Abstract
Background/Objectives
Visuospatial problems are common in Parkinson’s disease (PD) and likely stem from dysfunction in dopaminergic pathways and consequent disruption of cortical functioning. Characterizing the motor symptoms at disease onset provides a method of observing how dysfunction in these pathways influences visuospatial cognition. We examined two types of motor characteristics: body side (left or right) and type of initial symptom (tremor or symptom other than tremor).
Methods
31 non-demented patients with PD, 16 with left-side onset (LPD) and 15 with right-side onset (RPD), as well as 17 healthy control participants (HC). The PD group was also divided by type of initial motor symptom, 15 having tremor as the initial symptom and 16 having an initial symptom other than tremor. Visuospatial function was assessed with the Clock Drawing Test.
Results
Of the four Clock Drawing scoring methods used, the Rouleau method showed sensitivity to subgroup differences. As predicted, the LPD and non-tremor subgroups, but not the other subgroups, performed more poorly than the HC group.
Conclusion
The findings provide further evidence for differences in cognition between these subtypes of PD and highlight the importance of considering disease subtypes when examining cognition.
Keywords: functional laterality, parkinsonian, spatial behavior, cognition, neuropsychology
Visuospatial problems are frequently reported by patients with Parkinson’s disease (PD) [1] and significantly interfere with quality of life [2–4]. These symptoms likely originate from depletion of dopamine (DA) in the substantia nigra (SN) and the consequent disruption of neural circuits including those with nodes in the prefrontal and posterior parietal cortices [5–6]. Recent studies have supported earlier work indicating that several aspects of visuospatial functioning are impaired in PD, including deficits in spatial navigation [7], mental rotation [8], hierarchical pattern perception [9], facial emotion recognition [10], visuospatial working memory [11, 12], and visuospatial planning [13]. Not all PD patients show impairments to the same degree, consistent with views that PD may encompass multiple subtypes [14].
Disruption of dopaminergic pathways from the midbrain to basal ganglia and cortical areas results in particular cognitive consequences, which may explain the heterogeneity of cognitive dysfunction in PD. Clinical characteristics at disease onset, such as side of motor symptom onset (left or right) or type of initial motor symptom (tremor or akinesia/rigidity/posture/gait), may suggest a method for observing how dysfunction in these pathways influences cognition. For example, dysfunction in pathways predominantly affecting the right hemisphere impairs performance on visuospatial tasks, especially those requiring processing the overall figure rather than individual target features [7, 9]. PD patients typically present with a dominant type of motor symptom (e.g., tremor or rigidity) that primarily affects one side of the body, as described in Parkinson’s original essay [15]. As the disease progresses, symptoms appear on the initially non-affected side, but the initial side often remains more affected throughout the disease course [16].
Regarding laterality, side of motor symptom onset is associated with asymmetric DA depletion in the SN [17], as well as asymmetric up-regulation of postsynaptic striatal D2 receptors [18, 19]. These asymmetric changes in DA and its regulation result in asymmetric effects on cortical areas [5, 6]. Visuospatial performance may be especially affected either by disruption of connections between the SN and the parietal lobes [20] or indirectly as a downstream consequence of frontal dysfunction [21, 22]. Multiple reports examining visuospatial functioning by side of motor symptom onset have suggested that visuospatial problems in PD result from disruptions in networks including the parietal lobes [7, 9, 23]. Schendan and colleagues [9] investigated the relation between motor symptom onset and performance on a visuospatial task that was sensitive to posterior parietal lobe functioning but not to dorsolateral prefrontal cortex functioning (hierarchical pattern perception) and found dissociations in task performance as a function of side of motor symptom onset. These findings indicate that PD patients with motor symptoms starting on the left side of the body (LPD; predominant right hemisphere dysfunction) are at greater risk for visuospatial problems than patients with symptoms starting on the right side of the body (RPD; predominant left hemisphere dysfunction), likely reflecting in LPD the greater loss of DA and changes in DA regulation in the right SN and basal ganglia and their effects on the right posterior parietal lobe.
The types of initial and dominant motor symptoms are also associated with asymmetric DA depletion within midbrain structures and, through connections with the cortex, may have differing impacts on cognition and quality of life [24]. Single-photon emission computed tomography (SPECT) studies consistently report negative correlations between DA uptake in the striatum and indicators of each of the cardinal symptoms of PD: rigidity, bradykinesia, and disorders of gait, balance and posture [17, 25–26], with the exception of tremor, suggesting that tremor dominant and non-tremor dominant PD result from dysfunction of different neural systems. These findings have been supported by a SPECT study [27] indicating greater presynaptic DA binding in tremor-dominant PD than in non-tremor dominant PD, despite similar disease stage (Hoehn and Yahr stage I: unilateral symptoms). Perhaps even more compelling, a post-mortem study [28] used high performance liquid chromatography with electrochemical detection to determine DA concentrations within subregions of the globus pallidus in PD patients and a control group. Specific differences in DA levels were found within subregions of the internal globus pallidus between tremor-dominant and non-tremor dominant patients. The non-tremor PD group (n=6) had DA loss within the dorsal rostral (~80%) and ventral rostral (~71%) subdivisions of this region. By contrast, DA loss was observed only in the dorsal rostral region in the tremor-dominant PD participants (n=2) and to a lesser degree (~45%). The results with this small sample suggest that the ventral region of the internal globus pallidus plays a role in non-tremor dominant but not in tremor-dominant PD.
It is unknown how these biochemical differences by initial symptom relate to cognition. Cross-sectional [29–31] and community-based longitudinal studies [32–34] indicate that non-tremor dominant PD is associated with greater prevalence and incidence of overall cognitive impairments, likely stemming from greater posterior dysfunction as indicated by performance on neuropsychological tests. For example, Williams-Gray and colleagues [34] reported that the increased risk of developing dementia in non-tremor PD (4.1 times greater) is further increased (to 5.3 times) in non-tremor patients with baseline problems copying intersecting pentagons, a visuospatial task. These reports have been limited in scope, however, focusing primarily on overall cognition as measured by versions of the Mini-Mental State Examination and the scales for outcomes in PD (SCOPA-COG). Whereas multiple reports relate global cognitive decline to type of motor symptom, the cognitive profile of early stage non-demented patients with respect to presenting symptom is largely unknown. One report [35] indicated greater executive dysfunction in PD patients without tremor as the initial symptom, as assessed by the Wisconsin Card Sorting Test. Executive dysfunction in this PD subgroup implicates prefrontal impairment, consistent with observations of abnormal synchronous oscillations in the basal ganglia that provide “noisy” input to the frontal lobes, in turn leading to akinesia and rigidity but not tremor [36]. This study [35] also reported an interaction between side and type of initial symptom on visuospatial functioning, with right-onset tremor patients performing better than left-onset tremor patients on the Hooper Visual Organization Test and Judgment of Line Orientation. Overall, the combination of biochemical, epidemiological and neuropsychological studies suggest that the type of initial and dominant motor symptom may characterize subgroups of PD associated with dysfunction of different neural circuits. Patients with tremor as the initial motor symptom may be at less risk of developing cognitive impairment than patients with an initial motor symptom other than tremor.
The present study examined cognitive performance in non-demented patients with PD divided into subgroups by side of onset and initial motor symptom. Because many visuospatial tests are able to distinguish side-of-onset subgroups (as reviewed by Cronin-Golomb [37]) and because visuospatial dysfunction appears to predict overall cognitive difficulties in non-tremor onset PD [34] we focused on this domain of cognitive functioning. We used the Clock Drawing Test (CDT), a standard neuropsychological measure sensitive to visuospatial impairment and frequently used in clinical settings [38].
Our primary goal was to examine the potential effect of side and type of initial motor symptom on CDT performance. Based on the current literature, we expected LPD participants to perform more poorly than RPD and healthy control participants, as the spatial demands of the task would not be met by the dysfunctional right hemisphere. Similarly, we expected PD patients with an initial cardinal symptom other than tremor to perform poorly relative to PD patients first presenting with tremor and relative to healthy control participants, reflecting reported differences in the relation between dopamine and type of initial motor symptom as well as greater cognitive dysfunction in this subtype.
A secondary goal was to determine the sensitivity of various CDT scoring systems to subtle cognitive dysfunction in PD. Four scoring systems were selected from more than a dozen reported in the literature. The Sunderland method [39] was chosen for its frequency of use. The Clock Drawing Interpretation Scale (CDIS) [40] and the Rouleau method [41] were chosen for the capacity to identify problems within each of the three main features of the clock drawing (clock face, numbers and hands). Finally, the Ten Point Clock Test (TPCT) [42] was selected for its ease of use in clinical settings.
Methods
Participants
Participants included 31 patients with idiopathic PD and 17 healthy control adults (HC), all right-handed. PD patients were recruited from the Parkinson’s Disease Clinic at the Boston Medical Center and local support groups. HC participants were recruited from the community.
Exclusion criteria for both groups included co-existing serious chronic medical illnesses (including psychiatric or neurological), history of intracranial surgery, traumatic brain injury, alcoholism or other drug abuse, or eye disease or abnormalities, as well as use of psychoactive medications besides antidepressants and anxiolytics in the PD group and use of any psychoactive medications in the HC group. Most participants (24/31 PD and 13/17 HC) underwent a neuro-ophthalmological examination to rule out ocular disorders. There were no differences in basic visual functioning between those who did and did not receive this examination (e.g., letter-identification contrast sensitivity, t(6.47) =.07, p = 0.54).
Review of PD participants’ medical records confirmed side of disease onset and disease duration. PD participants were asked to provide a detailed description of their initial and current dominant symptom. Patients who indicated the presence of tremor at onset were categorized as tremor-onset. All other were considered non-tremor onset. “Non-tremor” is described elsewhere as the akinesia/rigidity subtype or the postural instability/gait difficulty subtype [36]. The majority of patients reported using dopamine agonists (26) and/or levodopa (24). Seven used monoamine oxidase inhibitors and/or catechol-O-methyltransferase inhibitors (2 MAOI alone, 2 COMT alone, 2 both), 6 used amantadine, and 11 used anticholinergic agents. Thirteen reported using antidepressant or anti-anxiety medication. All participants were tested in the “on” state with dosage optimized by their physician.
Side of onset
The HC, LPD (n=16), and RPD groups (n=15) were matched for age (F (2, 47) = .43, MSE = 30.52, p=.66), education (F (2, 47) = .27, MSE = 1.99, p=.77), and male:female ratio. None were demented, with all obtaining scores of 27 or above on the Mini-Mental State Examination (MMSE) and 139 or above on the Dementia Rating Scale (DRS). LPD and RPD patients endorsed more depressive symptoms than did HC on the Beck Depression Inventory II (BDI-II) (F (2, 44) = 10.22, MSE = 290.73, p<.01), with no difference between LPD and RPD (t (26) = 0.59, p=.56). The PD subgroups had similar mild bilateral motor symptoms (as indicated by a median stage II Hoehn and Yahr score [43]; Mann-Whitney U = 94, p = .14) and similar duration of illness (t (29) = 1.5, p=.14). Use of DA agonists was similar between groups with 13/16 LPD and 13/15 RPD participants on these medications (X2 [1, n=31]=.17, p=.68). Likewise, use of levodopa was similar between groups with 13/16 LPD and 11/15 RPD participants on these medications (X2 [1, n=31]=.28, p=.60). Three LPD and 3 RPD participants were on amantadine (X2 [1, n=31]=.01, p=.93). Seven LPD and 4 RPD participants were on anticholinergic agents (X2 [1, n=31]=.98, p=.32).
Type of initial symptom
The HC, PD tremor (n=15) and non-tremor (n=16) groups did not differ on age (F (2, 47) = 0.17, MSE = 12.25, p=.85), education (F (2, 47) = 0.28, MSE = 2.06, p=.76), overall cognitive status as indicated by the MMSE (F (2, 47) = 0.10, MSE = .098, p=.91) and DRS (F (2, 42) = 1.70, MSE = 2.53, p=.20), or male:female ratio. Tremor and non-tremor patients endorsed more depressive symptoms on the BDI-II than did HC participants (F (2, 44) = 9.92, MSE = 285.03, p<.01), with no difference between the PD subgroups (t (19.0) = .30, p= 0.77). Severity of motor symptoms was similar in the subgroups with a median of stage II Hoehn and Yahr (Mann-Whitney U = 104.5, p = 0.37). The duration of illness did not differ between subgroups (t (29) = .63, p=.53). Use of DA agonists was similar between groups with 14/15 tremor and 12/16 non-tremor participants on these medications (X2 [1, n=31]=1.9, p=.17). Likewise, use of levodopa was similar between groups with 13/15 Tremor and 11/16 non-tremor participants on these medications (X2 [1, n=31]=.23, p=.23). Three tremor and 3 non-tremor participants were on amantadine (X2 [1, n=31]=.01, p=.93). Six tremor and 5 non-tremor participants were on anticholinergic agents (X2 [1, n=31]=1.17, p=.28).
Side of onset and type of initial symptom
In the LPD subgroup, there were nine patients with and seven without tremor as the initial symptom. In the RPD subgroup, there were six patients with and nine without tremor as the initial symptom.
Measures and Procedure
Participants provided informed consent for the protocol approved by the Boston University Charles River Campus Institutional Review Board. The research was conducted in accordance with the Helsinki Declaration.
Each participant was provided a blank 8.5 × 11″ piece of white paper and the following instructions: “I would like you to draw a clock including the numbers and set the hands to 10 after 11.” Clocks were analyzed according to the following methods.
The Sunderland method [39] is a categorical ranking system in which drawings are assigned to one of ten previously identified clock representations. A “10” represents a well-drawn clock and “1” indicates a non-interpretable drawing. The 1–5 range primarily reflects the accuracy of the clock numbers and face. The 6–10 range reflects the placement of the hands, with the clock face (outer contour) and numbers generally intact. Hands placed at 11:10 were considered in the correct position.
The Clock Drawing Interpretation Scale (CDIS) [40] consists of 20 items that indicate the presence or absence of commonly observed errors. Inter-item correlations empirically identified three factors, including general clock features, placement of numbers, and placement of hands. An overall score is calculated by summing all items.
The Ten Point Clock Test (TPCT) [42] was developed to provide a quick evaluation of clock drawings in tertiary care settings, based on the placement of numbers and hands. The clock is divided into eighths and a point is given for each non-anchor number drawn in the appropriate segment. An additional point is awarded for the accurate drawing of each hand, indicating the time of 11:10. “10” represents a perfect drawing.
The Rouleau method [41] separately assesses the drawing of the clock face, numbers and hands. The face is scored according to the severity of distortion on a 0–2 point scale with “2” indicating gross distortion. Clock numbers are rated by severity of error in spatial arrangement on a 0–4 point scale (“0” worst). The hands are judged a 0–4 point scale (“0” worst) by placement and length.
Two trained raters blind to clinical diagnosis (PD vs. HC; and within the PD group, side and type of onset) independently analyzed each drawing using each scoring method.
Results
Inter-rater reliability was determined by mean intraclass coefficients (ICC) using a two-way random effects model with absolute agreement. A high degree of reliability was established for all four scoring systems: Sunderland (ICC=.97), CDIS (ICC=.85), TPCT (ICC=.91) and Rouleau (ICC=.96).
Data from the two raters were averaged. Non-parametric Kruskal-Wallis tests were conducted to examine potential group effects. When indicated, the Mann-Whitney test was used for follow-up analyses and a more conservative alpha level of .01 was adopted. No group differences emerged for the Sunderland, CDIS, or TPCT methods. Significant group differences emerged using the Rouleau method.
Rouleau method
PD compared to NC
Mann-Whitney U tests were performed to examine effects of group (alpha .05). A significant effect of group was observed for the numbers subscale (U = 144.5, p<.01), but not the hands (U = 248.5, p=.69) or clock face (U = 224.0, p=.272). The HC group performed better (M = 3.74, SD = .56) than the PD group (M = 3.24, SD = .63). The effect size was medium to large (r = −.40).
Side of onset
Kruskal-Wallis tests were performed to examine side of onset (alpha .05). A significant effect of group was observed for the numbers subscale (H (2) = 8.34, p<.02), but not the hands (H (2) = .16, p=.92) or clock face (H (2) = 2.5, p=.28). Post-hoc analyses (alpha .01) revealed a significant difference on the numbers subscale between the HC and LPD groups (U=65.0, p<.005), with HC performing better (HC M = 3.74, SD = .56; LPD M = 3.16, SD = .65). The effect size was medium to large (r=−.49).
Type of initial symptom
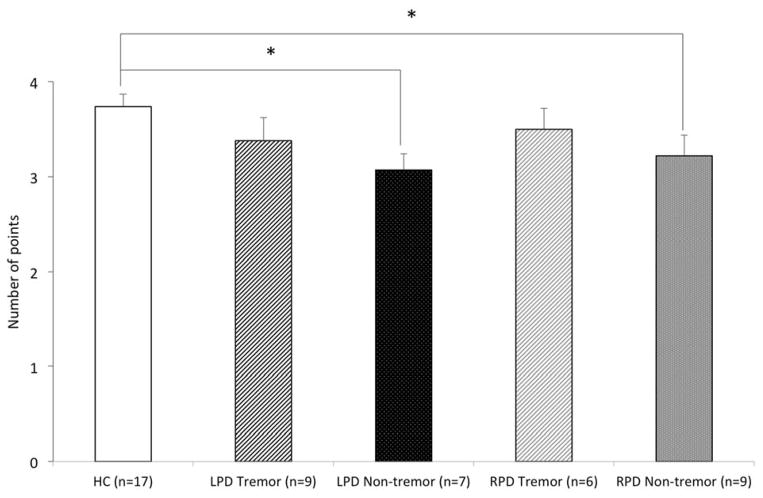
A significant effect of group was observed for the numbers subscale (H (2) = 8.75, p<.02), but not hands (H (2) = .69, p=.71) or face (H (2) = 1.46, p=.48). Post-hoc analyses (alpha .01) revealed a significant difference on the numbers subscale between the HC and non-tremor group (U = 63.5, p<.004), with HC performing better (HC M = 3.74, SD = .56; non-tremor M = −3.16, SD = .57) (Figure 2). The effect size was large (r = −.50).
Figure 2.
Mean score for each of the three scales on the Rouleau scoring system by type of initial symptom. Error bars represent standard errors of the mean. The non-tremor onset patients performed significantly more poorly than HC, p<.01. The tremor-onset patients did not differ from HC.
Interaction of side of onset and type of initial symptom
Because of small sample sizes (LPD: 9 with, 7 without tremor; RPD: 6 with, 9 without tremor), the following analyses should be considered preliminary. A significant effect of group was observed for the numbers subscale (H (4) = 9.58, p<.05), but not hands (H (4) = 1.70, p=.79) or face (H (4) = 2.70, p=.61). Post-hoc analyses (using an alpha of .05 given the preliminary nature of the data and small sample) revealed significant group differences between the HC and LPD non-tremor subgroup (U = 21.5, p<.006), HC and LPD tremor subgroup (U = 43.5, p<.04), as well as between HC and RPD non-tremor subgroup (U = 42.0, p< .03. Medium to large effect sizes were observed for HC and LPD non-tremor (r = .−56), HC and LPD tremor (r = .−40), and HC and RPD non-tremor (r = .−42). A general pattern across the poorly performing subgroups included a tendency to draw the numbers on the right side of the clock too closely together, resulting in relatively larger gaps between numbers on the left side of the clock.
Discussion
Our results revealed significant PD subgroup differences in clock drawings by side and type of initial motor symptom. Consistent with our hypotheses, LPD (inferred right hemisphere dysfunction) and those without tremor as the initial motor symptom performed significantly worse than well-matched healthy adults. These group differences were observed when clocks were scored according to the Rouleau scoring system [41], but were not observed with other methods, reflecting the sensitivity of this system to spatial arrangements among clock features. This finding accords with those of Lee and colleagues [44] and Matsuoka and colleagues [45] in Alzheimer’s disease, who reported that Rouleau scores correlated more strongly than did other-system scores with right-parietal structure (i.e. grey matter volume) and function (i.e. cerebral glucose metabolism). It also accords with the wider literature on visuospatial dysfunction in PD by subgroup using measures that are generally not as common in clinical use as is the CDT (e.g., 7, 9, 23, 35, 37). Our examination of the relative sensitivity of scoring methods on the CDT was motivated by the lack of a single standard scoring system on this test that is very widely used in PD and in other neurodegenerative and other neurological conditions. The fact that the other CDT scoring systems were not sensitive to group differences does not controvert our finding with the Rouleau—rather, it provides information, valuable in the clinic, that those other scoring systems should not be used if the aim is to understand visuospatial function in PD, especially in regard to subgroups.
Our findings suggest that the Rouleau system is particularly sensitive to the type of errors seen in non-demented patients with mild to moderate PD. Each of the clock elements (face, numbers, and hands) is scored by severity of errors ranging from mild to gross impairment. By contrast, the other methods score the presence or absence of errors typically seen in demented populations. For example, a clock feature may be absent or a number drawn more than 45 degrees off course. In our high-functioning sample, these types of errors were rare, resulting in ceiling performance on these scales. The PD-related effects using the Rouleau method were exclusive to the numbers subscale, which emphasizes errors in spatial arrangement of numbers, suggesting that overall drawing difficulties did not explain the findings.
Appreciation of the spatial relations among objects relies on the dorsal visual stream extending from the striate cortex to the parietal lobes [46]. The CDT has long been used as a measure of visuospatial and parietal-lobe functioning [74, 48], and the role of the right parietal lobe has often been emphasized because of its relevance to unilateral spatial neglect [49]. Of particular importance to the present study, Tranel and colleagues [50] observed that patients with right basal ganglia lesions (without PD) showed problems with spatial organization and number placement on the CDT, but not other type of errors, including hand placement. These findings are remarkably consistent with those of the present study, in which LPD (greater right basal ganglia dysfunction) showed impairments in aligning the numbers, but did not show difficulties with hand setting. These visuospatial deficits were observed in LPD but not RPD, suggesting relatively selective disruption of right-hemisphere circuits including the midbrain, basal ganglia, and parietal lobe.
When analyzing clock drawings by type of initial symptom, we found that the subgroup without tremor had more problems aligning numbers than did control participants. Group differences were not observed on for the clock face or hands, indicating that general drawing difficulties did not explain the findings. Relative to tremor-dominant PD, non-tremor dominant PD is associated with greater DA loss in the midbrain even when there are similar motor severity scores [28]. In those who present with non-tremor symptoms but not with tremor, clinical symptom severity correlates with severity of the DA deficiency in the striatum and with disease progression [36]. In light of the known relation between right parietal-lobe functioning and CDT performance, the specific visuospatial problems observed in non-tremor PD patients likely reflect greater DA loss interacting with the dysfunction of the right-hemisphere cortico-striato-thalamic circuit, as inferred by side of onset.
Limitations of the present study include the use of a single measure, the CDT, to investigate visuospatial cognition by PD subtype, as well as the relatively small sample size. While investigating performance by PD subtype on this measure alone was justified by previous research, subtype analysis should be conducted to examine cognition across multiple domains (e.g., visuospatial function, learning, memory, executive function; see [51] for a recent example of investigation of the latter). To minimize the effect of sample size, we used non-parametric analyses and a conservative alpha for post-hoc analyses and found medium to large effect sizes.
In sum, clinical characteristics at PD onset provide a method of observing how disruption of select dopaminergic circuits influences cognition in non-demented patients. This study examined the relation between visuospatial cognition and side and type of initial motor symptom. As expected, patients with motor symptoms starting on the left side of the body, as well as non-tremor patients, performed more poorly than healthy control participants. These deficits likely stem from substantial DA loss within the right midbrain and basal ganglia, which may disrupt connections to the right parietal lobe. The findings highlight the importance of considering subtypes when examining cognition in PD.
Figure 1.
Mean score for each of the three scales on the Rouleau scoring system by side of onset. Error bars represent standard errors of the mean. The LPD patients performed more poorly than HC, p<.01. The RPD patients did not differ from HC.
Figure 3.
Mean score for the numbers subscale (Rouleau scoring system) by side and type of initial symptom. Error bars represent standard errors of the mean. The LPD and RPD non-tremor subgroups each performed significantly more poorly than HC, p<.05.
Table 1.
Participant Characteristics
| HC (n=17) | PD (n=31)* | ||||
|---|---|---|---|---|---|
|
| |||||
| Side of onset
|
Type of initial symptom
|
||||
| LPD (n=16) | RPD (n=15) | T (n=15) | NT (n=16) | ||
| Age (yrs) | 61.5 (9.0) | 60.0 (8.6) | 62.8 (7.8) | 62.3 (8.1) | 60.5 (8.5) |
| Female: Male | 9:6 | 9:7 | 9:6 | 8:7 | 9:7 |
| Education (yrs) | 16.4 (2.7) | 16.6 (2.6) | 17.0 (2.9) | 16.6 (2.4) | 17.0 (3.0) |
| Hoehn & Yahr | N/A | 2.0 (2–3) | 2.0 (2–4) | 2.0 (2–3) | 2.0 (2–4) |
| Duration of illness (yrs) | N/A | 6.8 (3.6) | 4.9 (3.1) | 5.5 (2.7) | 6.3 (4.0) |
| MMSE | 29.4 (0.9) | 29.4 (1.1) | 29.1 (1.0) | 29.2 (1.1) | 29.3 (1.1) |
| DRS | 143.5 (1.1) | 142.6 (1.5) | 143.1 (1.4) | 143.5 (1.1) | 142.8 (1.5) |
| BDI-II | 1.8 (2.3) | 9.9 (7.7) | 8.5 (5.3) | 8.8 (3.4) | 9.5 (8.4) |
Values represent means and standard deviations except Hoehn & Yahr values which are reported in median and range.
MMSE - Mini-Mental State Examination; DRS – Dementia Rating Scale; BDI-II - Beck Depression Inventory II
LPD – Left-side motor symptom onset; RPD – Right-side motor symptom onset
T – Initial symptom tremor; NT – Initial symptom non-tremor
Same patients grouped by side of onset and type of initial symptom
Table 2.
Clock Drawing Test Scoring System (Rouleau et al., 1996)
| A. Integrity of the clock face (maximum: 2 points) | |
| Score: | |
| 2 | Present without gross distortion |
| 1 | Incomplete or some distortion (including size) |
| B. Presence and sequencing of the numbers (maximum: 4 points) | |
| Score: | |
| 4 | All present in the right order and at most minimal error in the spatial arrangement |
| 3 | All present but errors in spatial arrangement |
| 2 | - Numbers missing or added but no gross distortions of the remaining numbers |
| - Numbers placed in counterclockwise direction | |
| - Numbers all present but gross distortion in spatial layout (i.e. hemineglect, numbers outside the clock) | |
| 1 | Missing or added numbers and gross spatial distortions |
| 0 | Absence or poor representation of numbers |
| C. Presence and placement of the hands (maximum: 4 points) | |
| Score: | |
| 4 | Hands are in correct position and the size difference is respected |
| 3 | Slight errors in the placement of the hands or no representation of size difference between hands |
| 2 | Major errors in the placement of the hands (significantly out of course including 10 to 11) |
| 1 | Only one hand or poor representation of 2 hands |
| 0 | No hands or preservation on hands |
Acknowledgments
This work was supported by the National Institute of Neurological Disorders and Stroke under Grants R01 NS050446; R01 NS052914; and R01 NS067128. It was conducted in the Vision and Cognition Laboratory of the Department of Psychological and Brain Sciences, Boston University. We thank all of the individuals who participated in this study. Our recruitment efforts were supported, with our gratitude, by Marie Saint-Hilaire, MD, and Cathi Thomas RN, MSN, of Boston University Medical Center Neurology Associates, and by Boston area Parkinson Disease support groups. We thank Karishma Circelli, MD, for reviewing a draft of the manuscript. Thomas Laudate, PhD, and Bruce Reese, MA, provided expert technical support.
Footnotes
Disclosure Statement: None of the authors report any financial interest or benefit arising from the direct application of this research. The authors have no conflict of interest to report.
References
- 1.Davidsdottir S, Cronin-Golomb A, Lee A. Visual and spatial symptoms in Parkinson’s disease. Vision Res. 2005;45(10):1285–1296. doi: 10.1016/j.visres.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Amick MM, Grace J, Ott BR. Visual and cognitive predictors of driving safety in Parkinson’s disease patients. Arch Clin Neuropsychol. 2007;22(8):957–967. doi: 10.1016/j.acn.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klepac N, Trkulja V, Relja M, Babic T. Is quality of life in non-demented Parkinson’s disease patients related to cognitive performance? A clinic-based cross-sectional study. Eur J Neurol. 2008;15(2):128–133. doi: 10.1111/j.1468-1331.2007.02011.x. [DOI] [PubMed] [Google Scholar]
- 4.Uc EY, Rizzo M, Anderson SW, Sparks JD, Rodnitzky RL, Dawson JD. Impaired navigation in drivers with Parkinson’s disease. Brain. 2007;130(Pt 9):2433–2440. doi: 10.1093/brain/awm178. [DOI] [PubMed] [Google Scholar]
- 5.Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Brain Res Rev. 2000a;31(2–3):236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- 6.Middleton FA, Strick PL. Basal ganglia output and cognition: evidence from anatomical, behavioral, and clinical studies. Brain Cogn. 2000b;42(2):183–200. doi: 10.1006/brcg.1999.1099. [DOI] [PubMed] [Google Scholar]
- 7.Davidsdottir S, Wagenaar R, Young D, Cronin-Golomb A. Impact of optic flow perception and egocentric coordinates on veering in Parkinson’s disease. Brain. 2008;131(Pt 11):2882–2893. doi: 10.1093/brain/awn237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amick MM, Grace J, Chou KL. Body side of motor symptom onset in Parkinson’s disease is associated with memory performance. J Int Neuropsychol Soc. 2006;12(5):736–740. doi: 10.1017/S1355617706060875. [DOI] [PubMed] [Google Scholar]
- 9.Schendan HE, Amick MM, Cronin-Golomb A. Role of a lateralized parietal-basal ganglia circuit in hierarchical pattern perception: evidence from Parkinson’s disease. Behav Neurosc. 2009;123(1):125–136. doi: 10.1037/a0013734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark US, Neargarder S, Cronin-Golomb A. Specific impairments in the recognition of emotional facial expressions in Parkinson’s disease. Neuropsychologia. 2008;46(9):2300–2309. doi: 10.1016/j.neuropsychologia.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kemps E, Szmalec A, Vandierendonck A, Crevits L. Visuo-spatial processing in Parkinson’s disease: evidence for diminished visuo-spatial sketch pad and central executive resources. Parkinsonism Relat Disord. 2005;11(3):181–186. doi: 10.1016/j.parkreldis.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 12.Possin KL, Filoteo JV, Song DD, Salmon DP. Spatial and object working memory deficits in Parkinson’s disease are due to impairment in different underlying processes. Neuropsychology. 2008;22(5):585–595. doi: 10.1037/a0012613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Altgassen M, Phillips L, Kopp U, Kliegel M. Role of working memory components in planning performance of individuals with Parkinson’s disease. Neuropsychologia. 2007;45(10):2393–2397. doi: 10.1016/j.neuropsychologia.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 14.Miller IN, Neargarder S, Risi MM, Cronin-Golomb A. Frontal and posterior subtypes of neuropsychological deficit in Parkinson’s disease. Behav Neurosci. 2013;127(2):175–183. doi: 10.1037/a0031357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parkinson J. An Essay on the Shaking Palsy. London: Sherwood, Neely & Jones; 1817. [Google Scholar]
- 16.Lee CS, Schulzer M, Mak E, Hammerstad JP, Calne S, Calne DB. Patterns of asymmetry do not change over the course of idiopathic parkinsonism: implications for pathogenesis. Neurology. 1995;45(3 Pt 1):435–439. doi: 10.1212/wnl.45.3.435. [DOI] [PubMed] [Google Scholar]
- 17.Seibyl JP, Marek KL, Quinlan D, Sheff K, Zoghbi S, Zea-Ponce Y, Baldwin RM, Fussell B, Smith EO, Charney DS, van Dyck C. Decreased single-photon emission computed tomographic [123I]beta-CIT striatal uptake correlates with symptom severity in Parkinson’s disease. Annals of Neurology. 1995;38(4):589–598. doi: 10.1002/ana.410380407. [DOI] [PubMed] [Google Scholar]
- 18.Knable MB, Jones DW, Coppola R, Hyde TM, Lee KS, Gorey J, Weinberger DR. Lateralized differences in iodine-123-IBZM uptake in the basal ganglia in asymmetric Parkinson’s disease. J Nucl Med. 1995;36(7):1216–1225. [PubMed] [Google Scholar]
- 19.Verstappen CC, Bloem BR, Haaxma CA, Oyen WJ, Horstink MW. Diagnostic value of asymmetric striatal D2 receptor upregulation in Parkinson’s disease: an [123I]IBZM and [123I]FP-CIT SPECT study. Eur J Nucl Med Mol Imaging. 2007;34(4):502–507. doi: 10.1007/s00259-006-0258-4. [DOI] [PubMed] [Google Scholar]
- 20.Clower DM, Dum RP, Strick PL. Basal ganglia and cerebellar inputs to ‘AIP’. Cereb Cortex. 2005;15(7):913–920. doi: 10.1093/cercor/bhh190. [DOI] [PubMed] [Google Scholar]
- 21.Bondi MW, Kaszniak AW, Bayles KA, Vance KT. Contributions of frontal system dysfunction to memory and perceptual abilities in Parkinson’s disease. Neuropsychology. 1993;7(1):89–102. [Google Scholar]
- 22.Zgaljardic DJ, Borod JC, Foldi NS, Mattis P. A review of the cognitive and behavioral sequelae of Parkinson’s disease: relationship to frontostriatal circuitry. Cogn Behav Neurol. 2003;16(4):193–210. doi: 10.1097/00146965-200312000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Amick MM, Schendan HE, Ganis G, Cronin-Golomb A. Frontostriatal circuits are necessary for visuomotor transformation: mental rotation in Parkinson’s disease. Neuropsychologia. 2006;44(3):339–349. doi: 10.1016/j.neuropsychologia.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Appleman ER, Stavitsky K, Cronin-Golomb A. Relation of subjective quality of life to motor symptom profile in Parkinson’s disease. Parkinsons Dis. 2011:Article ID 472830. doi: 10.4061/2011/472830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brucke T, Asenbaum S, Pirker W, Djamshidian S, Wenger S, Wöber C, Müller C, Podreka I. Measurement of the dopaminergic degeneration in Parkinson’s disease with [123I] beta-CIT and SPECT. Correlation with clinical findings and comparison with multiple system atrophy and progressive supranuclear palsy. J Neural Transm Suppl. 1997;50:9–24. [PubMed] [Google Scholar]
- 26.Shinotoh H, Uchida Y, Ito H, Harrori T. Relationship between striatal [123I]beta-CIT binding and four major clinical signs in Parkinson’s disease. Ann Nucl Med. 2000;14(3):199–203. doi: 10.1007/BF02987860. [DOI] [PubMed] [Google Scholar]
- 27.Spiegel J, Hellwig D, Samnick S, Jost W, Möllers MO, Fassbender K, Kirsch CM, Dillmann U. Striatal FP-CIT uptake differs in the subtypes of early Parkinson’s disease. J Neural Transm. 2007;114(3):331–335. doi: 10.1007/s00702-006-0518-2. [DOI] [PubMed] [Google Scholar]
- 28.Rajput AH, Sitte HH, Rajput A, Fenton ME, Pifl C, Hornykiewicz O. Globus pallidus dopamine and Parkinson motor subtypes: clinical and brain biochemical correlation. Neurology. 2008;70(16 Pt 2):1403–1410. doi: 10.1212/01.wnl.0000285082.18969.3a. [DOI] [PubMed] [Google Scholar]
- 29.Oh JY, Kim YS, Choi BH, Sohn EH, Lee AY. Relationship between clinical phenotypes and cognitive impairment in Parkinson’s disease (PD) Arch Gerontol Geriatr. 2009;49(3):351–354. doi: 10.1016/j.archger.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 30.Williams LN, Seignourel P, Crucian GP, Okun MS, Rodriguez RL, Skidmore FM, Foster PS, Jacobson CE, 4th, Romrell J, Bowers D, Fernandez HH. Laterality, region, and type of motor dysfunction correlate with cognitive impairment in Parkinson’s disease. Mov Disord. 2007;22(1):141–145. doi: 10.1002/mds.21220. [DOI] [PubMed] [Google Scholar]
- 31.Zetusky WJ, Jankovic J. Laterality and symptom association in Parkinson’s disease. Arch Neurol. 1985;42(12):1132–1133. doi: 10.1001/archneur.1985.04060110010001. [DOI] [PubMed] [Google Scholar]
- 32.Taylor JP, Rowan EN, Lett D, O’Brien JT, McKeith IG, Burn DJ. Poor attentional function predicts cognitive decline in patients with non-demented Parkinson’s disease independent of motor phenotype. J Neurol Neurosurg Psychiatry. 2008;79(12):1318–1323. doi: 10.1136/jnnp.2008.147629. [DOI] [PubMed] [Google Scholar]
- 33.Uc EY, McDermott MP, Marder KS, Anderson SW, Litvan I, Como PG, Auinger P, Chou KL, Growdon JC Parkinson Study Group DATATOP Investigators. Incidence of and risk factors for cognitive impairment in an early Parkinson disease clinical trial cohort. Neurology. 2009;73(18):1469–1477. doi: 10.1212/WNL.0b013e3181bf992f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams-Gray CH, Foltynie T, Brayne CE, Robbins TW, Barker RA. Evolution of cognitive dysfunction in an incident Parkinson’s disease cohort. Brain. 2007;130(Pt 7):1787–1798. doi: 10.1093/brain/awm111. [DOI] [PubMed] [Google Scholar]
- 35.Katzen HL, Levin BE, Weiner W. Side and type of motor symptom influence cognition in Parkinson’s disease. Mov Disord. 2006;21(11):1947–1953. doi: 10.1002/mds.21105. [DOI] [PubMed] [Google Scholar]
- 36.Zaidel A, Arkadir D, Israel Z, Bergman H. Akineto-rigid vs. tremor syndromes in Parkinsonism. Curr Opin Neurol. 2009;22(4):387–393. doi: 10.1097/WCO.0b013e32832d9d67. [DOI] [PubMed] [Google Scholar]
- 37.Cronin-Golomb A. Parkinson’s disease as a disconnection syndrome. Neuropsychol Rev. 2010;20(2):191–208. doi: 10.1007/s11065-010-9128-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rubin LA, Barr WB, Burton LA. Assessment practices of clinical neuropsychologists in the United States and Canada: a survey of INS, NAN, and APA Division 40 members. Arch Clin Neuropsychol. 2005;20(1):33–65. doi: 10.1016/j.acn.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 39.Sunderland T, Hill JL, Mellow AM, Lawlor BA, Gundersheimer J, Newhouse PA, Grafman JH. Clock drawing in Alzheimer’s disease. A novel measure of dementia severity. J Am Geriatr Soc. 1989;37(8):725–729. doi: 10.1111/j.1532-5415.1989.tb02233.x. [DOI] [PubMed] [Google Scholar]
- 40.Mendez MF, Ala T, Underwood KL. Development of scoring criteria for the clock drawing task in Alzheimer’s disease. J Am Geriatr Soc. 1992;40(11):1095–1099. doi: 10.1111/j.1532-5415.1992.tb01796.x. [DOI] [PubMed] [Google Scholar]
- 41.Rouleau I, Salmon DP, Butters N. Longitudinal analysis of clock drawing in Alzheimer’s disease patients. Brain Cogn. 1996;31(1):17–34. doi: 10.1006/brcg.1996.0022. [DOI] [PubMed] [Google Scholar]
- 42.Manos PJ, Wu R. The ten point clock test: a quick screen and grading method for cognitive impairment in medical and surgical patients. Int J Psychiatry Med. 1994;24(3):229–244. doi: 10.2190/5A0F-936P-VG8N-0F5R. [DOI] [PubMed] [Google Scholar]
- 43.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17(5):427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 44.Lee DY, Seo EH, Choo IH, Kim SG, Lee JS, Lee DS, Jhoo JH, Kim KW, Youn JC, Woo JI. Neural correlates of the Clock Drawing Test performance in Alzheimer’s disease: a FDG-PET study. Dement Geriatr Cogn Disord. 2008;26(4):306–313. doi: 10.1159/000161055. [DOI] [PubMed] [Google Scholar]
- 45.Matsuoka T, Narumoto J, Shibata K, Okamura A, Nakamura K, Nakamae T, Yamada K, Nishimura T, Fukui K. Neural correlates of performance on the different scoring systems of the clock drawing test. Neurosci Lett. 2011;487(3):421–425. doi: 10.1016/j.neulet.2010.10.069. [DOI] [PubMed] [Google Scholar]
- 46.Ungerleider LG, Haxby JV. ‘What’ and ‘where’ in the human brain. Cur Opin in Neurobiol. 1994;4(2):157–165. doi: 10.1016/0959-4388(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 47.Battersby WS, Bender MB, Pollack M, Kahn RL. Unilateral spatial agnosia (inattention) in patients with cerebral lesions. Brain. 1956;79(1):68–93. doi: 10.1093/brain/79.1.68. [DOI] [PubMed] [Google Scholar]
- 48.Borod JC, Goodglass H, Kaplan E. Normative data on the Boston Diagnostic Aphasia Examination, parietal lobe battery, and the Boston Naming Test. J Clin Neuropsychol. 1980;2:209–216. [Google Scholar]
- 49.Newcombe F, Ratcliff G, Damasio H. Dissociable visual and spatial impairments following right posterior cerebral lesions: clinical, neuropsychological and anatomical evidence. Neuropsychologia. 1987;25(1B):149–161. doi: 10.1016/0028-3932(87)90127-8. [DOI] [PubMed] [Google Scholar]
- 50.Tranel D, Rudrauf D, Vianna EP, Damasio H. Does the Clock Drawing Test have focal neuroanatomical correlates? Neuropsychology. 2008;22(5):553–562. doi: 10.1037/0894-4105.22.5.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jaywant A, Musto G, Neargarder S, Stavitsky Gilbert K, Cronin-Golomb A. The effect of Parkinson’s disease subgroups on verbal and nonverbal fluency. J Clin Exp Neuropsychol. 2014;36:278–89. doi: 10.1080/13803395.2014.889089. [DOI] [PMC free article] [PubMed] [Google Scholar]