Abstract
Objective
Acute coronary syndrome (ACS) encompasses ST segment elevation myocardial infarction (STEMI), with generally high thrombus burden and non-ST segment elevation ACS (NSTE-ACS), with lower thrombus burden. In the setting of percutaneous coronary intervention (PCI) for ACS, bivalirudin appears superior to unfractionated heparin (UFH), driven by reduced major bleeding. Recent trials suggest that the benefit of bivalirudin may be reduced with use of transradial access and evolution in antiplatelet therapy. Moreover, a differential role of bivalirudin in ACS cohorts is unknown.
Methods
A meta-analysis of randomised trials comparing bivalirudin and UFH in patients with ACS receiving PCI, with separate analyses in STEMI and NSTE-ACS groups. Overall estimates of treatment effect were calculated with random-effects model.
Results
In 5 trials of STEMI (10 358 patients), bivalirudin increased the risk of acute stent thrombosis (ST) (OR 3.62; CI 1.95 to 6.74; p<0.0001) compared with UFH. Bivalirudin reduced the risk of major bleeding only when compared with UFH plus planned glycoprotein IIb/IIIa inhibitors (GPI) (OR 0.49; CI 0.36 to 0.67; p<0.00001). In 14 NSTE-ACS trials (25 238 patients), there was no difference between bivalirudin and UFH in death, myocardial infarction or ST. However, bivalirudin reduced the risk of major bleeding compared with UFH plus planned GPI (OR 0.52; CI 0.43 to 0.62; p<0.00001), or UFH plus provisional GPI (OR 0.68; CI 0.46 to 1.01; p=0.05). The reduction in major bleeding with bivalirudin was not related to vascular access site.
Conclusions
Bivalirudin increases the risk of acute ST in STEMI, but may confer an advantage over UFH in NSTE-ACS while undergoing PCI, reducing major bleeding without an increase in ST.
Key questions.
What is already known about this subject?
The use of bivalirudin during percutaneous coronary intervention (PCI) has been the subject of much debate recently.
What does this study add?
Our meta-analysis should help clinicians when selecting a periprocedural anticoagulant in different acute coronary syndrome (ACS) cohorts undergoing PCI (ie, in ST segment elevation myocardial infarction (STEMI) vs non-ST segment elevation (NSTE) ACS).
How might this impact on clinical practice?
This will probably have a great impact on clinical practice as this meta-analysis suggests that in patients with STEMI, with generally high thrombus burden and with the increasing use of transradial approach and more potent oral antiplatelet therapy, bivalirudin does not appear to be superior to unfractionated heparin, especially with provisional use of glycoprotein IIb/IIIa inhibitors (GPI) as currently recommended. In comparison, in patients with NSTE-ACS with lower thrombus burden, our meta-analysis suggests that bivalirudin may be superior to unfractionated heparin with provisional GPI use with regard to bleeding risk, but does not reduce risk of ischaemic events.
Background
Bivalirudin is an intravenous direct thrombin inhibitor that is widely used in the setting of percutaneous coronary intervention (PCI) for acute coronary syndrome (ACS).1 2 Randomised trials have demonstrated that bivalirudin is superior to unfractionated heparin (UFH) in reducing net adverse cardiac events, mainly due to a reduction in major bleeding.3–5 This is an important consideration since bleeding related to PCI has been associated with significant deleterious short-term and long-term consequences.6–8 However, over the past decade, the rates of major or clinically significant bleeding have decreased markedly as a result of innovations in PCI, including the increasing use of transradial vascular access and modifications in adjunct pharmacotherapy. With respect to the latter, introduction of potent oral antiplatelet agents have decreased the routine use of glycoprotein IIb/IIIa inhibitors (GPI) in ACS, with current opinion favouring their use in patients with large thrombus burden, insufficient oral antiplatelet therapy, or as a bailout for complications.9 Perhaps, as a result of this evolution in clinical practice, recent trials suggest that the previous benefit observed with bivalirudin may be substantially reduced in the contemporary PCI setting.10 11
Several trials of bivalirudin have reported a small increase in the risk of myocardial infarction (MI) and/or stent thrombosis (ST) in patients with ACS undergoing PCI.4 5 12 13 However, the study designs were not directly comparable as some of these trials used routine GPI in the UFH arm compared with provisional GPI use in the bivalirudin arm.5 12 13 Thus, the net clinical benefit of bivalirudin use is likely to depend on the relative risk of ischaemic versus bleeding complications.
The main beneficial effect of potent antithrombotic therapy would be expected in the setting of large thrombus burden. Since ACS comprises of both ST segment elevation MI (STEMI), with generally high thrombus burden and non-ST segment elevation ACS (NSTE-ACS), with lower thrombus burden,14 there is a need to assess the differential clinical benefit of bivalirudin versus UFH in these two groups. We, therefore, sought to evaluate the effects of bivalirudin compared with UFH on ischaemic and bleeding outcomes, with particular focus on the differential role in patients predominantly with STEMI versus NSTE-ACS.
Methods
The study was designed according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) Statement.15 Inclusion criteria were randomised controlled trials comparing the effects of bivalirudin and UFH, including those with multiple arms. Trials that involved a bolus of low-molecular-weight heparin or fondaparinux at baseline and before randomisation were also included. Trials were restricted to those studying patients predominantly with STEMI or NSTE-ACS, who underwent PCI. Since ACS encompasses a wide spectrum of disease, the trials referred to as STEMI also included some patients with non-STEMI (NSTEMI), and NSTE-ACS trials also included patients with stable angina undergoing elective PCI. However, similar treatment strategies were employed for all patients in these trials. Exclusion criteria were as follows: studies that used fibrinolytic therapy, studies that used balloon angioplasty only, studies that primarily addressed dosing or timing issues, small studies including ≤50 patients, studies that included patients with stable angina only, and studies that reported no clinical outcomes (see online supplementary figure S1).
The PubMed/Medline, Embase, Scopus and Cochrane Central Register of Controlled Trials databases were searched until March 2015, without language restrictions. Eligible studies were identified with the following search terms: bivalirudin, Angiomax, Hirulog, heparin, anticoagulation, primary percutaneous coronary intervention, percutaneous coronary intervention, coronary intervention, coronary angioplasty, stent, acute coronary syndrome, myocardial infarction, unstable angina, randomly, random and randomised controlled trial. We also searched reference lists of the retrieved articles to identify other eligible studies. Online oral presentations and expert slide presentations were also examined.
Two investigators independently reviewed all titles, or titles and abstracts from the search results to identify articles according to fulfilment of inclusion criteria. Selected trials were compared, and disagreement was resolved by team discussion and consensus. Data extraction was carried out independently and in duplicate by the study investigators. Results of data extraction were then compared, and discrepancies resolved by discussion. If results were incomplete or unclear, the study authors were contacted. Articles finally selected for the review were checked to avoid inclusion of data published in duplicate. Relevant data was collected on baseline characteristics, and clinical outcomes at baseline and end of follow-up. We investigated efficacy end points of death, MI (including Q wave and non-Q wave), early ST including acute (<24 h) and subacute (≥24 h to 30 days), all up to 30 days posthospitalisation. Major bleeding at 30 days posthospitalisation was the safety end point of interest. The primary outcomes and definition of major bleeding for each trial is listed in online supplementary table S1.
Relevant studies were stratified according to the population studied into predominantly STEMI versus predominantly NSTE-ACS. For the purposes of the meta-analysis, results are presented for each ACS cohort independently. Where both ACS cohorts were studied with distinct results, the respective groups were analysed separately. Likewise, for trials in which there were three arms based on GPI use, major bleeding comparisons were analysed separately.
Statistical analysis
Outcomes are presented as ORs with 95% CIs. Pooled OR were calculated using a random-effects model by the method of DerSimonian and Laird.16 Heterogeneity was assessed using χ2 and I2 tests. All analyses were performed with the intention-to-treat principle. We stratified results by key trial characteristics, including type of ACS (predominantly STEMI vs predominantly NSTE-ACS) and use of GPIs (predominantly planned in the UFH arm vs provisional in the bivalirudin arm, provisional in both arms, or planned in both arms). In sensitivity analysis, where possible, we included only trials which included patients predominantly with NSTEMI. We did meta-regression to examine the following relations: (1) the log-transformed OR of the effect of bivalirudin on major bleeding and log-transformed OR of the effect of bivalirudin on mortality, (2) the log-transformed OR of the effect of bivalirudin on major bleeding and where possible, the trial reported percentage of the use of transradial approach.
Publication bias was minimised by a comprehensive literature search. In addition, a graphical display (funnel plot) of the size of the treatment effect against the precision of the trial (1/SE) was used to investigate publication bias. All tests were two-sided, and statistical significance was fixed at 0.05 level. Analysis was carried out using Review Manager Software (RevMan V.5.3) and Stata V.11.2 (StataCorp, College Station, Texas, USA).
Results
We identified 32 randomised trials comparing the effects of bivalirudin and UFH, and involving patients with ACS undergoing PCI. Of these, 19 trials involving 35 596 patients, that met the inclusion and exclusion criteria, were included in the meta-analysis. The methodological quality of included studies is described in online supplementary table S2. The primary outcomes of each trial are listed in online supplementary table S1. Most trials used a major bleeding definition based on either REPLACE-2 or ACUITY. There was no evidence of publication bias having a significant effect on the results (eg, see online supplementary figures S2 and S3).
The characteristics of individual STEMI trials are listed in table 1. Five trials enrolled 10 358 patients predominantly with STEMI,5 10 11 13 17 in which 82–97% underwent PCI. The characteristics of individual NSTE-ACS trials are listed in table 2. Four trials predominantly enrolled patients with NSTEMI,4 12 18 19 and 10 trials predominantly enrolled patients with urgent or elective PCI for unstable or stable angina.3 20–28 In total, across the 14 NSTE-ACS trials, 24 979 (98.9%) of 25 238 patients underwent PCI. Bivalirudin doses were similar across all included trials (intravenous bolus of 0.75 mg/kg followed by an infusion of 1.75 mg/kg/h). UFH doses ranged between 50 U/kg, as in TENACITY24 and PROTECT-TIMI 30,18 and up to 140 U/kg, as in ISAR-REACT 3,22 with a median dose of 70 U/kg across all included trials.
Table 1.
Characteristics of predominantly STEMI studies
| Study | Patients (n) | PCI (%) | Bivalirudin (n) | UFH (n) | Bivalirudin design | UFH design | Female (%) | Mean age (year) | Follow-up (days) | GPI use (%) |
Transradial (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bivalirudin | UFH | |||||||||||
| HORIZONS-AMI, 200813 | 3602 | 93 | 1800 | 1802 | Intravenous bolus of 0.75 mg/kg followed by an infusion of 1.75 mg/kg/h | Intravenous bolus of 60 U/kg with subsequent boluses targeted to ACT of 200–250 s | 23 | 60 | 30 | 8 | 98 | 6 |
| EUROMAX, 20135 | 2218 | 86 | 1089 | 1109 | Intravenous bolus of 0.75 mg/kg followed by an infusion of 1.75 mg/kg/h | Intravenous bolus of 100 U/kg without GPI or 60 U/kg with GPI | 24 | 62 | 30 | 12 | 69 | 47 |
| Heat-PPCI, 201410 | 1812 | 82 | 905 | 907 | Intravenous bolus of 0·75 mg/kg followed by infusion of 1·75 mg/kg/h. A rebolus of 0·3 mg/kg was administered if ACT values were <225 s | Intravenous bolus of 70 U/kg with subsequent boluses if ACT values were <200 s | 28 | 63 | 28 | 13 | 15 | 81 |
| BRIGHT*, 201517 | 2178 | 97 | 729 | 1449 | Intravenous bolus of 0.75 mg/kg, followed by a continuous infusion of 1.75 mg/kg/h If ACT was <225 s, an additional bolus of 0.3 mg/kg was added |
Intravenous bolus of 100 U/kg without GPI or 60 U/kg with GPI, with subsequent boluses if ACT values were <200 s | 18 | 58 | 30 | 4 | 53 | 79 |
| BRAVE 4, 201411 | 548 | 92 | 271 | 277 | Intravenous bolus of 0.75 mg/kg followed by an infusion of 1.75 mg/kg/h | Intravenous bolus of 70–100 U/kg with subsequent boluses according to ACT | 23 | 61 | 30 | 3 | 6 | <1 |
*The trial involved three arms: bivalirudin alone, UFH alone, and UFH plus GPI.
ACT, activated clotting time; GPI, glycoprotein IIb/IIIa inhibitors; PCI, percutaneous coronary intervention; STEMI, ST segment elevation myocardial infarction; UFH, unfractionated heparin.
Table 2.
Characteristics of predominantly NSTE-ACS studies
| Study | Patients (n)/population | PCI (%) | Bivalirudin (n) | UFH (n) | Bivalirudin design | UFH design | Female (%) | Mean age (year) | Follow-up (days) | GPI use (%) |
Transradial (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bivalirudin | UFH | |||||||||||
| REPLACE-2, 20033 | 6010 Elective or urgent PCI |
98 | 2999 | 3011 | Intravenous bolus of 0.75 mg/kg, followed by a continuous infusion of 1.75 mg/kg/h | Intravenous bolus of 65 U/kg | 26 | 63 | 30 | 7 | 97 | 0 |
| REPLACE-1, 200420 | 1056 Elective or urgent PCI |
100 | 532 | 524 | Intravenous bolus of 0.75 mg/kg, followed by a continuous infusion of 1.75 mg/kg/h | Intravenous bolus of 60–70 U/kg adjusted to achieve and maintain an ACT of 200–300 s | 30 | 64 | 2 | 71 | 73 | 3 |
| PROTECT-TIMI 30, 200618 | 857 NSTEMI or UA |
100 | 284 | 573 | Intravenous bolus of 0.75 mg/kg, followed by a continuous infusion of 1.75 mg/kg/h with additional boluses to maintain ACT>200 s | Intravenous bolus of 50 U/kg adjusted to achieve and maintain an ACT of 200–250 s | 33 | 60 | 2 | 3 | 99 | NR |
| ACUITY-PCI*, 20074 | 7789 NSTEMI |
100 | 5228 | 2561 | Intravenous bolus of 0·1 mg/kg and an infusion of 0·25 mg/kg/h. An additional intravenous bolus of 0·5 mg/kg was administered before PCI, and the infusion was increased to 1·75 mg/kg/h |
Intravenous bolus of 60 U/kg followed by an infusion of 12 U/kg/h to achieve and maintain an ACT of 200–250 s | 27 | 63 | 30 | 53 | 97 | 6 |
| ARNO, 200821 | 850 UA or elective PCI |
86 | 425 | 425 | Intravenous bolus of 0.75 mg/kg, followed by a continuous infusion of 1.75 mg/kg/h | Intravenous bolus of 100 U/kg with or without additional boluses to achieve an ACT of 250–300 s | 24 | 69 | 30 | NR | NR | 2 |
| ISAR-REACT 3, 200822 | 4570 Elective or urgent PCI |
100 | 2289 | 2281 | Intravenous bolus of 0.75 mg/kg, followed by a continuous infusion of 1.75 mg/kg/h | Intravenous bolus of 140 U/kg followed by a placebo infusion for the duration of the procedure | 24 | 67 | 30 | <1 | <1 | 68 |
| NAPLES, 200923 | 335 Elective or urgent PCI |
100 | 167 | 168 | Intravenous bolus of 0.75 mg/kg, followed by a continuous infusion of 1.75 mg/kg/h with additional boluses to maintain ACT>250 s | Intravenous bolus of 70 U/kg with additional boluses to maintain ACT>250 s | 35 | 65 | 30 | 1 | 100 | 3 |
| TENACITY, 201124 | 383 Elective or urgent PCI |
100 | 185 | 198 | Intravenous bolus of 0.75 mg/kg, followed by a continuous infusion of 1.75 mg/kg/h | Intravenous bolus of 50 U/kg adjusted to achieve and maintain an ACT of 225 s | 27 | 63 | 30 | 100 | 100 | NR |
| ISAR-REACT 4, 201112 | 1721 NSTEMI |
100 | 860 | 861 | Intravenous bolus of 0.75 mg/kg, followed by a continuous infusion of 1.75 mg/kg/h | Intravenous bolus of 70 U/kg | 23 | 68 | 30 | 0 | 100 | <1 |
| ARMYDA-7 BIVALVE, 201225 | 401 Elective or urgent PCI |
93 | 198 | 203 | Intravenous bolus of 0.75 mg/kg, followed by a continuous infusion of 1.75 mg/kg/h | Intravenous bolus of 75 U/kg | 28 | 70 | 30 | 12 | 14 | 2 |
| Deshpande et al26 | 101 Elective or urgent PCI |
100 | 49 | 52 | Intravenous bolus of 0.75 mg/kg, followed by a continuous infusion of 1.75 mg/kg/h | Intravenous bolus of 70 U/kg followed by an infusion of 20 U/kg/h to achieve and maintain an ACT of 200–250 s | 13 | 56 | 30 | 100 | 100 | 0 |
| SWITCH III, 201319 | 100 NSTEMI |
98 | 51 | 49 | Intravenous bolus of 0.75 mg/kg, followed by a continuous infusion of 1.75 mg/kg/h | Intravenous bolus of 60 U/kg with subsequent boluses to maintain an ACT>200 s | 32 | 63 | 30 | 4 | 12 | 68 |
| Xiang et al27 | 218 UA or elective PCI |
100 | 110 | 108 | Intravenous bolus of 0.75 mg/kg, followed by a continuous infusion of 1.75 mg/kg/h If ACT was <225 s, an additional bolus of 0.3 mg/kg was added |
Intravenous bolus of 130 U/kg. If ACT was <225 s, an additional bolus of 300 U/kg was added | 17 | 58 | 30 | 1 | 4 | 25 |
| NAPLES III, 201528 | 837 Elective or urgent PCI |
100 | 418 | 419 | Intravenous bolus of 0.75 mg/kg, followed by a continuous infusion of 1.75 mg/kg/h If ACT was <250 s, an additional bolus of 0.3 mg/kg was added |
Intravenous bolus of 70 U/kg. If ACT was <250 s, an additional bolus of 20 U/kg was added | 48 | 78 | 30 | 1 | 1 | 1 |
*The trial involved three arms; bivalirudin alone, bivalirudin plus GPI, and UFH alone.
ACT, activated clotting time; GPI, glycoprotein IIb/IIIa inhibitors; NR, not reported; NSTE-ACS, non-ST segment elevation acute coronary syndrome; NSTEMI, non-ST segment elevation myocardial infarction; PCI, percutaneous coronary intervention; UFH, unfractionated heparin, UA, unstable angina.
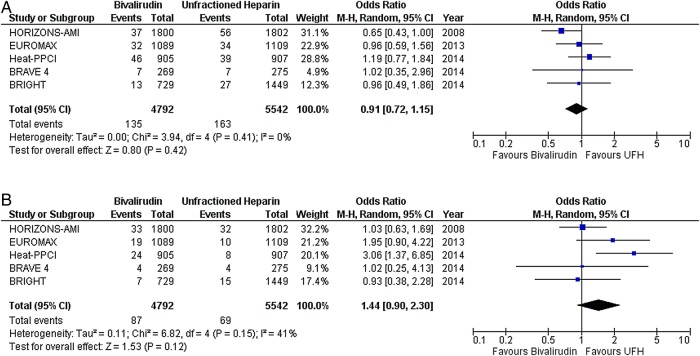
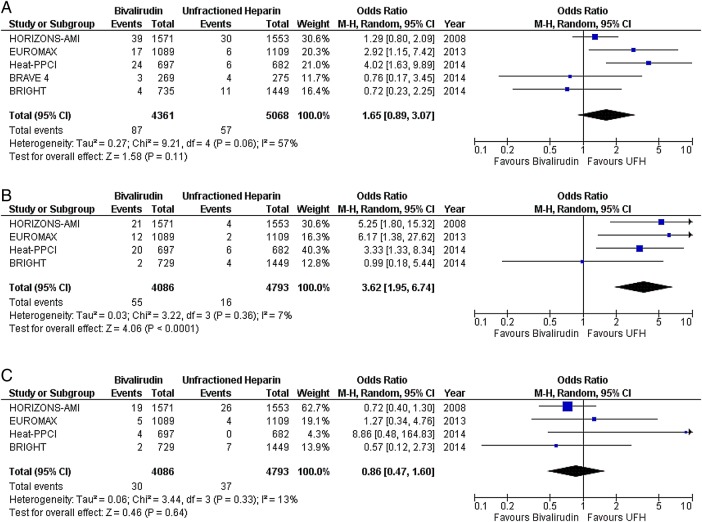
In STEMI, there was no difference in the risk of death (OR 0.91; CI 0.72 to 1.15; p=0.42) (figure 1A), MI (OR 1.44; CI 0.90 to 2.30; p=0.12) (figure 1B), any ST (OR 1.65; CI 0.89 to 3.07; p=0.11) (figure 2A), or subacute ST (OR 0.86; CI 0.47 to 1.60; p=0.64) (figure 2C) between the two drugs. As compared with UFH, bivalirudin increased the risk of acute ST (OR 3.62; CI 1.95 to 6.74; p<0.0001) (figure 2B). Major bleeding was less with bivalirudin plus provisional GPI compared with UFH plus planned GPI (OR 0.49; CI 0.36 to 0.67; p<0.00001) (figure 3A). However, there was no difference in the risk of major bleeding when both drugs were combined with provisional GPI (OR 0.98; CI 0.59 to 1.63; p=0.93) (figure 3B).
Figure 1.
Death, myocardial infarction with bivalirudin versus unfractionated heparin (UFH) in predominantly ST segment elevation myocardial infarction (STEMI) studies; (A) death, (B) myocardial infarction.
Figure 2.
Any, acute and subacute stent thrombosis with bivalirudin versus unfractionated heparin (UFH) in predominantly ST segment elevation myocardial infarction (STEMI) studies; (A) any stent thrombosis, (B) acute stent thrombosis (<24 h), and (C) subacute stent thrombosis (≥24 h to 30 days).
Figure 3.
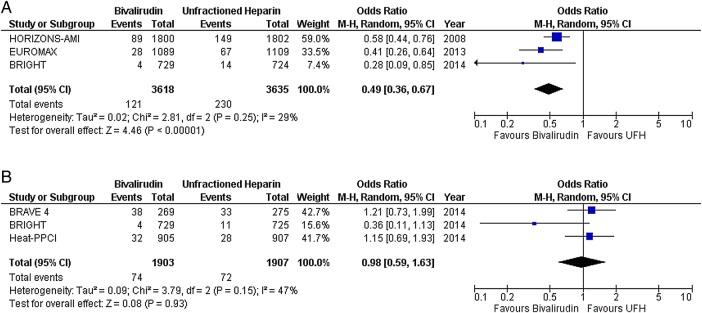
Major bleeding with bivalirudin versus unfractionated heparin (UFH) in predominantly ST segment elevation myocardial infarction (STEMI) studies; (A) glycoprotein IIb/IIIa inhibitors (GPI) predominantly provisional in the bivalirudin arm versus planned use in the heparin arm, and (B) provisional GPI use in both arms.
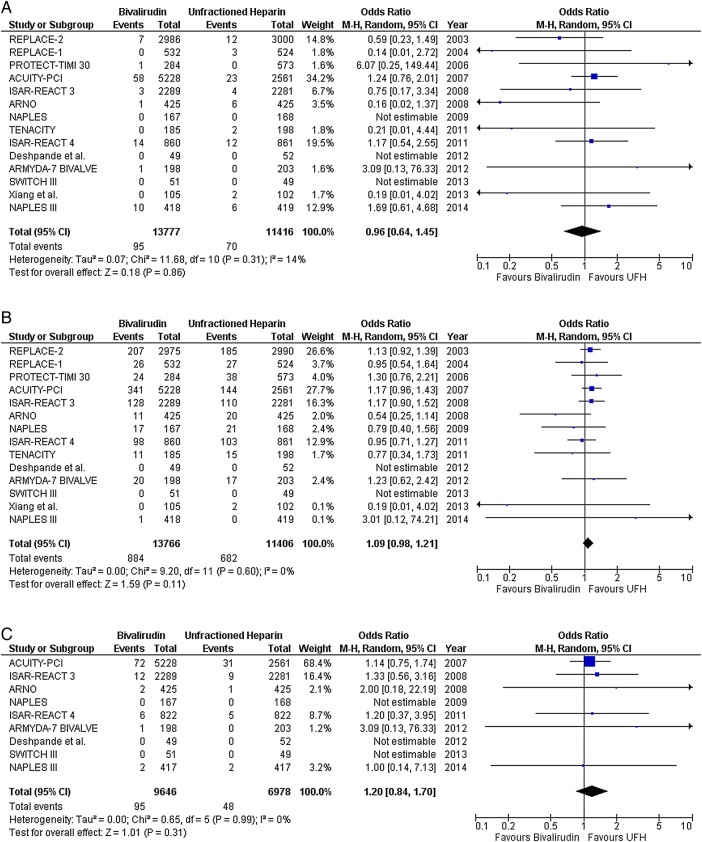
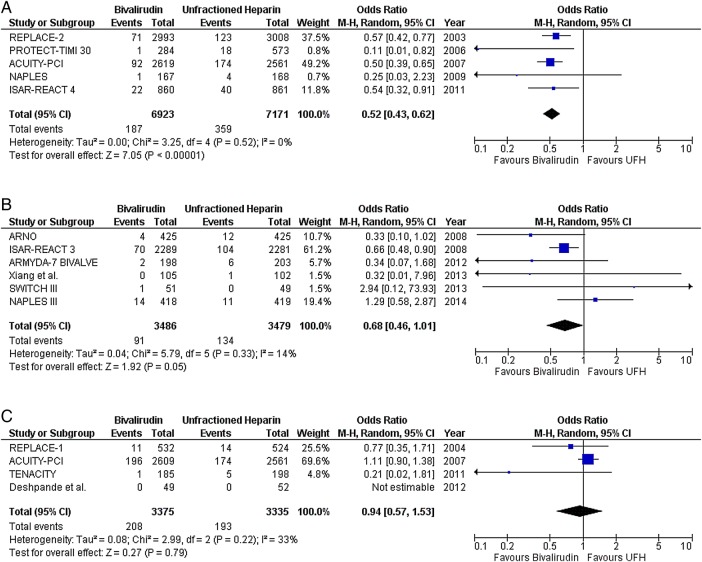
In NSTE-ACS, there was no difference between bivalirudin and UFH in the risk of death (OR 0.96; CI 0.64 to 1.45; p=0.86), MI (OR 1.09; CI 0.98 to 1.21; p=0.11), or any ST (OR 1.20; CI 0.84 to 1.70; p=0.31) (figure 4). Two NSTE-ACS trials reported no significant difference between bivalirudin and UFH in the risk of acute and subacute ST.21 25 Bivalirudin plus provisional GPI reduced the risk of major bleeding compared with UFH plus planned GPI (OR 0.52; CI 0.43 to 0.62; p<0.00001) (figure 5A). Also, bivalirudin reduced the risk of major bleeding compared with UFH, with provisional use of GPI in both arms (OR 0.68; CI 0.46 to 1.01; p=0.05) (figure 5B). However, there was no difference in the risk of major bleeding when either drug was combined with planned GPI (OR 0.94; CI 0.57 to 1.53; p=0.79) (figure 5C). The results observed in NSTE-ACS were also observed within the group of trials that enrolled patients predominantly with NSTEMI (see online supplementary figure S4).
Figure 4.
Death, myocardial infarction and any stent thrombosis with bivalirudin versus unfractionated heparin (UFH) in predominantly non-ST segment elevation acute coronary syndrome (NSTE-ACS) studies; (A) death, (B) myocardial infarction, and (C) any stent thrombosis.
Figure 5.
Major bleeding with bivalirudin versus unfractionated heparin (UFH) in predominantly non-ST segment elevation acute coronary syndrome (NSTE-ACS) studies; (A) glycoprotein IIb/IIIa inhibitors (GPI) predominantly provisional in the bivalirudin arm versus planned use in the heparin arm, (B) provisional GPI use in both arms, and (C) planned GPI use in both arms.
There was no significant relation between the reduction in bleeding with bivalirudin and mortality across all trials (p=0.96; see online supplementary figure S5). Also, there was no significant relation between the reduction in bleeding with bivalirudin and the use of transradial approach across all trials (p=0.49; see online supplementary figure S6), in predominantly STEMI trials (p=0.61), or in predominantly NSTE-ACS trials (p=0.15).
Discussion
The use of bivalirudin during PCI has been the subject of much debate recently. Most evidence of benefit was obtained when bivalirudin monotherapy was compared with a combination therapy of UFH plus GPI. Therefore, the differing benefits seen with bivalirudin and UFH in PCI may be in part related to the differential rates of GPI use. In this meta-analysis of 19 trials involving 35 596 patients, we found that bivalirudin may confer an advantage over UFH in patients with NSTE-ACS undergoing PCI, while this was not so in patients with STEMI. This difference is attributable to substantially increased risk of acute ST in STEMI-treated patients and not in NSTE-ACS. Moreover, bivalirudin use was associated with a significant reduction in major bleeding in NSTE-ACS but not in STEMI, when compared with UFH and provisional GPI use in both arms. There was no significant difference in the incidence of death, MI, or any ST between bivalirudin and UFH treated patients for both types of ACS.
The potential advantage of bivalirudin on bleeding risk was significantly affected by the strategy for concomitant GPI use. Overall, bivalirudin monotherapy consistently reduced the risk of bleeding in both ACS cohorts compared with UFH plus planned GPI. In contrast, when the use of GPI was provisional in bivalirudin and UFH arms, a benefit of bivalirudin use was observed in NSTE-ACS but not in STEMI. This may be in part due to the fact that the studies in NSTE-ACS included ISAR-REACT 322 (figure 5B), in which a very high bolus dose of UFH (140 U/kg) was used, resulting in more bleeding outcomes in the UFH arm. Also, the transradial approach was used more in STEMI than in NSTE-ACS trials (43% vs 15%, p=0.09), an approach associated with overall reduced bleeding29 30 and therefore, perhaps negating the reduction in bleeding that may have been observed with bivalirudin had a transfemoral approach been used. However, in contrast to what one might expect, our meta-analysis suggests that the relative reduction in major bleeding with bivalirudin was probably non-vascular access site related and did not seem to depend on the transradial approach use across all included trials (p=0.49), or in independent ACS cohorts (STEMI (p=0.61) versus NSTE-ACS (p=0.15)). In five NSTE-ACS studies, when GPI was planned in both arms, there was no difference in the risk of major bleeding.
Our findings are further supported by the recently conducted MATRIX trials, which showed that transradial compared with transfemoral access reduced net adverse clinical events through a reduction in all-cause mortality and major bleeding in 8404 patients with ACS undergoing PCI.31 In a parallel trial to assess antithrombotic effects, about 95% of 7213 patients with ACS (56% STEMI and 44% NSTE-ACS) were also randomised to bivalirudin or UFH with provisional GPI use (4.6% in bivalirudin group vs 26% in UFH group).32 Reduction in all-cause mortality was seen with bivalirudin (1.7% vs 2.3%; risk ratio (RR) 0.71; 95% CI 0.51 to 0.99), which was probably due to a reduction in major bleeding (1.4% vs 2.5%; RR 0.55; 95% CI 0.39 to 0.78), importantly with an independent bivalirudin impact on bleeding not related to the vascular access site. The rate of definite ST was higher with bivalirudin compared with UFH (1.0% vs 0.6%; RR 1.71; 95% CI 1.00 to 2.93). In contrast to our findings, although there has not been yet a published subgroup analysis of the MATRIX antithrombotic trial, the authors state that the findings were consistent across various subgroups (ie, STEMI vs NSTE-ACS).
In theory, continued bivalirudin infusion after PCI might decrease the risk of acute ST in patients with STEMI. The MATRIX optical coherence tomography (OCT) substudy will provide data on whether prolonged bivalirudin infusion, compared with intraprocedural-only administration in patients undergoing PCI, reduces the initial increase in ST (residual thrombosis on stent struts) as evaluated by OCT at the end of PCI and at 3–5 days follow-up.33
Our findings, which incorporate data from recent trials, should help clinicians when selecting a periprocedural anticoagulant in different ACS cohorts undergoing PCI. In patients with STEMI, with generally high thrombus burden and with the increasing use of transradial approach and more potent oral antiplatelet therapy, bivalirudin does not appear to be superior to UFH, especially with provisional use of GPI as currently recommended. In comparison, in patients with NSTE-ACS with lower thrombus burden, our meta-analysis suggests that bivalirudin may be superior to UFH with provisional GPI use with regard to bleeding risk, but does not reduce risk of ischaemic events. The results of future trials of bivalirudin should provide more understanding of the clinical outcomes with this antithrombotic drug in different ACS cohorts undergoing PCI.
Study limitations
Our meta-analysis has several limitations. First, most trials in NSTE-ACS included a considerable proportion of patients with stable angina undergoing elective PCI. This cohort was also included in our study, as the trials did not report a separate analysis for the ACS cohort. That being said, similar treatment strategies were allowed for patients with stable angina in these trials. Second, we were unable to gain full access to the MATRIX antithrombotic trial32 subgroup results to include in the meta-analysis section. Third, UFH doses were different across the trials, which may have impacted on differential bleeding outcomes in the ACS cohorts studied. Fourth, the trials included had differences in vascular access, drug design and definitions. Finally, the results of this meta-analysis are derived from study-level data and not from patient-level data. Additionally, one trial had been presented but not published.21 Also, many of the trials included were open label with a potential for high performance bias. Finally, most of the large studies were conducted in developed western countries and thus, may not be generalisable to other healthcare settings.
Conclusions
Although in STEMI, bivalirudin is associated with an increased risk of acute ST, it may confer an advantage over UFH in patients with NSTE-ACS undergoing PCI, with a reduction in non-vascular access site major bleeding and without an increase in ST. Large randomised studies are needed to elucidate more precisely the optimal PCI-related antithrombotic regimen for patients presenting with STEMI and NSTE-ACS in the contemporary era.
Acknowledgments
The authors would like to thank Ashraf Nabhan, MD, and Keith Sullivan, PhD, for analytical support.
Footnotes
Contributors: MF and MS did the literature search. All the authors analysed the data, interpreted the findings, drafted the manuscript, and approved the manuscript submitted.
Competing interests: None declared.
Ethics approval: All included studies in this meta-analysis obtained ethics approval.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Windecker S, Kolh P, Alfonso F et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2014;35:2541–619. doi:10.1093/eurheartj/ehu278 [DOI] [PubMed] [Google Scholar]
- 2.Levine GN, Bates ER, Blankenship JC et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 2011;124:e574–651. doi:10.1161/CIR.0b013e31823ba622 [DOI] [PubMed] [Google Scholar]
- 3.Lincoff AM, Bittl JA, Harrington RA et al. Bivalirudin and provisional glycoprotein IIb/IIIa blockade compared with heparin and planned glycoprotein IIb/IIIa blockade during percutaneous coronary intervention: REPLACE-2 randomized trial. JAMA 2003;289:853–63. doi:10.1001/jama.289.7.853 [DOI] [PubMed] [Google Scholar]
- 4.Stone GW, White HD, Ohman EM et al. Bivalirudin in patients with acute coronary syndromes undergoing percutaneous coronary intervention: a subgroup analysis from the Acute Catheterization and Urgent Intervention Triage strategy (ACUITY) trial. Lancet 2007;369:907–19. doi:10.1016/S0140-6736(07)60450-4 [DOI] [PubMed] [Google Scholar]
- 5.Steg PG, van ‘t Hof A, Hamm CW et al. Bivalirudin started during emergency transport for primary PCI. N Engl J Med 2013;369:2207–17. doi:10.1056/NEJMoa1311096 [DOI] [PubMed] [Google Scholar]
- 6.Mehran R, Pocock S, Nikolsky E et al. Impact of bleeding on mortality after percutaneous coronary intervention results from a patient-level pooled analysis of the REPLACE-2 (randomized evaluation of PCI linking angiomax to reduced clinical events), ACUITY (acute catheterization and urgent intervention triage strategy), and HORIZONS-AMI (harmonizing outcomes with revascularization and stents in acute myocardial infarction) trials. JACC Cardiovasc Interv 2011;4:654–64. doi:10.1016/j.jcin.2011.02.011 [DOI] [PubMed] [Google Scholar]
- 7.Hermanides RS, Ottervanger JP, Dambrink JH et al. Incidence, predictors and prognostic importance of bleeding after primary PCI for ST-elevation myocardial infarction. EuroIntervention 2010;6:106–11. doi:10.4244/ [PubMed] [Google Scholar]
- 8.Manoukian SV. The relationship between bleeding and adverse outcomes in ACS and PCI: pharmacologic and nonpharmacologic modification of risk. J Invasive Cardiol 2010;22:132–41. [PubMed] [Google Scholar]
- 9.Giugliano RP, White JA, Bode C et al. Early versus delayed, provisional eptifibatide in acute coronary syndromes. N Engl J Med 2009;360:2176–90. doi:10.1056/NEJMoa0901316 [DOI] [PubMed] [Google Scholar]
- 10.Shahzad A, Kemp I, Mars C et al. Unfractionated heparin versus bivalirudin in primary percutaneous coronary intervention (HEAT-PPCI): an open-label, single centre, randomised controlled trial. Lancet 2014;384:1849–58. doi:10.1016/S0140-6736(14)60924-7 [DOI] [PubMed] [Google Scholar]
- 11.Schulz S, Richardt G, Laugwitz KL et al. Prasugrel plus bivalirudin vs. clopidogrel plus heparin in patients with ST-segment elevation myocardial infarction. Eur Heart J 2014;35:2285–94. doi:10.1093/eurheartj/ehu182 [DOI] [PubMed] [Google Scholar]
- 12.Kastrati A, Neumann FJ, Schulz S et al. Abciximab and heparin versus bivalirudin for non-ST-elevation myocardial infarction. N Engl J Med 2011;365:1980–9. doi:10.1056/NEJMoa1109596 [DOI] [PubMed] [Google Scholar]
- 13.Stone GW, Witzenbichler B, Guagliumi G et al. Bivalirudin during primary PCI in acute myocardial infarction. N Engl J Med 2008;358:2218–30. doi:10.1056/NEJMoa0708191 [DOI] [PubMed] [Google Scholar]
- 14.Hong YJ, Jeong MH, Choi YH et al. Differences in intravascular ultrasound findings in culprit lesions in infarct-related arteries between ST segment elevation myocardial infarction and non-ST segment elevation myocardial infarction. J Cardiol 2010;56:15–22. doi:10.1016/j.jjcc.2010.01.010 [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J et al. Preferred Reporting Items for Systematic Reviews and Meta-analyses: the PRISMA Statement. Open Med 2009;3:e123–30. [PMC free article] [PubMed] [Google Scholar]
- 16.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. doi:10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 17.Han Y, Guo J, Zheng Y et al. Bivalirudin vs heparin with or without tirofiban during primary percutaneous coronary intervention in acute myocardial infarction: the BRIGHT randomized clinical trial. JAMA 2015;313:1336–46. doi:10.1001/jama.2015.2323 [DOI] [PubMed] [Google Scholar]
- 18.Gibson CM, Morrow DA, Murphy SA et al. A randomized trial to evaluate the relative protection against post-percutaneous coronary intervention microvascular dysfunction, ischemia, and inflammation among antiplatelet and antithrombotic agents: the PROTECT-TIMI-30 trial. J Am Coll Cardiol 2006;47:2364–73. doi:10.1016/j.jacc.2005.12.077 [DOI] [PubMed] [Google Scholar]
- 19.Waksman R, Bertrand O, Driesman M et al. Bivalirudin versus unfractionated heparin during percutaneous coronary intervention in patients with non-ST-segment elevation acute coronary syndrome initially treated with fondaparinux: results from an international, multicenter, randomized pilot study (SWITCH III). J Interv Cardiol 2013;26:107–13. doi:10.1111/joic.12005 [DOI] [PubMed] [Google Scholar]
- 20.Lincoff AM, Bittl JA, Kleiman NS et al. Comparison of bivalirudin versus heparin during percutaneous coronary intervention (the Randomized Evaluation of PCI Linking Angiomax to Reduced Clinical Events [REPLACE]-1 trial). Am J Cardiol 2004;93: 1092–6. doi:10.1016/j.amjcard.2004.01.033 [DOI] [PubMed] [Google Scholar]
- 21.Antoniucci D. A randomized trial comparing bivalirudin with unfractionated heparin in patients undergoing elective PCI (ARNO Trial). Washington, DC, USA: Transcatheter Cardiovascular Therapeutics, 2008. [Google Scholar]
- 22.Kastrati A, Neumann FJ, Mehilli J et al. Bivalirudin versus unfractionated heparin during percutaneous coronary intervention. N Engl J Med 2008;359:688–96. doi:10.1056/NEJMoa0802944 [DOI] [PubMed] [Google Scholar]
- 23.Tavano D, Visconti G, D'Andrea D et al. Comparison of bivalirudin monotherapy versus unfractionated heparin plus tirofiban in patients with diabetes mellitus undergoing elective percutaneous coronary intervention. Am J Cardiol 2009;104:1222–8. doi:10.1016/j.amjcard.2009.06.035 [DOI] [PubMed] [Google Scholar]
- 24.Moliterno DJ, TENACITY Steering Committee and Investigators. A randomized two-by-two comparison of high-dose bolus tirofiban versus abciximab and unfractionated heparin versus bivalirudin during percutaneous coronary revascularization and stent placement: the tirofiban evaluation of novel dosing versus abciximab with clopidogrel and inhibition of thrombin (TENACITY) study trial. Catheter Cardiovasc Interv 2011;77:1001–9. doi:10.1002/ccd.22876 [DOI] [PubMed] [Google Scholar]
- 25.Patti G, Pasceri V, D'Antonio L et al. Comparison of safety and efficacy of bivalirudin versus unfractionated heparin in high-risk patients undergoing percutaneous coronary intervention (from the Anti-Thrombotic Strategy for Reduction of Myocardial Damage During Angioplasty-Bivalirudin vs Heparin study). Am J Cardiol 2012;110:478–84. doi:10.1016/j.amjcard.2012.04.017 [DOI] [PubMed] [Google Scholar]
- 26.Deshpande NV, Pratiti R, Admane P et al. Safety and efficacy of bivalirudin with glycoprotein IIb/IIIa for high-risk percutaneous coronary intervention. Indian Heart J 2012;64:444–8. doi:10.1016/j.ihj.2012.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiang DC, Gu XL, Song YM et al. Evaluation on the efficacy and safety of domestic bivalirudin during percutaneous coronary intervention. Chin Med J (Engl) 2013;126:3064–8. [PubMed] [Google Scholar]
- 28.Briguori C, Visconti G, Focaccio A et al. Novel approaches for preventing or limiting events (Naples) III trial: randomized comparison of bivalirudin versus unfractionated heparin in patients at increased risk of bleeding undergoing transfemoral elective coronary stenting. JACC Cardiovasc Interv 2015;8: 414–23. doi:10.1016/j.jcin.2014.10.015 [DOI] [PubMed] [Google Scholar]
- 29.Jolly SS, Amlani S, Hamon M et al. Radial versus femoral access for coronary angiography or intervention and the impact on major bleeding and ischemic events: a systematic review and meta-analysis of randomized trials. Am Heart J 2009;157:132–40. doi:10.1016/j.ahj.2008.08.023 [DOI] [PubMed] [Google Scholar]
- 30.Karrowni W, Vyas A, Giacomino B et al. Radial versus femoral access for primary percutaneous interventions in ST-segment elevation myocardial infarction patients: a meta-analysis of randomized controlled trials. JACC Cardiovasc Interv 2013;6:814–23. doi:10.1016/j.jcin.2013.04.010 [DOI] [PubMed] [Google Scholar]
- 31.Valgimigli M, Gagnor A, Calabró P et al. Radial versus femoral access in patients with acute coronary syndromes undergoing invasive management: a randomised multicentre trial. Lancet 2015;385:2465–76. doi:10.1016/S0140-6736(15)60292-6 [DOI] [PubMed] [Google Scholar]
- 32.Valgimigli M. Minimizing Adverse haemorrhagic events by TRansradial access site and systemic Implementation of angioX: bivalirudin vs. heparin clinical trial—MATRIX. San Diego, CA, USA: American College of Cardiology, 2015. [Google Scholar]
- 33.Picchi A, Limbruno U, Andò G et al. Optical coherence tomography appraisal of residual thrombus burden in patients with ST-segment elevation myocardial infarction undergoing intraprocedural versus post-stenting prolonged bivalirudin infusion. Rationale and design of the MATRIX (minimizing adverse haemorrhagic events by TRansradial access site and angioX) OCT substudy. EuroIntervention 2015;10:1311–17. doi:10.4244/EIJY15M02_10 [DOI] [PubMed] [Google Scholar]