Abstract
Background:
Nicotine is a major pharmacologically active substance in cigarette smoke. It is mainly metabolized in liver and causes devastating effects. Crocin is the chemical ingredient primarily responsible for the color of saffron. It has different pharmacological effects such as antioxidant and anticancer. This study was designed to evaluate the protective role of crocin against nicotine on the liver of mice.
Methods:
Forty-eight mice were equally divided into 8 groups; control (normal saline), nicotine (2.5 mg/kg), crocin (12.5, 25 and 50 mg/kg) and crocin plus nicotine treated groups. Saline, crocin, nicotine and crocin/nicotine (once a day) were intraperitoneally injected for 4 weeks. The liver weight and histology, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), and serum nitric oxide levels have been studied.
Results:
The results indicated that nicotine administration significantly decreased liver weight (48.37%) and increased the mean diameter of hepatocyte (239%), central hepatic vein (28.45%), liver enzymes level (ALP 29.43%, AST 21.81%, ALT 21.55%), and blood serum nitric oxide level (57.18%) compared to saline group (P < 0.05). However, crocin and crocin plus nicotine administration significantly boosted liver weight (49.54%) and decreased the mean diameter of hepatocyte (40.48%), central hepatic vein (15.44%), liver enzymes (ALP 22.02%, AST 19.05%, ALT 23.11%), and nitric oxide levels (35.80%) in all groups compared to nicotine group (percentages represent the maximum dose) (P < 0.05).
Conclusions:
Crocin showed its partly protective effect against nicotine-induced liver toxicity.
Keywords: Crocin, liver, mice, nicotine
INTRODUCTION
Cigarette smoke has enormous negative health consequences worldwide. [1] Although approximately 4000 components occur in the cigarette, nicotine is a highly toxic organic compound containing nitrogen and alkaloid which is mostly found in tobacco and it is responsible for some of the deleterious effects of smoking. [2] Tobacco smoking is the single most important risk factor for cancer and is responsible for about one-third of all cancer deaths. [3] Nicotine affects a variety of cellular processes ranging from altered gene expression. [4] Exposure to nicotine produces oxidative tissue injuries in the mouse, often resulting in a depletion of glutathione content and a decrease in the activity of some oxygen free radical scavengers, such as catalase and superoxide dismutase. [5] Liver is considered to be the major site of nicotine biotransformation and nicotine exerts a number of adverse physiological effects on the liver. [6] Nicotine is absorbed through the lungs with smoking and is rapidly metabolized in the liver which induces three major adverse effects on the liver: Direct or indirect toxic effects, immunological effects, and oncogenic effects. [7] Smoking causes liver cell injury and exerts genotoxic effect of rat liver. [8] Nicotine has been recognized to result in oxidative stress by inducing the generation of reactive oxygen species (ROS). These ROS in turn are capable of initiating and promoting oxidative damage in the form of lipid peroxidation. [9] Lipid peroxidation is known to cause cellular injury by inactivation of membrane enzymes and receptors. [10] A major pathway of nicotine metabolism is co-oxidation to cotinine, which is catalyzed by CYP2A6 in the liver. [11] Crocin is a carotenoid, obtained commercially from the dried trifid stigma of the culinary spice Crocus sativus L. (saffron) and is responsible for the red color of saffron. [12] Crocin can be isolated in pure form the saffron extract and directly crystallized. [13] Saffron, the spice contain many chemical substances like carbohydrates, minerals, mucilage, vitamins (especially riboflavin and thiamin) and pigments including crocin, anthocyanin, carotene, lycopene, and zigzantin. [14,15] Crocin has also shown various pharmacological activities such as antioxidant, anticancer, radical scavenging and genoprotective. [16,17,18] Crocin as anti-tumor functions has got a special place in pharmaceutics. [19] At pharmacological and high doses, crocin did not exhibit marked damages to all major organs of the body, and no mortality was seen by crocin in mice. [20] According to crocin effects and by attention to so far any article on has not been reported protective effect of crocin against nicotine treated (crocin is expected to partially counteract the toxic effects of nicotine on some liver parameters), therefore, the present study was conducted to analyze the protective effect of crocin on the damage induced by nicotine in liver of male mice.
METHODS
Animals
Forty-eight Balb/c male mice with a weight range of 27–30 g were purchased from Tehran Razi Institute. Animals were kept in the temperature of 22 ± 2°C, under controlled environmental conditions, 12/12 h light/dark cycle and free access to water and food. Maintenance and care of experimental animals comply with National Institutes of Health guidelines. [21]
Chemicals
Crocin (digentiobiosyl 8,8’- diapocarotene-8,8’- oate; C44 H64O24) powder was purchased (Merk-Germany). The powder was diluted by normal saline (0.9%) to prepare different doses. Furthermore, the nicotine (CAS Name: 3-[(2S)-1-Methyl-2-pyrrolidinyl] pyridine) was purchased from Merk-Germany and diluted by normal saline (0.9%) for administration [22] [Figure 1].
Figure 1.
Structure of nicotine and crocin
Experimental protocol
The mice were randomly divided into 8 groups (n = 6). (1) Control group (normal saline; 1 ml DW/daily); (2) nicotine treated group (2.5 mg/kg);[23] (3) nicotine + crocin 12.5 mg/kg treated group; (4) nicotine + crocin 25 mg/kg treated group; (5) nicotine + crocin 50 mg/kg treated group; (6) crocin 12.5 mg/kg treated group; (7) crocin 25 mg/kg treated group and (8) crocin 50 mg/kg treated group. Nicotine intraperitoneally (IP) administrated once a day for 4 weeks. [23] Crocin and nicotine + crocin were given in the same way to animals. [22]
Liver weight and collection of blood serum
At the end of the experimental period, all animals were deeply anesthetized with ether. Blood was collected from the right ventricle of the heart, serum separated and stored at −80°C for measurement of the nitric oxide. They were then killed and sacrificed. Livers were removed and weighed on a microbalance sensitive to 0.001 mg (Precisa 125A, Switzerland) and recorded. [24]
Histological analysis
For the histological evaluation of the hepatic structures, the lower 1 cm long part of the right lobe of the liver in transverse pieces was removed, washed in saline and was fixed in 10% formalin at room temperature for 72 h. After fixing the tissue, it was thoroughly washed under running water and dehydrated in ascending concentration ethanol, cleared in xylene, and then embedded in soft paraffin. Thin sections (5 μm) were cut using a microtome (Leica RM 2125, Leica Microsystems Nussloch GmbH, Germany) and stained with Hematoxylin and Eosin. The preparation was examined an Olympus BX-51T-32E01 research microscope connected to a DP12 Camera with 3.34-million pixel resolution and Olysia Bio software (from: Olympus Optical Co. Ltd., Tokyo, Japan). [25]
Morphometric measurements
For each hepatocyte, the total cellular area was measured. The outline of each hepatocyte was measured after taking an image with a ×40 objective. The longest and shortest axis were measured in the drawing of each hepatocyte in order to estimate the mean diameter (mean axis). At least 50 hepatocytes from each zone (total 100) were measured in each liver. A separate measurement for central hepatic vein was performed, using the same methodology. [26,27]
Biochemical analysis
Liver was minced and homogenized (10% w/v) in ice-cold 0.1 M sodium phosphate buffer (pH-7.4). The homogenate was centrifuged at 10,000 rpm for 15-20 min at 4°C twice to get enzyme fraction. The resultant supernatant was used for various biochemical assays. Activities of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were assayed by the method of Reitman and Frankel [28] Activities of alkaline phosphatase (ALP) were determined according to the protocol described in laboratory practical manual. [29]
Griess assay
Nitric oxide was measured based on Griess colorimetric assay. Accordingly, NEED, sulfonamide solutions, and nitrite standards were prepared. To measure Nitrite concentration in serum, after de-freezing the serum samples, 100 μl of the sample serum was deproteinized by zinc sulfate and transferred to the wells. 100 μl chloride vanadium, 50 μl sulfonamide, and 50 μl NEED solutions were added afterward. The samples were incubated in the temperature of 30°C in darkness. Samples’ optical density was measured by ELISA reader (Hyperion, Germany) at the wavelength of 540 nm. [30]
Statistical analysis
All the quantitative data were presented as mean ± standard deviation. One-way analysis of variance followed by the least significant difference post-hoc test were performed to determine the statistical significance between different groups using Statistical Package for the Social Sciences, version 16.0, SPSS Inc, Chicago, Illinois, USA P < 0.05 was considered significant.
RESULTS
Liver weight
In the present study, the effective dose of nicotine (2.5 mg/kg) for 4 weeks caused a significant decrease in the liver weight of the mice compared to saline group (P < 0.05). Moreover, liver weight was significantly increase in treated animals with crocin and crocin plus nicotine in all doses in comparison with nicotine group (P < 0.05) [Figure 2].
Figure 2.
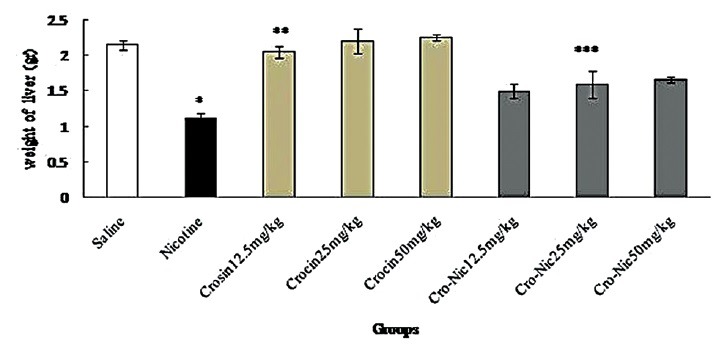
Forty-eight mice were equally divided into 8 groups. *Significant decrease of liver weight in nicotine group compared to saline group (P < 0.05). **Significant increase in all groups of crocin compared to nicotine group (P < 0.05). ***Significant increase in all groups of crocin-nicotine compared to nicotine group (P < 0.05). The numbers above the columns of the chart are mean ± standard error of triplicate experiments
Morphometric measurements
The mean diameter of hepatocytes and central hepatic vein were significantly increased in nicotine administration group in comparison with saline group administration (P < 0.05). Further, crocin and crocin plus nicotine caused a significant decrease in the mean diameter of hepatocytes and central hepatic vein in all treated groups in comparison with nicotine group administration (P < 0.05) [Figures 3 and 4].
Figure 3.
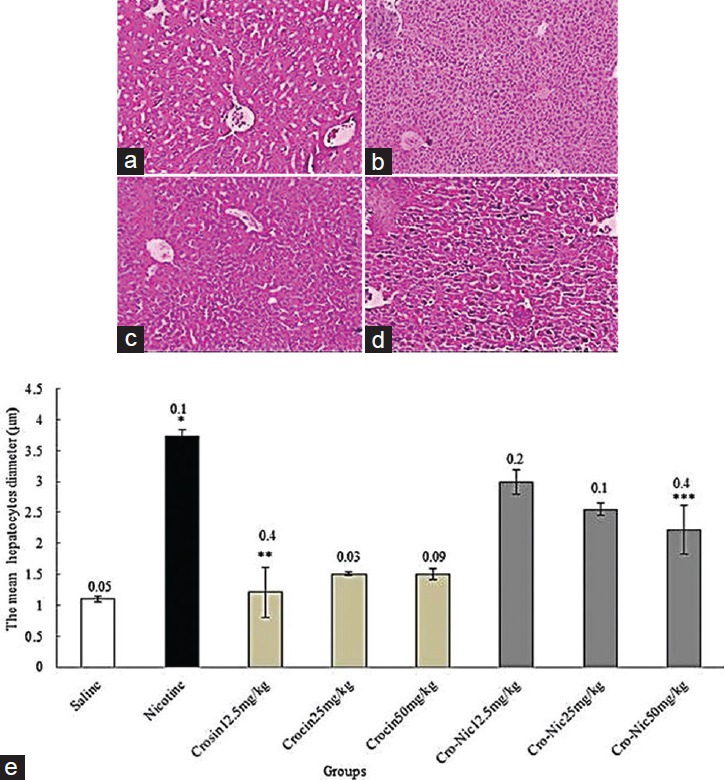
Forty-eight mice were equally divided into 8 groups. (a) Saline group; (b) nicotine group; (c) crocin 50 mg/kg; (d) crocin (50 mg/kg) plus nicotine (×100); (e) *Significant increase of the mean hepatocytes diameter in nicotine group compared to saline group (P < 0.05). **Significant decrease in all groups of crocin administration compared to nicotine group (P < 0.05). ***Significant decrease in all groups of crocin-nicotine administration compared to nicotine group (P < 0.05). The numbers above the columns of the chart are mean ± standard error of triplicate experiments
Figure 4.
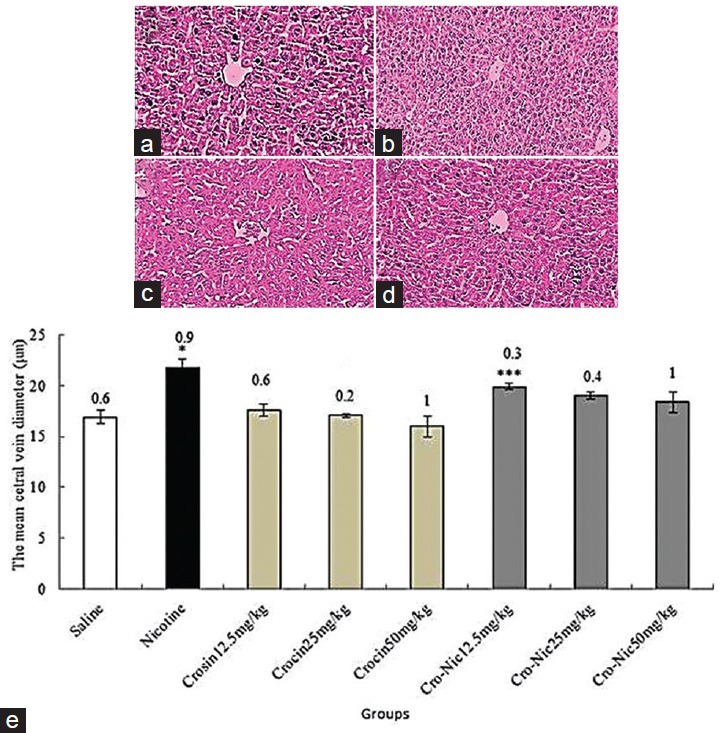
Forty-eight mice were equally divided into 8 groups. (a) Saline group; (b) nicotine group; (c) crocin 50 mg/kg; (d) crocin (50 mg/kg) plus nicotine (×100); (e) *Significant increase of the mean central hepatic vein diameter in nicotine group compared to saline group (P < 0.05). **Significant decrease in all groups of crocin administration compared to nicotine group (P < 0.05). ***Significant decrease in all groups of crocin-nicotine administration compared to nicotine group (P < 0.05). The numbers above the columns of the chart are mean ± standard error of triplicate experiments
Biochemical analysis
Nicotine (2.5 ml/kg) caused a significant increase in the mean of ALT, AST, and ALP enzymes compared to saline group (P < 0.05). In addition, the mean of ALT, AST, and ALP enzymes decreased significantly in crocin and crocin-nicotine in all groups administration compared to nicotine group (P < 0.05) [Figures 5-7].
Figure 5.
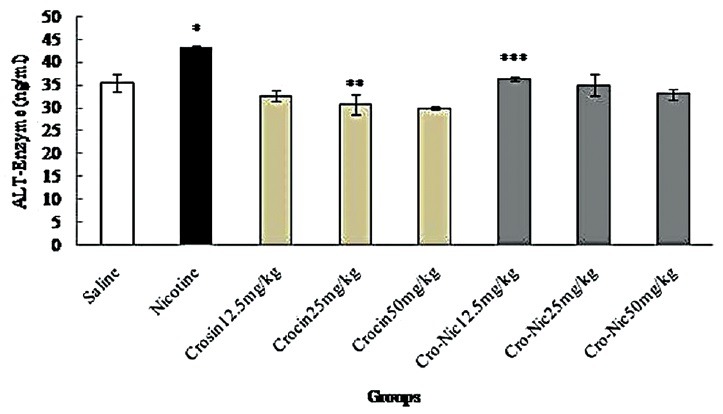
Forty-eight mice were equally divided into 8 groups. *Significant increase of alanine aminotransferase enzyme in nicotine group compared to saline group (P < 0.05). **Significant decrease in all groups of crocin compared to nicotine group administration (P < 0.05). ***Significant decrease in all groups of crocin-nicotine compared to nicotine group (P < 0.05). The numbers above the columns of the chart are mean ± standard error of triplicate experiments
Figure 7.
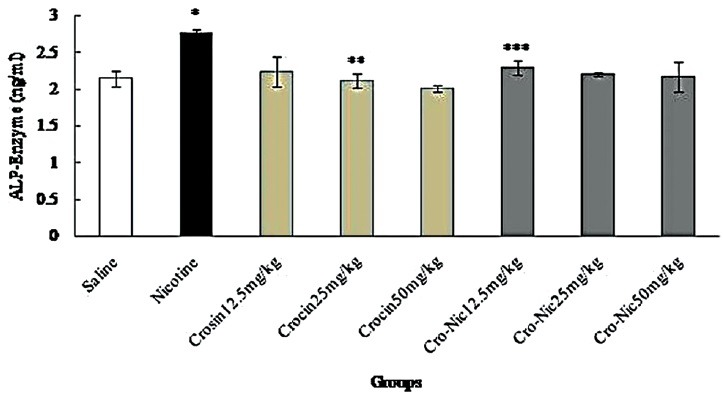
Forty-eight mice were equally divided into 8 groups. *Significant increase of alkaline phosphatase enzyme in nicotine group compared to saline group (P < 0.05). **Significant decrease in all groups of crocin compared to nicotine group administration (P < 0.05). ***Significant decrease in all groups of crocin-nicotine compared to nicotine group (P < 0.05). The numbers above the columns of the chart are mean ± standard error of triplicate experiments
Figure 6.
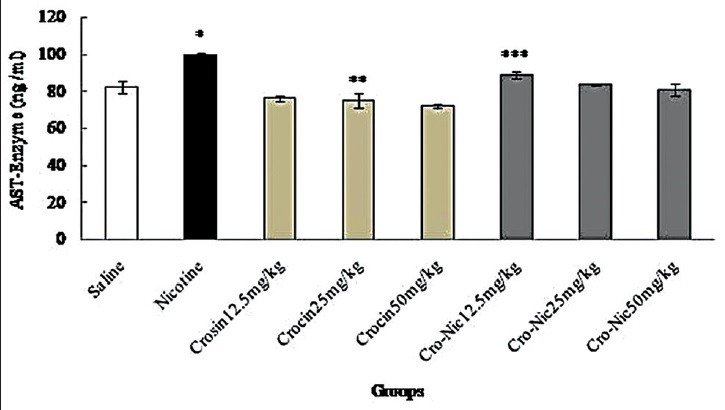
Forty-eight mice were equally divided into 8 groups. *Significant increase of aspartate aminotransferase enzyme in nicotine group compared to saline group (P < 0.05). **Significant decrease in all groups of crocin compared to nicotine group administration (P < 0.05). ***Significant decrease in all groups of crocin-nicotine compared to nicotine group (P < 0.05). The numbers above the columns of the chart are mean ± standard error of triplicate experiments
Nitric oxide
The mean of nitric oxide in blood serum increased significantly in nicotine (2.5 ml/kg) group administration compared to saline group (P < 0.05). Furthermore, the mean of nitric oxide in blood serum decreased significantly in crocin and crocin-nicotine in all groups compared to nicotine group [Figure 8].
Figure 8.
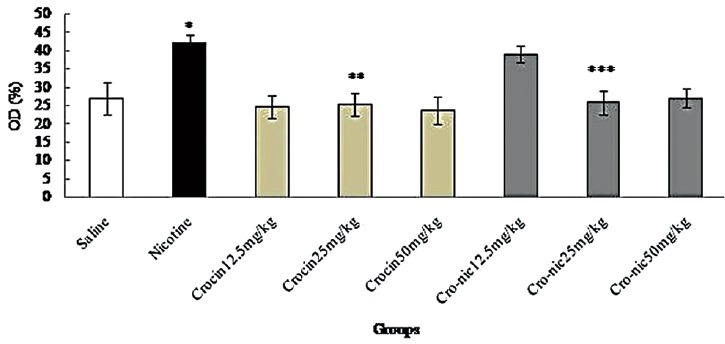
Forty-eight mice were equally divided into 8 groups. *Significant increase of nitric oxide in nicotine group compared to saline group (P < 0.05). **Significant decrease in all groups of crocin administration compared to nicotine group (P < 0.05). ***Significant decrease in all groups of crocin-nicotine administration compared to nicotine group (P < 0.05). The numbers above the columns of the chart are mean ± standard error of triplicate experiments
DISCUSSION
In the recent years, there has been an increasing awareness that certain naturally occurring compounds in plants have protective effects against carcinogens and endogenous mutagens. [18] Nicotine causes oxidative damage to the liver, it is a potential oxidant, which is capable of producing free radicals. [5] In this study, the protective role of crocin against nicotine-induced damages on the male mice liver was investigated. Treatment with different doses of the crocin was well-tolerated by all the animals, as there were no toxic effects observed by direct visual observation of the animals throughout the experiment. In the present study, the IP injection of nicotine resulted in the decrease of liver weight. It seems that nicotine can increase the release of neurotransmitters, including dopamine and serotonin, which are inhibitors food intake. [31] Mice treated with crocin and nicotine plus crocin showed significant increase liver weight. Liver weight growth might be associated with the appetite decreasing effects of nicotine that is overcome by crocin appetizing property. [13] This is in agreement with the results of the study conducted by Razavi et al., who reported that crocin increase body weight. [32] It seems that nicotine administration causes liver damage and metabolic dysfunction in the mice and thereby reduction of liver weight. [33] The results of histopathological studies obtained in this study indicated the increasing diameter of hepatocytes and the central vein of the liver due to nicotine administration in the study groups. Apparently, the size change of hepatic cells and liver's central vein can be the result of an increase in the metabolic activity of cells to excrete toxin from the body during the detoxification process. [34] The free radicals induced by nicotine metabolism cause lipid peroxidation, reaction with DNA and membrane proteins and consequently cell damage through various ways. [35] Antioxidant compounds like crocin may exert an inhibitory effect on cytochrome P450, prevent further nicotine metabolism and reduce the production of free radicals, consequently. [36] The findings of the present study are in line with the results of the study carried out by Suner et al., in which they showed nicotine administration resulted in the increased size and vascularity of choroidal neovascularization. [37] Nicotine can induce the production of free radicals and consequently oxidative stress, which is one of the most important causes of liver cells’ death. [38] In the present research, nicotine administration caused an increase in some liver enzymes, whereas, treatment of animals with crocin decreased these enzymes. Liver performance indices such as ALP, ALT, and AST are widely used to evaluate the liver damage. [39] Necrosis or cell membrane damage can trigger the release of these enzymes into the blood circulation. [39] It seems that the increased level of serum enzymes indicate cellular leakage, structural damage, and performance dysfunction of membrane markers in the liver due to nicotine administration. [40] Nicotine can also cause lipid peroxidation by affecting the membrane of hepatocytes, which, in turn, causes a change in membrane permeability and lipid degeneration and accumulation in the liver cells. [41] Free radicals seem to change the enzymatic activity and necrosis by attacking polyunsaturated fatty acids and alkylating groups of proteins and other cellular macromolecules. [41] The reduction of these enzymes due to crocin administration in the present study can be indicative of the protective effects of crocin on the liver owing to antioxidant activities and reduction of oxidative stress of the crocin compounds against nicotine toxicity. [42] The results obtained in this study confirm the findings of Balakrishnan and Menon, indicating that nicotine administration can significantly increase marker enzymes in liver, and hesperidin administration, as an antioxidant, can exert protective effects on liver cells. [43] In the present research, the findings obtained from the measurement of nitric oxide level present in blood serum revealed a significant increase between the group receiving nicotine and saline group; while crocin administration decreased nitric oxide level. Nitric oxide is a free radical produced in the mammalian cells and interferes with the regulation of physiological processes, whose increase is followed by induction of various diseases. [44] The hydroxyl radicals produced by nitric oxide seem to interfere with the pathogenic process and liver toxicity. [45] Nicotine can trigger the release of noradrenaline in paraventricular nucleus and amygdale through a direct effect on the nuclei of the solitary tract and N-methyl-D aspartate, which consequently stimulates the production of nitric oxide and neuronal noradrenergic activity. [46] Nicotine absorption in the body seems to be accompanied by increasing levels of serum nitric oxide and oxidative stress. [46] Antioxidants like crocin can destroy nitric oxide system (protein enzymes, substrates, and cofactors) and reduce nitric oxide production. [45] The results of the study conducted by Zhao and Sharp, showed that nicotine can decrease angiogenesis in gastric mucosa via inhibiting the production of nitric oxide and halting the production of new cells,[47] which are in contrast with the findings obtained in this study. On the other hand, the results of the present study confirm the findings of the study by Muller et al., in which an increase in peroxynitrite formation was reported as a result of cigarette smoke, which is a factor known for damaging DNA. [48]
CONCLUSIONS
The findings of this study showed that crocin may improve some of liver damage in mice treated with nicotine. Therefore, crocin could be useful for protection of the liver in persons who were exposed to nicotine. The antioxidant effects of crocin may be a major reason for its positive impact on liver parameters. However, further studies are required to define its exact mechanism of action.
ACKNOWLEDGEMENTS
We sincerely and gratefully thank the Kermanshah University of Medical Sciences for financial support of this project (No. 93207).
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Ruger P, Ng Y. Ethics and social value judgments in public health. In: Anthony J, editor. Encyclopedia of Health Economics. San Diego: Elseveir Publication; 2014. pp. 287–91. [Google Scholar]
- 2.Jana K, Samanta PK, De DK. Nicotine diminishes testicular gametogenesis, steroidogenesis, and steroidogenic acute regulatory protein expression in adult albino rats: Possible influence on pituitary gonadotropins and alteration of testicular antioxidant status. Toxicol Sci. 2010;116:647–59. doi: 10.1093/toxsci/kfq149. [DOI] [PubMed] [Google Scholar]
- 3.John U, Hanke M. Tobacco smoking- and alcohol drinking-attributable cancer mortality in Germany. Eur J Cancer Prev. 2002;11:11–7. doi: 10.1097/00008469-200202000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Zhang S, Day I, Ye S. Nicotine induced changes in gene expression by human coronary artery endothelial cells. Atherosclerosis. 2001;154:277–83. doi: 10.1016/s0021-9150(00)00475-5. [DOI] [PubMed] [Google Scholar]
- 5.Li S, Lu D, Zhang Y, Zhang Y. Long-term treatment of hydrogen-rich saline abates testicular oxidative stress induced by nicotine in mice. J Assist Reprod Genet. 2014;31:109–14. doi: 10.1007/s10815-013-0102-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Zayadi AR. Heavy smoking and liver. World J Gastroenterol. 2006;12:6098–101. doi: 10.3748/wjg.v12.i38.6098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hukkanen J, Jacob P, 3rd, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57:79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- 8.Bandyopadhyaya G, Sinha S, Chattopadhyay BD, Chakraborty A. Protective role of curcumin against nicotine-induced genotoxicity on rat liver under restricted dietary protein. Eur J Pharmacol. 2008;588:151–7. doi: 10.1016/j.ejphar.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 9.Perlemuter G, Davit-Spraul A, Cosson C, Conti M, Bigorgne A, Paradis V, et al. Increase in liver antioxidant enzyme activities in non-alcoholic fatty liver disease. Liver Int. 2005;25:946–53. doi: 10.1111/j.1478-3231.2005.01126.x. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y, Yang L, Tao K, Vizcaychipi MP, Lloyd DM, Sun X, et al. Protective effects of hydrogen enriched saline on liver ischemia reperfusion injury by reducing oxidative stress and HMGB1 release. BMC Gastroenterol. 2014;14:12. doi: 10.1186/1471-230X-14-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chenoweth MJ, Zhu AZ, Sanderson Cox L, Ahluwalia JS, Benowitz NL, Tyndale RF. Variation in P450 oxidoreductase (POR) A503V and flavin-containing monooxygenase (FMO)-3 E158K is associated with minor alterations in nicotine metabolism, but does not alter cigarette consumption. Pharmacogenet Genomics. 2014;24:172–6. doi: 10.1097/FPC.0000000000000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohamadpour AH, Ayati Z, Parizadeh MR, Rajbai O, Hosseinzadeh H. Safety Evaluation of Crocin (a constituent of saffron) Tablets in Healthy Volunteers. Iran J Basic Med Sci. 2013;16:39–46. [PMC free article] [PubMed] [Google Scholar]
- 13.Khayatnouri M, Safavi SE, Safarmashaei S, Babazadeh D, Mikailpourardabili B. The effect of saffron orally administration on spermatogenesis index in rat. Adv Environ Biol. 2011;5:1514–21. [Google Scholar]
- 14.Fernández J. Anticancer properties of saffron, Crocus sativus Linn. Adv Phytomed. 2006;2:313–30. [Google Scholar]
- 15.Assimopoulou AN, Sinakos Z, Papageorgiou VP. Radical scavenging activity of Crocus sativus L. extract and its bioactive constituents. Phytother Res. 2005;19:997–1000. doi: 10.1002/ptr.1749. [DOI] [PubMed] [Google Scholar]
- 16.Hosseinzadeh H, Shamsaie F, Mehri S. Antioxidant activity of aqueous and ethanolic extracts of Crocus sativus L. stigma and its bioactive constituent, crocin and safranal. Pharmacogn Mag. 2009;5:419–24. [Google Scholar]
- 17.Aung HH, Wang CZ, Ni M, Fishbein A, Mehendale SR, Xie JT, et al. Crocin from Crocus sativus possesses significant anti-proliferation effects on human colorectal cancer cells. Exp Oncol. 2007;29:175–80. [PMC free article] [PubMed] [Google Scholar]
- 18.Hosseinzadeh H, Abootorabi A, Sadeghnia HR. Protective effect of Crocus sativus stigma extract and crocin (trans-crocin 4) on methyl methanesulfonate-induced DNA damage in mice organs. DNA Cell Biol. 2008;27:657–64. doi: 10.1089/dna.2008.0767. [DOI] [PubMed] [Google Scholar]
- 19.Nair SC, Kurumboor SK, Hasegawa JH. Saffron chemoprevention in biology and medicine: A review. Cancer Biother. 1995;10:257–64. doi: 10.1089/cbr.1995.10.257. [DOI] [PubMed] [Google Scholar]
- 20.Hosseinzadeh H, Shariaty MV, Sameni AK, Vahabzadeh M. Acute and sub-acute toxicity of crocin, a constituent of Crocus sativus L. (saffron), in mice and rats. Pharmacologyonline. 2010;2:943–51. [Google Scholar]
- 21.Jalili C, Salahshoor MR, Pourmotabbed A, Moradi S, Roshankhah SH, Darehdori AS, et al. The effects of aqueous extract of Boswellia serrata on hippocampal region CA1 and learning deficit in kindled rats. Res Pharm Sci. 2014;9:351–8. [PMC free article] [PubMed] [Google Scholar]
- 22.Lari P, Abnous K, Imenshahidi M, Rashedinia M, Razavi M, Hosseinzadeh H. Evaluation of diazinon-induced hepatotoxicity and protective effects of crocin. Toxicol Ind Health. 2015;31:367–76. doi: 10.1177/0748233713475519. [DOI] [PubMed] [Google Scholar]
- 23.Gawish AM, Ramadan S, Hassan AM, Issa AM. Morphometrical, histopathological, and cytogenetical ameliorating effects of green tea extract on nicotine toxicity of the testis of rats. J Cytol Histol. 2010;1:105. [Google Scholar]
- 24.Basu S, Haldar N, Bhattacharya S, Biswas S, Biswas M. Hepatoprotective activity of Litchi chinensis Leaf against paracetamol-induced liver damage in rats. Middle East J Sci Res. 2014;20:292–6. [Google Scholar]
- 25.Hematológico E. Action of trivalent chromium on rat liver structure. Histometric and haematological studies. Int J Morphol. 2006;24:197–203. [Google Scholar]
- 26.Zaitoun A, Apelqvist G, Al-Mardini H, Gray T, Bengtsson F, Record C. Quantitative studies of liver atrophy after portacaval shunt in the rat. J Surg Res. 2006;131:225–32. doi: 10.1016/j.jss.2005.11.587. [DOI] [PubMed] [Google Scholar]
- 27.Sala M, Komesu M, Lopes R, Maia Campos G. Karyometric study of basal cell carcinoma. Braz Dent J. 1994;5:11–4. [PubMed] [Google Scholar]
- 28.Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 29.Sadashivam S, Manickam A. 2nd ed. India: New Delhi publication; 1996. Biochemical Methods; pp. 121–4. [Google Scholar]
- 30.Khazaei M, Zarei M, Sharifi MR, Pourshanazari AA. The effect of maintenance and reversal of DOCA-Salt hypertension on extravasation of macromolecules and serum nitric oxide concentration in male rats. Pathophysiology. 2011;18:201–6. doi: 10.1016/j.pathophys.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 31.Yang ZJ, Blaha V, Meguid MM, Oler A, Miyata G. Infusion of nicotine into the LHA enhances dopamine and 5-HT release and suppresses food intake. Pharmacol Biochem Behav. 1999;64:155–9. doi: 10.1016/s0091-3057(99)00111-2. [DOI] [PubMed] [Google Scholar]
- 32.Razavi M, Hosseinzadeh H, Abnous K, Motamedshariaty VS, Imenshahidi M. Crocin restores hypotensive effect of subchronic administration of diazinon in rats. Iran J Basic Med Sci. 2013;16:64–72. [PMC free article] [PubMed] [Google Scholar]
- 33.Sharif S, Farasat T, Fatima N, Farooq A, Naz S. Effect of nicotine on hematology, lipid profile and liver enzymes in adult male mice (Mus musculus), parameters. Adv Anim Vet Sci. 2014;2(4):222–5. [Google Scholar]
- 34.Sakr S, Saber A. Ameliorative effect of ginger (Zingiber officinale) on mancozeb fungicide induced liver injury in albino rats. Aust J Basic Appl Sci. 2007;1:650–6. [Google Scholar]
- 35.Cordova C, Netto C, Yunes R. Protective properties of butanolic extract of the Calendula officinalis L. against lipid peroxidation of rat liver microsomes and action as free radical scavenger. Redox Rep. 2002;7:95–102. doi: 10.1179/135100002125000325. [DOI] [PubMed] [Google Scholar]
- 36.Zaragoza A, Sarrion D. Potentiation of hioacetamide hepatotoxicity by phenobarbita retreatment in rats, inducibility of FAD monooxygenase system and age effect. Chem Biol Interact. 2000;124:87–101. doi: 10.1016/s0009-2797(99)00147-7. [DOI] [PubMed] [Google Scholar]
- 37.Suner I, Espinosa-Heidmann D, Marin-Castano M, Hernandez E, Pereira-Simon S, Cousins S. Nicotine increases size and severity of experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2004;45:311–7. doi: 10.1167/iovs.03-0733. [DOI] [PubMed] [Google Scholar]
- 38.Larrauri J, Saura-Calixto F. Free radical scavenging capacity in the aging of selected red Spanish wines. J Agric Food Chem. 1999;47:1603–6. doi: 10.1021/jf980607n. [DOI] [PubMed] [Google Scholar]
- 39.Drotman R. Serum enzymes are indications of chemical induced liver damage. Drug Chem Toxicol. 1978;1:163–71. doi: 10.3109/01480547809034433. [DOI] [PubMed] [Google Scholar]
- 40.Wang JQ, Li J, Zou YH, Cheng WM, Lu C, Zhang L, et al. Preventive effects of total flavonoids of Litsea coreana leve on hepatic steatosis in rats fed with high fat diet. J Ethnopharmacol. 2009;121:54–60. doi: 10.1016/j.jep.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 41.Khorsandi LS. The protective effect of turmeric (Curcuma longa) extract on acetaminophen induced liver damage in mice. J Zanjan Univ Med Sci Health Serv. 2006;14:23–9. [Google Scholar]
- 42.Xiang M, Qian ZY, Zhou CH, Liu J, Li WN. Crocetin inhibits leukocyte adherence to vascular endothelial cells induced by AGEs. J Ethnopharmacol. 2006;107:25–31. doi: 10.1016/j.jep.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 43.Balakrishnan A, Menon VP. Protective effect of hesperidin on nicotine induced toxicity in rats. Indian J Exp Biol. 2007;45:194–202. [PubMed] [Google Scholar]
- 44.Qureshi M. In-vitro antioxidant and in-vivo hepatoprotective activity of Leucas ciliata Leaves. Rec Nat Prod. 2010;2:124–30. [Google Scholar]
- 45.Hon WM, Lee KH, Khoo HE. Nitric oxide in liver diseases: Friend, foe, or just passerby? Ann N Y Acad Sci. 2002;962:275–95. doi: 10.1111/j.1749-6632.2002.tb04074.x. [DOI] [PubMed] [Google Scholar]
- 46.Lallemand F, Ward RJ, Dravolina O, De Witte P. Nicotine-induced changes of glutamate and arginine in naive and chronically alcoholized rats: An in vivo microdialysis study. Brain Res. 2006;1111:48–60. doi: 10.1016/j.brainres.2006.06.083. [DOI] [PubMed] [Google Scholar]
- 47.Zhao R, Sharp BM. Nicotine-induced norepinephrine release in hypothalamic paraventricular nucleus and amygdale is mediated by N-methyl-D-aspartate receptors and nitric oxide in the nucleus tractus solitarius. J Pharmacol Exp Ther. 2007;320:837–44. doi: 10.1124/jpet.106.112474. [DOI] [PubMed] [Google Scholar]
- 48.Muller T, Haussmann HJ, Schepers G. Evidence for peroxynitrite as an oxidative stress-inducing compound of aqueous cigarette smoke fractions. Carcinogenesis. 1997;18:295–301. doi: 10.1093/carcin/18.2.295. [DOI] [PubMed] [Google Scholar]


