Abstract
Multi-organ dose evaluations and the effects of heterogeneous tissue dose calculations have been retrospectively evaluated following irradiation to the whole thorax and lung in non-human primates. A clinical-based approach was established to evaluate actual doses received in the heart and lungs during whole thorax lung irradiation. Anatomical structure and organ densities have been introduced in the calculations to show the effects of dose distribution through heterogeneous tissue. Mean organ doses received by non-human primates undergoing whole thorax lung irradiations were calculated using a treatment planning system that is routinely used in clinical radiation oncology. The doses received by non-human primates irradiated following conventional dose calculations have been retrospectively reconstructed using computerized tomography based, heterogeneity-corrected, dose calculations. The use of dose volume descriptors for irradiation to organs at risk and tissue exposed to radiation is introduced. Mean and partial-volume doses to lung and heart are presented and contrasted. The importance of exact dose definitions is highlighted, and the relevance of precise dosimetry to establish organ-specific dose response relationships in NHP models of acute and delayed effects of acute radiation exposure is emphasized.
Keywords: Mean organ dose, dose-volume effects, heterogeneity corrections, DRR – Dose response relationship, WTLI- whole thorax lung irradiation
INTRODUCTION
Results of radiobiological studies are often described using some form of a quantitative dose response relationship (DRR). The DRR portrays radiobiological effect or endpoint that commonly varies quite sensitively as a function of radiation dose. This dose-response sensitivity calls for radiation dosimetry that is both precise and accurately defined (Kazi et al. 2014, DesRosiers et. al. 2013). A whole thorax lung irradiation (WTLI) model in the non-human primate (NHP) with medical management has been developed to study radiation induced lung injury and its treatment. The stated “dose” of radiation to the thoracic cavity is normally defined at a point at mid-depth in the thorax, often at the level of the xiphoid process (Garofalo et al. 2014a). Dose to a point in tissue has been calculated traditionally assuming certain conditions, e.g. homogeneous media (water or tissue), normal incidence on a flat surface, and uniform intensity across the beam. When clinical treatment-planning systems are used for computerized tomography (CT)-based dose calculations, the CT density data is generally overridden and replaced with water density in order to retain dose-calculation consistency with prior studies of lung injury.
Previously reported lung injury has been equated to dose prescribed and defined at a single point, which may or may not be reflective of the actual dose received throughout the whole lung. To determine the true DRR for lung injury it is necessary to more accurately determine the dose delivered to the whole lung volume, using the densities of lung, and densities of other irradiated tissues in the calculations. In this study three dimensional (3D) dose calculations have been performed using tissue heterogeneity corrections to estimate lung and heart doses corresponding to a prescribed mid-thorax dose. All calculations were completed using a commercial clinical treatment planning system (Eclipse version 11.0, Varian Medical Systems, Palo Alto, California) which utilizes a (AAA-version 10.0.28) dose-computation anisotropic analytical algorithm validated for use in heterogeneous media (Fogliata et al. 2007). This approach permits precise measurement of specific volume of organ tissue and the dose received within that volume. CT defined anatomical structure dose information and the comparison of resultant pathology allows for the establishment of organ specific DRRs.
MATERIALS AND METHODS
Animals, model and dose
A WTLI model was developed to evaluate the efficacy of medical countermeasures (MCM) in mitigating radiation-induced lung injury. The model was also used to assess combined organ injury to the heart since it is included in the exposure area. The WTLI model is an organ-targeted “laboratory model” designed to avoid acute hematopoietic and gastrointestinal sub-syndromes while allowing for evaluation and characterization of potentially lethal radiation-induced lung damage. A previous WTLI study established the DRR for the pulmonary injury characteristic of the delayed effects of acute radiation exposure (DEARE) (ref Garofalo 2014a). The DRR was established by delivering the dose to a point mid-depth in the thorax using a homogeneous (water) calculation technique. It was determined that the LD 70/180 (lethal dose to 70% of the population in 180 days) is 1074 cGy [1040, 1132] – with the 95% confidence intervals shown in brackets (Garofalo et al. 2014a).
Male Chinese Rhesus macaques (n=80) were used, with ages ranging from approximately 2.5 to 6 years, and body weights (BW) from approximately 5.0 kg to 10.0 kg at the time of irradiation. NHP housing and care was performed in accordance with the Animal Welfare Act at the University of Maryland's Association for Assessment and Accreditation of Laboratory Animal Care-accredited animal facility. All NHP were administered medical management according to an Institutional Animal Care and Use Committee-approved protocol. Prior to CT or irradiation, the NHP were acclimated to a “supine restraint device”. Animals were sedated and restrained during the irradiation procedure to assure that they remained still within the irradiation field. A Lucite neck plate prevented excessive movement of the head, and soft Velcro straps secured the limbs and body to prevent motion. Sedation agents (ketamine [Ketaset; Fort Dodge, IA] and xylazine [Anased, Fort Dodge]) were administered intramuscularly (IM) prior to the CT or irradiation procedure to minimize stress and anxiety in the animals. Each animal had an identification tattoo which was confirmed prior to all procedures. (MacVittie et. al 2012, Garofalo et al. 2014a)
Initial Dose Calculation
The NHP received baseline CT scans in the exposure position (supine in the restraint device) using a General Electric Lightspeed Helical CT scanner (GE Healthcare Waukesha, WI 53188). Sedated NHP were placed in a supine position in the restraint device so that the spine and body alignment was as straight as possible. A small metal fiducial marker (BB) was placed on the skin surface of the sternum at the level of the xiphoid process. Ink marks were drawn on the skin to show the placement of the BB for the radiation delivery set up. In the CT scanner, AP and lateral scout images were taken, to define the area for CT data capture. The area scanned extended superiorly from the thoracic inlet to mid-abdomen inferiorly and laterally to include the restraint device. This volume allows for full coverage of both lungs with a 2-cm margin. Inclusion of tissue in the CT data beyond the 2-cm margin allows for internal scattered radiation to be accounted for in the calculation. The slice thickness of the CT images is 1.25 mm. The CT gantry was positioned with no tilt at 0 degrees, and a technique of 120 kV, 300 mA, and helical rotation with a 0.5 pitch was used to capture the CT data. The CT images were reconstructed and exported from the scanner to the treatment planning system. The planning system contains well characterized CT and x-ray beam data which allowed for creation of beam arrangements, organ delineation and dose evaluation
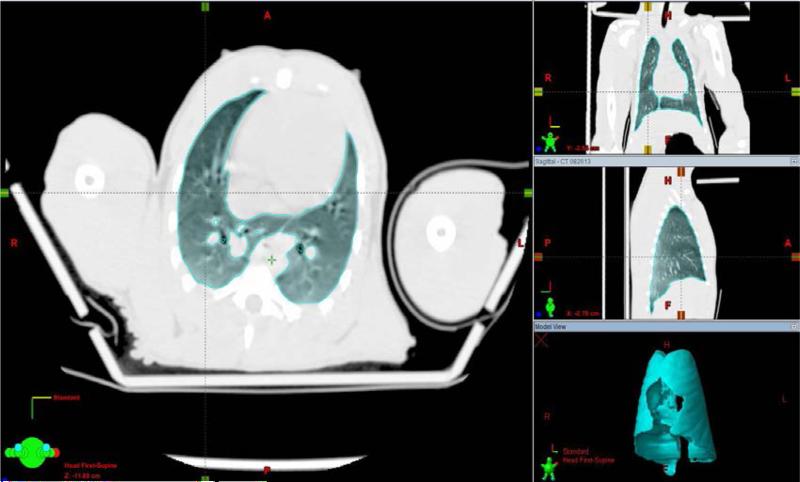
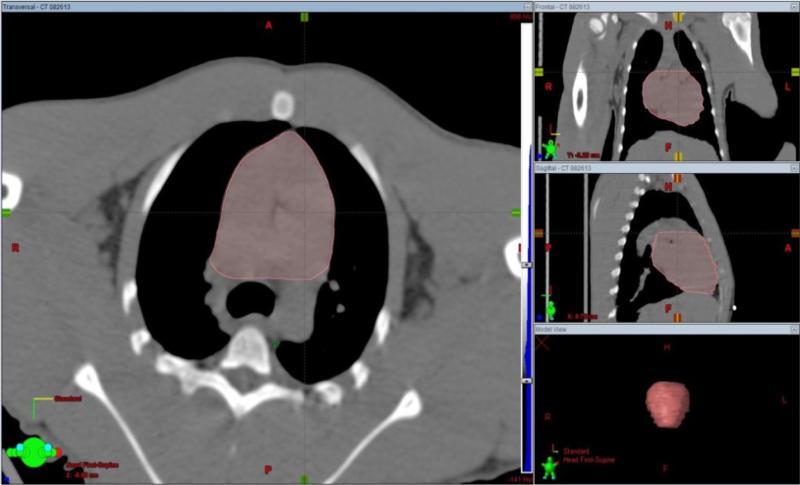
To determine the location of the center of the radiation beam, the planner outlined both lungs on each slice of the CT images. Auto-contouring methods using the Hounsfield values and manual edits of the volumes were used as needed (Fig 1). A 2-cm expansion contour was created around the lung volume to be used as the Planning Target Volume (PTV) (Fig 2). The PTV is designed to include the lung volume and the areas within the irradiated volume into which they might move during the time that it takes to deliver the radiation dose. The use of a PTV mitigates the effects of motion during breathing or possible motion of the NHP. It also allows for minor differences in reproducing the original set-up position of the NHP in the restraining device. The borders of the radiation-field openings are collimated such that the edges of the irradiation area encompass the widest portions of the PTV volume and define the outline of the anterior field. The central axis of the radiation beam is positioned centrally (superior/Inferior and right/left) in this volume. Along the central axis, the beam's isocenter is positioned at mid-depth in the body. (Fig 3).
Fig. 1.
Lungs are “auto contoured” (using CT numbers for lung) and then manually edited to create the lung volumes. The resulting total volume is shown in the bottom-right portion of the figure.
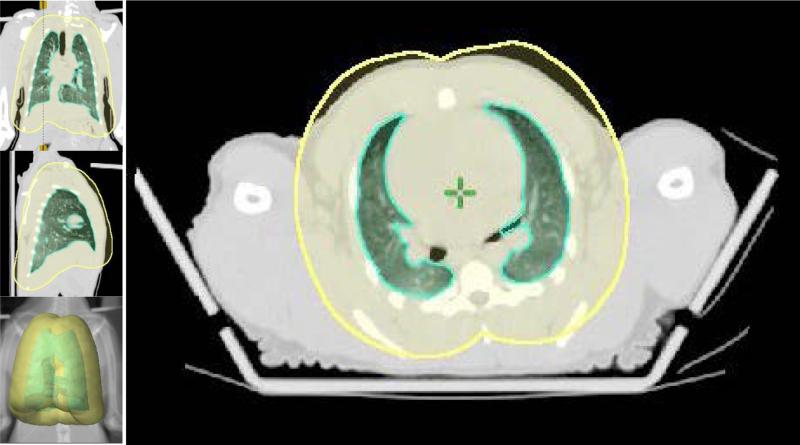
Fig. 2.
The PTV, a 2 centimeter, three dimensional, expanded volume is created around the lung to define the thorax area which is to be irradiated. The lung volume and the PTV are shown in the bottom right portion of the figure.
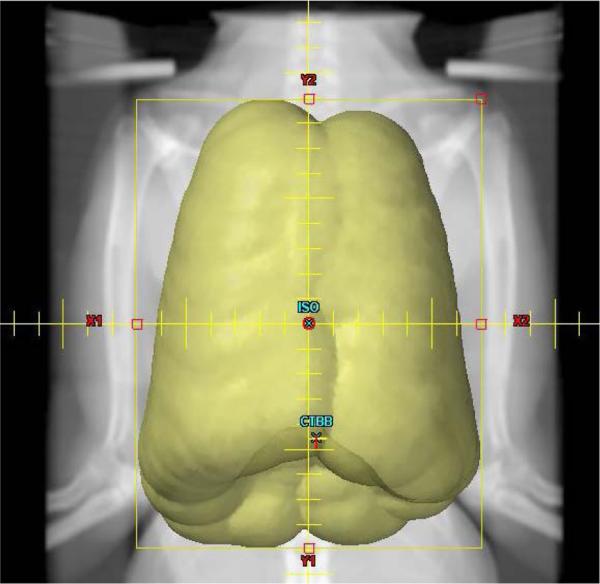
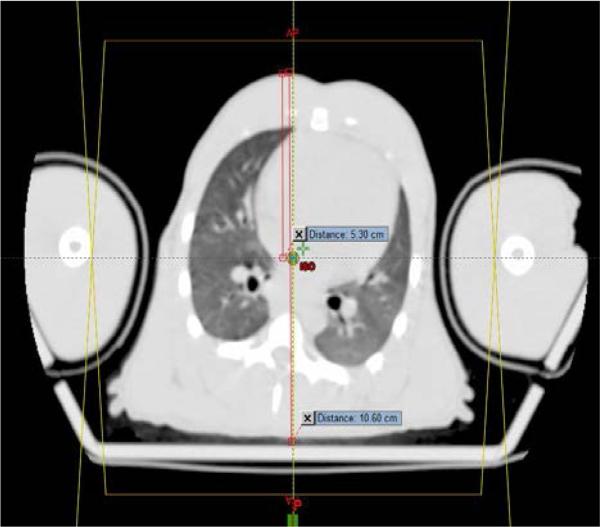
Fig. 3a 3b.
The radiation fields are created and centered to encompass the PTV (Fig 3a). The isocenter of each beam is positioned at mid depth in the NHP along the central axis of the radiation beam (Fig 3b). The prescribed 1074 cGy dose is delivered to this point.
Once the radiation-field parameters and the mid-depth point in the body was established, the prescribed dose of 1074 cGy to the midline isocenter point using 6 MV x rays was entered into the planning system. The prescribed dose was divided in two sessions, with one half of the dose being planned for delivery from the anterior field and the second half to be delivered using an opposing posterior field. Using two opposing beams distributes the dose more evenly throughout the thorax. The linear-accelerator (Varian True Beam, Varian Medical Systems, Palo Alto, California) monitor unit (MU) settings that are needed to deliver the prescribed dose were then calculated. The prescription point for the calculation was in the center of the fields at mid-depth in the thorax, assuming a uniform water-equivalent density. Prior to delivery of the radiation, the planned field arrangement and NHP position was reproduced on the linear accelerator and portal x-ray images were taken to ensure that field placement was consistent with the Eclipse plan (Fig 4). Radiation was delivered utilizing 6-MV x-rays of a medical linear accelerator. The dose-rate for the radiation delivery was 80cGy/minute at the prescription point.
Fig. 4.
A portal x-ray image of the area to be irradiated is taken prior to the exposure. The digital image is taken on the linear accelerator in order to confirm the NHP and beam positioning is correct and to assure inclusion of the whole lung and PTV in the radiation field.
Retrospective evaluation of doses to lungs and heart
In order to more accurately and precisely evaluate the radiation-dose distributions in the irradiated organs and more properly relate these doses to observed biological changes, the irradiation plans were re-calculated using an anisotropic analytical algorithm (AAA - version 10.0.28), in the Eclipse planning system, version 11.0, incorporating heterogeneity corrections. The fields were weighted using the MU settings calculated initially and subsequently used for delivery of the prescribed dose.
For this retrospective study, the doses to the heart and whole lung have been evaluated. All contours were defined by the same planner in order to create consistency in anatomical definitions. The heart is contoured, with the superior border outlined up to the level at which the large vessels begin to appear on the axial images. This level is also where the heart volume begins to narrow (Fig 5). The lungs were contoured as described previously. Organ doses are reported as “mean dose” - the mathematical average of the doses received by each voxel (volume element) within the contoured organ volume.
Fig. 5.
The heart is contoured in order to evaluate the dose. The borders and anatomy, including a defined top border is consistent for all NHPs.
RESULTS
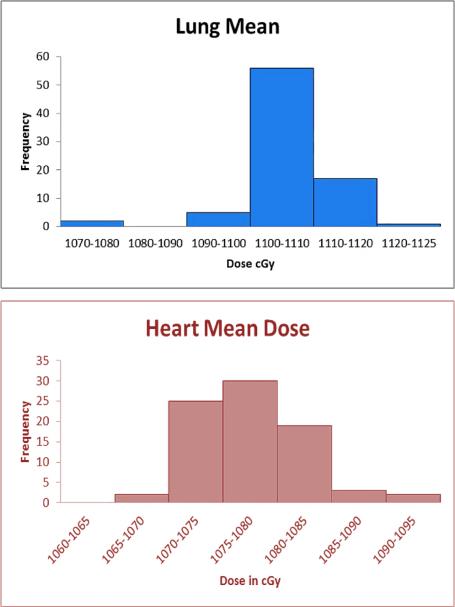
Organ dose statistics averaged over all 80 NHP in the cohort are shown in Table 1. The resulting data is fairly tightly distributed, as shown by the small standard deviation in the mean doses to heart and lung (coefficients of variation less than 1%), indicating similar results among all animals in the cohort. The frequency histograms graphically demonstrate the relatively small dispersion of the data. (Fig 6)
Table 1.
Organ Volumes and Organ Dose Statistics (calculated in heterogeneous media)
| Organ | Average Volume cm3 | Ave. Mean Dose | Standard Deviation | Average Dose to 95% of Volume | % Volume at Rx Dose | Ave. Min Dose | Ave. Max Dose |
|---|---|---|---|---|---|---|---|
| Heart | 64 cc | 1077 cGy | 4.7 cGy | 1050 cGy | 55% | 1033 cGy | 1127 cGy |
| Lung | 253 cc | 1105 cGy | 6.5 cGy | 1065 cGy | 91% | 1003 cGy | 1167 cGy |
Fig. 6.
Frequency distribution of mean lung and heart doses across n=80 animals in the cohort.
The mean lung dose averaged across all animals (1105 cGy) is approximately 3% greater than the ‘prescribed’ dose of 1074 cGy. This was expected since the retrospective dose calculations shown here incorporate the reduced density of the lung, while the prescribed (and originally calculated) dose was delivered using dose calculations that assumed uniform unit (water) density. The minimum dose received by 95% of the lung volume was 1065 cGy, within 1% of prescribed, and the prescribed dose of 1074 cGy was delivered, on average, to 90% of the lung volume. The difference between the average minimum and maximum doses to the lung was on the order of 15%.
The mean heart dose averaged over the cohort (1077 cGy) is essentially equal to the prescribed dose. This too was expected since heterogeneity corrections in the region of the mediastinum are minimal or close to water equivalent. The dose spread in the heart was roughly 9%. The lower percentage of volume at prescription is most probably due to the smaller volume of heart and to the dose gradients that are typically observed in the area of the lung-mediastinum interface (Fig 7).
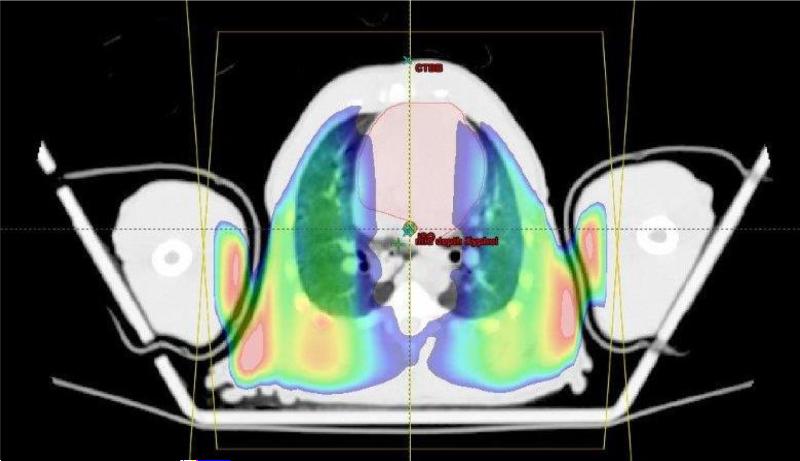
Fig. 7.
Dose gradient within the heart and lungs at the level of the sternum shown in an axial view for one NHP. Dose levels are displayed in a color-wash. Doses demonstrated range from 1070 cGy (blue) to 1170 cGy (orange). Note the dose deficit in the heart caused by the increased attenuation by the bones of the sternum and spine, and the increased dose to tissues underlying the less dense lung.
DISCUSSION
Dose-Volume Concepts
It has been shown that fairly comprehensive and precise calculations of dose delivered to the organs of irradiated primates are feasible. Treatment planning systems that are routinely used in human patient radiotherapy can be easily employed for dose calculations in irradiated NHPs. Doses to organ volumes can be computed, beyond simply estimating doses to discrete points. Heterogeneity corrections can be incorporated into calculations and dose-volume statistics can be extracted and reported as deemed appropriate.
The clinical effects of radiation therapy are commonly quantified using a volume versus dose frequency distribution called the Dose Volume Histogram (DVH). The DVH, as a quantitative descriptor of radiation dose delivered to an irradiated volume, has been associated with tumor control and normal-tissue complication probabilities since the early 1980s (Lyman 1985). The DVH, in its most common form, is a graphical representation of the dose received by a specified volume of an identified organ. It is a plot of organ volume (y-axis) as a function of the minimum dose (x-axis) received by that volume. The DVH allow for evaluation of dose, and subsequently dose-effect, in organs when they are not uniformly irradiated.
The dose distributions showed (Fig. 7) that dose gradients exist within the organs of the irradiated NHPs.
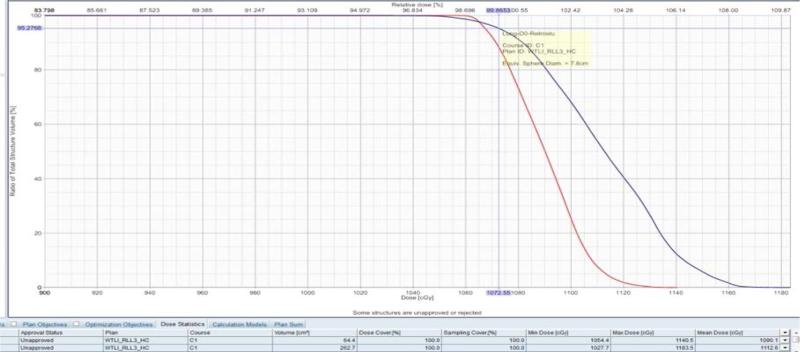
Although graphical dose-distribution representations help to describe dose deposition anatomically, it fails to provide a quantitative description for dose and associated anatomy volume. A quantitative descriptor for the lung and heart DVHs of a single NHP is shown in (Fig.8). Note that the doses to these organs can vary quite significantly. The dose to the lung in this example varies from a minimum of 1050 cGy to a maximum of roughly 1165 cGy. Any dose within this continuum could be selected as a descriptor of lung damage. Notice, however, that the lung volume receiving the prescribed dose of 1074 cGy in this particular NHP is approximately 95% of its total lung volume. This is a finding of some significance, as will be discussed in the next section. Mean lung dose – the mathematical average dose received by all volume elements in the lung – has also been associated with biological effects, e.g. radiation pneumonitis in lung cancer patients (Marks et al. 2009, Rancati et al 2003).
Fig. 8.
A representative Dose-Volume Histogram (DVH) of the total lung (blue - rightmost) and heart (red - leftmost) volumes of one of the NHP of the cohort. Plotted on the y-axis is volume in percent of total, and plotted on the x-axis is minimum dose (cGy) received. A point along the graph of the histogram represents the minimum dose received by that volume. Indicated on the lung (blue-rightmost) histogram is the dose (approximately 1074 Rx cGy) received by (approximately) 95% of the lung volume.
Dose-Volume Prescriptions
An important step, when moving from point-dose to dose-volume dosimetry in radiobiology, is quantifying the relationship between prior and newer dose prescriptions. A similar situation existed in clinical radiotherapy for lung cancer when heterogeneity corrections were being introduced for patients on clinical trials. In a clinical dosimetry study conducted at MD Anderson Cancer Center in Houston, radiation-oncology investigators determined that similar radiation doses are delivered when comparing traditional point-dose prescriptions in homogenous media to dose-volume prescriptions in heterogeneous media (Frank et al. 2003). According to their report, equivalence was achieved by prescribing to a 95% volume, the so called D95, and this prescription practice is now a common procedure in clinical treatment of lung cancer. The data presented here also supports this finding, suggesting that the D95 parameter should be tracked in studies of radiobiological animal models of the lungs or other organ systems, thus preserving previously reported radiobiological results while incorporating newer dosimetry methods.
Importance of Specificity of Dose Prescription
The amount of radiation delivered to NHP exposed to WTLI is acutely dependent upon the specificity of the dose prescription and calculation point. In this and prior WTLI studies (Garofalo et al 2014b), dose was prescribed to midline thorax. The point to which the prescribed dose was calculated and delivered in this study was a point at mid-depth along the central axes of the lung radiation fields. This point may not necessarily lie within the axial plane of the xiphoid process, the plane on which mid-thorax dose can be commonly prescribed. In this study, the doses at points which are mid-depth in both the axial plane at the level of the xiphoid process and the axial plane of the radiation-fields central axes were compared. Assuming homogenous density, the difference between the doses at these two points was on average 1.3%. Under heterogeneous conditions, the difference was 2.3%. Although these differences could be considered small, they are definitely not negligible, particularly in light of the fact that they can be minimized through more precise definitions of dose prescription and more detailed descriptions of calculation assumptions. Previous studies (MacVittie et al. 2012, Farese et al. 2013, Plett et al. 2012, and Jackson et.al 2014) have shown a rapid increase or decline in mortality with only a few percent difference in dose when plotted as a DRR. Given this very sensitive dependence of the biological response on dose, it is imperative that dose prescription and calculation be more specifically defined to minimize dose spread, taking into account homogeneous versus heterogeneous dose calculations and dose at mid-depth at the level of the xiphoid versus dose at mid-depth at the level of the center of the lungs. It is also important for the intensities of the radiation beams to be properly characterized, and for these characteristics to be explicitly accounted for in dose calculations. The differences in dose reported here apply specifically to the 6 MV beams used. The use of other beams could lead to further differences.
Lastly, the importance of an effective collaboration between physics and radiobiology investigators is imperative for adequate design and conduct of protocols dependent on accurate and consistent delivery of the prescribed dose. The NHP in this study were subjected to treatment planning and irradiation under dedicated physics guidance. The therapy unit used for irradiation was thoroughly characterized and rigorously tested. Radiation beam properties were well suited for NHP irradiation with good beam uniformity and favorable penetrability. The unit's beam data has been properly parameterized in dose calculation systems and was thoroughly verified. Strict quality-assurance measures were applied to the unit's performance and its use, and to dose calculation methods. Procedural differences were kept to a minimum. Care was taken in the immobilization and positioning of subjects. Imaging was used to verify field placement. Dose prescriptions and calculations were established and evaluated by the physics staff in close coordination with radiobiology investigators, with continuation of prior dosimetry baselines always taken into consideration. Only under these conditions can uncertainties in dose and dose effects be minimized.
CONCLUSIONS
Highly accurate and precise radiation dosimetry is feasible in NHP radiobiological studies. Given the acute dependence of radiobiological effect on dose, it is important to acknowledge and use the advanced means of radiation-dose measurement, calculation, and delivery. Radiobiological effects due to radiation doses to specific organs can be and should be more precisely defined. As an extension to this retrospective dose-reconstruction study, a second study to determine the dose to other organs, such as kidney and bone marrow, in total-body and partial-body (as opposed to WTLI) irradiation is in progress. The conduct of similar studies have the potential to further refine the organ-specific dose response relationships that form the basis of the link between acute radiation effects and the multiple organ injury characteristic of delayed effects of acute radiation exposure.
Acknowledgments
This work has been funded in whole or in part with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, and MCART Radiation/Nuclear Medical countermeasure Product development support services, under Contract No. HHSN272201000046C and Aeolus/BARDA, Advanced development of AEOL 10150 as a medical countermeasure for pulmonary injury associated with ARS and DEARE, Contract NO. HHSO0100201100007C. We acknowledge the continuous discussion, insight and constructive critique of colleagues at Aeolus Inc., BARDA and NIAID. These include John McManus, Brian May, David Cassatt, and Bert Maidment as well as the insight and ongoing support of the Preclinical Radiobiology Laboratory (PRL), the PRL imaging core and of the Division of Medical Physics, Department of Radiation Oncology, in the University of Maryland School of Medicine.
Footnotes
Conflicts of Interest and Source of Funding:
Authors declare there are no conflicts of interest:
References
- DesRosiers M, DeWerd L, Deye J, Lindsay P, Murphy M, Mitch M, Macchiarini F, Stodajinovic S, Stone H. The importance of dosimetry standardization in radiobiology. NIST Jour. Res. 2013;118:403–418. doi: 10.6028/jres.118.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farese A, Cohen M, Katz B, Smith C, Gibbs A, Cohen D, MacVittie T. Filgrastim Improves survival Lethally Irradiated Nonhuman Primates. Radiation Research Journal. 2013;179:89–100. doi: 10.1667/RR3049.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogliata A, Vanetti E, Albers D, Brink C, Clivio A, Knoos T, Nicolini G, Cozzi L. On the dosimetric behavior of photon dose calculation algorithms in the presence of simple heterogeneities: comparison with Monte Carlo calculations. Phys. Med. Biol. 2007;52:1363–1385. doi: 10.1088/0031-9155/52/5/011. [DOI] [PubMed] [Google Scholar]
- Frank S, Forster K, Stevens C, Cox J, Komaki R, Liao Z, Tucker S, Wang X, Steadham R, Brooks C, Starkschall G. Treatment planning for lung cancer: traditional homogeneous point-dose prescription compared with heterogeneity-corrected dose-volume prescription. Int. J. Rad. Onc. Biol. Phys. 2003;56(5):1308–1318. doi: 10.1016/s0360-3016(03)00337-7. [DOI] [PubMed] [Google Scholar]
- Garofalo M, Bennett A, Farese A, Ward A, Taylor C, Cui W, Gibbs A, Lasio G, Jackson W, MacVittie T. The delayed pulmonary syndrome following acute high-dose irradiation: a rhesus macaque model. Health Phys. 2014a;106(1):56–72. doi: 10.1097/HP.0b013e3182a32b3f. [DOI] [PubMed] [Google Scholar]
- Garofalo M, Ward A, Farese A, Bennett A, Taylor C, Cui W, Gibbs A, Prado K, MacVittie T. A pilot study in rhesus macaques to assess the treatment efficacy of a small molecular weight catalytic metalloporphyrin antioxidant (AEOL 10150) in mitigating radiation-induced lung damage. Health Phys. 2014b;106(1):73–83. doi: 10.1097/HP.0b013e3182a4d967. [DOI] [PubMed] [Google Scholar]
- Jackson I, Xu P, Nguyen G, Down J, Johnson C, Katz B, Hadley C, Vujaskovic Z. Characterization of the Dose Response Relationship for Lung Injury following Acute Radiation Exposure in Three Well-established Murine Strains: Developing an Interspecies Bridge to Link Animal Models with Human Lung Health Phys. 2014;106(1):48–55. doi: 10.1097/HP.0b013e3182a32ccf. [DOI] [PubMed] [Google Scholar]
- Kazi A, MacVittie T, Lasio G, Lu W, Prado K. The MCART radiation physics core: the quest for radiation dosimetry standardization. Health Phys. 2014;106(1):97–105. doi: 10.1097/HP.0b013e3182a2a987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyman J. Complication probability as assessed from dose-volume histograms. Rad. Res. 1985;104(2s):S13–S19. [PubMed] [Google Scholar]
- MacVittie T, Farese A, Bennett A, Gelfond D, Shea T, Tudor G, Booth C, McFarland E, Jackson W. The acute gastrointestinal subsyndrome of the acute radiation syndrome: a rhesus macaque model. Health Phys. 2012;103:411–426. doi: 10.1097/HP.0b013e31826525f0. [DOI] [PubMed] [Google Scholar]
- Marks L, Bentzen S, DEasy J, Kong F, Bradley J, Vogelius I, El Naqa I, Hubbs J, Lebesque J, Timmerman R, Martel M, Jackson A. Radiation dose volume effects in the lung. Int. J. Rad. Onc. Biol. Phys. 2010;76(S3):70–76. doi: 10.1016/j.ijrobp.2009.06.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plett P, Sampson C, Chua H, Joshi M, Booth C, Gough A, Johnson C, Katz B, Farese A, Parker J, MacVittie T, Orschell C. Establishing a murine model of the hematopoietic syndrome of the acute radiation syndrome. Health Phys. 2012;103:343–355. doi: 10.1097/HP.0b013e3182667309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rancati T, Ceresoli G, Gagliardi G, Schipani S, Cattaneo G. Factors predicting radiation pneumonitis in lung cancer patients: a retrospective study. Radioth. Oncol. 2003;67(3):275–283. doi: 10.1016/s0167-8140(03)00119-1. [DOI] [PubMed] [Google Scholar]