Abstract
Objectives
This study aimed to assess the role of SLC5A8 expression in the survival of pancreatic cancer.
Methods
We determined SLC5A8 expression in pancreatic ductal adenocarcinoma and adjacent non–neoplastic pancreas (NNP) obtained from 110 patients who underwent pancreatectomy. Formalin-fixed paraffin-embedded core sections in a tissue microarray were immunostained using polyclonal anti-SLC5A8 antibody, and a semiquantitative measure of SLC5A8 expression was determined.
Results
SLC5A8 expression was low in 56% (62/110) of pancreatic cancers as compared to NNP that had low expression in only 9% (10/107) of specimens (P < 0.0001). All cells expressing SLC5A8 did so in the cytoplasm, whether they are neoplastic or not. Nuclear expression of SLC5A8 occurred in 38% (42/110) of cancers, but it was uncommon in NNP (7%, 8/107) (P < 0.0001). Kaplan-Meier estimates showed that survival in patients whose cancers had low SLC5A8 expression, and/or nuclear expression, was significantly worse than in patients whose cancers had none of these abnormalities (P = 0.02). For the 88 patients whose cancers had abnormal SLC5A8 expression, median survival was 1.4 years, as compared to 3.9 years in patients whose cancers both expressed high levels of SLC5A8 and lacked nuclear expression.
Conclusions
SLC5A8 nuclear translocation and loss of expression are associated with poor outcome in pancreatic ductal adenocarcinoma.
Keywords: pancreatic cancer, SLC5A8, tumor suppressor gene, survival, tissue microarray
Pancreatic cancer has the lowest long-term survival rate of any cancer, with no more than 5% of all pancreatic cancer patients surviving for 5 years.1 Most pancreatic cancers are ductal adenocarcinomas. The prognosis of patients with pancreatic ductal adenocarcinoma (PDA) has remained unchanged despite significant advances in our knowledge of the molecular biology and treatment of many other cancers. If the outcome of patients with pancreatic cancer is to improve, we need to identify new biomarkers for early diagnosis and prognosis, as well as molecular targets for treatment.
Abnormal expression of the tumor suppressor gene SLC5A8 may be useful as a prognostic biomarker and is potentially of interest for the development of pancreatic cancer treatments. SLC5A8 belongs to the solute-linked carrier gene family 5 (SLC5), a family of 12 sodium-coupled transporters for a number of chemicals.2,3 SLC5A8 is a sodium-coupled transporter for nicotinate and analogs,4 lactate,5 and of particular interest, the short-chain fatty acids butyrate and pyruvate, which are known to induce tumor apoptosis through histone deacetylase (HDAC) inhibition.2,6,7 Evidence suggests that SLC5A8 functions as a tumor suppressor gene whose silencing may contribute to carcinogenesis and tumor progression.2,8 We and others have reported that loss of SLC5A8 expression resulting from DNA hypermethylation in the promoter region is associated with prognostic features in cancers of the brain,9 colon,8,10–12 thyroid,13–16 stomach,17 breast,7 lung,18 prostate,19 and head and neck,20 as well as acute myeloid leukemia.21
In a previous study, we showed by methylation-specific polymerase chain reaction that SLC5A8 CpG island methylation was infrequent in non–neoplastic pancreas (NNP) (11%) but common in pancreatic cancer (70%), a finding consistent with tumor-specific loss of SLC5A8 expression.22 Using bisulfite sequencing analysis, we also observed that pancreatic cancer cell lines that did not express SLC5A8 were densely methylated in the promoter region. SLC5A8 expression in these cell lines was restored by treatment with the demethylating agent 5-aza-deoxycytidine, implying that SLC5A8 expression is suppressed by aberrant DNA methylation in pancreatic cancer.22 In colon and thyroid cancer, hypermethylation in the promoter region of SLC5A8 has been found to be associated with disease progression features, including target tissue invasion, lymphangiogenesis, multifocality, and advanced stage.10,14 However, little is known about the prognostic significance of SLC5A8 expression in PDA. Our aim in this study was to characterize SLC5A8 expression in pancreatic cancer by immunohistochemistry and seek an association of abnormal expression with poor survival.
MATERIALS AND METHODS
We previously reported the outcomes of a series of 137 patients with resectable stage IA to IIB PDA who underwent pancreatectomy at the Moffitt Cancer Center from January 1987 through December 2006, and survived at least 30 days after surgery.23,24 The present report is focused on 110 of these pancreatic cancer patients who had archived fixed surgical specimens available for this study. The histologic diagnosis and characteristics in each case were confirmed by an expert review of stained sections cut from fixed surgical specimens. Data from the Moffitt Cancer Registry, the patient's medical record, and the Social Security Death Index were reviewed to determine the patient's clinicopathological characteristics, tumor stage, survival of each patient after surgery, whether the patient was deceased as of most recent knowledge, and if there was evidence of disease at that time. During the period in which our series of patients underwent surgery, the standard practice at our institution was to give adjuvant treatment. The study was approved by the University of South Florida Institutional Review Board.
Immunohistochemistry
Tissue microarrays were prepared using cores obtained from formalin-fixed, paraffin-embedded surgical specimens. Duplicate 0.6-mm diameter core sections were taken from PDA within the specimens, as well as from adjacent NNP. Four-micrometer-thick sections were cut by microtome (Leica Microsystems, Inc, Bannockburn, IL) and transferred to adhesive-coated slides. Microarray slides were deparaffinized by heating at 55°C for 30 minutes and by three 5-minute washes with xylene. Tissues were rehydrated by a series of 5-minute washes in 100%, 95%, and 80% ethanol and distilled water. Antigen retrieval was accomplished by heating the tissues at 95°C for 30 minutes in 10 mmol/L sodium citrate (pH 6.0). Tissue microarrays were immunostained using the avidin-biotin-peroxidase method. After blocking with universal blocking serum (DAKO Diagnostic, Mississauga, Ontario, Canada) for 30 minutes, tissue arrays were incubated with a polyclonal anti-SLC5A8 antibody (provided by VG) at 4°C overnight. Then, sections were incubated with biotin-labeled secondary antibody and streptavidin-peroxidase for 30 minutes each (DAKO Diagnostic). Tissues were developed with a 3,3′-diaminobenzidine substrate (Vector Laboratories, Burlington, Ontario, Canada) and counterstained with hematoxylin. Negative controls were created by omitting the anti-SLC5A8 antibody during primary antibody incubation. Human duodenal normal tissue cores were used as a positive control on the tissue microarrays.
A semiquantitative measure of SLC5A8 expression was determined as follows: a single pathologist (DC) blinded to tissue origins scored stained tissue sections for intensity of SLC5A8 immunostain and percent of cells stained. Intensity of stain was ranked subjectively on a scale of 0 to 3, where “0” represents the absence of staining and “3” is maximal staining. The percent of cells stained was estimated on a scale of 0 to 3, with 0 for 0%, 1 for 1% to 33%, 2 for 34% to 66%, and 3 for greater than 66% of cells stained. SLC5A8 expression score for each core section was calculated as the product of immunostain intensity and the percent of cells stained. Thus, the SLC5A8 score could take on only the values 0, 1, 2, 3, 4, 6, and 9. In case of a disagreement between duplicate cores, the higher of the 2 scores was used as the measure of SLC5A8 expression. Nuclear SLC5A8 expression was considered present in a tissue specimen if the nuclei of at least 5% of cells in the specimen were stained.
Statistical Analysis
Proportions were compared by Fisher exact test. The sign test was used to compare median expression scores in paired specimens. Disease-specific survival was estimated by the Kaplan-Meier method using death with evidence of disease as the end point. The log-rank test for equality of survival functions was used to compare Kaplan-Meier survival curves. A multivariable Cox proportional hazard model was used to determine if the hazard of death posed by a single variable at any given time after surgery was different after adjusting for other covariables.
RESULTS
We determined SLC5A8 expression by immunostain in PDA obtained from the surgical specimens of 110 patients who underwent pancreatectomy with curative intent (stages IA-IIB). Both PDA and adjacent NNP tissues were stained in 98 of these patients. In an additional 12 patients, only PDA tissues were stained, making a total of 110 PDA stained. In 9 more patients, only NNP tissues taken from areas adjacent to PDAwere stained, making a total of 107 NNP tissues stained. The 110 patients whose cancer tissues were stained for SLC5A8 consisted of 61 women and 49 men; median age was 68 years (range, 25–78 years). Table 1 shows American Joint Committee on Cancer staging by anatomic extent (AJCC Cancer Staging Manual, 6th ed.) and histological characteristics for these patients.
TABLE 1.
Histopathological Characteristics of 110 Patients Who Underwent Resection of PDA With Curative Intent
| n (%) | |
|---|---|
| American Joint Committee on Cancer Stage Group | |
| IA—tumor ≤2 cm, confined to pancreas, no positive nodes | 6 (5) |
| IB—tumor >2 cm, confined to pancreas, no positive nodes | 17 (15) |
| IIA—local extrapancreatic extension without celiac axis or superior mesenteric artery involvement, no positive nodes | 38 (35) |
| IIB—regional node metastasis | 49 (45) |
| T Classification | |
| T 1—tumor ≤2 cm, confined to pancreas | 10 (9) |
| T 2—tumor >2 cm, confined to pancreas | 23 (21) |
| T 3—local extrapancreatic extension without celiac axis or superior mesenteric artery involvement | 77 (70) |
| N Classification | |
| N 0—no nodal metastases | 60 (55) |
| N 1—regional node metastases | 50 (45) |
| Histologic differentiation | |
| Well | 27 (25) |
| Moderate | 64 (58) |
| Poor | 19 (17) |
| Lymphatic invasion | 72 (65) |
| Venous invasion | 41 (37) |
| Perineural invasion | 90 (82) |
SLC5A8 Expression
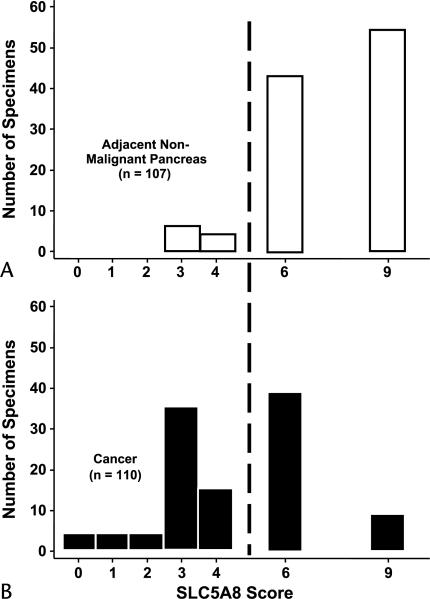
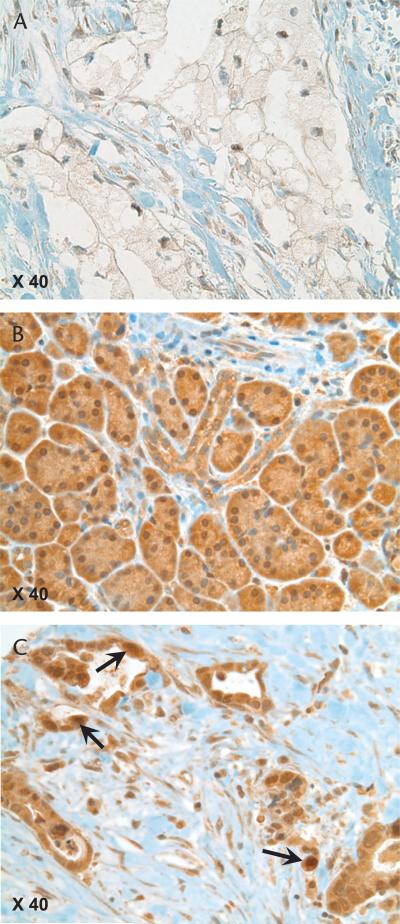
The frequency distribution of SLC5A8 product scores for the 107 NNP tissues shows that most scores were either 6 or 9 (Fig. 1A). SLC5A8 expression in the 110 PDA tissues (Fig. 1B) was compared to the normally high level of expression in NNP tissues (Fig. 1A). Pancreatic ductal adenocarcinomas were 6 times as likely to exhibit low SLC5A8 expression as NNPs. SLC5A8 expression was low (score ≤4) in 56% (62/110) of PDAs, in contrast to NNPs, which had scores of 4 or less in only 9% (10/107) of tissues (P < 0.0001). When only the 98 paired PDAs and adjacent NNPs were considered, SLC5A8 expression was down-regulated in 73% of PDAs as compared to their paired adjacent NNPs, not different in 18%, and overexpressed in 9%. The median SLC5A8 expression score of 4 in the 98 PDAs was significantly less than the median score of 6 in the paired NNPs tissues (P < 0.0001). The immunostained tissue shown in Figure 2A is an example of the loss of SLC5A8 expression that is most typical in PDA, in contrast to the normally high expression of SLC5A8 found in NNP (Fig. 2B).
FIGURE 1.
Frequency distribution of SLC5A8 expression scores in PDA and adjacent NNP. SLC5A8 expression in NNP (A) is normally high with scores exceeding 4, as indicated by the dashed line, whereas down-regulation of SLC5A8 expression is common in PDA (B).
FIGURE 2.
Immunostain for SLC5A8 in PDA and adjacent NNP. SLC5A8 expression is commonly low or even absent in PDA (A) but normally high in NNP (B). All cells expressing SLC5A8 did so in the cytoplasm, whether neoplastic or not. Nuclear expression occurred in a significant minority of PDAs (C) but was relatively uncommon in NNPs. Arrows identify several nuclei prominently stained for SLC5A8.
Nuclear Expression of SLC5A8
Individual cells expressing SLC5A8 in the nucleus did so in the cytoplasm as well, whether PDA or NNP. Nuclear SLC5A8 expression, defined as immunostaining in at least 5% of cells in an individual tissue, was 5 times more likely in PDAs than in NNPs. Nuclear SLC5A8 expression occurred in 38% (42/110) of PDAs, whereas it was relatively uncommon in NNPs (7%, 8/107) (P < 0.0001). Figure 2C shows nuclear SLC5A8 expression in an immunostained pancreatic cancer specimen. In cancer, nuclear expression of SLC5A8 was twice as likely to occur if the SLC5A8 score was within the normally high range observed in NNP. Specifically, nuclear SLC5A8 expression was present in 54% (26/48) of cancers with an SLC5A8 score higher than 4, but in only 26% (16/62) of cancers with a low score of 4 or less (P < 0.01).
SLC5A8 Expression and Survival
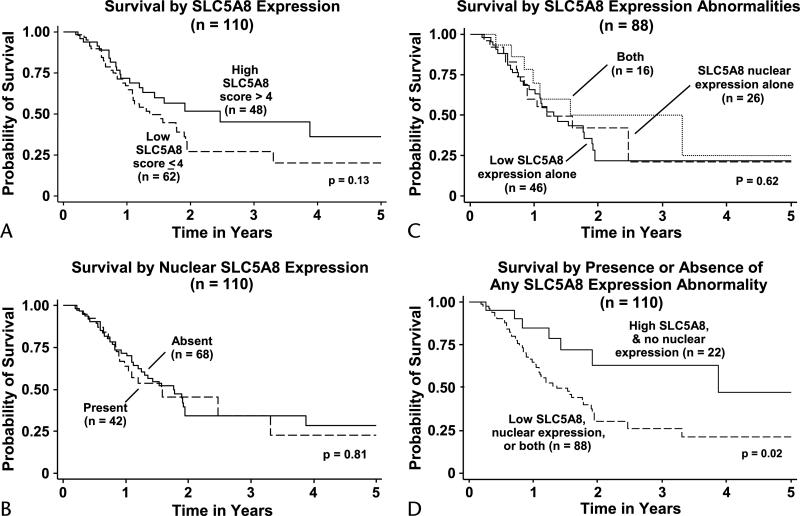
The disease-specific Kaplan-Meier survival estimate was determined for all 110 patients who underwent pancreatectomy. Median survival time was found to be 1.59 years after surgery, with the probability of surviving 3 years being 0.34 (95% confidence interval [CI], 0.23–0.46). Kaplan-Meier survival estimates by level of cancer SLC5A8 expression are shown in Figure 3A. Survival was worse in patients whose cancer SLC5A8 expression fell below the high levels normally found in NNPs (P = 0.13, log-rank test for difference in survival curves). Patients with low SLC5A8 expressing PDAs (scores ≤4) had a median survival of 1.36 years, whereas those with higher SLC5A8 expressing PDAs had a 2.47-year median survival. The probability of surviving 3 years after surgery in patients whose PDAs had low SLC5A8 expression was 0.27 (95% CI, 0.14–0.41), as compared to 0.45 (95% CI, 0.26–0.63) in those with high SLC5A8 expression.
FIGURE 3.
Kaplan-Meier survival estimates showed that (A) survival was worse for patients whose PDAs exhibited loss of SLC5A8 expression than in those with cancers expressing SLC5A8 at the high levels normally found in NNP (P = 0.13). B, Survival seemed to be independent of nuclear SLC5A8 expression if only nuclear expression was considered. C, Survival did not differ between patients whose PDAs had low SLC5A8 expression alone, nuclear expression alone, or both. D, Survival in patients whose cancers exhibited either or both of these abnormalities of SLC5A8 expression was worse than for patients whose PDAs had high SLC5A8 expression and lacked nuclear SLC5A8 expression, which is the SLC5A8 expression pattern typical of NNP (P = 0.02).
Nuclear Expression of SLC5A8 and Survival
Kaplan-Meier analysis for all 110 cancer patients showed apparent independence of survival from nuclear SLC5A8 expression, if only nuclear expression were considered. Survival in the 42 patients with cancers exhibiting nuclear expression of SLC5A8 did not differ from that in the 68 patients whose tumors lacked nuclear expression (P = 0.81) (Fig. 3B).
In a subset analysis (Fig. 3C and D), we considered how both level of SLC5A8 expression and the presence or absence of nuclear expression might influence survival. Survival did not differ between patients whose cancers had low SLC5A8 expression alone (SLC5A8 ≤4), nuclear SLC5A8 expression alone, or both (P = 0.62) (Fig. 3C). The outcomes of patients in all 3 of these categories were pooled, and a single survival curve determined for comparison with the survival curve of patients whose cancers had neither low SLC5A8 expression nor the presence of nuclear SLC5A8 expression (Fig. 3D). Survival was significantly worse in patients whose PDAs exhibited loss of SLC5A8 expression alone, nuclear SLC5A8 expression alone, or both (P = 0.02). For the 88 patients whose cancers had these abnormalities in SLC5A8 expression, median survival was 1.4 years, in contrast to a median survival of 3.9 years in the 22 patients whose cancers both expressed high levels of SLC5A8 and lacked nuclear expression. In the probability of surviving 3 years in patients with abnormal PDA, SLC5A8 expression was 0.26 (95% CI, 0.15–0.40), as compared to 0.63 (95% CI, 0.34–0.82) in those patients whose cancers had patterns of SLC5A8 expression typical of NNP.
SLC5A8 and Histopathologic Characteristics
Three histopathologic characteristics previously identified as being predictive of poor survival after pancreatectomy in our patient population are poor differentiation, local extrapancreatic extension (AJCC T3 classification), and histologic lymphatic invasion.24 Survival for the 30 patients (27% of the 110 patients) who had positive surgical margins did not from those patients with negative margins (P = 0.52 by log-rank test). We first determined whether any of the 3 characteristics predicting poor survival were associated with abnormal SLC5A8 expression as characterized by loss of SLC5A8 expression alone, nuclear SLC5A8 expression alone, or both. No association was found between abnormal SLC5A8 expression and poor tumor differentiation (P = 0.76), local extrapancreatic extension (P = 1.00), or lymphatic invasion (P = 0.46). We next used a multivariable Cox proportional hazard model to determine whether adjusting for these 3 histopathologic characteristics would change the worse survival predicted by the Kaplan-Meier estimate for abnormal SLC5A8 expression in PDA (Fig. 3D). The univariable hazard ratio of 2.49 (95% CI, 1.12–5.55) indicates that death with evidence of disease is approximately 2.5 times as likely at any given time after surgery if SLC5A8 expression is abnormal. After adjusting for the 3 histopathologic characteristics, the resulting hazard ratio of 2.41 (95% CI, 1.07–5.43) was not significantly different from the univariable hazard ratio.
DISCUSSION
SLC5A8 is regarded as a tumor suppressor gene whose expression is silenced by epigenetic changes in a number of cancers, and in a previous study, we found evidence that this is the case in pancreatic adenocarcinoma as well.22 In the present study, we extended our earlier work by first using immunohistochemistry to characterize SLC5A8 expression in pancreatic cancer, and then we sought evidence that abnormal expression might be associated with shorter survival. Although all cells expressing SLC5A8 did so in the cytoplasm, we found that nuclear translocation and loss of expression were common in PDA, but uncommon in NNP. Survival in patients whose cancers had either or both abnormalities of SLC5A8 expression was decidedly worse than in patients whose cancers had neither abnormality. Previous studies suggested an importance of translocation between nucleus and cytoplasm on target proteins, such as A-catenin25 and inhibitor of growth gene 4 (ING4).26 Results of ING4 study suggested that the down-regulation of nuclear ING4 may be correlated with tumorigenesis, progression, and tumor differentiation in head and neck cancer, whereas overexpression of cytoplasmic ING4 may be involved in malignant progression. Li et al26 suggested that nuclear ING4 may modulate the transactivation of target genes, promoting apoptosis and cell cycle arrest through interactions with p300 and p21. However, further study is warranted to evaluate the biological role of nuclear SLC5A8 in progression of pancreatic cancer. Our findings are consistent with other studies in colon, thyroid, lung, breast, prostate, and oral cancers reporting evidence for loss of SLC5A8 expression by epigenetic silencing and an association with tumor progression.
The proposed function of SLC5A8 as a tumor suppressor gene is based not only on reversible hypermethylation in the promoter region, but also on its association with tumor progression and survival in cancers of the colon and thyroid. Li and colleagues8 reported that 60% of colon cancers were methylated in the promoter region of SLC5A8, whereas only 5% of normal epithelial specimens were methylated. Moreover, DNA methylation seems to be an early event in carcinogenesis, as approximately 60% of precursor lesions were methylated in the SLC5A8 promoter region.8 Paroder and colleagues11 suggested that SLC5A8 expression is associated with survival in patients with colon cancer. A recent study in colon cancer reported frequent loss of heterozygosity in the region 12q13-24 that contains SLC5A8.27 In thyroid cancer, 26% to 50% of tumor tissues were methylated in the SLC5A8 promoter region.13–15 Porra and colleagues13 observed that SLC5A8 was methylated in 90% of papillary thyroid carcinomas. Thyroid cancer cell lines which do not express SLC5A8 were densely methylated in the promoter region and reactivated by 5-aza treatment.14 Hu and colleagues14 reported that aberrant methylation of SLC5A8 is associated with progression of thyroid cancer, although these results were not replicated in another study.15
Further studies indicate that SLC5A8 is frequently down-regulated in other cancers, although these studies did not attempt to seek an association with survival or features of tumor progression. Ueno and colleagues17 reported that 30% (23/71) of gastric tumor tissues were methylated in the SLC5A8 promoter, whereas 83% (10/12) of gastric cancer cell lines were found to be methylated. SLC5A8 was silenced in 9 of 10 methylated gastric cancer cell lines, and reactivated by 5-aza in all 9 silenced cell lines.17 Thangaraju and colleagues7 reported on transformed breast cancer cells lines that did not express SLC5A8, but in which expression was reactivated by 5-aza treatment. The same group observed that SLC5A8 expression was down-regulated in 27 of 30 breast tumor tissues. Recent studies in acute myeloid leukemia suggest that SLC5A8 is epigenetically silenced.21 We previously reported that SLC5A8 is frequently silenced in prostate and pancreatic tumors, likely by DNA methylation.19,22
Few studies have reported that subcellular location of a protein may affect disease prognosis. For example, loss of heterozygosity at 18q, the locus of Smad4, is a common mechanism for inactivation of Smad4 in pancreatic cancer.28 Kloth and colleagues29 reported that weak cytoplasmic and absent nuclear expression of Smad4 were associated with poor survival in cervical cancer patients. In another study, nuclear expression of maspin, a member of the serine protease inhibitors family, has been associated with tumor status30,31 and a better prognosis in pancreatic cancer.32 Cao and colleagues32 observed that patients whose pancreatic cancers expressed maspin in the nucleus survived longer than those whose tumors expressed maspin in only the cytoplasm.
Although we found that nuclear translocation of SLC5A8 is associated with worse survival in pancreatic cancer, we know of no previous studies indicating that the subcellular location of SLC5A8 might be important in determining the protein's function or the status of the tumor. The cell membrane and cytoplasm is the cellular compartment in which SLC5A8 activity normally resides, however, we can only speculate that perhaps a subset of the protein may be translocated into the nucleus to serve as a carrier for pyruvate (HDAC inhibition factor). Nuclear and cytoplasmic SLC5A8 proteins might have different biologic activities as well.
Ganapathy and colleagues have proposed a mechanism for tumor suppression by SLC5A8.2,3,7,33 SLC5A8 mediates sodium-coupled, electrogenic transport of monocarboxylates such as short-chain fatty acids. Pyruvate, one of the substrates for SLC5A8, is an HDAC inhibitor known to induce apoptosis in various tumors. Therefore, the transport function of SLC5A8 may affect the acetylation of histone and, hence, gene expression in cells. SLC5A8 activity in transport of pyruvate into cells may explain its apparent role as a tumor suppressor.2,3 These results are further supported by another study which identified HDAC1 and HDAC3 as targets for 3-bromopyruvate which is transported into cells by SLC5A8.34 Zhang and colleagues recently identified a 295-bp region which is essential for basal promoter activity35 and reported that SLC5A8 expression is regulated through the Smad signaling pathway.36
We previously confirmed a perception that tumor staging based on anatomic extent alone is an insufficient predictor of survival after pancreatectomy for PDA.24 In a logical extension of the anatomic staging system, we found that a simple combination of histopathologic characteristics reflecting aggressiveness of tumor biology was predictive of survival after pancreatectomy.24 We suggested that future studies may show molecular staging to be of value in predicting survival in PDA. The decidedly worse survival in patients whose cancers had SLC5A8 nuclear translocation, loss of expression or both suggests that abnormal of SLC5A8 expression may be a useful prognostic biomarker for survival after pancreatectomy for PDA. Further study of the biological role of SLC5A8 in tumor progression may lead to development of strategies for improving pancreatic cancer treatment.
ACKNOWLEDGMENTS
The authors thank the staff of the Tissue Procurement facilities at H. Lee Moffitt Cancer Center for excellent technical assistance.
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Ganapathy V, Thangaraju M, Gopal E, et al. Sodium-coupled monocarboxylate transporters in normal tissues and in cancer. AAPS J. 2008;10:193–199. doi: 10.1208/s12248-008-9022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ganapathy V, Thangaraju M, Prasad PD. Nutrient transporters in cancer: relevance to Warburg hypothesis and beyond. Pharmacol Ther. 2009;121:29–40. doi: 10.1016/j.pharmthera.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Gopal E, Miyauchi S, Martin PM, et al. Transport of nicotinate and structurally related compounds by human SMCT1 (SLC5A8) and its relevance to drug transport in the mammalian intestinal tract. Pharm Res. 2007;24:575–584. doi: 10.1007/s11095-006-9176-1. [DOI] [PubMed] [Google Scholar]
- 5.Martin PM, Gopal E, Ananth S, et al. Identity of SMCT1 (SLC5A8) as a neuron-specific Na+-coupled transporter for active uptake of L-lactate and ketone bodies in the brain. J Neurochem. 2006;98:279–288. doi: 10.1111/j.1471-4159.2006.03878.x. [DOI] [PubMed] [Google Scholar]
- 6.Gupta N, Martin PM, Prasad PD, et al. SLC5A8 (SMCT1)-mediated transport of butyrate forms the basis for the tumor suppressive function of the transporter. Life Sci. 2006;78:2419–2425. doi: 10.1016/j.lfs.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 7.Thangaraju M, Gopal E, Martin PM, et al. SLC5A8 triggers tumor cell apoptosis through pyruvate-dependent inhibition of histone deacetylases. Cancer Res. 2006;66:11560–11564. doi: 10.1158/0008-5472.CAN-06-1950. [DOI] [PubMed] [Google Scholar]
- 8.Li H, Myeroff L, Smiraglia D, et al. SLC5A8, a sodium transporter, is a tumor suppressor gene silenced by methylation in human colon aberrant crypt foci and cancers. Proc Natl Acad Sci U S A. 2003;100:8412–8417. doi: 10.1073/pnas.1430846100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hong C, Maunakea A, Jun P, et al. Shared epigenetic mechanisms in human and mouse gliomas inactivate expression of the growth suppressor SLC5A8. Cancer Res. 2005;65:3617–3623. doi: 10.1158/0008-5472.CAN-05-0048. [DOI] [PubMed] [Google Scholar]
- 10.Dong SM, Lee EJ, Jeon ES, et al. Progressive methylation during the serrated neoplasia pathway of the colorectum. Mod Pathol. 2005;18:170–178. doi: 10.1038/modpathol.3800261. [DOI] [PubMed] [Google Scholar]
- 11.Paroder V, Spencer SR, Paroder M, et al. Na(+)/monocarboxylate transport (SMCT) protein expression correlates with survival in colon cancer: molecular characterization of SMCT. Proc Natl Acad Sci U S A. 2006;103:7270–7275. doi: 10.1073/pnas.0602365103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thangaraju M, Cresci G, Itagaki S, et al. Sodium-coupled transport of the short chain fatty acid butyrate by SLC5A8 and its relevance to colon cancer. J Gastrointest Surg. 2008;12:1773–1781. doi: 10.1007/s11605-008-0573-0. discussion 1781–1782. [DOI] [PubMed] [Google Scholar]
- 13.Porra V, Ferraro-Peyret C, Durand C, et al. Silencing of the tumor suppressor gene SLC5A8 is associated with BRAF mutations in classical papillary thyroid carcinomas. J Clin Endocrinol Metab. 2005;90:3028–3035. doi: 10.1210/jc.2004-1394. [DOI] [PubMed] [Google Scholar]
- 14.Hu S, Liu D, Tufano RP, et al. Association of aberrant methylation of tumor suppressor genes with tumor aggressiveness and BRAF mutation in papillary thyroid cancer. Int J Cancer. 2006;119:2322–2329. doi: 10.1002/ijc.22110. [DOI] [PubMed] [Google Scholar]
- 15.Schagdarsurengin U, Gimm O, Dralle H, et al. CpG island methylation of tumor-related promoters occurs preferentially in undifferentiated carcinoma. Thyroid. 2006;16:633–642. doi: 10.1089/thy.2006.16.633. [DOI] [PubMed] [Google Scholar]
- 16.Xing M. Gene methylation in thyroid tumorigenesis. Endocrinology. 2007;148:948–953. doi: 10.1210/en.2006-0927. [DOI] [PubMed] [Google Scholar]
- 17.Ueno M, Toyota M, Akino K, et al. Aberrant methylation and histone deacetylation associated with silencing of SLC5A8 in gastric cancer. Tumour Biol. 2004;25:134–140. doi: 10.1159/000079145. [DOI] [PubMed] [Google Scholar]
- 18.Park J, Brena RM, Gruidl M, et al. CpG island hypermethylation profiling of lung cancer using restriction landmark genomic scanning (RLGS) analysis. Cancer Biomark. 2005;1:193–200. doi: 10.3233/cbm-2005-12-307. [DOI] [PubMed] [Google Scholar]
- 19.Park JY, Zheng W, Kim D, et al. Candidate tumor suppressor gene SLC5A8 is frequently down-regulated by promoter hypermethylation in prostate tumor. Cancer Detect Prev. 2007;31:359–365. doi: 10.1016/j.cdp.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Bennett KL, Karpenko M, Lin MT, et al. Frequently methylated tumor suppressor genes in head and neck squamous cell carcinoma. Cancer Res. 2008;68:4494–4499. doi: 10.1158/0008-5472.CAN-07-6509. [DOI] [PubMed] [Google Scholar]
- 21.Whitman SP, Hackanson B, Liyanarachchi S, et al. DNA hypermethylation and epigenetic silencing of the tumor suppressor gene, SLC5A8, in acute myeloid leukemia with the MLL partial tandem duplication. Blood. 2008;112:2013–2016. doi: 10.1182/blood-2008-01-128595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park JY, Helm JF, Zheng W, et al. Silencing of the candidate tumor suppressor gene solute carrier family 5 member 8 (SLC5A8) in human pancreatic cancer. Pancreas. 2008;36:e32–e39. doi: 10.1097/MPA.0b013e3181630ffe. [DOI] [PubMed] [Google Scholar]
- 23.Helm JF, Centeno BA, Coppola D, et al. Outcomes following resection of pancreatic adenocarcinoma: 20-year experience at a single institution. Cancer Control. 2008;15:288–294. doi: 10.1177/107327480801500403. [DOI] [PubMed] [Google Scholar]
- 24.Helm J, Centeno BA, Coppola D, et al. Histologic characteristics enhance predictive value of American Joint Committee on Cancer staging in resectable pancreas cancer. Cancer. 2009;115:4080–4089. doi: 10.1002/cncr.24503. [DOI] [PubMed] [Google Scholar]
- 25.Wong SC, Lo ES, Lee KC, et al. Prognostic and diagnostic significance of beta-catenin nuclear immunostaining in colorectal cancer. Clin Cancer Res. 2004;10:1401–1408. doi: 10.1158/1078-0432.ccr-0157-03. [DOI] [PubMed] [Google Scholar]
- 26.Li XH, Kikuchi K, Zheng Y, et al. Downregulation and translocation of nuclear ING4 is correlated with tumorigenesis and progression of head and neck squamous cell carcinoma. Oral Oncol. 2011;47:217–223. doi: 10.1016/j.oraloncology.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Aytekin T, Ozaslan M, Cengiz B. Deletion mapping of chromosome region 12q13-24 in colorectal cancer. Cancer Genet Cytogenet. 2010;201:32–38. doi: 10.1016/j.cancergencyto.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 28.Hahn SA, Schutte M, Hoque AT, et al. DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science. 1996;271:350–353. doi: 10.1126/science.271.5247.350. [DOI] [PubMed] [Google Scholar]
- 29.Kloth JN, Kenter GG, Spijker HS, et al. Expression of Smad2 and Smad4 in cervical cancer: absent nuclear Smad4 expression correlates with poor survival. Mod Pathol. 2008;21:866–875. doi: 10.1038/modpathol.2008.62. [DOI] [PubMed] [Google Scholar]
- 30.Maass N, Hojo T, Ueding M, et al. Expression of the tumor suppressor gene Maspin in human pancreatic cancers. Clin Cancer Res. 2001;7:812–817. [PubMed] [Google Scholar]
- 31.Oh YL, Song SY, Ahn G. Expression of maspin in pancreatic neoplasms: application of maspin immunohistochemistry to the differential diagnosis. Appl Immunohistochem Mol Morphol. 2002;10:62–66. doi: 10.1097/00129039-200203000-00011. [DOI] [PubMed] [Google Scholar]
- 32.Cao D, Zhang Q, Wu LS, et al. Prognostic significance of maspin in pancreatic ductal adenocarcinoma: tissue microarray analysis of 223 surgically resected cases. Mod Pathol. 2007;20:570–578. doi: 10.1038/modpathol.3800772. [DOI] [PubMed] [Google Scholar]
- 33.Ganapathy V, Gopal E, Miyauchi S, et al. Biological functions of SLC5A8, a candidate tumour suppressor. Biochem Soc Trans. 2005;33:237–240. doi: 10.1042/BST0330237. [DOI] [PubMed] [Google Scholar]
- 34.Thangaraju M, Karunakaran SK, Itagaki S, et al. Transport by SLC5A8 with subsequent inhibition of histone deacetylase 1 (HDAC1) and HDAC3 underlies the antitumor activity of 3-bromopyruvate. Cancer. 2009;115:4655–4666. doi: 10.1002/cncr.24532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y, Bao YL, Wu Y, et al. Identification and characterization of the human SLC5A8 gene promoter. Cancer Genet Cytogenet. 2010;196:124–132. doi: 10.1016/j.cancergencyto.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Bao YL, Yang MT, et al. Activin A induces SLC5A8 expression through the Smad3 signaling pathway in human colon cancer RKO cells. Int J Biochem Cell Biol. 2010;42:1964–1972. doi: 10.1016/j.biocel.2010.08.007. [DOI] [PubMed] [Google Scholar]