU was injected into pregnant mothers at E11.5, E13.5, or E15.5. BrdU injected at E11.5 labeled neurons distributed in the subplate in WT mice (arrowheads), but BrdU-labeled cells were detected in layer 6 in cKO mice. BrdU injected at E13.5 labeled neurons concentrated in layer 6 in WT mice, but E13.5 BrdU-labeled cells were spread to L2/3 in cKO mice. BrdU injected at E15.5 labeled many neurons in L2/3 in WT, but only a few superficially in L2/3 in cKO. (C and C′) Quantification of results from B and B′ indicating the location of BrdU-positive neurons in P7 WT and cKO cortices labeled at indicated times (n = 3). In a 100-μm-wide radial column, BrdU-labeled neurons were counted at 100-μm intervals from the subplate (defined as 0) to the pial surface. The percentage of BrdU-positive cells was calculated by determining the number of BrdU-positive cells in a 100 × 100 μm box divided by the total number of BrdU-positive cells in the entire radial column. (Scale bar, 100 μm.)
Neurogenesis and Radial Glia Differentiation Initiate Earlier in Lhx2 cKO.
To demonstrate that the loss of Lhx2 leads to increased neurogenesis during early cortical development, we analyzed the production of divisions by cortical progenitors derived from E13.5 WT and cKO dTel. We cultured progenitors at clonal density for 24 h to allow them to divide once to form two-cell pairs. We observed significantly fewer two-cell pairs formed from cells from the cKO compared with WT dTel (Fig. 2A). We also stained the two-cell pairs with antibodies against TuJ1 (neuron-specific class III b-tubulin) to label neurons and Sox2 (sex-determining region Y-related HMG box 2) to label progenitors. Pairs were identified as symmetric proliferative (P–P) divisions to form two Sox2-positive progenitors, symmetric neurogenic (N–N) divisions to form two TuJ1-positive neuronal cells, or asymmetric (P–N) divisions with one Sox2-positive and one TuJ1-positive cell. In WT and cKO cortices, the percentage of N–N pairs was comparable. In WT cortex, the majority of the two-cell pairs were P–P, indicating proliferative division of most progenitors at this stage. We found that in cKO cortex, the percentage of P–P and P–N pairs was significantly decreased and increased, respectively (Fig. 2B). This finding agreed with our previous report that Lhx2 deletion by Nestin-cre enhances neurogenesis, with an increased number of cortical progenitors exciting cell cycle at early developmental stages (16).
Fig. 2.
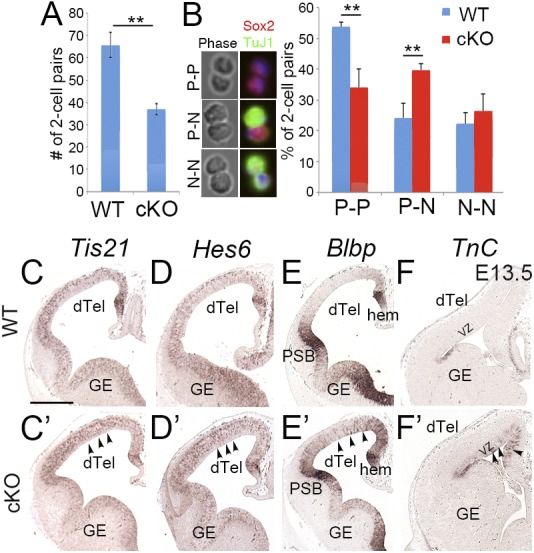
Lhx2 maintains proliferative division in progenitor cells and regulates the timing of radial glia differentiation. (A) Cortical progenitors from E13.5 WT and cKO dTel were cultured at clonal density for 24 h and then evaluated for cell pair formation. cKO formed significantly fewer pairs than WT (n = 3; P < 0.01). (B) Immunostaining of two-cell pairs for Sox2 and TuJ1. P–P, a pair with two Sox2-positive cells; N–N, a pair with two TuJ1-positive cells; and P–N pairs exhibit one of each. Quantitative results demonstrated that in the cKO cortex, the number of P–P pairs decreased significantly (n = 3; P < 0.01), and the number of P–N pairs increased significantly (n = 3; P < 0.01), whereas the number of N–N pairs is not significantly different (n = 3; P = 0.3435). (C–F′) In situ hybridization for Tis21, Hes6, Blbp, and TnC on coronal sections of E12.5 (C–E′) and E13.5 (F and F′) WT and cKO dTel. (C–D′) Tis21 and Hes6 expression in the dTel increased in the cKO cortex (arrowheads). (E and E′) At E12.5, Blbp is expressed in the cortical hem (hem) and the pallial-subpallial boundary (PSB) in WT dTel. Blbp expression is increased in the cKO (arrowheads). (F and F′) At E13.5, TnC expression is up-regulated in the cKO dTel VZ (arrowheads). (Scale bar, 200 μm.) GE, ganglionic eminence.
To provide additional evidence to support that the deletion of Lhx2 leads to increased neurogenic progenitors, we examined the expression of neurogenic progenitor markers, such as Tis21 (Btg2) and Hes6 (19–21), in E12.5 WT and cKO cortices. We found significantly increased expression of Tis21 and Hes6 in the dTel VZ in Lhx2 cKO relative to WT (Fig. 2 C, C′, D, and D′ and SI Appendix, Fig. S3), an effect consistent with increased neurogenesis in the cKO.
Our results suggested that neurogenesis initiates earlier in Lhx2 cKO, and thus we further examined whether the differentiation of radial glia, the neurogenic progenitors, occurs earlier in cKO. In the developing dTel, we defined radial glial cells (RGCs) with RGC markers Blbp (brain lipid-binding protein; or Fabp7, fatty acid binding protein 7), Glast (glial high-affinity glutamate transporter; or Slc1a3, solute carrier family 1 member 3), and TnC (Tenasin-C) (22–27). We found that all these RGC markers are precociously up-regulated in the VZ of cKO dTel. (Fig. 2 E, E′, F, and F′ and SI Appendix, Figs. S3 and S4). The precocious expression of radial glial marker genes suggested that the neurogenic RGCs are precociously generated in the Lhx2 cKO. Together with the previous results, we concluded that the deletion of Lhx2 in the cortical progenitors by Nestin-cre results in an earlier neurogenic RGC differentiation and an earlier initiation of neurogenesis.
The smaller cortex phenotype could alternatively be the consequence of increased cell death in the cKO cortices. To test this hypothesis, we examined whether the deletion of Lhx2 causes increased cell death. We performed TUNEL analyses on E11.5, E12.5, E13.5, and E15.5 WT and cKO embryos. In general, very few apoptotic cells are present in the developing WT dTel, and we did not detect a measurable increase of apoptotic cells in the Lhx2 cKO dTel (SI Appendix, Fig. S5). As the decreased cortical size is apparent by E13.5, we concluded that cell death is unlikely to account for the change in cortical size in cKO.
dTel of Lhx2 cKO Mice Shows Misregulated β-Cat Transcriptional Activity.
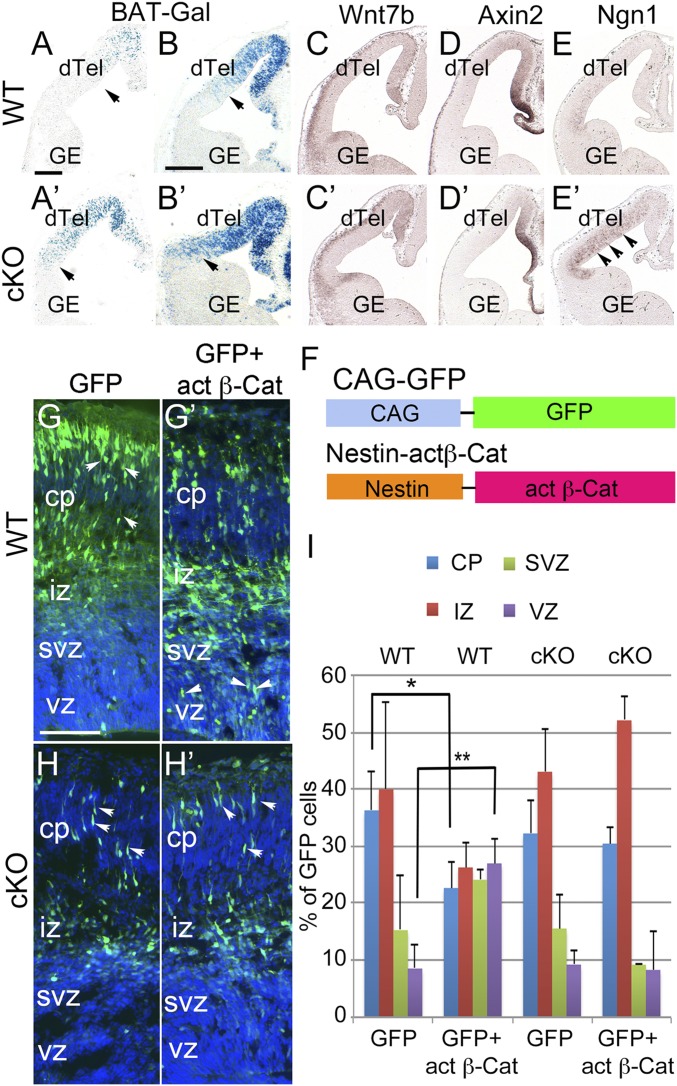
Canonical Wnt signaling reportedly governs whether cortical progenitors undergo proliferation or differentiation (28). To establish a readout for activity of canonical Wnt signaling, we crossed Lhx2 cKO mice with the BAT-Gal reporter line, in which the β-Gal gene is regulated by TCF binding sites such that the reporter is activated by accumulated β-Cat in the nucleus (29). Given the stage-dependent function of β-Cat in inducing proliferation or differentiation, we examined BAT-Gal reporter expression at several stages from E11.5 to E13.5 in the WT and cKO cortices. In WT cortices, LacZ-positive cells were distributed in a high/medial to low/lateral gradient in the dTel VZ, which correlates with the location of Wnt-expressing cortical hem cells located in the most medial part of the cortex (30). The number of LacZ-positive cells increased in the cKO dTel VZ from E11.5 to E13.5, and these LacZ-positive cells were distributed throughout the entire VZ of the cKO dTel, even in the most lateral part (Fig. 3 A, A′, B, and B′ and SI Appendix, Fig. S6). Thus, with the BAT-Gal reporter, we found that the transcriptional activity of canonical Wnt signaling is dramatically increased in the Lhx2 cKO. This is unexpected, given that the precocious neurogenesis we observed in Lhx2 cKO is similar to the phenotype of β-Cat deletion (9, 10).
Fig. 3.
Wnt/β-Cat signaling is misregulated and fails to maintain progenitor proliferation in the dTel of Lhx2 cKO mice. (A and B′) LacZ staining of coronal sections from E11.5 (A and A′) and E12.5 (B and B′) WT and cKO cortices with the BAT-Gal reporter. In WT mice, β-Gal expression shows a high/medial to low/lateral gradient and is undetectable in lateral dTel. In cKO mice, β-Gal expression is greatly increased, especially in the lateral dTel. Arrowheads indicate the extent of the distribution of LacZ-positive cells. (C–E′) In situ hybridization of Wnt7b, Axin2, and Ngn1 on coronal sections of E12.5 WT and cKO cortices. The expression level and pattern of Wnt7b and Axin2 are maintained in the cKO, whereas the expression of Ngn1 is up-regulated in the cKO (arrowheads). (F) Constructs used for in utero electroporation. (Top) CAG-GFP contains a ubiquitously active CAG promoter and GFP. (Bottom) Nestin-actβCat contains a Nestin enhancer in front of the Hsp68 promoter driving expression of the active form of β-Cat in the neural progenitor cells. (G and H′) Immunostaining for GFP on coronal sections of E15.5 WT (G and G′) and cKO (H and H′) brains electroporated at E13.5 with CAG-GFP alone (G and H) or CAG-GFP together with Nestin-actβCat (G′ and H′). (G) Most GFP-expressing cells are distributed in cortical plate (cp) (arrowheads) and the intermediate zone (iz) in brains transfected with CAG-GFP alone. (G′) Many GFP-expressing cells remain in the ventricular zone (vz) (arrowheads) in CAG-GFP+Nestin-actβCat-cotransfected WT brains. (H and H′) In either GFP alone (H) or CAG-GFP+Nestin-actβCat (H′) transfected cKO brains, most GFP-expressing cells are distributed in the intermediate zone and cortical plate (arrowheads), rather than in the ventricular zone. (I) Histogram showing the percentage of GFP+ cells in cp, iz, svz, and vz in a 100-μm-wide column of dTel. When actβCat is transfected in the WT, GFP+ cells located in the cortical plate are significantly reduced (n = 3; P < 0.05), whereas GFP+ cells located in the ventricular zone are significantly increased (n = 3; P < 0.01). (Scale bars, 100 μm, A, A′, G, H′; 200 μm, B–E′.) GE, ganglionic eminence.
We then examined whether the deletion of Lhx2 affects the expression of components in Wnt/β-Cat signaling pathway in a similar way as the BAT-Gal reporter. We examined the expression of genes involved in Wnt signaling during early cortical development in the cKO, including Wnt ligands Wnt3a and Wnt7b; Wnt antagonists Sfrp1 and Sfrp2; and Wnt downstream factors Axin2, Lef1, CyclinD1, and Ngn1 (11, 31). We found no significant difference in the expression of most of these genes in the Lhx2 cKO dTel compared with WT from E11.5 to E13.5 (Fig. 3 C, C′, D, and D′ and SI Appendix, Fig. S7). However, we did detect a dramatic up-regulation of Ngn1 (Fig. 3 E and E′), a reported Wnt downstream target driving neuronal differentiation (11). Further, the expression of Sfrp2 at the pallium–subpallium boundary is decreased in cKO at E13.5 (SI Appendix, Fig. S7). We found that in Lhx2 cKO, the high level of Wnt/β-Cat signaling transcriptional activity fails to increase the expression of many of the Wnt downstream genes, such as Axin2 and CyclinD1. We thus hypothesized that Lhx2 is required for the function of Wnt signaling.
Wnt/β-Cat Signaling Fails to Maintain Cortical Progenitor in the dTel of Lhx2 cKO Mice.
To examine the requirement for Lhx2 in the Wnt/β-Cat signaling pathway to promote cortical progenitor proliferation, we compared the function of active β-Cat in WT and cKO cortical progenitors. We generated a Nestin-actβCat construct, in which a stabilized β-Cat with first 47-amino acid truncation is driven by the nestin enhancer and the hsp68 basal promoter (32) (Fig. 3F) to drive transcriptionally active β-Cat expression in VZ progenitors. In addition, we generated a CAG (CMV early enhancer/chicken β-actin promoter)-GFP construct, in which a GFP reporter is driven by a constitutively active CAG promoter (Fig. 3F). In E13.5 dTel, we electroporated the CAG-GFP construct with or without the Nestin-actβCat construct and then analyzed distribution of GFP-expressing cells as the transfected cells at E15.5. In WT cortices transfected with CAG-GFP alone, most GFP-expressing cells were distributed in the intermediate zone and cortical plate, but not in the proliferative zone, VZ/SVZ (Fig. 3 G and I). However, after cotransfection with the Nestin-actβCat construct in WT, a significantly increased number of transfected cells remained in the VZ and decreased number of transfected cells migrated to the cortical plate (Fig. 3 G′ and I). To ensure that the increased number of the active β-Cat transfected cells in the VZ was not a result of migration defects of these cells, we stained them with a neuronal marker, TuJ1. We found that these VZ located cells do not express the neuronal marker (SI Appendix, Fig. S8). We thus concluded that the expression of active β-Cat in WT cortical progenitors maintains these progenitors in the proliferating zone.
We also electroporated CAG-GFP alone into the cKO dTel at E13.5, and we found, similar to in WT dTel, that most GFP-expressing cells were distributed in the intermediate zone and cortical plate at E15.5 (Fig. 3 H and I). When both CAG-GFP and Nestin-actβCat constructs were electroporated into the cKO dTel, most GFP-labeled cells still migrated to the cortical plate and did not stay in the VZ/SVZ (Fig. 3 H′ and I). Our results here showed that in the absence of Lhx2, the cKO cortical progenitors do not remain in the proliferating zone in response to active β-Cat.
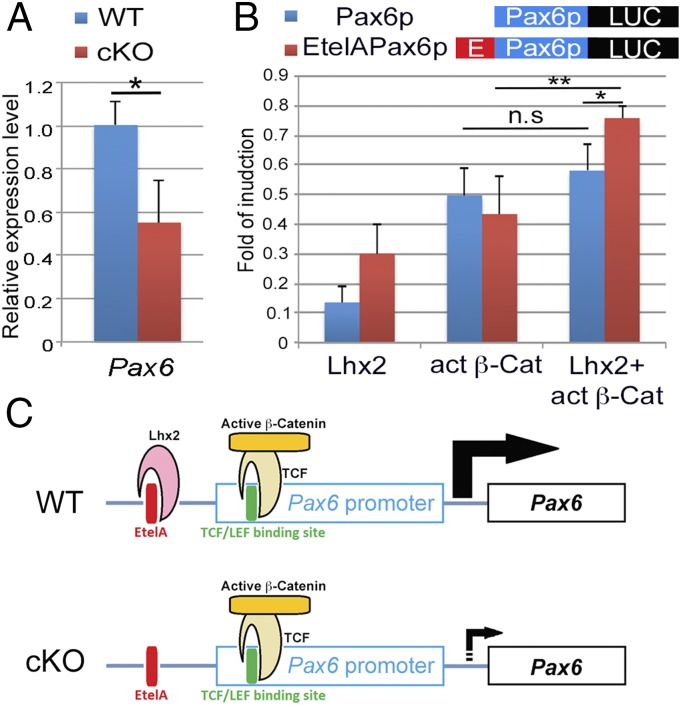
Our results demonstrated that Lhx2 is required for the function of β-Cat to promote cortical progenitor proliferation. We hypothesized that Lhx2 could act together with β-Cat to regulate the expression of genes governing progenitor proliferation. Pax6 is likely to be one of such genes, as it is a key regulator for cortical progenitor proliferation and neurogenesis (33). Further, in cortical progenitors, the β-Cat/TCF complex was shown to activate Pax6 transcription by binding to the Pax6 promoter (34), and Lhx2 was also reported to directly regulate Pax6 expression by binding to its enhancers (35, 36). We first confirmed that Pax6 is down-regulated in the cKO dTel at E12.5 (Fig. 4A). We then performed luciferase reporter assays to analyze the function of Lhx2 and active β-Cat on regulating Pax6 expression. We compared the induction level of Lhx2, active β-Cat or Lhx2 and active β-Cat together on Pax6p, in which luciferase gene is under the control of the Pax6 promoter containing a β-Cat/TCF binding site (34), and EtelAPax6p, in which luciferase gene is under the control of the Pax6 promoter and a Pax6 enhancer containing a Lhx2 binding site (35). We found that EtelAPax6p can be induced by active β-Cat, as well as by Lhx2, while Pax6p can be induced by active β-Cat, but not by Lhx2. When both Lhx2 and active β-Cat were cotransfected, EtelAPax6p can be further induced to reach a significantly higher level than Pax6p (Fig. 4B). These results suggested that Lhx2 and active β-Cat could collaboratively induce Pax6 expression (Fig. 4C).
Fig. 4.
Lhx2 and β-Cat collaboratively induce Pax6 expression. (A) Quantitative RT-PCR to compare the relative expression level of Pax6 in E12.5 WT and cKO dTel. The expression of Pax6 is significantly down-regulated in the cKO (P < 0.05; n = 5). (B) The reporter constructs, Pax6p-Luc (containing Pax6 promoter) and EtelAPax6p-Luc (containing Pax6 promoter and a Pax6 enhancer, E, as indicated). Reporter activity without cotransfection of effectors was set as 1, and results are presented as fold of induction. The activity of Pax6p cannot be induced by Lhx2, but it can be induced by active βCat, and there is no further induction when Lhx2 and active βCat were both added (n.s, not significant). EtelAPax6p-Luc activity can be induced by Lhx2 and active βCat, individually. Lhx2 and active βCat together can further induce the activity of EtelAPax6p (P < 0.01; n = 3). (C) Model for Lhx2 and active βCat collaboratively regulate Pax6 expression.
Timing of Neurogenesis Affects the Cortical Size.
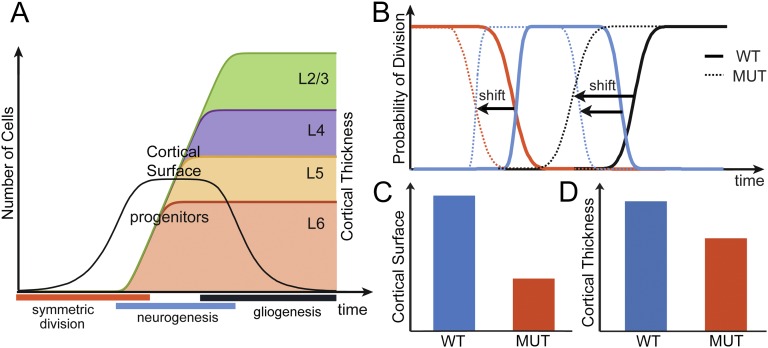
Our experimental data reveal that the deletion of Lhx2 leads to a significantly smaller cortex, which is associated with a precocious initiation of cortical neurogenesis. To demonstrate that this early neurogenesis can indeed account for the phenotype we observed in Lhx2 cKO, we developed a parsimonious mathematical model that simulates the sequence of divisions of progenitors during cortical neurogenesis (see details in SI Appendix). The model reproduces the sequences of progenitor divisions during corticogenesis (2); namely (i) symmetric division into two progenitors (proliferative phase); (ii) asymmetric division into a progenitor and an intermediate progenitor, itself generating two neurons; (iii) asymmetric division into a progenitor and a neuron (ii and iii are neurogenesis phase); and (iv) loss of capacity to generate neurons (gliogenesis phase). When a neuron is generated, it is considered to belong to a specific layer according to the classical radial inside-out migration of neurons to the distinct layers, depending on their generation time (17, 37). An example of the evolution of the number of progenitors and neuronal cells as a function of time in the model is provided in Fig. 5A.
Fig. 5.
Theoretically deciphering the effect of Lhx2 deletion. (A) Evolution of the number of progenitors (black line) and number of neurons in each layer as a function of time. Symmetric division (red) duplicates the number of progenitors, neurogenesis (blue) gives rise to neurons, and gliogenesis (black) decreases the pool of progenitors. (B) Comparison between fitted models to WT and cKO (MUT, dotted lines). In cKO, the precocious end of symmetric division and neurogenesis leads to decreased cortical surface (C) and thickness (D).
We used the model to investigate the causal relationship of the smaller and thinner cortex and the temporal shift of neurogenesis program in Lhx2 cKO (as shown in Fig. 1). The size of the cortical surface is directly related to the number of the progenitors lining the ventricular zone. As the number of progenitors is an exponential function of the duration of the proliferative phase, a shorter duration of the progenitor symmetric division period reduced the surface size of the cKO cortex (Fig. 5 B and C and SI Appendix, Fig. S9). The thickness of the cortex is related to the number of neurons generated in a radial column and is governed by the duration of the neurogenesis phase (SI Appendix, Fig. S9). To generate a smaller and thinner cortex, we found that the durations of proliferative phase and the neurogenesis phases in Lhx2 cKO are shorter (Fig. 5 B, C, and D).
In addition, the mathematical model provided us with further information on how to generate a thinner cortex with a disproportional change in the thickness of deep and superficial layers, as in the cKO cortices (as shown in SI Appendix, Fig. S1). We found that an early initiation of gliogenesis during the neurogenesis phase would lead to a more dramatic reduction of neuronal numbers in superficial layers than in deep layers. We tested this prediction experimentally and confirmed that the gliogenesis indeed initiates earlier in the cKO (SI Appendix, Figs. S9 and S10).
Discussion
In this study, we uncovered biological mechanisms regulating the timing of neurogenesis. We found that Lhx2 deletion by Nestin-cre leads to a shift of the sequential process of neurogenesis to an earlier time. We thus concluded that Lhx2 maintains cortical progenitors in proliferative state and delays the initiation of neurogenic differentiation at early developmental stages. This finding is consistent with the fact that Lhx2 expression in cortical progenitors decays during cortical development and that Lhx2 is expressed in a pattern opposite to that of neurogenesis: it forms a high/medial to low/lateral and high/caudal to low/rostral gradient, whereas neurogenesis initiates from the rostrolateral cortex, where Lhx2 is expressed at the lowest level (38).
Further, we identified that Lhx2 is involved in the Wnt/β-Cat signaling pathway. This pathway is known to play important roles in regulating cortical neurogenesis; however, it was shown to induce both progenitor proliferation and differentiation, two contradicting events (28). It has been difficult to analyze β-Cat function in knockout animals because mutants also show defects in cell adhesion and tissue integrity. Ectopic expression of Dkk1, a negative regulator of Wnt signaling pathway, in the developing cortex was used to reduce Wnt signaling activity while maintaining β-Cat function in cortical progenitors. It confirmed that Wnt signaling plays a positive role in the expansion of progenitors (39). Recently, the role of β-Cat in cortical development was studied elegantly by constructing a mutant form of β-Cat with normal cell–cell adhesion activity, but defective transcriptional activity (40). Interestingly, mice harboring this mutant form of β-Cat exhibited a phenotype similar to what we observe in Lhx2 cKO, including a temporal shift of neurogenesis and fewer neurons in both deep and upper cortical layers (40).
As the deletion of Lhx2 resembles the loss of Wnt/β-Cat signaling activity, we expected to find decreased β-Cat transcriptional activity in Lhx2 cKO. However, with BAT-Gal reporter, we found an increase in β-Cat transcriptional activity in Lhx2 cKO dTel (Fig. 3). Interestingly, we observed a discrepancy between the expression of the BAT-Gal reporter, a faithful reporter for Wnt/β-Cat signaling pathway in many systems (9, 29, 39, 41, 42), and the known Wnt/β-Cat downstream genes, such as Axin2 and CyclinD1 (SI Appendix, Fig. S7). Only Ngn1, a neurogenic Wnt/β-Cat downstream gene (28), is up-regulated in the Lhx2 cKO (Fig. 3). We further confirmed that the loss of Lhx2 impairs cortical progenitor self-renewal promoted by Wnt/β-Cat signaling by showing that the expression of stabilized β-Cat maintains transfected cells in the ventricular zone in WT, but not in Lhx2 cKO (Fig. 3). Thus, we proposed that Lhx2 is involved in directing the activity of the Wnt/β-Cat signaling pathway to promote progenitor proliferation, probably in a similar way as Axin, a component in the Wnt/β-Cat signaling pathway, directing the proliferation or differentiation choice in the intermediate progenitors (43).
During cortical development, patterning centers express signaling molecules such as Fgfs and Wnts to establish expression patterns of transcription factors and regulate cortical neurogenesis and patterning. Several patterning transcription factors, including Emx2 (empty spiracles homeobox 2), COUP-TF1 (chicken ovalbumin upstream promoter transcription factor 1 or NR2F1), Pax6 (paired box 6), and Sp8 (trans-acting transcription factor 8), were shown to collaboratively regulate the proliferation and differentiation of cortical progenitors and cortical arealization (44). In cortical progenitors, these transcription factors establish a regulatory network, such as the reciprocal regulation between COUP-TF1 and Sp8 (45), to coordinate multiple signaling pathways. For example, COUP-TF1 regulates progenitor proliferation and differentiation (46) and also plays an important role in regulating cortical areal patterning (47). Similar to these patterning transcription factors, Lhx2 controls multiple aspects of cortical development. It is likely these different functions of Lhx2 are coordinated in cortical progenitors by interacting with other transcription factors during cortical neurogenesis. Further studies of genetic machinery regulated by Lhx2 and the interactions between Lhx2 and other transcription factors are needed to understand the mechanisms of the temporal regulation of neurogenesis.
Cortical size expansion and the increase in cortical neuronal number suggest that regulation of neurogenesis has changed over evolution (48, 49). The mathematical model of cortical neurogenesis we developed provided insights on how the timing of different phases in neurogenesis affect the size and composition of the cortex. Our findings suggested that the intricate interplay between Lhx2 and Wnt/β-Cat signaling pathway, by modifying the timing of neurogenesis, appears to be a key regulatory mechanism in cortical evolution and may function in cortical developmental disorders, such as microcephaly.
Materials and Methods
The different mouse lines and antibodies used in this study, along with their sources and the detailed protocols for cell culture, luciferase reporter assays, and in utero electroporation, are described in SI Appendix, Materials and Methods. In situ hybridization and immunostainings were performed as described previously (14, 16).
Supplementary Material
Acknowledgments
We thank Dr. Dennis O’Leary for providing the Lhx2 floxed allele, Nestin-cre, and BAT-Gal mouse lines; Dr. Paola Bovolenta for Axin2 and CyclinD1 in situ probes; and Dr. Chin-Ying Tai for stabilized β-Cat constructs. We also thank Dr. Hwai-Jong Cheng for advice and members of the S.-J.C. laboratory for their help. This work was supported by NHRI-EX102, 103-10260NI (National Health Research Institutes), AS-104-TP-B09-2 (Academia Sinica), and the Institute of Cellular and Organismic Biology of Academia Sinica.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. J.L.R.R. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1507145112/-/DCSupplemental.
References
- 1.Fishell G, Kriegstein AR. Neurons from radial glia: The consequences of asymmetric inheritance. Curr Opin Neurobiol. 2003;13(1):34–41. doi: 10.1016/s0959-4388(03)00013-8. [DOI] [PubMed] [Google Scholar]
- 2.Florio M, Huttner WB. Neural progenitors, neurogenesis and the evolution of the neocortex. Development. 2014;141(11):2182–2194. doi: 10.1242/dev.090571. [DOI] [PubMed] [Google Scholar]
- 3.Götz M, Huttner WB. The cell biology of neurogenesis. Nat Rev Mol Cell Biol. 2005;6(10):777–788. doi: 10.1038/nrm1739. [DOI] [PubMed] [Google Scholar]
- 4.Noctor SC, Martínez-Cerdeño V, Kriegstein AR. Distinct behaviors of neural stem and progenitor cells underlie cortical neurogenesis. J Comp Neurol. 2008;508(1):28–44. doi: 10.1002/cne.21669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miyata T, Kawaguchi D, Kawaguchi A, Gotoh Y. Mechanisms that regulate the number of neurons during mouse neocortical development. Curr Opin Neurobiol. 2010;20(1):22–28. doi: 10.1016/j.conb.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Clevers H, Loh KM, Nusse R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science. 2014;346(6205):1248012. doi: 10.1126/science.1248012. [DOI] [PubMed] [Google Scholar]
- 7.Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297(5580):365–369. doi: 10.1126/science.1074192. [DOI] [PubMed] [Google Scholar]
- 8.Wrobel CN, Mutch CA, Swaminathan S, Taketo MM, Chenn A. Persistent expression of stabilized beta-catenin delays maturation of radial glial cells into intermediate progenitors. Dev Biol. 2007;309(2):285–297. doi: 10.1016/j.ydbio.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Machon O, et al. A dynamic gradient of Wnt signaling controls initiation of neurogenesis in the mammalian cortex and cellular specification in the hippocampus. Dev Biol. 2007;311(1):223–237. doi: 10.1016/j.ydbio.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 10.Mutch CA, Schulte JD, Olson E, Chenn A. Beta-catenin signaling negatively regulates intermediate progenitor population numbers in the developing cortex. PLoS One. 2010;5(8):e12376. doi: 10.1371/journal.pone.0012376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirabayashi Y, et al. The Wnt/beta-catenin pathway directs neuronal differentiation of cortical neural precursor cells. Development. 2004;131(12):2791–2801. doi: 10.1242/dev.01165. [DOI] [PubMed] [Google Scholar]
- 12.Inestrosa NC, Varela-Nallar L. Wnt signalling in neuronal differentiation and development. Cell Tissue Res. 2015;359(1):215–223. doi: 10.1007/s00441-014-1996-4. [DOI] [PubMed] [Google Scholar]
- 13.Bulchand S, Grove EA, Porter FD, Tole S. LIM-homeodomain gene Lhx2 regulates the formation of the cortical hem. Mech Dev. 2001;100(2):165–175. doi: 10.1016/s0925-4773(00)00515-3. [DOI] [PubMed] [Google Scholar]
- 14.Chou SJ, Perez-Garcia CG, Kroll TT, O’Leary DD. Lhx2 specifies regional fate in Emx1 lineage of telencephalic progenitors generating cerebral cortex. Nat Neurosci. 2009;12(11):1381–1389. doi: 10.1038/nn.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mangale VS, et al. Lhx2 selector activity specifies cortical identity and suppresses hippocampal organizer fate. Science. 2008;319(5861):304–309. doi: 10.1126/science.1151695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chou SJ, O’Leary DD. Role for Lhx2 in corticogenesis through regulation of progenitor differentiation. Mol Cell Neurosci. 2013;56:1–9. doi: 10.1016/j.mcn.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Molyneaux BJ, Arlotta P, Menezes JR, Macklis JD. Neuronal subtype specification in the cerebral cortex. Nat Rev Neurosci. 2007;8(6):427–437. doi: 10.1038/nrn2151. [DOI] [PubMed] [Google Scholar]
- 18.Leone DP, Srinivasan K, Chen B, Alcamo E, McConnell SK. The determination of projection neuron identity in the developing cerebral cortex. Curr Opin Neurobiol. 2008;18(1):28–35. doi: 10.1016/j.conb.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arai Y, et al. Neural stem and progenitor cells shorten S-phase on commitment to neuron production. Nat Commun. 2011;2:154. doi: 10.1038/ncomms1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iacopetti P, et al. Expression of the antiproliferative gene TIS21 at the onset of neurogenesis identifies single neuroepithelial cells that switch from proliferative to neuron-generating division. Proc Natl Acad Sci USA. 1999;96(8):4639–4644. doi: 10.1073/pnas.96.8.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gratton MO, et al. Hes6 promotes cortical neurogenesis and inhibits Hes1 transcription repression activity by multiple mechanisms. Mol Cell Biol. 2003;23(19):6922–6935. doi: 10.1128/MCB.23.19.6922-6935.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hartfuss E, Galli R, Heins N, Götz M. Characterization of CNS precursor subtypes and radial glia. Dev Biol. 2001;229(1):15–30. doi: 10.1006/dbio.2000.9962. [DOI] [PubMed] [Google Scholar]
- 23.Anthony TE, Klein C, Fishell G, Heintz N. Radial glia serve as neuronal progenitors in all regions of the central nervous system. Neuron. 2004;41(6):881–890. doi: 10.1016/s0896-6273(04)00140-0. [DOI] [PubMed] [Google Scholar]
- 24.Feng L, Hatten ME, Heintz N. Brain lipid-binding protein (BLBP): A novel signaling system in the developing mammalian CNS. Neuron. 1994;12(4):895–908. doi: 10.1016/0896-6273(94)90341-7. [DOI] [PubMed] [Google Scholar]
- 25.Hegedus B, et al. Neurofibromatosis-1 regulates neuronal and glial cell differentiation from neuroglial progenitors in vivo by both cAMP- and Ras-dependent mechanisms. Cell Stem Cell. 2007;1(4):443–457. doi: 10.1016/j.stem.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 26.Anthony TE, Heintz N. Genetic lineage tracing defines distinct neurogenic and gliogenic stages of ventral telencephalic radial glial development. Neural Dev. 2008;3:30. doi: 10.1186/1749-8104-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Götz M, Bolz J, Joester A, Faissner A. Tenascin-C synthesis and influence on axonal growth during rat cortical development. Eur J Neurosci. 1997;9(3):496–506. doi: 10.1111/j.1460-9568.1997.tb01627.x. [DOI] [PubMed] [Google Scholar]
- 28.Bielen H, Houart C. The Wnt cries many: Wnt regulation of neurogenesis through tissue patterning, proliferation, and asymmetric cell division. Dev Neurobiol. 2014;74(8):772–780. doi: 10.1002/dneu.22168. [DOI] [PubMed] [Google Scholar]
- 29.Maretto S, et al. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci USA. 2003;100(6):3299–3304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grove EA, Tole S, Limon J, Yip L, Ragsdale CW. The hem of the embryonic cerebral cortex is defined by the expression of multiple Wnt genes and is compromised in Gli3-deficient mice. Development. 1998;125(12):2315–2325. doi: 10.1242/dev.125.12.2315. [DOI] [PubMed] [Google Scholar]
- 31.Katoh M. WNT signaling in stem cell biology and regenerative medicine. Curr Drug Targets. 2008;9(7):565–570. doi: 10.2174/138945008784911750. [DOI] [PubMed] [Google Scholar]
- 32.Zimmerman L, et al. Independent regulatory elements in the nestin gene direct transgene expression to neural stem cells or muscle precursors. Neuron. 1994;12(1):11–24. doi: 10.1016/0896-6273(94)90148-1. [DOI] [PubMed] [Google Scholar]
- 33.Manuel MN, Mi D, Mason JO, Price DJ. Regulation of cerebral cortical neurogenesis by the Pax6 transcription factor. Front Cell Neurosci. 2015;9:70. doi: 10.3389/fncel.2015.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gan Q, et al. Pax6 mediates ß-catenin signaling for self-renewal and neurogenesis by neocortical radial glial stem cells. Stem Cells. 2014;32(1):45–58. doi: 10.1002/stem.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hou PS, et al. LHX2 regulates the neural differentiation of human embryonic stem cells via transcriptional modulation of PAX6 and CER1. Nucleic Acids Res. 2013;41(16):7753–7770. doi: 10.1093/nar/gkt567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shetty AS, et al. Lhx2 regulates a cortex-specific mechanism for barrel formation. Proc Natl Acad Sci USA. 2013;110(50):E4913–E4921. doi: 10.1073/pnas.1311158110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greig LC, Woodworth MB, Galazo MJ, Padmanabhan H, Macklis JD. Molecular logic of neocortical projection neuron specification, development and diversity. Nat Rev Neurosci. 2013;14(11):755–769. doi: 10.1038/nrn3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suter B, Nowakowski RS, Bhide PG, Caviness VS. Navigating neocortical neurogenesis and neuronal specification: a positional information system encoded by neurogenetic gradients. J Neurosci. 2007;27(40):10777–10784. doi: 10.1523/JNEUROSCI.3091-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Solberg N, Machon O, Krauss S. Effect of canonical Wnt inhibition in the neurogenic cortex, hippocampus, and premigratory dentate gyrus progenitor pool. Dev Dyn. 2008;237(7):1799–1811. doi: 10.1002/dvdy.21586. [DOI] [PubMed] [Google Scholar]
- 40.Draganova K, et al. Wnt/β-catenin signaling regulates sequential fate decisions of murine cortical precursor cells. Stem Cells. 2015;33(1):170–182. doi: 10.1002/stem.1820. [DOI] [PubMed] [Google Scholar]
- 41.Mutch CA, Funatsu N, Monuki ES, Chenn A. Beta-catenin signaling levels in progenitors influence the laminar cell fates of projection neurons. J Neurosci. 2009;29(43):13710–13719. doi: 10.1523/JNEUROSCI.3022-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ivaniutsin U, Chen Y, Mason JO, Price DJ, Pratt T. Adenomatous polyposis coli is required for early events in the normal growth and differentiation of the developing cerebral cortex. Neural Dev. 2009;4:3. doi: 10.1186/1749-8104-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fang WQ, Chen WW, Fu AK, Ip NY. Axin directs the amplification and differentiation of intermediate progenitors in the developing cerebral cortex. Neuron. 2013;79(4):665–679. doi: 10.1016/j.neuron.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 44.O’Leary DD, Chou SJ, Sahara S. Area patterning of the mammalian cortex. Neuron. 2007;56(2):252–269. doi: 10.1016/j.neuron.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 45.Borello U, et al. Sp8 and COUP-TF1 reciprocally regulate patterning and Fgf signaling in cortical progenitors. Cereb Cortex. 2014;24(6):1409–1421. doi: 10.1093/cercor/bhs412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Faedo A, et al. COUP-TFI coordinates cortical patterning, neurogenesis, and laminar fate and modulates MAPK/ERK, AKT, and beta-catenin signaling. Cereb Cortex. 2008;18(9):2117–2131. doi: 10.1093/cercor/bhm238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Armentano M, et al. COUP-TFI regulates the balance of cortical patterning between frontal/motor and sensory areas. Nat Neurosci. 2007;10(10):1277–1286. doi: 10.1038/nn1958. [DOI] [PubMed] [Google Scholar]
- 48.Kaas JH. The evolution of brains from early mammals to humans. Wiley Interdiscip Rev Cogn Sci. 2013;4(1):33–45. doi: 10.1002/wcs.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kriegstein A, Noctor S, Martínez-Cerdeño V. Patterns of neural stem and progenitor cell division may underlie evolutionary cortical expansion. Nat Rev Neurosci. 2006;7(11):883–890. doi: 10.1038/nrn2008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.