Abstract
A 77-year-old man presented with a chronic lesion located in the left penoscrotal area. Apart from pruritus, bleeding and an occasional discharge from this area, he also reported reduced appetite and weight loss. Examination revealed an ulcerated skin lesion attached to a firm subcutaneous mass. Wide local excision of the lesion revealed invasive adenocarcinoma on a background of extramammary Paget's disease. Staging studies showed disseminated metastatic disease within the lymph nodes, and liver and bone metastases. He was treated with carboplatin and paclitaxel chemotherapy initially, but then continued only on Carboplatin chemotherapy due to side effects from Paclitaxel. Eleven weeks after the start of his chemotherapy, his restaging imaging showed reduced lymphadenopathy, unchanged liver metastasis and sclerosis of bone metastasis. With completion of chemotherapy, repeat imaging showed stable disease. The patient is currently on follow-up.
Background
Extramammary Paget's disease of the male genitalia is a rare disease. It is commonly misdiagnosed as eczema and treated conservatively in the primary care setting. This case highlights the importance of specialist referral when a lesion occurs in the penoscrotal area and is unresponsive to conventional topical treatment. Specialist review and biopsy of the lesion in this case led to a diagnosis of extramammary Paget's disease. Patients with this condition should be monitored and managed in tertiary centres with multidisciplinary expertise in this rare skin disease.
Case presentation
A 77-year-old man was referred to the urology department as a result of a lesion at the base of his penis, extending to the left penoscrotal area, and an elevated prostate specific antigen (PSA). The skin lesion was present for at least a year. It was associated with erythaema, itchiness, bleeding and discharge. On examination, the lesion appeared to be ulcerated, with suspicious features for neoplasia, and was attached to an underlying mass. It measured about 3×4 cm with surrounding erythaema (figure 1). Palpable lymph nodes in both inguinal regions were present and the patient also reported reduced appetite and weight loss.
Figure 1.
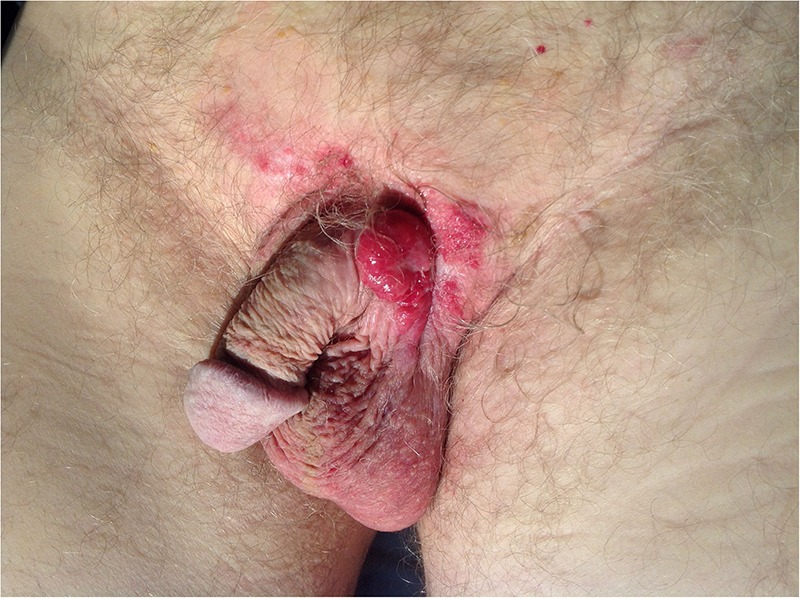
Malignant appearing lesion measuring 3×4 cm in the left penoscrotal area, with surrounding erythaema.
He had a history of benign prostate hyperplasia, prostatitis and an elevated PSA. Three years earlier, his PSA was 15.5 ng/mL and he refused to undergo a prostate biopsy. But following antibiotic treatment, his PSA levels reduced and he wished to proceed with PSA surveillance alone, without any interventional procedure to exclude a prostate adenocarcinoma. His PSA levels at the time of referral to the urology department were 7.42 ng/mL. He also had a past history of malaria and trigeminal neuralgia. He was a non-smoker and a retired professional bird watcher and mechanical engineer. There was no relevant family history.
The patient was referred to a tertiary centre with expertise in penile cancer, as this was initially thought to be a squamous cell carcinoma. A decision was made for a wide local excision of the suspicious lesion and a scrotal flap to cover the defect.
Investigations
A staging CT scan was performed, which revealed disseminated metastatic disease with suspicious lymphadenopathy in the bilateral inguinal areas and iliac lymph nodes together with liver metastases. There were no pulmonary metastases.
An ultrasound scan confirmed abnormal looking lymph nodes in both inguinal regions with the largest at 10 mm in the right groin. There was suspicion of metastatic involvement of all nodes and a fine-needle aspiration cytology (FNAC) was performed from the right groin node.
The FNAC indicated a metastatic epithelial malignant tumour with features that were not typical of a squamous cell carcinoma. Unfortunately, it was not possible to perform immunocytochemistry on the FNAC sample. The patient then underwent a wide local excision of the left penoscrotal mass to allow histological assessment of the possible primary lesion.
A positron emission tomography-CT (PET-CT) was also performed to quantify the degree of metastatic disease. It confirmed the liver metastases (figure 2) and inguinal lymphadenopathy (figure 3), and, additionally, extensive lymphadenopathy with metastases in the para-aortic, aortic, abdominal, periportal, paraoesophageal and supraclavicular nodes. There were also metastatic deposits within the bones, specifically the ribs and spine, but with no evidence of cord compression. There was no tracer uptake in the gastrointestinal or urinary tract.
Figure 2.
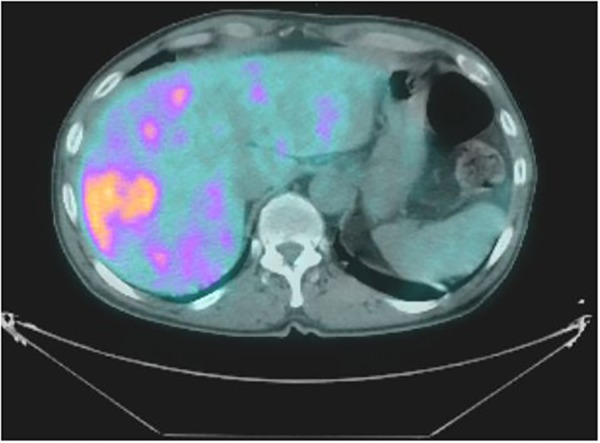
PET-CT image showing fluorodeoxyglucose (FDG)-avid liver metastases. PET-CT, positron emission tomography-CT.
Figure 3.
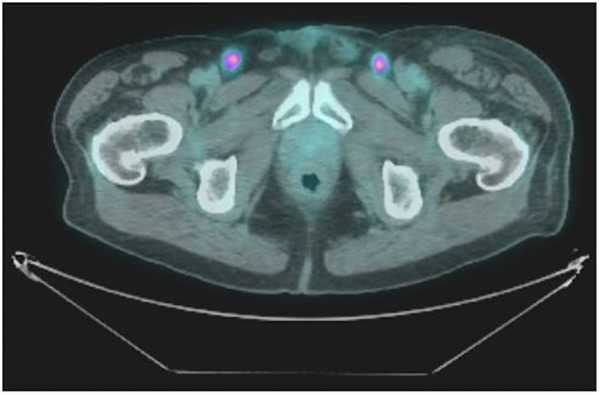
PET-CT Image showing bilateral FDG-avid inguinal lymph nodes. PET-CT, positron emission tomography-CT.
Differential diagnosis
The histology from the penoscrotal lesion revealed extramammary Paget's disease with an invasive adenocarcinoma (figure 4).
Figure 4.
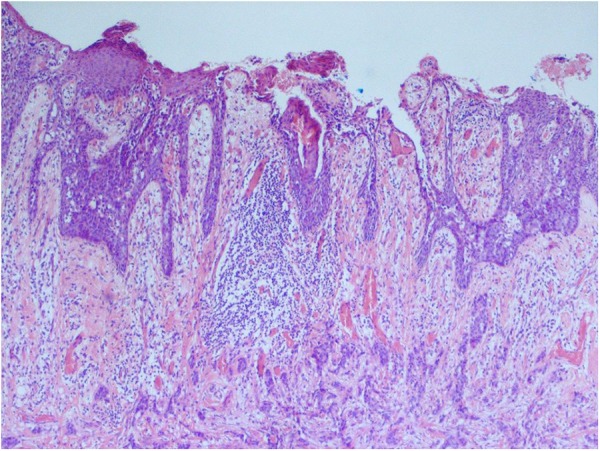
Surface extramammary Paget's disease with underlying invasive adenocarcinoma (×40 magnification).
Microscopically, there were malignant cells with oval nuclei, prominent nucleoli, irregular chromatin and moderate amounts of eosinophilic cytoplasm infiltrating the dermis in nests and sheets (figure 5). There was a widespread formation of glandular structures, giving a cribriform appearance, and some nests also had central necrosis (figure 6). There were also several foci suspicious for lymphovascular invasion. The epidermis overlying the lesion was largely ulcerated but the adjacent epidermis showed a Pagetoid spread of the malignant cells.
Figure 5.
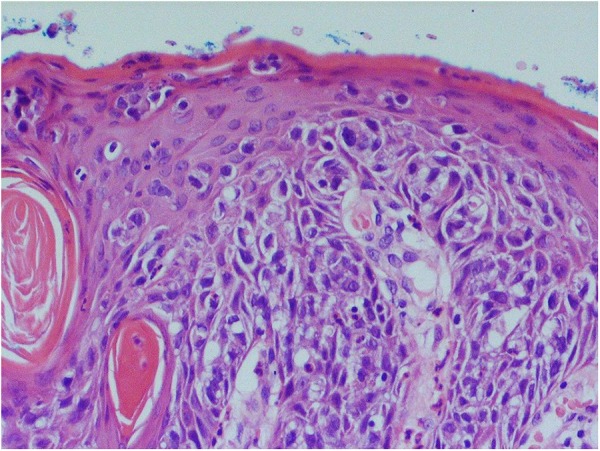
Surface extramammary Paget's disease composed of groups of atypical vacuolated cells infiltrating through the epidermis (×200 magnification).
Figure 6.
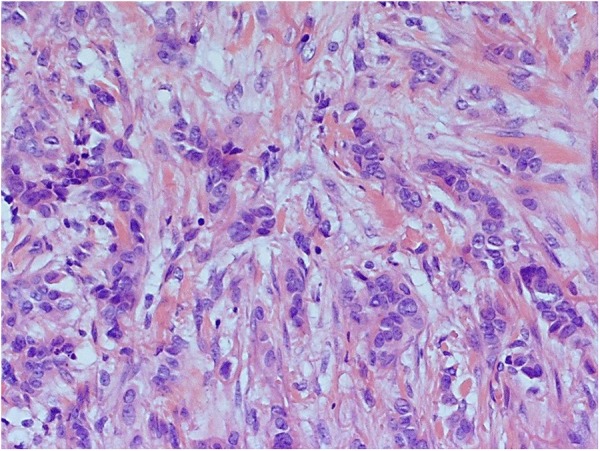
Invasive tumour composed of nests of poorly differentiated adenocarcinoma (×200 magnification).
The histology showed features of extramammary Paget's disease, and to confirm, immunohistochemistry was performed; the tumour cells were positive for cytokeratin 7 (CK7), which is diagnostic for this disease (figures 7 and 8).
Figure 7.
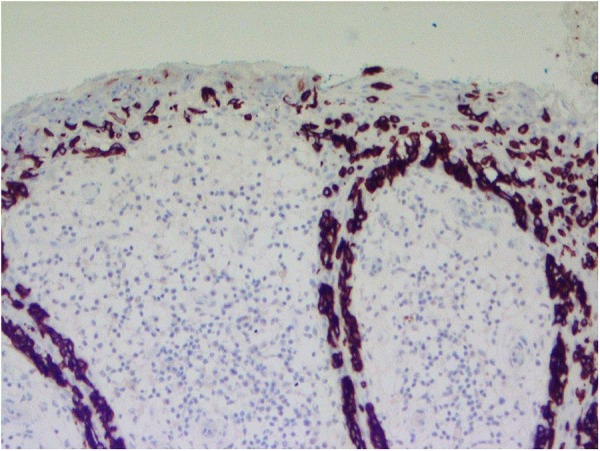
CK7 immunostain highlights the atypical cells but not the background skin (×200 magnification). CK7, cytokeratin 7.
Figure 8.
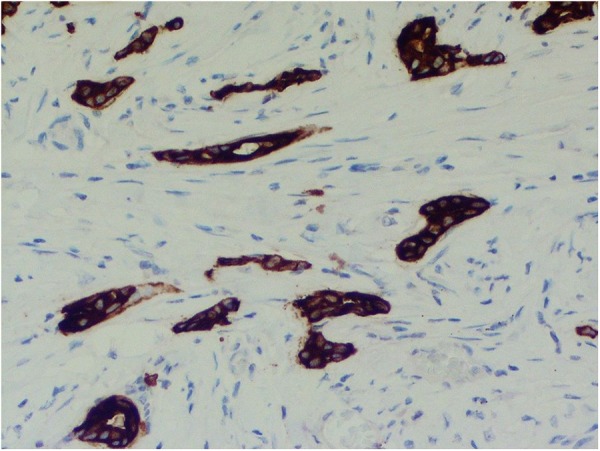
CK7 immunostain highlights the invasive tumour and shows occasional gland formation (×200 magnification). CK7, cytokeratin 7.
Further immunohistochemistry was positive for carcinoembryonic antigen (CEA) and epithelial membrane antigen (EMA) staining. PSA, prostate-specific membrane antigen, S-100 protein, melan-A, human melanoma black-45 marker, chromogranin-A, synaptophysin, and CD-56 and CD-20 testing were negative, strengthening the diagnosis of extramammary Paget's disease and excluding metastatic disease from prostate adenocarcinoma and melanoma.
Treatment
A multidisciplinary meeting reviewed this case and as there was no tracer uptake in the bowel or urinary tract on PET-CT to indicate a second primary cancer causing the liver metastases, a decision was made not to perform any further diagnostic tests and to proceed with palliative chemotherapy treatment due to already disseminated disease.
The patient started carboplatin and paclitaxel chemotherapy. Human epidermal growth factor receptor 2 (HER 2), oestrogen receptor (ER) and progesterone receptor (PR) biomarkers were also requested to assess the feasibility for other chemotherapy agents.
After his 2nd cycle of chemotherapy, the patient developed neutropaenia and, later, a deep vein thrombosis in his left leg. He started prophylactic antibiotics and a treatment dose of low molecular weight heparin. He received granulocyte-colony stimulating factor as a prophylaxis with his chemotherapy treatments but due to chemotherapy-related sensory neuropathy, the paclitaxel was stopped and the patient continued his chemotherapy cycles with carboplatin alone.
ER and PR status were negative. HER 2 status showed +1 staining in the invasive component of the tumour and +2 staining in the in situ Paget's disease. This meant that, ultimately, HER 2 status was negative, as +1 and +2 staining are not sufficiently predictive to classify cases.
Outcome and follow-up
Eleven weeks after the first cycle of chemotherapy, the patient had his first restaging CT scan, which showed reduced lymphadenopathy and sclerosis of rib and spinal metastases, indicating some response to treatment. The liver metastases though were unchanged. A restaging CT scan at the end of the 16th week of carboplatin chemotherapy showed stable disease.
One month after his last chemotherapy cycle, the patient did not report any additional side effects other that tiredness and alopecia. The sensory neuropathy he developed due to paclitaxel was slowly resolving. He currently remains active and independent in his daily life despite awareness of the poor prognosis of his disease.
Discussion
Invasive extramammary Paget's disease (EMPD) is a rare intraepithelial neoplasm with an incidence of 6 per million person-years as calculated by a Surveillance, Epidemiology and End Results (SEER) register analysis in 2011.1 It affects apocrine gland-bearing areas, especially the vulvar, perianal skin and axilla. Penoscrotal EMPD accounts for 14% of reported cases.2–5 The first description of penoscrotal EMPD was by Crocker, in 1889.3 From 1973 to 2007, SEER analysis found 197 cases of scrotal and 82 cases of penile EMPD.1
It is frequently misdiagnosed as other benign skin conditions such as seborrhoeic dermatitis, contact dermatitis, lichen sclerosus, Bowen disease, eczema, psoriasis or a superficial fungal infection.2 3 6 It usually appears as a well-circumscribed erythaematous pruritic plaque that can also erode, bleed and form crusts. It can sometimes be present for over a decade before it undergoes malignant transformation.4
Diagnosis of EMPD is preceded by a high index of suspicion and diagnostic biopsy with immunohistochemistry.2 6 Immunohistochemical tests show a positive staining for CK7, CEA and EMA, as in our case. Some cases have also reported positivity in other markers as well, such as low-molecular-weight cytokeratins (CAM 5.2) and prolactin-induced protein.2 3 6–8 Staining, however, is consistently negative for high-molecular-weight CKs, CK 20, A100 and prostate markers.2 3 8
Once diagnosis is confirmed, these cases should be managed in a multidisciplinary team setting. Once a treatment plan is set, further investigations are necessary, as EMPD is also associated with cancers of the urethra, bladder, prostate, colon, vagina, cervix or endometrium; one case of hepatocellular carcinoma has also been reported.2 3 6 7 Some publications have even reported the coexistence of EMPD with squamous cell carcinoma, especially in the vulva.6 Penoscrotal EMPD is mostly associated with cancers of the urinary and gastrointestinal tract in approximately 11% of cases, therefore PET-CT or endoscopic investigations are necessary to exclude this presentation.2 3 5
Surgical excision of the affected area remains the most widely accepted treatment option. Wide local excision with immediate reconstruction combined with frozen section of the resection margins or Mohs micrographic surgery can reduce the risk of residual disease and therefore diminish local recurrence.2 3 5 Recurrence rates range from 16–25% and dermal invasion is thought to be present in up to 25% of primary intraepidermal EMPD.3 8 9 Invasive EMPD usually develops as an adenocarcinoma and, once lymphovascular invasion occurs, prognosis is poor.2 3
Other treatment options such as carbon dioxide Laser vaporisation and photodynamic therapy can only be used after invasive disease is excluded.6 10 Radiotherapy treatment for penoscrotal EMPD lacks experience as it has only been used for vulvar disease.6 10 Topical agents such as 5-fluorouracil (5-FU) and 5% imiquimod cream have been used in small lesions with success, but close surveillance is required.5 9
Surgical lymphadenectomy is indicated in cases of infiltrative disease or suspicious metastasis to the groins without evidence of spread to the pelvic nodes.9 We proceeded directly to chemotherapy and avoided lymphadenectomy due to the presence of distant metastases.
Unfortunately, not many effective therapies for metastatic disease have been reported.1 Chemotherapy regimens reported in the literature are: systemic 5-FU with mitomycin C, carboplatin plus 5-FU and leucovorin, low dose 5-FU and cisplatin, docetaxel, mitomycin C plus epirubicin, vincristine and cisplatin, and 5-FU alone.2 10 Establishing whether HER2, ER and PR factors are expressed in the immunohistochemistry is important and can assist in selecting chemotherapy agents. In one case report, the HER 2 protein was overexpressed and a combination chemotherapy with trastuzumab and paclitaxel was effective.11
Owing to the rare incidence of this disease, no clear diagnostic and treatment guidelines are available yet. Long-term follow-up in a specialised centre is required once diagnosis of EMPD is confirmed, to monitor for progression to invasive carcinoma as well as other EMPD-associated malignancies.6 7
Learning points.
This case highlights the importance of patients contacting their family doctor when any abnormal lesion occurs in the penoscrotal area.
In the early stages of extramammary Paget's disease, the areas affected appear similarly to eczema. The family doctor should seek for specialist input such as a dermatologist with experience in genital dermatological diseases.
Early diagnosis of extramammary Paget's disease is important, as it has the potential to expand to adjacent areas, making treatment more challenging when it involves a larger area of skin.
Treatment options available are surgical excision, topical chemotherapy or immunotherapy, phototherapy and laser. If it develops invasive features, management becomes more challenging and can progress to metastatic disease leading to a less favourable prognosis for the patient.
Acknowledgments
The authors wish to thank the Urology Multidisciplinary Team, who managed all aspects of this patient's care.
Footnotes
Contributors: MC wrote the case report, and obtained consent from the patient for the use of images and publication of the case report. PK contributed in the histopathology section of this article as well as in editing figures. AMi and AMu contributed in the care of this patient, and AMu also edited the final version of the article.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Master VA, Herrel L, Johnson TV et al. Extramammary Paget's disease of the penis and anogenital area: seer analysis. J Clin Oncol 2011;29 (Suppl 7):Abstract 220, http://meetinglibrary.asco.org/content/72169–104 [Google Scholar]
- 2.Beleznay KM, Levesque MA, Gill S. Response to 5-fluorouracil in metastatic extramammary Paget disease of the scrotum presenting as pancytopenia and back pain. Curr Oncol 2009;16:81–3. 10.3747/co.v16i5.374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ekwueme KC, Zakhour HD, Parr NJ. Extramammary Paget's disease of the penis: a case report and review of the literature. J Med Case Rep 2009;3:4 10.1186/1752-1947-3-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Isrow D, Oregel KZ, Cortes J et al. Advanced extramammary Paget's disease of the groin, penis and scrotum. Clin Med Insights Oncol 2014;8:87–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanitakis J. Mammary and extramammary Paget's disease. J Eur Acad Dermatol Venereol 2007;21:581–90. [DOI] [PubMed] [Google Scholar]
- 6.Quinn AM, Sienko A, Basrawala Z et al. Extramammary Paget disease of the scrotum with features of Bowen disease. Arch Pathol Lab Med 2004;128:84–6. [DOI] [PubMed] [Google Scholar]
- 7.Li Y-C, Lu L-Y, Yang Y-T et al. Extramammary Paget's disease of the scrotum associated with hepatocellular carcinoma. J Chin Med Assoc 2009;72:542–6. 10.1016/S1726-4901(09)70425-3 [DOI] [PubMed] [Google Scholar]
- 8.Parada D, Moreira O, López C et al. Extramammary Paget's disease of scrotum: a case with local lymph node metastasis. Arch Esp Urol 2005;58:85–9. 10.4321/S0004-06142005000100015 [DOI] [PubMed] [Google Scholar]
- 9.Londero AP, Bertozzi S, Salvador S et al. A review of extramammary Paget's disease: clinical presentation, diagnosis, management and prognosis. J Med Med Sci 2013;4:134–48. [Google Scholar]
- 10.Moretto P, Nair VJ, El Hallani S et al. Management of penoscrotal extramammary Paget disease: case series and review of the literature. Curr Oncol 2014;20:e311–20. 10.3747/co.20.1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanawa F, Inozume T, Harada K et al. A case of metastatic extramammary Paget's disease responding to trastuzumab plus paclitaxel combination therapy. Case Rep Dermatol 2011;3:223–7. 10.1159/000333002 [DOI] [PMC free article] [PubMed] [Google Scholar]


