Abstract
Background
Antigen presenting cells play a pivotal role in the adaptive immune response in hypersensitivity pneumonitis (HP). It was hypothesised that lymphangiogenesis is involved in the pathophysiology of HP via cell transport.
Objective
To determine the clinical significance of lymphangiogenic factors in HP.
Methods
Levels of vascular endothelial growth factors (VEGF)-A, VEGF-C, VEGF-D and CCL21 in the serum and bronchoalveolar lavage fluid (BALF) were measured in 29 healthy volunteers, 14 patients with idiopathic pulmonary fibrosis (IPF) and 26 patients with HP by ELISA. Additionally, immunohistochemical analyses were performed using lung specimens of patients with HP (n=8) and IPF (n=10).
Results
BALF VEGF-D levels were significantly elevated in patients with HP compared to the other groups. BALF VEGF–D levels in patients with HP correlated significantly with the BALF total cell and lymphocyte counts (r=0.485, p=0.014 and r=0.717, p<0.0001, respectively). BALF VEGF-C and CCL21 levels were increased in patients with HP compared to healthy volunteers, but not patients with IPF. BALF CCL21 levels were negatively correlated with the forced expiratory volume in 1 s percentage and diffuse capacity of the lung for carbon monoxide (r=−0.662, p=0.007 and r=−0.671, p=0.024, respectively). According to the immunohistochemical analyses, CCL21 was expressed in the lymphatic endothelium in both conditions and CCR7+ cells were aggregated around lymphatics in patients with HP, but not in patients with IPF.
Conclusions
Lymphangiogenic factors might be associated with the inflammatory and functional severity of HP. The increased BALF VEGF-D levels were associated with lymphatic alveolitis intensity, and CCL21 with lung function impairment.
Keywords: Allergic Alveolitis
Key messages.
Vascular endothelial growth factor (VEGF)-D and CCL21 in bronchalveolar lavage fluid (BALF) is elevated in hypersensitivity pneumonitis (HP), and correlated with inflammatory and functional severities of HP.
VEGF-D might be a useful marker for the diagnoses of HP and assessment of HP severity.
CCR7-CCL21 signals are related to HP pathogenesis.
Introduction
Hypersensitivity pneumonitis (HP) is a pulmonary disease caused by the inhalation of an antigen to which the patient has been previously sensitised. Acute-onset and subacute-onset diseases represent the most active forms of this condition, which is often complicated by moderate to severe respiratory failure.1 Pulmonary function test results are characterised by a restrictive functional pattern that is occasionally complicated by obstructive impairment.2 3 Furthermore, remarkable increases have been noted in the total cell and lymphocyte counts in bronchoalveolar lavage fluid (BALF).4 The characteristic histopathological feature is a granulomatous interstitial bronchiolocentric pneumonitis that mainly involves lymphocytes and histiocytes.5–7 In the host, the presentation of causative antigens is of pivotal importance to HP pathogenesis.8–10 Interactions between the causative antigens and antigen-presenting cells regulate the differentiation of CD4+ T cells into various effector subsets in the regional lymph nodes.1 Crucially, only very few individuals with previous antigen sensitisation develop clinically detectable disease. A 2-hit hypothesis has been suggested to interpret this intriguing observation; specifically, antigen exposure acts as the inducing factor, whereas genetic and environmental factors act as promoting risk factors.1
Lymphatic vessels are the principal conduits for the transport of soluble antigens and antigen-presenting cells from peripheral tissues to the lymph nodes in order to mount immune responses.11 Lymphangiogenesis occurs in various pathological conditions, including inflammation, wound healing and tumour metastasis, and it promotes immune responses. Vascular endothelial growth factor (VEGF)-C and VEGF-D generally induce lymphangiogenesis, and macrophages are a major source of these mediators in inflamed tissues.12–15 VEGF-A also substantially participates in lymphangiogenesis, although this factor typically induces angiogenesis.16 Recently, there has been increasing evidence that lymphatic endothelial cells express a variety of chemokines and cell surface markers.17–19 CCL21 (also known as the secondary lymphoid chemokine), which is exclusively secreted by lymphatic endothelial cells in afferent lymphatic vessels, attracts activated antigen-presenting cells that express the CCL-21 receptor CCR7, and CCL21-CCR7-mediated signalling can function as an active regulator of immune responses.20 21
Lymphangiogenic factors have been recently investigated in some lung diseases, including lung cancer, lymphangioleiomyomatosis and pulmonary sarcoidosis, for which the pathogenic roles of lymphangiogenesis and clinical adaption have been explored.18 22–24 However, limited information is available about the role of lymphangiogenesis in HP. It would be rational to hypothesise that lymphangiogenesis is involved in the development of this extrinsic allergic disease. In the present study, we aimed to clarify the clinical significance of lymphangiogenic factors in acute and subacute-onset HP by quantifying the expression of the three lymphatic VEGFs and CCL21 and evaluating the correlations of these factors with HP severity.
Materials and methods
Materials
We retrospectively examined the serum and BALF samples from 26 patients with acute or subacute-onset HP who had never been treated for this condition; samples from patients with chronic HP were excluded from this study. We used serum and BALF samples from 14 patients with idiopathic pulmonary fibrosis (IPF) and 29 healthy volunteers as controls. HP was diagnosed in all patients according to the criteria proposed by Richerson et al.25 The samples were obtained from the archives of the Departments of Pulmonary Medicine, Allergy and Rheumatology at Iwate Medical University, Japan. The use of all samples in this study was approved by The Ethical Committees of Iwate Medical University (IRB: H25-1). Informed consent was obtained from all patients and volunteers.
Pulmonary function tests
The vital capacity (VC), forced expiratory volume in 1 s (FEV1), diffuse capacity of the lung for carbon monoxide (DLco), and total lung capacity (TLC) were measured according to the American Thoracic Society guidelines.26 These values were also expressed as percentages of the predicted normal values calculated according to sex, weight and age.27 The values are shown as means±SDs. Six patients with HP did not participate in all pulmonary function tests.
Processing of serum and BALF samples
Serum samples were obtained by centrifuging peripheral blood at 3000 rpm for 15 min at 4°C. BALF samples were obtained from both patients with IPF and HP and normal healthy volunteers, according to previously described methods.18 Briefly, a flexible bronchoscope was wedged into a subsegmental bronchus in a predetermined region of interest based on radiographic findings. Bronchoalveolar lavage (BAL) was performed by instilling a total volume of 150 mL of normal saline in 50 mL aliquots, each of which was retrieved via low suction. The BALF mean (SD) recovery rates were 81% (8.9%) in healthy volunteers, 36% (5.2%) in patients with IPF and 47.7% (3.2%) in patients with HP; the recovery rate in each group satisfied the BAL criteria for interstitial lung disease as recommended by the American Thoracic Society.28 The cell pellets were resuspended in 1 mL of phosphate-buffered saline, and an aliquot was used to evaluate the total number of cells. Other aliquots were fixed, subjected to May-Giemsa staining and used for differential cell counting. The cell count values were recorded as means±SD. The BALF fractions were pooled and divided equally into two samples. One sample was sent to the clinical microbiology and cytology laboratory, and the other was placed on ice and transported to the research laboratory. The research sample was filtered through sterile gauze and centrifuged for 10 min at 500 g at 4°C. The resulting cell-free solution was aliquoted and frozen immediately at −80°C until thawed for cytokine ELISA analysis.
Immunoassay
The cytokine levels in the serum and BALF samples were determined by using VEGF-A, VEGF-C and VEGF-D Quantikine ELISA kits purchased from R&D Systems (Minneapolis, Minnesota, USA) and a CCL21 kit from Abcam (Cambridge, UK). The BALF levels of the 3 VEGF types were determined in samples that had been concentrated fivefold by using Minicon B15 concentrators (Merck Millipore, Billerica, Massachusetts, USA).18 Standard curves were plotted using known recombinant protein concentrations ranging from 0 to 1000 pg/mL. The lower limits of detection of the kits were as follows: VEGF-A, 15.6 pg/mL; VEGF-C, 109.0 pg/mL; VEGF-D, 125.0 pg/mL; and CCL21, 3.8 pg/mL. The standards and samples were analysed in duplicate. In particular, we performed spiking and recovery assay in BALF CCL21 using the BALF samples obtained from five healthy volunteers and the ELISA kit, because the measurement of CCL21 in BALF was not standardised. The recovery rate was 94.9% (±4.5%), indicating that the measurement of BALF CCL21 using this kit was valid.
Immunohistochemistry
We examined lung specimens of patients with HP (n=8), obtained by TBLB, and patients with IPF (n=10), obtained by video-assisted thoracic surgery. We used the primary antibodies against podoplanin (Angiobio, Del Mar, California, USA; dilution 1:100), CCL21 (R&D Systems; dilution 1:50) and CCR7 (R&D Systems; dilution 1:50). Podoplanin and CCL21 are antibodies immunized by mouse, whereas CCR7 is a goat antibody. The antigen-antibody reaction was visualised using 3,3′-diaminobenzidine tetrahydrochloride. Dual immunohistochemical staining with podoplanin and CCR7 was performed as described previously.18
Statistical analysis
A one-way analysis of variance followed by the Tukey test was used for analysing the variances among multiple groups. The Spearman rank correlation coefficient (r) was used to evaluate the relationship between (1) the levels of the three VEGF proteins and CCL21, and (2) the HP severity based on pulmonary function test results and BALF cell counts. The samples with levels below the lower detection limit values were included in the correlation coefficient analyses. A p value of <0.05 was considered statistically significant. Missing data were managed by removing data units.
Results
Patient characteristics
The characteristics and BALF data of the healthy volunteers, patients with IPF and patients with HP are summarised in table 1. The healthy volunteers were never-smokers and significantly younger relative to patients with HP and IPF (p<0.001). The sex distribution was not uniform among patients with IPF and HP. Among the three groups, the counts of total cells, macrophages and lymphocytes in BALF were significantly elevated in patients with HP (p<0.0001). The following clinical conditions were present in the 26 patients with HP: farmer's lung (n=15), mushroom lung (n=4), bird-related lung (n=4) and summer type (n=3). Among the 26 patients with HP, 7 and 19 patients had acute-onset and subacute-onset diseases, respectively.
Table 1.
Characteristics of healthy volunteers and patients with idiopathic pulmonary fibrosis (IPF) and hypersensitivity pneumonitis (HP)
| Healthy control | IPF | HP | p Value | |
|---|---|---|---|---|
| Subjects (n) | 29 | 14 | 26 | – |
| Age (years) | 25.9±7.51 | 68.1±1.7 | 61.7±2.51 | p<0.001 |
| Sex | ||||
| Female | 12 (41.4) | 6 (42.9) | 11 (42.3) | NS |
| Male | 17 (58.6) | 8 (57.1) | 15 (57.7) | NS |
| Smoker | 0 | 7 (50.0) | 9 (34.6) | |
| Type of disease onset | ||||
| Acute | ND | 0 | 7 (26.9) | – |
| Non-acute | ND | 14 (100) | 19 (73.1) | – |
| VC (% pred) | ND | 74.9±6.98 | 82.0±4.90 | NS |
| FEV1 (% pred) | ND | 82.7±1.43 | 79.3±3.73 | NS |
| TLC (% pred) | ND | 71.9±6.89 | 67.7±12.7 | NS |
| DLco (% pred) | ND | 66.8±6.02 | 67.9±6.60 | NS |
| Total cell counts (104/mm2) | 6.64±5.7 | 9.39±1.84 | 28.5±5.54 | p<0.001 |
| Differential cell count | ||||
| Macrophage (%) | 87.5±15.1 | 78.1±5.47 | 43.8±4.26 | p<0.001 |
| Lymphocyte (%) | 7.19±4.96 | 17.5±5.53 | 52.5±1.83 | p<0.001 |
| Neutrophil (%) | 0.74±0.80 | 2.58±0.39 | 2.06±0.41 | NS |
| Eosinophil (%) | 0.24±0.36 | 2.19±0.09 | 1.34±0.63 | NS |
| CD4/8 ratio | ND | 1.87±0.06 | 2.22±0.40 | NS |
Data are shown as means±SDs. Brackets indicate percentages.
p Values were calculated by comparing patients with HP with healthy volunteers and patients with IPF by using Tukey's test.
DLco, diffusing capacity of the lungs for carbon monoxide; FEV1, forced expiratory volume in 1 s; ND, not determined; NS, no statistical significance; TLC, total lung capacity; VC, vital capacity.
Characterisation of expression of the three VEGF types in patients with HP
Serum VEGF and BALF levels as measured by ELISA
The serum levels of the three VEGF types showed significant increases in patients with HP compared to healthy volunteers (figure 1). No differences were observed in the serum VEGF-C and VEGF-D levels between patients with HP and patients with IPF (figure 1). The BALF VEGF-A level did not differ among the three groups (figure 2A). The BALF VEGF-C and VEGF-D levels were significantly elevated in patients with HP compared to healthy volunteers. Furthermore, the BALF VEGF-D levels were significantly elevated in patients with HP compared to patients with IPF (figure 2C). To confirm the absence of an inflammatory gradient in patients with HP, we explored the correlation between the serum and BALF levels of the three growth factors. No correlation was detected for VEGF-A, VEGF-C and VEGF-D levels (r=0.29, p=0.15; r=0.014, p=0.941; and r=0.306, p=0.129, respectively).
Figure 1.
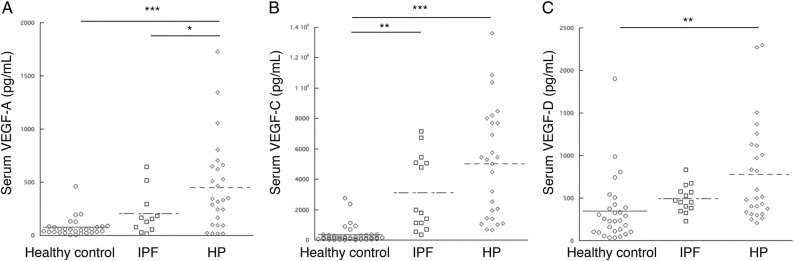
Comparison of the serum levels of the three vascular endothelial growth factor (VEGF) types in healthy volunteers and patients with idiopathic pulmonary fibrosis (IPF) and hypersensitivity pneumonitis (HP). (A) The serum VEGF-A level was significantly reduced in patients with IPF compared to healthy volunteers (p<0.05). (B) The serum VEGF-C levels were significantly elevated in patients with HP compared to those with the other groups (p<0.001). (C) The serum VEGF-D levels did not differ among the three groups. ***p<0.001, **p<0.01, *p<0.05.
Figure 2.

Comparison of the bronchoalveolar lavage fluid (BALF) levels of the three vascular endothelial growth factor (VEGF) types in healthy volunteers and patients with idiopathic pulmonary fibrosis (IPF) and hypersensitivity pneumonitis (HP). (A) There were no differences in the BALF VEGF-A levels among the three groups. (B, C) The BALF VEGF-C and VEGF-D levels were below the lower detection limits in many subjects from the three groups. The BALF VEGF-C level was significantly elevated in patients with HP compared to healthy volunteers; however, no difference was detected between patients with HP and patients with IPF. The BALF VEGF-D level was significantly elevated in patients with HP compared to patients in the other two groups. **p<0.01, *p<0.05.
Correlation between VEGF levels and HP severity
The serum levels of the three VEGF types were not correlated with the pulmonary function tests and cell counts in BALF samples. The BALF VEGF-D levels were correlated with the total cell and lymphocyte counts in BALF samples (r=0.485, p=0.014 and r=0.717, p<0.0001, respectively; table 2).
Table 2.
Correlation of bronchoalveolar lavage fluid (BALF) vascular endothelial growth factor (VEGF)-D and BALF CCL21 with disease severity in hypersensitivity pneumonitis
| BALF VEGF-D |
BALF CCL21 |
|||
|---|---|---|---|---|
| r Value | p Value | r Value | p Value | |
| VC (% pred) | 0.196 | NS | −0.306 | NS |
| FEV1 (% pred) | −0.369 | NS | −0.662 | 0.007 |
| TLC (% pred) | 0.061 | NS | −0.298 | NS |
| DLco (% pred) | 0.081 | NS | −0.671 | 0.024 |
| Total cell count (104/mm2) | 0.485 | 0.014 | 0.202 | NS |
| Macrophage (104/mm2) | 0.223 | NS | 0.333 | NS |
| Lymphocyte (104/mm2) | 0.717 | <0.0001 | 0.143 | NS |
| CD4/8 ratio | −0.029 | NS | 0.256 | NS |
BALF, bronchoalveolar lavage fluid; DLco, diffusion capacity of the lung for carbon monoxide; FEV1, forced expiratory volume in 1 s; NS, not statistically significant; TLC, total lung capacity; VC, vital capacity; VEGF-D, vascular endothelial growth factor-D.
Characteristics of CCL21 expression in HP
Quantification of serum and BALF CCL21 as measured by ELISA
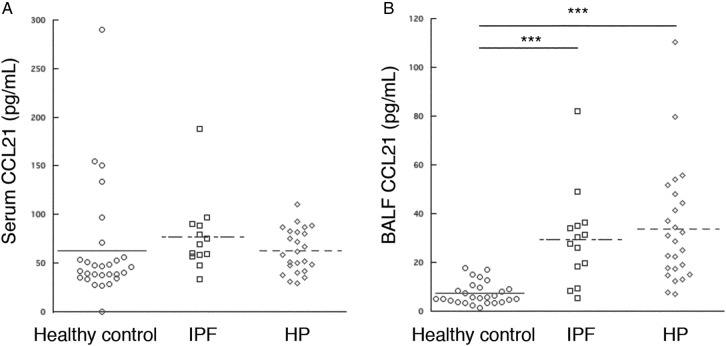
We also measured the levels of lymphovascular endothelial-related chemokine CCL21 in each group because the levels of lymphangiogenic factors, such as VEGF-D, were significantly elevated in patients with HP. No significant difference was observed in the serum CCL21 levels among the three groups (figure 3A). The BALF CCL21 levels were significantly increased in patients with HP and IPF compared to healthy volunteers; however, the BALF CCL21 levels were not different between patients with HP and IPF (figure 3B). No correlation was detected between the serum and BALF CCL21 levels (r=0.185, p=0.386).
Figure 3.
Comparison of the serum and bronchoalveolar lavage fluid (BALF) levels of CCL21 among healthy volunteers and patients with idiopathic pulmonary fibrosis (IPF) and hypersensitivity pneumonia (HP). (A) The serum CCL21 levels showed no difference among the three groups. (B) The BALF CCL21 levels were significantly elevated in patients with IPF and HP compared to healthy volunteers, although no difference was observed between patients with HP and patients with IPF. ***p<0.001.
Correlation between CCL21 levels and HP severity
The BALF CCL21 levels were negatively correlated with %FEV1.0 and %DLco (r=−0.662, p=0.007 and r=0.671, p=0.024, respectively); however, serum CCL21 levels were not correlated with any factors of disease severity (table 2). A strong correlation was observed between the BALF CCL21 and BALF VEGF-C levels (r=0.769, p<0.0001), but there was no correlation between the BALF CCL21 and BALF VEGF-D levels (r=0.266, p=0.501).
Immunohistochemical characterisation of CCL21 expression and CCR7+ cells in patients with HP and IPF
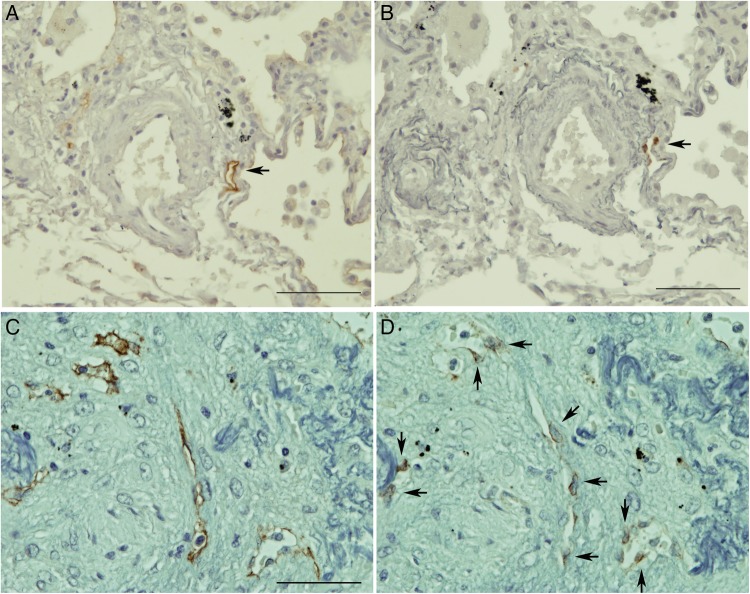
According to the results from the immunohistochemical analyses using serial sections and primary antipodoplanin and anti-CCL21 antibodies, CCL21 was exclusively expressed in podoplanin-positive lymphatic vessels in patients with HP (figure 4A, B). Similarly, CCL21 was also expressed in lymphatic vessels of massive fibrotic lesions in patients with IPF (figure 4C, D). However, CCR7+ (a receptor of CCL21) cells are numerously observed near lymphatic vessels in the HP sections (figure 5), but barely in the lung specimens obtained from patients with IPF (data not shown).
Figure 4.
Immunohistochemical characterisation of CCL21 expression. (AD) are serial sections stained for podoplanin (A and C) and CCL21 (B and D). CCL21 was exclusively expressed in the podoplanin+ existing lymphatic vessels in the HP sections (arrows in A and B). Scale bar, 100 µm. In IPF, CCL21 expression was detectable in the podoplanin+ newly formed lymphatic vessels in the massive fibrotic lesions HP (arrows in D). Scale bar, 100 µm. All sections were counterstained by resorcin-fuchsin and haematoxylin.
Figure 5.
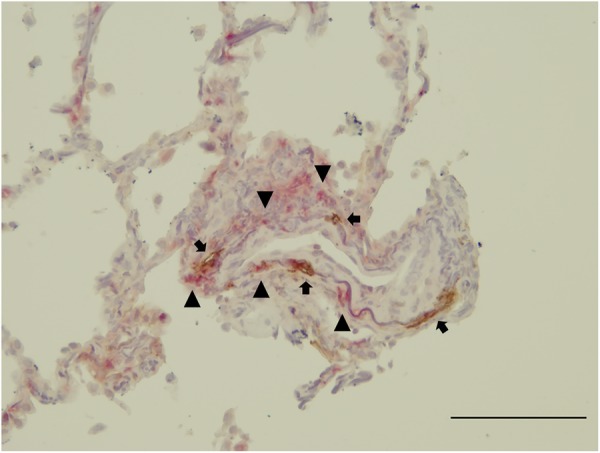
Immunohistochemical characterisation of CCR7 expression. CCR7+ cells (red, arrowheads) were observed around the podoplanin+ lymphatic in the HP sections (brown, arrows). Scale bar, 100 µm. The section was counterstained by resorcin-fuchsin and haematoxylin.
Comparison of the BALF lymphangiogenic factor levels among HP subtypes
No difference was observed in the BALF VEGF-D and CCL21 levels among the four subtypes in patients with HP (see online supplementary figure S1).
Discussion
No information is available regarding the involvement of lymphatic VEGFs in HP. In this study, the BALF VEGF-D levels were elevated in patients with HP compared to the other groups (ie, healthy controls and patients with IPF). Additionally, we demonstrated a significant correlation between the BALF VEGF-D levels and HP inflammatory severity. However, the serum VEGF-A levels were increased in patients with HP compared to patients with IPF, but no difference was detectable in the BALF VEGF-A levels. The BALF VEGF-C and CCL21 levels were increased in HP relative to healthy volunteers, although there was no difference between patients with HP and IPF. According to our findings, BALF VEGF-D among these lymphangiogenic factors is of importance in HP, and might be a useful marker for the diagnoses of HP and assessment of HP severity.
Although no difference was observed in the BALF levels between patients with HP and IPF, we could not ignore the relevance of VEGF-C and CCL21 in HP. There has been increasing experimental evidence that dendritic cells play a pivotal role in HP pathogenesis.8–10 In this study, the BALF CCL21 levels were more elevated in patients with HP compared to healthy volunteers, and BALF CCL21 expression was associated with the severity of HP. In addition, CCL21 was exclusively expressed in the lymphatic endothelium in our immunohistochemistry analyses, whereas CCR7+ cells numerously aggregated around lymphatic vessels. According to these findings, elevated CCL21 expression facilitates the trafficking of CCR7+ antigen-presenting cells into the regional lymph nodes in HP. VEGF-C/VEGF-D/vascular endothelial growth factor receptor-3 (VEGFR-3) signalling was also reported to regulate CCL21 production in a mouse cardiac allograft model, although VEGF-C and VEGF-D generally induce lymphangiogenesis by the proliferation of lymphatic endothelial cells via VEGFR-3 signalling.29 In that model, the inhibition of VEGF-C/VEGF-D/VEGFR-3 signalling produced a beneficial effect against the rejection response to allograft transplantation, along with decreased CCL21 expression. In this study, a strong correlation was observed between BALF CCL21 and BALF VEGF-C levels in patients with HP. Therefore, it is possible to presume that the elevated levels of lymphangiogenic factors in extrinsic allergic alveolitis facilitate lung CCL21 expression and consequently accelerate HP. It is important to note that the lymphangiogenic factors induce the expansion of passive conduits that transport inflammatory cells from the lungs into the regional lymph nodes, but might also actively regulate antigen-presenting cell trafficking during HP development. We could not elucidate whether VEGF-C and VEGF-D have different roles in the development of HP.
We observed a significant elevation in the BALF CCL21 levels in patients with IPF compared to healthy volunteers. The immunohistochemical expression of CCL21 was also detectable in lymphatic vessels within massive fibrotic lesions of IPF, but CCR7+ cells were barely observed in the IPF sections. IPF is characterised by spatial and/or temporal heterogeneity in inflammation and fibrosis.30 31 Marchal-Sommé et al32 reported the potential for dendritic cells to be involved in the pathogenesis of IPF, suggesting that IPF also represents an aspect of immune disease. However, Pierce et al33 reported that CCR7 was highly expressed on primary fibroblasts grown from IPF surgical lung biopsy specimens, which exhibited significant migratory and proliferative responses to CCL21 exposure. Additionally, Pierce et al34 reported that the intravenous administration of these fibroblasts into immunodeficient beige mice-induced pulmonary fibrosis and the immunoneutralisation of CCR7 or CCL21 could attenuate this experimental fibrosis. Furthermore, several investigators have suggested that active lymphangiogenesis was immunohistochemically associated with massive fibrogenesis in IPF.35 36 CCL21–CCR7 signalling appears to directly facilitate fibrogenesis in pulmonary fibrosis; however, the role of this signalling in the immune response in IPF was not determined.
The BALF CCL21 levels were negatively correlated with the %FEV1.0. In patients with HP, the results of the pulmonary function tests are usually characteriszed by a restrictive functional pattern.2 However, farmer's lung has been reported to complicate obstructive impairment.3 In this study, more than half of the patients with HP presented with farmer's lung. Therefore, the correlation of the CCL21 levels with the %FEV1.0, but not %VC, might be attributable to the patient composition in our study.
To the best of our knowledge, this was the first report in which a close relationship between lymphangiogenic factors and HP was determined. In particular, VEGF-D is important in HP and might be a marker for HP diagnoses and evaluation of HP severity. We were able to provide a new perspective for understanding the mechanism of HP pathogenesis and facilitate further investigations concerning lymphangiogenesis in this complex disorder. The relatively small sample sizes in this study may impose a limitation on the interpretation of our results. Independent replication of this study's findings would be required to certainly determine the relationships between BALF VEGF-D and CCL-21, and HP diagnosis and severity.
Acknowledgments
The authors would like to thank Mr Noriyuki Yamada, Mr Yuji Shibata and Mr Tomohito Hanasaka for their excellent assistance.
Footnotes
Contributors: MY designed and performed the experiments, analysed the data and wrote the manuscript. TM, HN, TS and KK provided research materials. MN assisted with the experiments. HK, MO and RE provided crucial ideas.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: Iwate Medical University.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Selman M, Pardo A, King TE Jr. Hypersensitivity pneumonitis: insights in diagnosis and pathobiology. Am J Respir Crit Care Med 2012;186:314–24. doi:10.1164/rccm.201203-0513CI [DOI] [PubMed] [Google Scholar]
- 2.Hapke EJ, Seal RM, Thomas GO et al. Farmer's lung. A clinical, radiographic, functional, and serological correlation of acute and chronic stages. Thorax 1968;23:451–68. doi:10.1136/thx.23.5.451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lalancette M, Carrier G, Laviolette M et al. Farmer's lung. Long-term outcome and lack of predictive value of bronchoalveolar lavage fibrosing factors. Am Rev Respir Dis 1993;148:216–21. doi:10.1164/ajrccm/148.1.216 [DOI] [PubMed] [Google Scholar]
- 4.Cormier Y, Bélanger J, LeBlanc P et al. Bronchoalveolar lavage in farmers’ lung disease: diagnostic and physiological significance. Br J Ind Med 1986;43:401–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hariri LP, Mino-Kenudson M, Shea B et al. Distinct histopathology of acute onset or abrupt exacerbation of hypersensitivity pneumonitis. Hum Pathol 2012;43:660–8. doi:10.1016/j.humpath.2011.06.001 [DOI] [PubMed] [Google Scholar]
- 6.Kawanami O, Basset F, Barrios R et al. Hypersensitivity pneumonitis in man. Light- and electron-microscopic studies of 18 lung biopsies. Am J Pathol 1983;110:275–89. [PMC free article] [PubMed] [Google Scholar]
- 7.Coleman A, Colby TV. Histologic diagnosis of extrinsic allergic alveolitis. Am J Surg Pathol 1988;2:514–18. doi:10.1097/00000478-198807000-00002 [DOI] [PubMed] [Google Scholar]
- 8.Bhan U, Newstead MJ, Zeng X et al. Stachybotrys chartarum-induced hypersensitivity pneumonitis is TLR9 dependent. Am J Pathol 2011;179:2779–87. doi:10.1016/j.ajpath.2011.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blanchet MR, Bennett JL, Gold MJ et al. CD34 is required for dendritic cell trafficking and pathology in murine hypersensitivity pneumonitis. Am J Respir Crit Care Med 2011;184:687–98. doi:10.1164/rccm.201011-1764OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daito H, Kikuchi T, Sakakibara T et al. Mycobacterial hypersensitivity pneumonitis requires TLR9-MyD88 in lung CD11b+ CD11c+ cells. Eur Respir J 2011;38:688–701. doi:10.1183/09031936.00177110 [DOI] [PubMed] [Google Scholar]
- 11.Alitalo K. The lymphatic vasculature in disease. Nat Med 2011;17:1371–80. doi:10.1038/nm.2545 [DOI] [PubMed] [Google Scholar]
- 12.Jeltsch M, Kaipainen A, Joukov V et al. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science 1997;276:1423–5. doi:10.1126/science.276.5317.1423 [DOI] [PubMed] [Google Scholar]
- 13.Karkkainen MJ, Haiko P, Sainio K et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol 2004;5:74–80. doi:10.1038/ni1013 [DOI] [PubMed] [Google Scholar]
- 14.Achen MG, Jeltsch M, Kukk E et al. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4). Proc Natl Acad Sci USA 1998;95:548–53. doi:10.1073/pnas.95.2.548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamashita M, Iwama N, Date F et al. Macrophages participate in lymphangiogenesis in idiopathic diffuse alveolar damage through CCL19-CCR7 signal. Hum Pathol 2009;40:1553–63. doi:10.1016/j.humpath.2009.03.021 [DOI] [PubMed] [Google Scholar]
- 16.Cursiefen C, Chen L, Borges LP et al. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest 2004;113:1040–50. doi:10.1172/JCI20465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kriehuber E, Breiteneder-Geleff S, Groeger M et al. Isolation and characterization of dermal lymphatic and blood endothelial cells reveal stable and functionally specialized cell lineages. J Exp Med 2001;194:797–808. doi:10.1084/jem.194.6.797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamashita M, Mouri T, Niisato M et al. Heterogeneous characteristics of lymphatic microvasculatures associated with pulmonary sarcoid granulomas. Ann Am Thorac Soc 2013;10: 90–7. doi:10.1513/AnnalsATS.201209-078OC [DOI] [PubMed] [Google Scholar]
- 19.Ålgars A, Karikoski M, Yegutkin GG et al. Different role of CD73 in leukocyte trafficking via blood and lymph vessels. Blood 2011;117:4387–93. doi:10.1182/blood-2010-11-321646 [DOI] [PubMed] [Google Scholar]
- 20.Serra HM, Eberhard Y, Martín AP et al. Secondary lymphoid tissue chemokine (CCL21) is upregulated in allergic contact dermatitis. Int Arch Allergy Immunol 2004;133:64–71. doi:10.1159/000076129 [DOI] [PubMed] [Google Scholar]
- 21.Kerjaschki D, Regele HM, Moosberger I et al. Lymphatic neoangiogenesis in human kidney transplants is associated with immunologically active lymphocytic infiltrates. J Am Soc Nephrol 2004;15:603–12. doi:10.1097/01.ASN.0000113316.52371.2E [DOI] [PubMed] [Google Scholar]
- 22.Glasgow CG, Avila NA, Lin JP et al. Serum vascular endothelial growth factor-D levels in patients with lymphangioleiomyomatosis reflect lymphatic involvement. Chest 2009;135:1293–300. doi:10.1378/chest.08-1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu KF, Zhang P, Tian X et al. The role of vascular endothelial growth factor-D in diagnosis of lymphangioleiomyomatosis (LAM). Respir Med 2013;107:263–8. doi:10.1016/j.rmed.2012.10.006 [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Meng X, Zeng H et al. Serum vascular endothelial growth factor-C levels: a possible diagnostic marker for lymph node metastasis in patients with primary non-small cell lung cancer. Oncol Lett 2013;6:545–9. doi:10.3892/ol.2013.1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richerson HB, Bernstein IL, Fink JN et al. Guidelines for the clinical evaluation of hypersensitivity pneumonitis. Report of the Subcommittee on Hypersensitivity Pneumonitis. J Allergy Clin Immunol 1989;84:839–44. [DOI] [PubMed] [Google Scholar]
- 26.Gardner RM, Hankinson JL. Standardization of spirometry—1987 ATS update (American Thoracic Society). J Occup Med 1988;30:272–3. [PubMed] [Google Scholar]
- 27.Miller MR, Hankinson J, Brusasco V. Standardisation of spirometry. Eur Respir J 2005;26:319–38. doi:10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 28.Meyer KC, Raghu G, Baughman RP et al. , American Thoracic Society Committee on BAL in Interstitial Lung Disease. An official American Thoracic Society clinical practice guideline: the clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am J Respir Crit Care Med 2012;185:1004–14. doi:10.1164/rccm.201202-0320ST [DOI] [PubMed] [Google Scholar]
- 29.Nykänen AI, Sandelin H, Krebs R et al. Targeting lymphatic vessel activation and CCL21 production by vascular endothelial growth factor receptor-3 inhibition has novel immunomodulatory and antiarteriosclerotic effects in cardiac allografts. Circulation 2010;121:1413–22. doi:10.1161/CIRCULATIONAHA.109.910703 [DOI] [PubMed] [Google Scholar]
- 30.Travis WD, Matsui K, Moss JE et al. Idiopathic nonspecific interstitial pneumonia: prognostic significance of cellular and fibrosing patterns. Survival comparison with usual interstitial pneumonia and desquamative interstitial pneumonia. Am J Surg Pathol 2000;24:19–33. doi:10.1097/00000478-200001000-00003 [DOI] [PubMed] [Google Scholar]
- 31.Katzenstein AL, Myers JL. Idiopathic pulmonary fibrosis: clinical relevance of pathologic classification. Am J Respir Crit Care Med 1998;157:1301–15. doi:10.1164/ajrccm.157.4.9707039 [DOI] [PubMed] [Google Scholar]
- 32.Marchal-Sommé J, Uzunhan Y, Marchand-Adam S et al. Dendritic cells accumulate in human fibrotic interstitial lung disease. Am J Respir Crit Care Med 2007;176:1007–14. doi:10.1164/rccm.200609-1347OC [DOI] [PubMed] [Google Scholar]
- 33.Pierce EM, Carpenter K, Jakubzick C et al. Idiopathic pulmonary fibrosis fibroblasts migrate and proliferate to CC chemokine ligand 21. Eur Respir J 2007;29:1082–93. doi:10.1183/09031936.00122806 [DOI] [PubMed] [Google Scholar]
- 34.Pierce EM, Carpenter K, Jakubzick C et al. Therapeutic targeting of CC ligand 21 or CC chemokine receptor 7 abrogates pulmonary fibrosis induced by the adoptive transfer of human pulmonary fibroblasts to immunodeficient mice. Am J Pathol 2007;170:1152–64. doi:10.2353/ajpath.2007.060649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.El-Chemaly S, Malide D, Zudaire E et al. Abnormal lymphangiogenesis in idiopathic pulmonary fibrosis with insights into cellular and molecular mechanisms. Proc Natl Acad Sci USA 2009;106:3958–63. doi:10.1073/pnas.0813368106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lara AR, Cosgrove GP, Janssen WJ et al. Increased lymphatic vessel length is associated with the fibroblast reticulum and disease severity in usual interstitial pneumonia and nonspecific interstitial pneumonia. Chest 2012;142:1569–76. doi:10.1378/chest.12-0029 [DOI] [PMC free article] [PubMed] [Google Scholar]