SUMMARY
Of the three DNA ligases present in all vertebrates, DNA ligase I (Lig1) has been considered essential for ligating Okazaki fragments during DNA replication and thereby essential for cell viability. Here, we report the striking finding that a Lig1-null murine B cell line is viable. Surprisingly, the Lig1-null cells exhibit normal proliferation and normal immunoglobulin heavy chain class switch recombination and are not hypersensitive to a wide variety of DNA damaging agents. These findings demonstrate that Lig1 is not absolutely required for cellular DNA replication and repair and that either Lig3 or Lig4 can substitute for the role of Lig1 in joining Okazaki fragments. The establishment of a Lig1-null cell line will greatly facilitate the characterization of DNA ligase function in mammalian cells, but the finding alone profoundly reprioritizes the role of ligase I in DNA replication, repair, and recombination.
INTRODUCTION
DNA ligases are involved in many aspects of DNA metabolism: replication, repair, and recombination (Ellenberger and Tomkinson, 2008). All eukaryotes have only two or three functional DNA ligases (I, III, and IV); all are ATP dependent. DNA ligases I (Lig1) and IV (Lig4) are conserved in all eukaryotes, whereas DNA ligase III (Lig3) is found only in vertebrates. The functions of these ligases have been studied for decades. It is generally believed that the primary function of Lig1 is to join Okazaki fragments during DNA replication. Lig3 (in complex with X-ray cross-complementation protein 1, XRCC1), is thought to be the primary ligase for DNA excision repair. Lig4 (in complex with XRCC4 and XRCC4-like factor, XLF) functions exclusively in the nonhomologous end-joining (c) pathway that repairs DNA double-strand breaks (DSBs).
Deletion of any of the three ligase genes is incompatible with mouse embryonic development (Frank et al., 2000; Petrini et al., 1995; Puebla-Osorio et al., 2006). In contrast, human or mouse cell lines lacking Lig4 are viable (Grawunder et al., 1998; Han and Yu, 2008). Recently, deletion of Lig3 in mouse embryonic stem cells (ESCs) was achieved by introducing a transgene encoding a mitochondria-targeted ligase, providing convincing evidence that the mitochondrial function of Lig3 is essential for cell viability, but its nuclear function is not (Simsek et al., 2011). Interestingly, Lig3-deficient cells are not hypersensitive to various DNA damaging agents. This unexpected finding calls into question the role of Lig3 in nuclear DNA repair, which was largely inferred from its interaction with XRCC1. It is possible that either Lig3 is not the primary ligase that functions in DNA excision repair, or that its function in DNA repair can be efficiently substituted by another ligase (e.g., Lig1). Thus, establishing a mammalian cell line that lacks Lig1 would be essential for clarifying the relative priority of the three ligases in various DNA transactions. Here, we report a Lig1-null mammalian cell line, which itself permits us to gain insight into its role in DNA repair, replication, and recombination.
RESULTS AND DISCUSSION
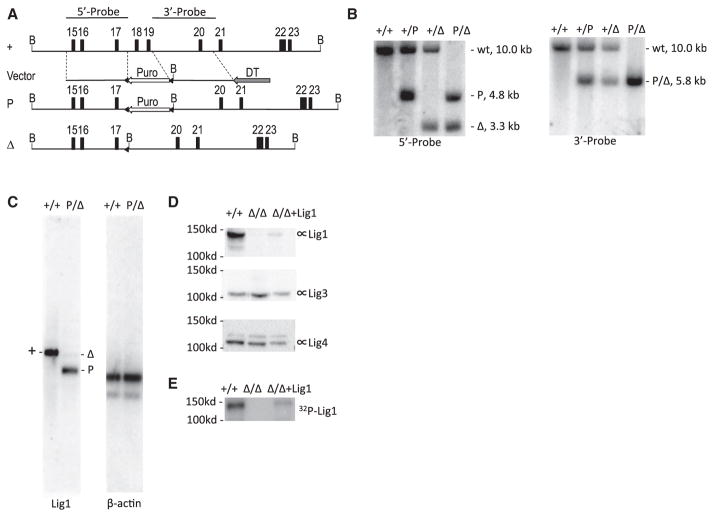
Lig1 was previously reported to be essential for the viability of mouse ESCs (Petrini et al., 1995). In light of a recent report that Lig1-deficient chicken DT40 cells are viable (Arakawa et al., 2012), we set forth to test the hypothesis that the cellular lethality caused by Lig1 deficiency is cell type specific. Gene targeting of Lig1 was performed in a mouse B cell line (CH12F3) that is capable of cytokine-induced class switch recombination (CSR) in vitro. The targeted deletion removes exons 18–19 of the Lig1 gene and causes a frameshift of all downstream exons (Figure 1A). The resulting mutant allele is very similar to that described in the previous study that demonstrated an essential role for Lig1 in mouse ESCs (Petrini et al., 1995). Successful gene targeting was confirmed by Southern blot analysis (Figure 1B). CH12F3 cell line has two alleles for Lig1, so two rounds of gene targeting were performed. Northern blot analysis of polyadenylated RNA isolated from Lig1P/Δ cells showed two mRNA species derived from the two targeted alleles, respectively (Figure 1C). The transcript derived from the P allele is relatively abundant, and its size indicates polyadenylation at the SV40 polyadenylation signal from the integrated puro cassette (Figure 1A). The transcript derived from the Δ allele is of very low abundance (Figure 1C), and its size is consistent with a transcript lacking exon 18–19 (Figure 1A). The low abundance of this transcript may be due to nonsense-mediated mRNA decay.
Figure 1. Gene Targeting of Lig1 in CH12F3 Cell Line.
(A) Genomic organization of the wild-type and targeted Lig1 allele. The map is drawn to scale. Exons are indicated by numbered boxes. Arrows indicate transcription orientations of expression cassettes of the puromycin-resistant gene (Puro) and diphtheria toxin A chain (DTA), respectively. B, BamHI restriction sites. Probes used in Southern blot analysis are depicted at the top. A “+” sign indicates the wild-type allele. “P” and “Δ” indicate targeted alleles with or without the Puro cassette, respectively. Triangles indicate loxP sites.
(B) Southern blot analysis of BamHI-digested genomic DNA from wild-type (+/+) and targeted cells (+/P, +/Δ, P/Δ). Genotypes, sizes of bands, and probes are indicated.
(C) Northern blot analysis of polyadenylated RNA. The entire Lig1 coding region sequence was used as a probe. The blot was stripped and stored for a month to allow radioisotype decay before reprobed with β-actin.
(D) Western blot analysis of the Lig1 protein level in wild-type (+/+), Lig1Δ/Δ and mouse Lig1 cDNA complemented Lig1Δ/Δ cells (Δ/Δ+Lig1) with a rabbit polyclonal antibody raised against GST-Lig1 protein (Proteintech 18051-1-AP). The blot was stripped and reprobed with a Lig3 antibody (BD Biosciences, 611876) and Lig4 antibody (Han et al., 2012), respectively, for loading control and protein size distinction.
(E) Adenylation of Lig1 in the cell extract with α-32P-ATP.
When mRNA from Lig1Δ/Δ cells (Figure S1A) were analyzed, only the low-abundance mRNA lacking exons 18–19 were detected (Figure S1B). RT-PCR using primers at the beginning and end of the Lig1 coding region sequences yields a barely detectable smaller-sized band from the Lig1Δ/Δ cells (Figure S1C), but not from the Lig1P/Δ cells, suggesting that the P-allele-derived transcript interferes with RT-PCR. Sanger sequencing of the RT-PCR product confirmed the absence of exons 18–19 in the residual mRNA derived from the Δ allele (Figure S1D). Neither of the transcripts support Lig1 protein synthesis as was shown by immunoblotting using two different antibodies that recognize different regions of Lig1 (Figures 1D and S1E). Finally, adenylation of Lig1 with α-32P-ATP in cell extract yields a radioactive band (~130 kDa, Figure 1E) matching the size of Lig1 (Figure 1D), whereas Lig3 and Lig4 migrates at ~100 kDa (Figure 1D). The radioactive band is absent in the extract prepared from Lig1Δ/Δ cells but reappears when Lig1Δ/Δ cells were transiently transfected with a expression vector carrying mouse Lig1 cDNA (Figure 1E). Adenylation of Lig3 or Lig4 was not detected under this assay condition. Based on these data, we conclude that the gene targeting generated null alleles on both chromosomes in Lig1P/Δ and Lig1Δ/Δ cells.
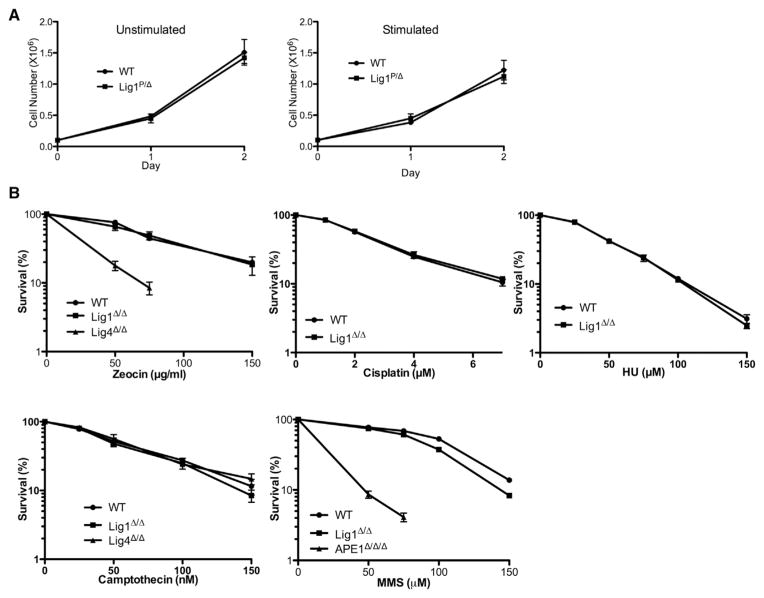
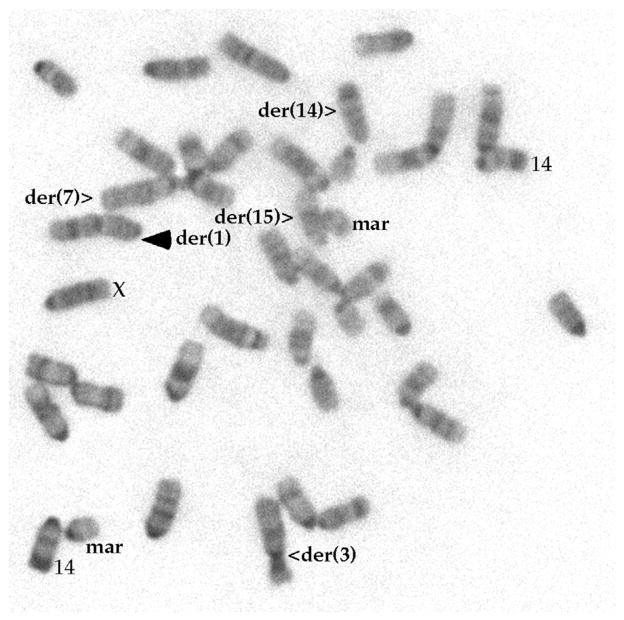
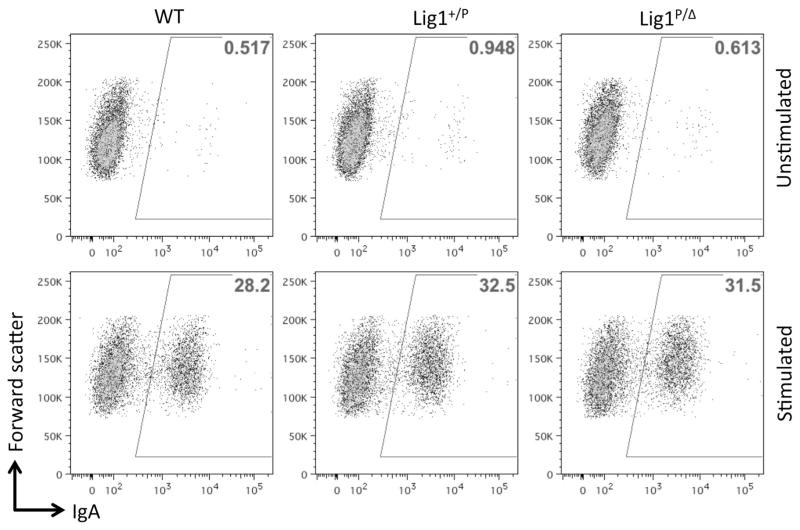
Lig1-null CH12F3 cells display a normal proliferative capacity, with or without cytokine stimulation (Figure 2A). Lig1-null cells also do not show increased sensitivity to a variety of DNA damaging agents, including zeocin, cisplatin, hydroxyurea, camptothecin, but are slightly more sensitive to methyl methane-sulfonate (Figure 2B), perhaps suggesting a redundant role for Lig1 in base excision repair. Cytogenetic analysis of Lig1-null cells reveals a stable near-diploid karyotype similar to that of the parental line (Figure 3). For the particular clone used in this study, a chromosomal rearrangement involving chromosome 1 was identified (Figure 3), but this rearrangement is stably transmitted to progeny cells; no obvious genomic instability has been observed in Lig1-null CH12F3 cells. Because Lig1 and Lig3 have been implicated in joining CSR-associated DSB in NHEJ-deficient cells, we measured CSR in Lig1-null cells and found that CSR is not reduced in the absence of Lig1 (Figure 4). No difference was observed between Lig1P/Δ and Lig1Δ/Δ cells with regard to cell proliferation, drug sensitivities, and CSR.
Figure 2. Proliferation and Drug Sensitivity of Lig1-Null CH12F3 Cells.
(A) Live cell counts of wild-type (WT) and Lig1-null (P/Δ) cells in cultures with (stimulated) or without (unstimulated) cytokines (see Experimental Procedures) for inducing CSR.
(B) Sensitivity of wild-type (WT) and Lig1-null (Δ/Δ) cells to several DNA damaging agents. Error bars represent SEM of three independent experiments.
Figure 3. A Metaphase of Lig1-Null Cell.
All the abnormal chromosomes, two copies of the normal chromosome 14, and the sex chromosome are as marked. The der(1) is the only abnormality (marked with solid arrowhead) in comparison with the parental cells, which has a chromosome count of 41.
Figure 4. CSR of Lig1-Null CH12F3 Cells.
Representative FACS analysis of CSR by surface staining of IgA after 72 hr of growth with (stimulated) or without (unstimulated) cytokines (see the Experimental Procedures). Numbers in the boxed areas indicate percentages.
Lig1 is conserved throughout evolution and generally considered indispensable for cell viability due to its well-characterized role in DNA replication. Although in one previous study, viable cells were obtained upon targeting exons 23–27 of the Lig1 gene (Bentley et al., 1996, 2002), there is some uncertainty whether the gene targeting strategy generated a hypomorphic allele because the catalytic region (e.g., active site K583 in exon 18) was not removed (Ellenberger and Tomkinson, 2008). In fact, in another study, deletion of the catalytic domain of Lig1 was found to be lethal in mouse ESCs (Petrini et al., 1995). The lack of phenotype upon disrupting the Lig1 gene in CH12F3 cell line was unexpected but is consistent with a recent report that Lig1 deletion is compatible with viability in chicken DT40 cells (Arakawa et al., 2012). Thus, although all three DNA ligases are essential for mouse embryogenesis, none of them is absolutely required for somatic cell growth.
Lymphocyte antigen receptor gene rearrangements, including V(D)J and class switch recombination, require the generation and repair of programmed DNA DSBs (Alt et al., 2013). Normally, these DSBs are repaired by the ubiquitous NHEJ pathway that is dependent on the ligase complex XRCC4/Lig4. However, CSR can occur in NHEJ-deficient cells at a reduced level via a poorly defined mechanism often termed alternative end-joining (A-EJ) (Boboila et al., 2010a, 2010b; Han and Yu, 2008; Yan et al., 2007). In Lig4-null cells, A-EJ must be mediated by Lig1 and/or Lig3. Initially, Lig3 (in complex with XRCC1) was thought be the primary ligase that functions in A-EJ. However, it was recently shown that neither a substantial depletion of Lig3 by small hairpin RNA (Boboila et al., 2012), nor ablation of XRCC1 gene in Lig4 or XRCC4 deficient cells inhibits A-EJ during CSR (Boboila et al., 2012; Han et al., 2012). These data imply that Lig1 may play a significant role in A-EJ during CSR. Because Lig1 deficiency alone does not inhibit CSR, these data suggest either (1) that Lig1 and Lig3 function interchangeably during A-EJ (Paul et al., 2013), or (2) that Lig1 or Lig3-mediated A-EJ only contributes to CSR when NHEJ is compromised. A definitive answer to that awaits targeted disruption of Lig1 or Lig3 gene in Lig4-null cells.
EXPERIMENTAL PROCEDURES
Reagents
Lig1 antibody (18051-1-AP) was purchased from Proteintech Group. Lig1 (sc-20222) and β-actin (sc-47778) antibodies were purchased from Santa Cruz Biotechnology. Primers for amplifying Lig1 coding region sequences, Lig1F 5′ ATGAGAAAAAAAGAGCAAGAGAGG 3′ and Lig1R 5′ TTAATAGTCTTCAACGTCGGAGT 3′ were purchased Sigma-Aldrich.
Cell Culture and CSR Assay
CH12F3 cells were cultured in RPMI1640 medium supplemented with 10% fetal bovine serum and 50 μM of beta-mercaptoethanol. For CSR assay, healthy CH12F3 cells were seeded at 5 × 104 cells/ml in the presence of 1 μg/ml anti-CD40 (eBioscience 16-0402-86), 5 ng/ml of IL-4 (R&D Systems 404-ML), and 0.5 ng/ml TGF-β1 (R&D Systems 240-B) and grown for 72 hr. Cells were stained with a FITC-conjugated anti-mouse IgA (BD Biosciences 559354) and analyzed on a LSR II flow cytometer (BD Biosciences). CSR efficiency is determined as the percentage of IgA-positive cells.
Lig1 Gene Targeting
Two 2 kb DNA fragments were PCR amplified from CH12F3 genomic DNA and cloned into a targeting vector as homology blocks for gene targeting (Figure 1A). Gene targeting procedures have been described previously (Han and Yu, 2008). Five targeted clones (+/P) were obtained from 192 puromycin resistant clones screened. One of them was randomly picked for Puro cassette excision and second round of gene targeting. Three Lig1-null clones were obtained in the second round of gene targeting from more than 20 targeted clones. One of them was picked for further studies.
Drug Sensitivity and MTT Assay
Cells were seeded at 1 × 105/ml per well in a 24-well plate, and various drugs were added at different concentrations. After 48 hr of growth, cell viability was determined by a colorimetric assay that measures the metabolic conversion of thiazolyl blue tetrazolium bromide (MTT) in the mitochondria of living cells as described previously (Han and Yu, 2008).
Adenylation Assay
Twenty million cells were resuspended in 500 μl of adenylation assay buffer (60 mM Tris Cl, 10 mM MgCl2, 5 nM DTT, 5 μg/ml BSA) and lysed by three cycles of freezing and thawing. The cell lysate was centrifuged at 21,000 × g for 15 min at 4°C. The supernatant (cell extract) was transferred to a different tube, and protein concentration was determined by the Bradford method (Bio-Rad). For the adenylation assay, 6 μg of cell extract was incubated with 10 μCi of α-32P-ATP (3,000 Ci/mmol) in a total volume of 10 μl in adenylation buffer for 15 min at room temperature. The reaction mixture was resolved on an 8% SDS-PAGE gel and imaged on a Typhoon 9200 phosphoimager (GE Healthcare).
Karyotype of Metaphase Chromosomes
Proliferating cells were harvested for cytogenetic analysis with G banding using the standard method (Hsieh, 1997).
Supplementary Material
Acknowledgments
This work is supported by a National Institutes of Health grant (R01 AI081817) to K.Y.
Footnotes
Supplemental Information includes one figure and can be found with this article online at http://dx.doi.org/10.1016/j.celrep.2014.03.024.
AUTHOR CONTRIBUTIONS
L.H. performed the gene targeting and Southern and northern blot analysis. S.M. performed ligase adenylation, cell proliferation, drug sensitivity, and class-switch assays. C.-l.H. performed the karyotype analysis. K.Y. designed and supervised all experiments and wrote the manuscript.
References
- Alt FW, Zhang Y, Meng FL, Guo C, Schwer B. Mechanisms of programmed DNA lesions and genomic instability in the immune system. Cell. 2013;152:417–429. doi: 10.1016/j.cell.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa H, Bednar T, Wang M, Paul K, Mladenov E, Bencsik-Theilen AA, Iliakis G. Functional redundancy between DNA ligases I and III in DNA replication in vertebrate cells. Nucleic Acids Res. 2012;40:2599–2610. doi: 10.1093/nar/gkr1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley D, Selfridge J, Millar JK, Samuel K, Hole N, Ansell JD, Melton DW. DNA ligase I is required for fetal liver erythropoiesis but is not essential for mammalian cell viability. Nat Genet. 1996;13:489–491. doi: 10.1038/ng0896-489. [DOI] [PubMed] [Google Scholar]
- Bentley DJ, Harrison C, Ketchen AM, Redhead NJ, Samuel K, Waterfall M, Ansell JD, Melton DW. DNA ligase I null mouse cells show normal DNA repair activity but altered DNA replication and reduced genome stability. J Cell Sci. 2002;115:1551–1561. doi: 10.1242/jcs.115.7.1551. [DOI] [PubMed] [Google Scholar]
- Boboila C, Jankovic M, Yan CT, Wang JH, Wesemann DR, Zhang T, Fazeli A, Feldman L, Nussenzweig A, Nussenzweig M, Alt FW. Alternative end-joining catalyzes robust IgH locus deletions and translocations in the combined absence of ligase 4 and Ku70. Proc Natl Acad Sci USA. 2010a;107:3034–3039. doi: 10.1073/pnas.0915067107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boboila C, Yan C, Wesemann DR, Jankovic M, Wang JH, Manis J, Nussenzweig A, Nussenzweig M, Alt FW. Alternative end-joining catalyzes class switch recombination in the absence of both Ku70 and DNA ligase 4. J Exp Med. 2010b;207:417–427. doi: 10.1084/jem.20092449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boboila C, Oksenych V, Gostissa M, Wang JH, Zha S, Zhang Y, Chai H, Lee CS, Jankovic M, Saez LM, et al. Robust chromosomal DNA repair via alternative end-joining in the absence of X-ray repair cross-complementing protein 1 (XRCC1) Proc Natl Acad Sci USA. 2012;109:2473–2478. doi: 10.1073/pnas.1121470109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenberger T, Tomkinson AE. Eukaryotic DNA ligases: structural and functional insights. Annu Rev Biochem. 2008;77:313–338. doi: 10.1146/annurev.biochem.77.061306.123941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank KM, Sharpless NE, Gao Y, Sekiguchi JM, Ferguson DO, Zhu C, Manis JP, Horner J, DePinho RA, Alt FW. DNA ligase IV deficiency in mice leads to defective neurogenesis and embryonic lethality via the p53 pathway. Mol Cell. 2000;5:993–1002. doi: 10.1016/s1097-2765(00)80264-6. [DOI] [PubMed] [Google Scholar]
- Grawunder U, Zimmer D, Fugmann S, Schwarz K, Lieber MR. DNA ligase IV is essential for V(D)J recombination and DNA double-strand break repair in human precursor lymphocytes. Mol Cell. 1998;2:477–484. doi: 10.1016/s1097-2765(00)80147-1. [DOI] [PubMed] [Google Scholar]
- Han L, Yu K. Altered kinetics of nonhomologous end joining and class switch recombination in ligase IV-deficient B cells. J Exp Med. 2008;205:2745–2753. doi: 10.1084/jem.20081623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L, Mao W, Yu K. X-ray repair cross-complementing protein 1 (XRCC1) deficiency enhances class switch recombination and is permissive for alternative end joining. Proc Natl Acad Sci USA. 2012;109:4604–4608. doi: 10.1073/pnas.1120743109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh CL. Cell Biology: A Laboratory Handbook. New York: Academic Press; 1997. Basic Cytogenetic Techniques: Culture, Slidemaking and Banding. [Google Scholar]
- Paul K, Wang M, Mladenov E, Bencsik-Theilen A, Bednar T, Wu W, Arakawa H, Iliakis G. DNA ligases I and III cooperate in alternative non-homologous end-joining in vertebrates. PLoS ONE. 2013;8:e59505. doi: 10.1371/journal.pone.0059505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrini JH, Xiao Y, Weaver DT. DNA ligase I mediates essential functions in mammalian cells. Mol Cell Biol. 1995;15:4303–4308. doi: 10.1128/mcb.15.8.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puebla-Osorio N, Lacey DB, Alt FW, Zhu C. Early embryonic lethality due to targeted inactivation of DNA ligase III. Mol Cell Biol. 2006;26:3935–3941. doi: 10.1128/MCB.26.10.3935-3941.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simsek D, Furda A, Gao Y, Artus J, Brunet E, Hadjantonakis AK, Van Houten B, Shuman S, McKinnon PJ, Jasin M. Crucial role for DNA ligase III in mitochondria but not in Xrcc1-dependent repair. Nature. 2011;471:245–248. doi: 10.1038/nature09794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan CT, Boboila C, Souza EK, Franco S, Hickernell TR, Murphy M, Gumaste S, Geyer M, Zarrin AA, Manis JP, et al. IgH class switching and translocations use a robust non-classical end-joining pathway. Nature. 2007;449:478–482. doi: 10.1038/nature06020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.