Abstract
Background
Chromosome 17q21.31 microdeletion syndrome is a multisystem genomic disorder caused by a recurrent 600-kb-long deletion, or haploinsufficiency of the chromatin modifier gene KANSL1, which maps to that region. Patients with KANSL1 intragenic mutations have been reported to display the major clinical features of 17q21.31 microdeletion syndrome. However, they did not exhibit the full clinical spectrum of this disorder, which might indicate that an additional gene or genes, located in the 17q21.31 locus, might also be involved in the syndrome’s phenotype.
Methods
Conventional and molecular karyotypes were performed on a female patient with intellectual disability, agenesis of the corpus callosum, heart defects, hydronephrosis, hypotonia, pigmentary skin anomalies and facial dysmorphic features. FISH analysis was conducted for chromosomal breakpoint localization. qRT-PCR was applied for the comparative gene expression of KANSL1 gene in the patient and a control group.
Results
Herein, we present the first report of disruption and haploinsufficiency of the KANSL1 gene, secondary to a t(1;17)(q12;q21)dn chromosomal translocation in a girl that also carried a de novo ~289-kb deletion on 16p11.2. KANSL1 gene expression studies and comparative clinical analysis of patients with 17q21.31 deletions and intragenic KANSL1 gene defects indicate that KANSL1 dysfunction is associated with the full spectrum of the 17q21.31 microdeletion syndrome, which includes characteristic facial features, hypotonia, intellectual disability, and structural defects of the brain, heart and genitourinary system, as well as, musculoskeletal and neuroectodermal anomalies. Moreover, we provide further evidence for the overlapping clinical phenotype of this condition with the cardio-facio-cutaneous (CFC) syndrome.
Conclusions
KANSL1 gene haploinsufficiency is necessary and sufficient to cause the full spectrum of the 17q21.31 microdeletion syndrome. We hypothesize that the KANSL1 gene might have an effect on the Ras/mitogen-activated protein kinase (MAPK) pathway activity, which is known to be deregulated in the CFC syndrome. This pathway has a crucial role in the development of the heart and craniofacial morphology, as well as the skin, eye, brain and musculoskeletal systems.
Keywords: 17q21.31 Microdeletion syndrome, Chromosomal rearrangement, Genotype-phenotype association, KANSL1, RASopathies
Background
The 17q21.31 microdeletion syndrome (del17q21.31), also known as the Koolen-De Vries Syndrome, is characterized by distinctive facial features, hypotonia, intellectual disability and friendly/amiable behavior [1, 2]. Over 50 % of the cases also present with structural defects of the brain (agenesis of the corpus callosum, ventricle dilatation), heart (aortic and/or pulmonary stenosis, ventricular and atrial septal defects) and/or genitourinary system. Additional signs include musculoskeletal features and hair, dental and pigmentary skin anomalies [2–6] that, in some cases, resemble those seen in the cardio-facio-cutaneous (CFC) syndrome.
The critical deleted region of the del17q21.31 syndrome was initially defined as a 424-kb segment [2], and later narrowed down to 160–274 kb containing MAPT, STH and KANSL1 genes [5, 7]. Recent reports of small atypical deletions and heterozygous intragenic mutations in KANSL1 demonstrated that haploinsufficiency of this gene is responsible for the major clinical signs of the syndrome [8, 9]. However, with the exception of a ventricular septal defect that spontaneously corrected, structural defects were not present in patients with intragenic KANSL1 mutations. Therefore, it is plausible that additional genes at the 17q21.31 locus might account for the severity of the clinical phenotype of the del17q21.31 syndrome.
Analysis of apparently balanced chromosomal abnormalities associated with developmental disorders has been a successful approach to gene discovery, as the abnormal associated phenotype can be caused by hidden genomic defects at the molecular level [10, 11]. Herein, we report on the first patient to be described to have a de novo (1;17) translocation that truncates the KANSL1 gene. In addition, we also reviewed the phenotype of cases with 17q21.31 microdeletions and KANSL1 intragenic defects, in an attempt to further define the full clinical spectrum of the syndrome and understand the molecular mechanisms associated with deleterious KANSL1 alleles.
Patient and methods
Patient
The proband for our study was a girl born after a 36-week gestation complicated by fetal hydrocephaly. She weighed 1860 g, measured 45.5 cm and had a head circumference (OFC) of 30.6 cm. She presented with facial dysmorphic features, hypotonia, dilatation of cerebral ventricles, agenesis of the corpus callosum, aortic stenosis, bicuspid aorta and bilateral hydronephrosis. During the neonatal period she had severe feeding difficulties and frequent urinary infections that were caused by vesico-ureteral reflux and required surgical treatment. Her psychomotor milestones were delayed. She walked at 4 years, had language difficulties and needed special education. Facial characteristics are shown in Fig. 1a. She had a long face, short and upslanting palpebral fissures, hypertelorism, epicanthal folds, ptosis of the eyelids, iris heterochromia, broad nasal bridge, bulbous nose with thick, hypoplastic nares, long chin and absence of permanent lateral incisors. Her hair was coarse and thick. She also presented numerous nevi, café au lait spots and hypopigmented areas. At the age of 13 years, she showed severe thoracic kyphosis, moderate/severe intellectual disability, speech and deficit attention disorders, and a compulsive appetite that required strict diet control. Continuous cardiac and renal evaluations demonstrated mild pulmonary stenosis, peripheral cyanosis, vasomotor liability and increased size of the left ventricle, as well as a chronic renal disease with hypertension and hyperproteinuria. At her last examination (19 years of age) her weight, height and OFC were at the 90th, 10th and 50th percentiles, respectively.
Fig. 1.
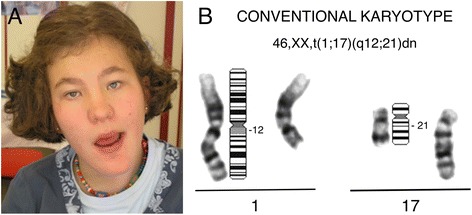
Individual with t(1;17)dn. a Facial features of the patient. b G-banding partial karyotype showing the apparently balanced translocation between chromosomes 1 and 17
The Ethics Committee at our institution (Comité Ético de Investigación Clínica de Navarra—CEIC) approved this study. Written informed consent was obtained from the patient’s parents.
Conventional and molecular karyotype
Metaphase chromosomes were obtained from cultured peripheral lymphocytes of our patient and her parents, and a standard GTL-banding karyotype was performed for conventional cytogenetic analysis. Genomic DNA was extracted from peripheral blood cells of the patient for array-based Comparative Genomic Hybridization (aCGH) studies (Agilent Technologies). The patient was studied with a 105-K platform and a mixture of 1 μg test Cy5-labeled DNA and 1 μg male reference (Promega) Cy3-labeled DNA was used in the hybridization. The software Genomic Workbench 7.0 was used for the bioinformatic analysis of the copy number variants (CNVs). An altered copy number of DNA was considered when a region contained a minimum of five consecutive probes. Locations are based on hg18/NCBI mapview build 36.
Fluorescence in situ hybridization (FISH)
Metaphase spreads were obtained from phytohaemagglutinin-stimulated peripheral blood lymphocytes of the patient and both parents. Bacterial artificial chromosome (BAC) clones were selected from the Centro de Regulación Genómica Genome Browser [12]. Plasmid DNA was extracted from clones using QIAprep Spin Miniprep Kit (Qiagen) and labeled with Spectrum Orange dUTP (SpO) or Spectrum Green dUTP (SpG) using the Nick Translation Reagent Kit (Abbott Molecular Inc.). FISH experiments were carried out according to standard procedures. The slides were examined using a Nikon Eclipse E400 with appropriate filters for Spectrum Orange, Spectrum Green and the UV Filter for the DAPI nuclear counterstain. The signals were recorded with a CCD camera and processed by ISIS v5.1 fluorescence imaging system (MetaSystems).
Gene expression assay
Quantitative reverse-transcription polymerase chain reaction (qRT-PCR) was performed with RNA extracted from the patient and five control individuals. Total RNA was isolated from whole blood using the QIAamp RNA Blood Mini kit (Qiagen), following the manufacturer’s instructions. cDNA synthesis was performed with 1 μg of RNA using a TaqMan Reverse Transcription Reagents kit (Invitrogen-Life Technologies) in a total volume of 50 μL. Primer and probe mixtures for KANSL1 (Hs00393805_m1, localized at exon boundary 7–8, NM_001193465.1) and the endogenous gene GAPDH (Hs99999905_m1, exon location 3, NM_002046.3) were supplied by Applied Biosystems. The PCR reactions were run in a 7300 Real Time PCR System (Applied Biosystems). All samples of cDNA (40 ng per well) were run in triplicate in 20 μL reaction volumes. The thermal cycling parameters were the standard conditions of the Real Time PCR System. Relative differences in transcript levels were quantified with the ΔΔCt method and data are reported as the fold-change in expression of the proband relative to the mean ± SEM of the control group.
Results
Balanced de novo translocation (1;17)
The patient’s karyotype analysis revealed the presence of an apparently balanced de novo translocation, designated 46,XX,t(1;17)(q12;q21)dn (Fig. 1b).
De novo microdeletion on the 16p11.2 atypical/distal region
The aCGH study detected an ~289-kb deletion on the 16p11.2 atypical/distal region, flanking the recurrent microdeletion/duplication locus (Fig. 2a). No genomic imbalances were observed at translocation breakpoints or other genomic regions. Additional FISH analysis using the RP11-264B17 BAC probe confirmed the de novo origin of the 16p11.2 distal deletion in the patient (Fig. 2b). Combining the results of the GTL-banding, FISH and aCGH experiments, we concluded that the patient had the karyotype 46,XX,t(1;17)(q12;q21)dn.ish del(16)(q11.2q11.2)(RP11-264B17-). arr[hg18] 16q11.2(28,732,295-29,021,443) × 1 dn.
Fig. 2.
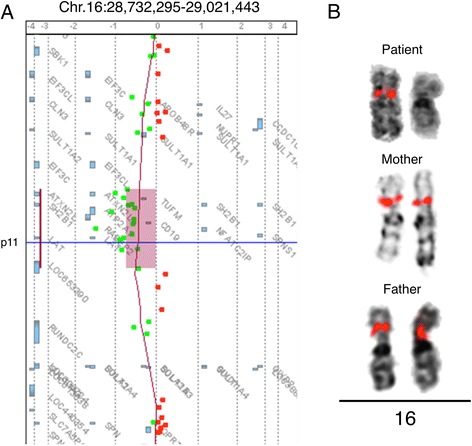
aCGH and FISH experiments on 16p11.2. a A detail of the patient’s aCGH analysis showing a ~289-kb deletion on the chromosome 16p11.2 distal region. b FISH experiments on the patient and both parents using RP11-264B17 (SpO) BAC clone. Only one signal was observed on 16p11.2 in the patient, confirming the deletion found in aCGH. Two hybridization signals were observed in each parent, indicating the de novo origin of the deletion. Location of RP11-264B17 probe based on hg18: 28786266–28936684
KANSL1 disruption and reduced gene expression
FISH analysis, using the overlapping BAC clones RP11-782E01 and RP11-86C01 on 17q21.31, revealed hybridization signals on both derivative chromosomes (1 and 17). Additional studies with flanking probes RP11-368D10 and RP11-259G18 showed one signal on der(17) and one on der(1) chromosomes, respectively. These results indicated that RP11-782E01 and RP11-86C01 BAC probes spanned the translocation breakpoint, placing the breakpoint within a 65.6-kb region of overlap at the 17q21.31 band, which would disrupt the KANSL1 gene (Fig. 3). This overlapping region contains a small segmental duplication, which might have contributed to a non-allelic homologous recombination event, favoring the chromosome disruption at this point.
Fig. 3.
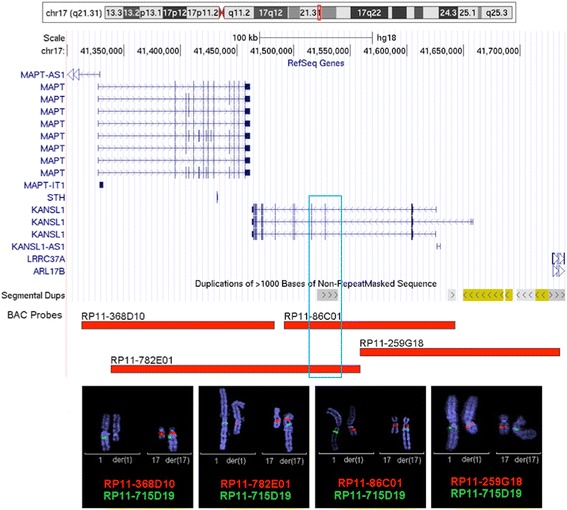
Mapping of the breakpoint on chromosome 17. Genomic context for the 17q21.31 breakpoint studied by FISH showing the genes (different transcript variants represented) and segmental duplications mapping in the region, and the position of the FISH probes RP11-368D10 (41315374–41484448), RP11-782E01 (41339059–41559177), RP11-86C01 (41493577–41644115) and RP11-259G18 (41559185–41734024). Coordinates are given according to the human reference sequence hg18 (adapted from the UCSC genome browser [20]). RP11-368D10 BAC probe gives red signal on chromosomes 17 and der(17). RP11-782E01 probe gives red signal on chromosomes 17, der(17) and der(1), overlapping the translocation breakpoint. RP11-86C01 probe gives signal on chromosomes 17, der(1) and der(17), overlapping the translocation breakpoint on KANSL1 gene. RP11-259G18 BAC probe gives signal on chromosomes 1 and der(1). RP11-715D19 probe (SpG) has been used as a guide on chromosomes 1 and der(17). The blue box represents the putative region for the KANSL1 gene disruption
To determine how KANSL1 gene expression was affected by the de novo translocation, transcript levels were assessed by qRT-PCR. The relative differences in transcript levels corresponding to KANSL1 in the patient were half of those observed in controls, correlating with the finding of the KANSL1 gene disruption in one chromosome (Fig. 4).
Fig. 4.
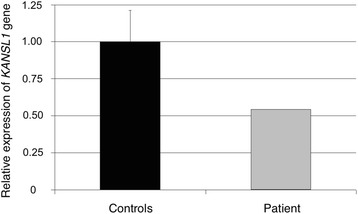
Quantification of KANSL1 gene expression levels by qRT-PCR. The expression level of KANSL1 gene on the patient bearing the t(1;17) (grey bar) was half that observed in the control group (black bar)
Discussion
We present the first report of haploinsufficiency of the KANSL1 gene caused by gene interruption, secondary to a de novo (1;17)(q12;q21) chromosomal translocation. The patient, who also carries an ~289-kb deletion flanking the recurrent 16p11.2 region, shows the complete phenotype of the 17q21.31 microdeletion syndrome, including characteristic facial features, hypotonia, intellectual disability, and structural defects of the brain, heart and genitourinary system, as well as musculoskeletal and neuroectodermal anomalies.
To analyze the association of the KANSL1 gene disruption with the patient’s phenotype, we compared the frequencies of signs previously described in cases with small (424 kb) and large (502–800 kb) 17q21.31 deletions with patients carrying a KANSL1 gene defect, including the present case. As shown in Table 1, the severity of clinical presentation of the 17q21.31 syndrome does not correlate with the size of the deletion. Moreover, our patient presented with most of the structural defects, facial features and cognitive characteristics previously described in patients with that syndrome. It could be argued, however, that her phenotype might be attributable, at least partially, to the presence of the 16p11.2 microdeletion. This deletion, which overlaps with that described in the 16p11.2 deletion syndrome (220 kb), includes nine genes (ATXN2L, TUFM, SH2B1, ATP2A1, RABEP2, CD19, NFATC2IP, SPNS1 and LAT). Interestingly, SH2B1 is known to be involved in leptin and insulin signaling [13] and has been reported as a predisposing factor for obesity [14]. In agreement with this hypothesis, our patient showed a compulsive appetite with difficulty in managing her weight that required strict diet control. Functions of other genes included in the 16p11.2-deleted region are currently unknown, but they do not seem to make a clear and significant contribution to the phenotype. Some authors have described cases with distal 16p11.2 deletion showing developmental delay, behavioral problems and unusual facial morphology. Although detailed clinical information may not have been available for most patients participating in large study cohorts, structural birth defects have not been previously observed associated with this genomic imbalance. Therefore, we conclude that although we can not rule out that the 289-kb microdeletion on distal 16p11.2 might contribute to the severity of the intellectual disability and/or the behavioral problems in our patient, there is currently no evidence indicating that other clinical manifestations can be attributable to the presence of this genomic imbalance. On the contrary, her facial appearance and the specific brain (agenesis of the corpus callosum), cardiac (valvulo-septal defects) and renal defects (dilation of the renal system) were very similar to those previously described in cases with 17q21.31 microdeletion syndrome.
Table 1.
Summary of clinical signs observed in patients with del17q21.31 (classical and larger sizes), KANSL1 mutations and KANSL1 disruption
| 17q21.31 deletion Frequency (%) | KANSL1 mutations | KANSL1 disruption | |||||
|---|---|---|---|---|---|---|---|
| Classical [2] | Largea | Zollino et al. [9] | Zollino et al. [9] | Koolen et al. [8] | Koolen et al. [8] | Moreno-Igoa et al. | |
| 424 kb | 502-810 kb | p.R606X | p.R929G fsX44 | c.916C>T p.(Gln306*) | c.1652+1G>A | t(1;17) | |
| Age (years) at last observation | 3 | 14 | 2, 11/12 | 13 | 17 | ||
| Sex | 9 M/13 F | 6 M/4 F | F | F | F | F | F |
| Growth | |||||||
| Intrauterine growth retardation | 27 | 10 | + | - | - | - | - |
| Short stature | 18 | 30 | - | ||||
| Microcephaly | 5 | - | - | ||||
| Neurological features | |||||||
| Hypotonia | 96 | 80 | + | + | + | + | + |
| Failure to thrive | 40 | + | + | + | + | + | |
| Developmental delay/intellectual disability | 100 | 100 | + | + | + | + | + |
| Speech disorder | 50 | + | + | + | |||
| Seizures | 60 | - | - | - | - | + | |
| Engaging or amiable personality | 89 | 50 | + | + | + | + | - |
| Behavioral disorder | 30 | + | |||||
| Facial dysmorphic features | |||||||
| Broad forehead | 68 | 40 | + | + | + | + | |
| Long face | 74 | 70 | - | + | + | ||
| Short palpebral fissures | 36 | 10 | - | + | + | ||
| Upslanting palpebral fissures | 68 | 70 | + | + | + | + | + |
| Ptosis | 50 | - | - | - | - | - | + |
| Epicanthal folds | 68 | 10 | + | + | + | + | + |
| “Pear” shaped nose | 82 | 40 | + | + | + | + | + |
| Large nasal bridge | 30 | + | + | + | |||
| Bulbous nasal tip | 95 | 90 | + | + | + | ||
| Long philtrum | 10 | + | + | + | |||
| Cleft palate | 9 | - | - | - | - | - | - |
| High/narrow palate | 50 | 10 | - | ||||
| Large/prominent ears | 59 | 80 | + | + | - | ||
| Broad chin | 42 | 10 | + | + | + | - | + |
| Ophthalmological features | |||||||
| Hypermetropia | 36 | - | - | + | - | ||
| Strabismo | 45 | 10 | - | + | - | ||
| Iris color defects (pale/heterochromia) | 45 | - | - | - | - | - | + |
| Congenital structural defects | |||||||
| Brain | 38 | 60 | - | - | - | - | + |
| Heart defects | 27 | 30 | - | - | - | + | + |
| Renal & urologic anomalies | 32 | 50 | - | - | - | - | + |
| Criptorchidism | 78 | 67 | |||||
| Musculoskeletal features | |||||||
| Slender fingers/hands | 61 | 20 | + | ||||
| Dislocation of the hip | 27 | 30 | - | + | - | + | - |
| Joint laxity | 30 | + | + | + | + | + | |
| Pectus deformity | 23 | 10 | - | - | - | - | - |
| Kiphosis/Scoliosis | 36 | 30 | - | - | - | - | + |
| Skin, hair, teeth | |||||||
| Abnormal hair texture | 55 | 30 | + | + | + | + | + |
| Skin pigmentary abnormalities | 50 | - | - | - | + | + | |
| Hipodontia | 20 | + | |||||
The wide range of defects affecting the skin, hair, irises and teeth present in our patient are of particular interest as they indicate a role of the KANSL1 gene in neuroectodermal developmental processes. Additionally, these neurocutaneous signs, in conjunction with the musculoskeletal and cardiac manifestations, including the myocardiopathy, overlap with the phenotype of CFC syndrome. This disorder belongs to a clinically defined group of genetic syndromes caused by germline mutations in genes that encode for components or regulators of the Ras/MAPK pathway, generically known as RASopathies [15]. The phenotypic similarity between the 17q21.31 deletion syndrome and some RASopathies might be indicative of the possible influence of the KANSL1 gene on the Ras-MAPK pathway activity, which has a crucial role in the development of the heart and craniofacial morphology, as well as the skin, eye, brain and musculoskeletal systems.
KANSL1 is a nuclear protein identified as a member of the non-specific lethal (NSL) complex. This histone acetyltransferase (HAT) complex also includes MOF, encoded by KAT8, and exerts its influence on gene expression through the acetylation of histone H4, mainly H4K16 [16]. KANSL1 is necessary and sufficient for regulating MOF acetyltransferase activity on nucleosome H4. Moreover, it is also required for the specific acetylation of p53 on K120, which is crucial for the differential and optimal transcription activation of p53 target genes, both in vivo and in vitro [17]. BTG2 (B-cell translocation gene 2) is an early growth response gene whose promoter contains p53-binding sites that is strongly regulated by p53 [18]. Interestingly, BTG2 is one of the mediators of the p53-dependent inhibition of H-Ras activity, which is involved in a variety of biological processes including cell growth, development, differentiation, senescence and cell death. This gene binds H-Ras (G12V) and represses its activity by reducing its GTP loading state, which, in turn, activates the Ras/MAPK signaling cascade and causes a reduction in the expression of a large number of downstream molecules [19]. Therefore, it is plausible that dysfunction of KANSL1 might perturb the activity of p53 transcription target genes, such as BTG2, producing an aberrant regulation of important downstream cascades of Ras. This mechanism could explain the overlapping phenotypic features observed in the KANSL1 haploinsufficiency phenotype and the RASopathies. Additional clinical and experimental studies will be needed to evaluate this hypothesis and further understand the molecular mechanisms associated with deleterious KANSL1 alleles.
Conclusions
We present the first report of haploinsufficiency of the KANSL1 gene caused by gene interruption, secondary to a chromosomal translocation, associated with the complete phenotype of the 17q21.31 microdeletion syndrome, including brain, cardiac and renal structural defects not previously described in patients bearing KANSL1 point mutations. This further demonstrates that dysfunction of the KANSL1 gene is necessary and sufficient to cause the full clinical spectrum of this syndrome. We also hypothesize that the KANSL1 gene might have an effect on the Ras/MAPK pathway activity, which is known to be deregulated in the CFC syndrome.
Acknowledgements
We gratefully acknowledge the collaboration of the patient and her parents. This work was supported by the Health Department of the Navarre Government (Ref. 90/2010).
Abbreviations
- aCGH
Array-based comparative genomic hybridization
- BAC
Bacterial artificial chromosome
- CCD
Charge-coupled device
- CFC
Cardio-facio-cutaneous
- CNV
Copy number variant
- DAPI
4′,6-diamidino-2-phenylindole
- dUTP
Deoxyuridine triphosphate
- FISH
Fluorescence in situ hybridization
- GTL-banding
Giemsa/Trypsin/Leishman-banding
- HAT
Histone acetyltransferase
- MAPK
Mitogen-activated protein kinase
- NSL
Non-specific lethal
- OFC
Occipital frontal circumference
- qRT-PCR
Quantitative reverse-transcription polymerase chain reaction
- SEM
Standard error of the mean
- SpG
Spectrum green
- SpO
Spectrum orange
- UV
Ultraviolet
Footnotes
Competing interests
The authors declare that they have no competing interest.
Authors’ contributions
MARA, MMI, APJ, ABA and BHC designed the experiment. MARA and CR analyzed the clinical data. MMI, APJ, ABA and BHC performed FISH analysis. BNE conducted the aCGH testing. MMI performed the qRT-PCR experiments. MARA and MMI prepared the draft manuscript. All authors contributed to discussion of the results and manuscript preparation. All authors read and approved the final manuscript.
Contributor Information
María Moreno-Igoa, Phone: +34 848 429990, Email: maria.moreno.igoa@navarra.es.
Blanca Hernández-Charro, Phone: +34 848 429990, Email: blanca.hernandez.charro@navarra.es.
Amaya Bengoa-Alonso, Phone: +34 848 429990, Email: amaya.bengoa.alonso@navarra.es.
Aranzazu Pérez-Juana-del-Casal, Phone: +34 848 429990, Email: aranzazu.perezjuana.delcasal@navarra.es.
Carlos Romero-Ibarra, Phone: +34 848 429853, Email: cromeroi@navarra.es.
Beatriz Nieva-Echebarria, Phone: +34 943 002823, Email: bnievae@euskalnet.net.
María Antonia Ramos-Arroyo, Phone: +34 848 429576, Email: ma.ramos.arroyo@navarra.es.
References
- 1.Shaw-Smith C, Pittman AM, Willatt L, Martin H, Rickman L, Gribble S, et al. Microdeletion encompassing MAPT at chromosome 17q21.3 is associated with developmental delay and learning disability. Nat Genet. 2006;38(9):1032–7. doi: 10.1038/ng1858. [DOI] [PubMed] [Google Scholar]
- 2.Koolen DA, Sharp AJ, Hurst JA, Firth HV, Knight SJ, Goldenberg A, et al. Clinical and molecular delineation of the 17q21.31 microdeletion syndrome. J Med Genet. 2008;45(11):710–20. doi: 10.1136/jmg.2008.058701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan TY, Aftimos S, Worgan L, Susman R, Wilson M, Ghedia S, et al. Phenotypic expansion and further characterisation of the 17q21.31 microdeletion syndrome. J Med Genet. 2009;46(7):480–9. doi: 10.1136/jmg.2008.065391. [DOI] [PubMed] [Google Scholar]
- 4.Wright EB, Donnai D, Johnson D, Clayton-Smith J. Cutaneous features in 17q21.31 deletion syndrome: a differential diagnosis for cardio-facio-cutaneous syndrome. Clin Dysmorphol. 2011;20(1):15–20. doi: 10.1097/MCD.0b013e32833e8f1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dubourg C, Sanlaville D, Doco-Fenzy M, Le Caignec C, Missirian C, Jaillard S, et al. Clinical and molecular characterization of 17q21.31 microdeletion syndrome in 14 French patients with mental retardation. Eur J Med Genet. 2011;54(2):144–51. doi: 10.1016/j.ejmg.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Terrone G, D’Amico A, Imperati F, Carella M, Palumbo O, Gentile M, et al. A further contribution to the delineation of the 17q21.31 microdeletion syndrome: central nervous involvement in two Italian patients. Eur J Med Genet. 2012;55(8–9):466–71. doi: 10.1016/j.ejmg.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Cooper GM, Coe BP, Girirajan S, Rosenfeld JA, Vu TH, Baker C, et al. A copy number variation morbidity map of developmental delay. Nat Genet. 2011;43(9):838–46. doi: 10.1038/ng.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koolen DA, Kramer JM, Neveling K, Nillesen WM, Moore-Barton HL, Elmslie FV, et al. Mutations in the chromatin modifier gene KANSL1 cause the 17q21.31 microdeletion syndrome. Nat Genet. 2012;44(6):639–41. doi: 10.1038/ng.2262. [DOI] [PubMed] [Google Scholar]
- 9.Zollino M, Orteschi D, Murdolo M, Lattante S, Battaglia D, Stefanini C, et al. Mutations in KANSL1 cause the 17q21.31 microdeletion syndrome phenotype. Nat Genet. 2012;44(6):636–8. doi: 10.1038/ng.2257. [DOI] [PubMed] [Google Scholar]
- 10.Nadal M, Valiente A, Domenech A, Pritchard M, Estivill X, Ramos-Arroyo MA. Hereditary neuropathy with liability to pressure palsies: two cases with a reciprocal translocation t(16;17)(q12;11.2) interrupting the PMP22 gene. J Med Genet. 2000;37(5):396–8. doi: 10.1136/jmg.37.5.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gribble SM, Prigmore E, Burford DC, Porter KM, Ng BL, Douglas EJ, et al. The complex nature of constitutional de novo apparently balanced translocations in patients presenting with abnormal phenotypes. J Med Genet. 2005;42(1):8–16. doi: 10.1136/jmg.2004.024141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centro de Regulación Genómica Genome Browser. [Barcelona, http://davinci.crg.es/] Accessed 13 Apr 2015.
- 13.Ren D, Li M, Duan C, Rui L. Identification of SH2-B as a key regulator of leptin sensitivity, energy balance, and body weight in mice. Cell Metab. 2005;2(2):95–104. doi: 10.1016/j.cmet.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Bachmann-Gagescu R, Mefford HC, Cowan C, Glew GM, Hing AV, Wallace S, et al. Recurrent 200-kb deletions of 16p11.2 that include the SH2B1 gene are associated with developmental delay and obesity. Genet Med. 2010;12(10):641–7. doi: 10.1097/GIM.0b013e3181ef4286. [DOI] [PubMed] [Google Scholar]
- 15.Tidyman WE, Rauen KA. The RASopathies: developmental syndromes of Ras/MAPK pathway dysregulation. Curr Opin Genet Dev. 2009;19(3):230–6. doi: 10.1016/j.gde.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cai Y, Jin J, Swanson SK, Cole MD, Choi SH, Florens L, et al. Subunit composition and substrate specificity of a MOF-containing histone acetyltransferase distinct from the male-specific lethal (MSL) complex. J Biol Chem. 2010;285(7):4268–72. doi: 10.1074/jbc.C109.087981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X, Wu L, Corsa CA, Kunkel S, Dou Y. Two mammalian MOF complexes regulate transcription activation by distinct mechanisms. Mol Cell. 2009;36(2):290–301. doi: 10.1016/j.molcel.2009.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rouault JP, Falette N, Guehenneux F, Guillot C, Rimokh R, Wang Q, et al. Identification of BTG2, an antiproliferative p53-dependent component of the DNA damage cellular response pathway. Nat Genet. 1996;14(4):482–6. doi: 10.1038/ng1296-482. [DOI] [PubMed] [Google Scholar]
- 19.Solomon H, Buganim Y, Kogan-Sakin I, Pomeraniec L, Assia Y, Madar S, et al. Various p53 mutant proteins differently regulate the Ras circuit to induce a cancer-related gene signature. J Cell Sci. 2012;125(Pt 13):3144–52. doi: 10.1242/jcs.099663. [DOI] [PubMed] [Google Scholar]
- 20.UCSC Genome Browser. [https://genome.ucsc.edu/cgi-bin/hgGateway] Accessed 13 Apr 2015.
- 21.Koolen DA, Dupont J, de Leeuw N, Vissers LE, van den Heuvel SP, Bradbury A, et al. Two families with sibling recurrence of the 17q21.31 microdeletion syndrome due to low-grade mosaicism. Eur J Hum Genet. 2012;20(7):729–33. doi: 10.1038/ejhg.2012.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wray CD. 17q21.31 microdeletion associated with infantile spasms. Eur J Med Genet. 2013;56(1):59–61. doi: 10.1016/j.ejmg.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 23.Dornelles-Wawruk H, Pic-Taylor A, Rosenberg C, Krepischi AC, Safatle HP, Ferrari I, et al. Complex phenotype associated with 17q21.31 microdeletion. Mol Syndromol. 2013;4(6):297–301. doi: 10.1159/000354120. [DOI] [PMC free article] [PubMed] [Google Scholar]


