Abstract
Manipulations of lethally-irradiated animals, such as for administration of pharmaceuticals, blood sampling, or other laboratory procedures, have the potential to induce stress effects that may negatively affect morbidity and mortality. To investigate this in a murine model of the hematopoietic acute radiation syndrome, 20 individual survival efficacy studies were grouped based on the severity of the administration (Admn) schedules of their medical countermeasure (MCM) into Admn 1 (no injections), Admn 2 (one to three injections), or Admn 3 (29 injections or six to nine oral gavages). Radiation doses ranged from LD30/30 to LD95/30. Thirty-day survival of vehicle controls in each group was used to construct radiation dose lethality response relationship (DRR) probit plots, which were compared statistically to the original DRR from which all LDXX/30 for the studies were obtained. The slope of the Admn 3 probit was found to be significantly steeper (5.190) than that of the original DRR (2.842) or Admn 2 (2.009), which were not significantly different. The LD50/30 for Admn 3 (8.43 Gy) was less than that of the original DRR (8.53 Gy, p<0.050), whereas the LD50/30 of other groups were similar. Kaplan-Meier survival curves showed significantly worse survival of Admn 3 mice compared to the three other groups (p=0.007). Taken together, these results show that stressful administration schedules of MCM can negatively impact survival, and that dosing regimens should be considered when constructing DRR to use in survival studies.
Keywords: Health effects, mice, radiation dose, radiation damage
INTRODUCTION
The growing threat of terrorist use of radiation and radiation accidents at nuclear power facilities highlights the need for medical countermeasures (MCM) against radiation, and appropriate animal models for testing such MCM. Over the past 10 years, the authors have developed and refined a total body irradiation (TBI) mouse model of the hematopoietic acute radiation syndrome (H-ARS) for efficacy testing of candidate MCM against radiation (Plett et al. 2012). This base H-ARS model, developed in 12 week old C57BL/6 mice, has been used extensively to a) test survival efficacy of more than 50 candidate MCM from the government or private industry in more than 150 efficacy screening assays, b) optimize the MCM dose and/or administration schedule of more than 20 MCM in more than 75 assays c) examine polypharmacy of more than 10 different MCM combinations, d) perform Good Laboratory Practices (GLP)-compliant survival studies, and d) perform Pharmacokinetic/Pharmacodynamic PK/PD studies (Shakhov et al. 2012, Hoggatt et al. 2013, Chua et al. 2014, Garrett et al. 2014, Plett et al. 2014). These data validate this H-ARS mouse model as a suitable model for efficacy testing of potential MCM and for qualification as a Drug Development Tool (DDT) by the FDA / Center for Drug Evaluation and Research (CDER).
The stability of the radiation Dose lethality Response Relationship (DRR) of the H-ARS model is essential for confidence in using the model for efficacy testing of candidate MCM. The DRR was established by irradiating mice at 6 different radiation doses ranging from 775 to 900 cGy, which would result in 0 to 100% lethality. These data were then used to establish the range of lethal doses to be utilized in the subsequent studies. Stability of the DRR is assessed by monitoring “drift” in the expected survival of control groups. For example, if mice are exposed the LD50/30 (i.e., the lethal dose for 50% of the population by day 30), it is reasonable to expect survival in the control vehicle-treated group to be 50% ± 20% (i.e., 40% to 60% survival). If survival exceeds ±20%, evaluation of the efficacy of the MCM can be difficult, especially if survival is higher than expected as this situation leaves little “room” for the candidate MCM to exhibit efficacy. Stability of the murine H-ARS DRR is sensitive to many factors, demanding extensive characterization of the model and any variables that can lead to drift in the expected survival of controls. Such variables include chronoradiosensitivity (daily, weekly, annual), support (antibiotics, wet feed), frequency of blood sampling, MCM dosing (volume, frequency, route), stress effects, caretakers (gender, experience, familiarity) characteristics of mice (strain, age, gender, weight, vendor, barrier, room), treatment of the mice (acclimation period, identification method, housing, vent rack, barrier cages, single or group housing), husbandry (nutritional status of food, bedding, enrichment, water pH, temperature, humidity, air changes, light:dark cycles), irradiation [source, dose rate, irradiation apparatus, geometry (partial body shielding vs. total body irradiation)], anesthesia, cage effects, water consumption post-irradiation, and euthanasia criteria (Plett et al. 2012). All these parameters are tightly controlled in the authors’ H-ARS model.
Another contributor to instability in the DRR is the use of inbred mice. While genetic uniformity and consistency make inbred mouse strains popular for medical research, DRR constructed with inbred strains are characteristically steep. The 95% confidence intervals (CI) around the calculated LDXX/30 in such DRR are considerably larger than CI of DRR constructed with genetically diverse animals, leading to a relatively large window of possible survival outcomes at a given LDXX/30. This observation has been previously acknowledged by Cerveny et al (Cerveny et al. 1989), where he notes: “the more inbred and homogenous the population, the steeper the slope of the lethality curve”. Others have made similar statements: “it seems possible to conclude that the doses giving between 90%-95% mortality in most animal experiments are about twice those giving 5%-10% mortality” (Baverstock and Ash 1983). The authors have previously reported the slope of the DRR in their H-ARS model in C57BL/6 mice to be 2.56, whereas the slope of the DRR in the H-ARS model generated with the genetically diverse non-human primates H-ARS model is 1.13 (Farese et al. 2012), illustrating the steepness of DRR of inbred animals.
The authors have previously documented that lethally irradiated mice are sensitive to handling and manipulation post-exposure, such that excessive handling can lead to increased lethality (Plett et al. 2012). Thirty-day survival in mice that were bled every 5th day post-LD90 for CBC analyses (~30uL per mouse via tail snips in mice immobilized within a plexiglass mouse restrainer) was significantly decreased compared to non-bled mice (3.2% versus 12.2%, p=0.008), as was mean and overall survival time (p≤0.031) (Plett et al. 2012). In addition, morbidity occurred earlier and was more prevalent in bled mice compared to non-bled (Plett et al. 2012). There appear to be two possible causes for the increased morbidity and mortality in bled mice: 1) the small amount of blood loss was sufficient to negatively affect their health in their weakened post-irradiation state, or 2) the added handling and manipulation of the mice during the blood draw negatively affected health in their weakened state. To fully understand the cause of the increased morbidity and mortality observed in bled mice, experiments in which control “un-bled” are similarly handled as bled mice would need to be performed. Regardless, these data show that lethally-irradiated mice are sensitive to stress effects that affect survival, and likely cannot undergo the same types or extent of manipulation in their weakened post-irradiation state that larger animals apparently can withstand, without impacting survival.
In addition to stress effects due to frequent blood sampling, the authors have also observed increased mortality in mice subjected to stressful MCM administration schedules that entail multiple daily injections or intrusive procedures such as multiple oral gavages. In such cases the LDXX/30 appears to be most negatively affected at higher radiation doses (≥ LD70/30) compared to lower radiation doses (i.e., LD30/30). Due to study-to-study drift in the DRR from such factors, 2 to 3 different doses of radiation (i.e., LD50/30, LD70/30 and/or LD90/30) are used in every efficacy screen (Plett et al. 2012, Chua et al. 2014, Plett et al. 2014).
To better define the DRR and effects that excessive handling and / or manipulation of the mice can have on the DRR, as well as to investigate the stability of the DRR over time, the authors examined drift in the DRR over a 2.7 year period in 20 independent studies (n=15-103 mice per group). To this end, new DRR probit curves were constructed using mice from “control, vehicle-treated” groups in MCM efficacy screening studies. All control group mice were administered vehicle using the same administration schedule as that of the candidate MCM. While some administration schedules consisted of relatively few doses and simple routes of administration [i.e., 1-2 subcutaneous (SQ) injections], others were more stressful, requiring up to 29 SQ injections or multiple oral gavages. Experiments were thus categorized based on the “severity” of their administration schedules, and new DRR probit curves were generated that reflected relatively “light” administration schedules and those that were more “stressful”. The new DRR were then compared statistically to the original DRR from which the LDXX/30 values for all the screening studies were obtained. The original DRR was generated in March 2012, and all MCM screening studies used in this analysis (n=20 studies) were performed in the subsequent 2.7 years.
MATERIALS AND METHODS
Mice
Specific pathogen free C57BL/6 mice (50/50 male/female; Jackson Laboratory, Bar Harbor, Maine) were received at 10 weeks of age, an age analogous to a “young adult” human. All studies are performed on mice of the same age to avoid age-related changes in radiosensitivity (Grahn and Hamilton 1957, Grahn 1958, Yuhas and Storer 1967, Casarett 1968). Weights ranged from 16.0-21.6gm in females and 19.6-28.2gm in males. Mice were uniquely identified by ear punch and/or tail marks, and acclimatized for 2 weeks prior to irradiation. All studies were approved by the Indiana University School of Medicine Institutional Animal Care and Use Committee. Given the short duration of these survival studies, a sentinel mouse program, which analyzes mice every 3-4 months, was not used.
Husbandry
Up to 5 mice per cage were housed in microisolator cages on sterilized, certified direct contact bedding (Alpha Dri) and provided sterilized certified commercial extruded lab rodent chow (Harlan 2018SXC) ad libitum in cage hoppers and acidified water (pH 2.0-3.0) in sipper tube bottles. Autoclaved acidified water was provided on days 1-30 post-total body irradiation (TBI) in sipper tubes and on days 4-30 in wet feed in a petri dish set on the cage bottom. Animal rooms on a 12-hour light/dark cycle were maintained at 21±3°C with 30-80% relative humidity and at least 10 air changes per hour of 100% conditioned fresh air. Mouse rooms were sanitized between studies.
Irradiation and dosimetry
Mice were placed in single chambers of a Plexiglas irradiation apparatus and were exposed to a single uniform total body dose of gamma radiation from a 137Cs radiation source at 0.97 – 1.03 Gy min-1 (Mark 1 Irradiator, JL Shepherd, San Fernando, CA). These dose rates represent the decay in the cesium source over the 2.7yr period that the studies were performed. In-house dosimetry verified dose homogeneity in the exposure field of the mice was 0.0-4.3% of calculated central dose. For the DRR studies, mice were irradiated with 8 doses (7.25, 7.50, 7.75, 8.00, 8.25, 8.50, 8.75, and 9.00 Gy), while for the Admn 1-3 studies mice were irradiated with doses between 8.53 and 9.27 Gy. The radiation source is stationary, while the irradiation apparatus is rotated during irradiation to ensure uniform exposure. To verify exposure doses, Landauer Inlight OSL nanodosimeters were placed in mouse phantoms and exposed along with the mice during every exposure. Nanodosimeters were read on a validated Landauer microStar reader calibrated with standard Dot dosimeters exposed with a NIST-traceable 137Cs source (Battelle Memorial Institute, WA). Reproducibility of individual dots was 3±1% with accuracy of 4±2%, well within the 10% industry standard for experimental radiation dosimetry. Dose output checks (using farmer-type ion chambers and a validated electrometer), and dose field uniformity checks by exposing film, are performed annually by an onsite medical physicist.
Health status monitoring
Irradiated mice were observed for morbidity and mortality twice daily by trained laboratory personnel and scored on a scale of zero to three for signs meeting the criteria for early euthanasia based on three parameters: the severity of hunched posture, squinted/closed eyes, and decreased activity, using our novel method as previously described (Plett et al. 2012). When the sum of the three scores equaled eight or nine, mice underwent humane euthanasia by CO2 inhalation followed by cervical dislocation. Using these criteria, approximately 50% of decedent mice undergo euthanasia, and approximately 50% are “found dead”. There were no differences in the distribution of mice undergoing euthanasia versus found dead in the current study (data not shown). Body weights, while useful for health status monitoring in some models, were not used in these studies to avoid any potentially negative effects on morbidity and mortality from the added stress of handling the weakened mice.
Study design
Twenty MCM screening studies or DRR stability studies performed over a period of 2.7 years were separated into three groups based on the “severity” of the administration (Admn) regimen of the MCM (Table 1). Admn 1 studies (n=5) were designed to test the stability of the LD50/30 dose of radiation calculated in DRR study, and were repeated periodically to observe possible drift in the DRR curve. As such, there were no injections or administration of any MCM or vehicle in Admn 1 studies, and all experiments used the LD50/30, which was calculated to be 8.53 Gy from the original DRR. Admn 2 (n=11) comprised studies where the particular MCM or vehicle was administered in one to three SQ or intramuscular (IM) injections, beginning on day 1 post-irradiation and ending on day 2, 3, or 5 post-TBI. Mice in Admn 2 studies were exposed to the LD50/30, LD70/30 (8.72 Gy), LD90/30 (9.04 Gy), or the LD96/30 (9.27 Gy). Admn 3 studies (n=4) comprised the most severe administration regimens, where the MCM / vehicles were administered via 29 consecutive SQ injections beginning on day 1 post-exposure, or six to nine every other day oral gavages beginning on day 1 post-exposure and continuing up to day 17 post-TBI. Mice in Admn 3 studies were exposed to the LD30/30 (8.34 Gy), LD50/30, or the LD70/30. In all studies, each cage of mice was randomized by a study statistician to a radiation exposure dose and individual mice were randomized to treatment groups so that vehicle mice and MCM mice resided in the same cage. Volumes of vehicles used in these studies were 50 uL for IM and 94 to 128 uL for SQ injections and oral gavages. Vehicle solutions consisted of: 20 mM Tris, pH 7.5, 200 mM NaCl, 10% glycerol; or 0.05% Tween-20; PlasmaLyte A solution; or 10 mM sodium acetate, pH 4.8, 140 mM NaCl; or 10 mM sodium acetate, 5% sorbitol, 0.003% polysorbate 20, pH 4.0; 160mmol/L sodium chloride solution (Normal saline; 0.9% NaCl, w/v); or 10 mM sodium phosphate, 4% mannitol, 1% sucrose pH 6.2; or Dextrose 5% in water (D5W), or 5% DMSO Polyethylene glycol (PEG)-200; or 10% EtOH Sesame oil: Cremophor RH40 (55:35 w/w); or 20 mM sodium phosphate monobasic; or 1% sucrose and 4% D-mannitol in Water for Injection, pH 6.5±0.1. No data from the MCM groups are presented herein.
Table 1.
Administration schedule, route, radiation dose, expected and actual LDXX/30, and number of mice in each Admn group.
| Group | Study Number | Days of vehicle administration | Route | Radiation dose (Gy) | Expected LDXX/30 | Actual LDXX/30 | Number of mice |
|---|---|---|---|---|---|---|---|
| Admn 1 | 1 | none | none | 8.53 | LD50/30 | LD52/30 | 15 |
|
| |||||||
| 2 | none | none | 8.53 | LD50/30 | LD68/30 | 44 | |
|
| |||||||
| 3 | none | none | 8.53 | LD50/30 | LD40/30 | 38 | |
|
| |||||||
| 4 | none | none | 8.53 | LD50/30 | LD67/30 | 103 | |
|
| |||||||
| 5 | none | none | 8.53 | LD50/30 | LD39/30 | 74 | |
|
| |||||||
| Admn 2 | 6 | 1 | SQ | 8.53 | LD50/30 | LD35/30 | 20 |
| 8.72 | LD70/30 | LD55/30 | 20 | ||||
|
| |||||||
| 7 | 1, 3, & 5 | SQ | 8.53 | LD50/30 | LD55/30 | 20 | |
| 8.72 | LD70/30 | LD75/30 | 20 | ||||
|
| |||||||
| 8 | 1 & 5 | IM | 8.53 | LD50/30 | LD47/30 | 17 | |
| 8.72 | LD70/30 | LD67/30 | 18 | ||||
| 9.04 | LD90/30 | LD78/30 | 18 | ||||
|
| |||||||
| 9 | 1 | SQ | 8.53 | LD50/30 | LD40/30 | 20 | |
| 8.72 | LD70/30 | LD90/30 | 20 | ||||
|
| |||||||
| 10 | 1 | SQ | 8.53 | LD50/30 | LD95/30 | 20 | |
| 8.72 | LD70/30 | LD55/30 | 20 | ||||
|
| |||||||
| 11 | 2 | SQ | 8.53 | LD50/30 | LD70/30 | 20 | |
| 8.72 | LD70/30 | LD90/30 | 20 | ||||
|
| |||||||
| 12 | 1 & 2 | SQ | 8.53 | LD50/30 | LD59/30 | 17 | |
| 8.72 | LD70/30 | LD71/30 | 17 | ||||
| 9.04 | LD90/30 | LD88/30 | 17 | ||||
|
| |||||||
| 13 | 1 | SQ | 8.72 | LD70/30 | LD65/30 | 20 | |
| 9.27 | LD96/30 | LD95/30 | 20 | ||||
|
| |||||||
| 14 | 1 | SQ | 9.27 | LD96/30 | LD90/30 | 20 | |
|
| |||||||
| 15 | 3 | SQ | 8.53 | LD50/30 | LD45/30 | 20 | |
| 8.72 | LD70/30 | LD75/30 | 20 | ||||
|
| |||||||
| 16 | 1, 2, & 3 | SQ | 8.53 | LD50/30 | LD40/30 | 20 | |
| 8.72 | LD70/30 | LD60/30 | 20 | ||||
|
| |||||||
| Admn 3 | 17 | 1, 3, 5, 7, 9, & 11 | gavage | 8.53 | LD50/30 | LD63/30 | 40 |
| 8.72 | LD70/30 | LD90/30 | 39 | ||||
|
| |||||||
| 18 | Daily on days 1 thru 29 | SQ | 8.53 | LD50/30 | LD95/30 | 20 | |
| 8.72 | LD70/30 | LD100/30 | 20 | ||||
|
| |||||||
| 19 | 1, 3, 5, 7, 9, & 11 | gavage | 8.34 | LD30/30 | LD30/30 | 20 | |
| 8.53 | LD50/30 | LD65/30 | 20 | ||||
|
| |||||||
| 20 | 1, 3, 5, 7, 9, 11, 13, 15, & 17 | gavage | 8.34 | LD30/30 | LD35/30 | 20 | |
| 8.53 | LD50/30 | LD70/30 | 20 | ||||
Statistical analyses
Probit fits of mortality (DRR curves) were made using generalized linear models with a probit link, and comparisons of these fits were made using differences in deviance as a chi-square statistic. LD50/30 comparisons were made using standard errors and covariances of slopes and intercepts of probit fits (Wald test). The log rank test was used to compare survival curves [Kaplan-Meier (KM) fits plotted]. Statistical comparisons and plots (figures) were made using the R software (http://cran.r-project.org/).
The KM survival curves for Admn 1, Admn 2, and Admn 3 used day 30 survival data for mice exposed to 8.53 Gy only (expected LD50/30). This radiation dose was not used in constructing the DRR, so the dose closest to 8.53 Gy (ie, 8.50 Gy) was used for the DRR KM survival curve. It was assumed that a 3 cGy offset would not contribute an appreciable difference in the survival curve. All survival data within these radiation doses were plotted, without regard to route of administration. Probit analyses used day 30 survival data across all radiation doses.
RESULTS
The original DRR was constructed with 164 mice randomized into 10 groups of 13 to 23 mice/group. Each group was exposed to a different radiation dose ranging from 7.25 to 9.00 Gy in increments of 0.25 Gy. Survival on day 30 was used to construct the DRR shown in Fig. 1, which defined the following LDXX/30 (±95% CI) doses of radiation: LD30/30 = 8.34 (±10.81) Gy, LD50/30 = 8.53 (±10.63) Gy, LD70 = 8.72 (±13.68) Gy, LD90 = 9.04 (±22.21) Gy, and LD96/30 = 9.27 (±30.54) Gy. These LDXX/30 were used in subsequent MCM screening assays, of which the control vehicle-treated groups were used herein to investigate the stability of the original DRR and the effect that handling and manipulation of the mice have on survival predicted from the original DRR.
Figure 1. Probit plots.
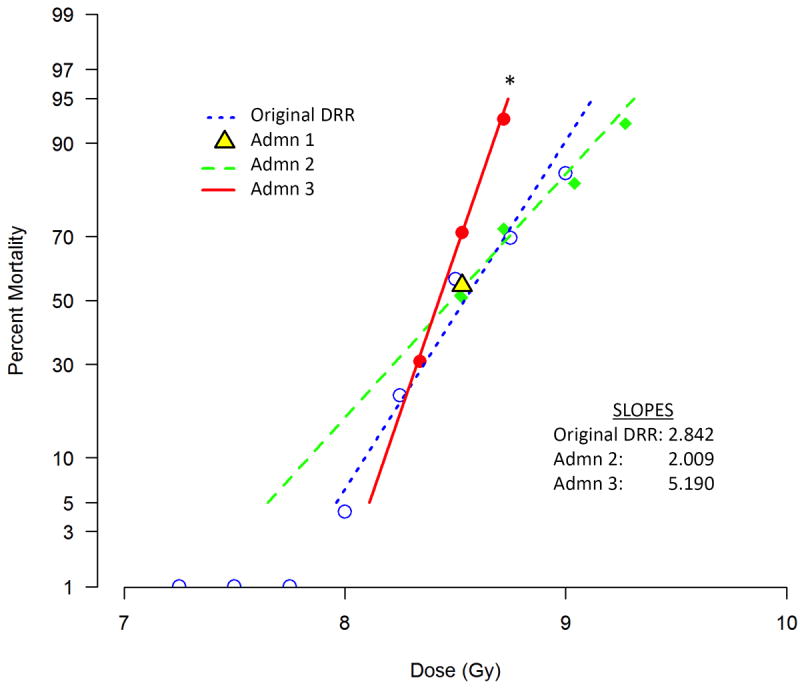
Vehicle-treated control groups from 20 individual survival efficacy studies were divided into 3 groups based on the severity of the administration (Admn) schedule of their MCM: Admn 1 (n=5 studies, 274 total mice) underwent no injections, Admn 2 (n=11 studies, 444 total mice) underwent 1-3 SQ or IM injections, and Admn 3 (n=4 studies, 199 total mice) underwent 29 consecutive daily SQ injections or 6 to 9 every other day oral gavages. Thirty-day mortality of mice in each group at different radiation doses was used to construct the probit plots, with percent mortality on the y-axis and radiation dose on the x-axis. Admn 1 mice were exposed to only one radiation dose (LD50/30), thus a probit could not be constructed. Admn 2 mice were exposed to the LD50/30, LD70/30, LD90/30, and LD95/30. Admn 3 mice were exposed to the LD30/30, LD50/30, and LD70/30. All LDXX/30 values were derived from the original DRR probit shown on the figure, generated using 164 mice randomized into 10 radiation dose groups. The slopes of each probit are given on the figure; the slope of Admn 3 was significantly steeper than that of Admn 2 or the original DRR.
Admn 1, which is comprised of mice exposed to the LD50/30 (8.53 Gy) but not injected with any vehicle, is shown as a single point on Fig. 1 since these mice were exposed to only one radiation dose. Analyses of slopes for the original DRR, Admn 2, and Admn 3 mice showed (Chi-square test) that these slopes were statistically different (p=0.0013). A comparison of the slopes of the original DRR and Admn 2 (2.842 (±0.699 95%CI) and 2.009 (±0.836 95%CI), respectively) showed that they were not significantly different (p=0.120). In addition, there was no significant shift left or right of the Admn 2 probit compared to the original DRR (p=0.530). Thus, these two data sets are statistically indistinguishable and it can be concluded that the statistical difference in the overall comparison is due to the Admn 3 probit, which has a significantly steeper slope (5.190) compared those of the DRR and Admn 2 probits.
The LD50/30 value for the Admn 3 probit was found to be less than that of the original DRR probit (8.43 Gy versus 8.53 Gy, respectively, p<0.050, Table 2), illustrating that the stressful vehicle administration schedules of Admn 3 negatively impacted survival. The LD50/30 of the Admn 2 probit (8.48 Gy) was not statistically different than that of the DRR or of Admn 3 (p>0.050, unadjusted comparisons).
Table 2.
Estimated LDXX/30 values for original DRR, Admn2, and Admn3 groups
| Expected LDXX/30 | Original DRR Gy (±95% CI) | Admn 2 Gy (±95% CI) | Admn 3 Gy (±95% CI) |
|---|---|---|---|
| LD30/30 | 8.34 (0.11) | 8.22 (0.13) | 8.33 (0.06) |
| LD50/30 | 8.53 (0.10) | 8.48 (0.07) | 8.43 (0.04) |
| LD70/30 | 8.72 (0.14) | 8.74 (0.08) | 8.53 (0.04) |
| LD90/30 | 9.04 (0.12) | 9.12 (0.25) | 8.68 (0.09) |
| LD95/30 | 9.22 (0.28) | 9.30 (0.34) | 8.75 (0.12) |
Of interest, lethality in mice exposed to the highest doses in Admn 3 (LD70/30, 8.72 Gy) was more disparate from the original DRR than mice exposed to the lowest doses (i.e., LD30/30, 8.34 Gy). In the LD70/30 groups, lethality was 29-43% higher than the expected LD70/30 (actual LDXX/30 = LD90/30 and LD100/30, Table 1), while actual lethality in the LD30/30 groups was LD30/30 and LD35/30. These data suggest that mice exposed to higher doses of radiation are more negatively affected by stressful administration regimens than mice exposed to lower doses.
The Kaplan-Meier (KM) survival curves shown in Fig. 2 used day 30 survival data for mice exposed to 8.53 Gy only (LD50/30; Admn 1, Admn 2, and Admn 3), or 8.50 Gy in the case of the DRR, since the DRR did not contain a group exposed to 8.53 Gy. A log rank test comparing all four KM curves indicated differences among the groups (p=0.007), whereas a log rank test comparing DRR, Admn 1, and Admn 2 indicated no differences (p=0.882), illustrating that the KM of Admn 3 mice was statistically different from the other three KM curves.
Figure 2. Kaplan-Meier survival curves.
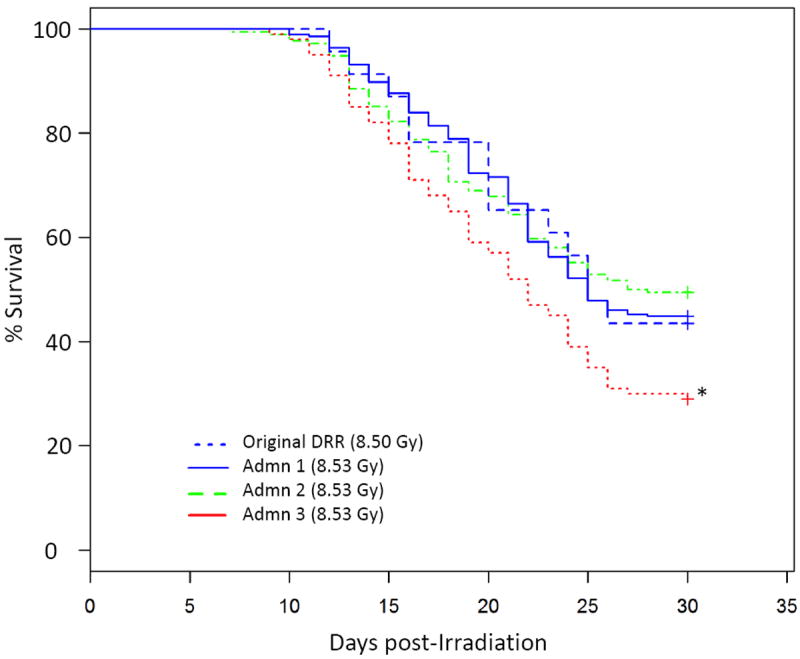
A description of the original DRR and Admn groups is given in the legend to Fig. 1. Thirty-day survival of mice exposed to 8.53 Gy (Admn 1, Admn 2, and Admn 3), or 8.50 Gy (original DRR), was used to construct Kaplan-meier survival curves. Survival in Admn 3 was significantly worse compared to the other groups (p=0.007).
DISCUSSION
These data illustrate the sensitivity of the mouse radiation dose lethality response relationship (DRR) to handling and manipulation of mice during the acute phase of the radiation response. These data further show that mice exposed to higher doses of radiation (LD70/30) are more susceptible to stress effects than mice exposed to lower doses (LD30/30). It is noteworthy that the actual LDXX/30 in mice exposed to the LD90/30 or higher in the Admn 2 group was usually very close to the expected LDXX/30, supporting the notion that stressful administration schedules (rather than drift in the DRR curve) are responsible for the increased lethality in the high radiation dose groups. The incremental effect of stress at higher radiation doses could be due to the effects of ARS on other organ systems, such as the gastrointestinal system, thus increasing the sensitivity of the mice to handling stress and infections.
These results build upon the authors’ previously published data documenting increased lethality in mice undergoing periodic (every 5th day) blood sampling during the first 30 days post-radiation exposure (Plett et al. 2012). While it is unknown whether the increased lethality in the Plett 2012 paper was due to loss of blood or increased handling necessary during the blood draw, data in the current paper suggest that handling alone during vehicle administration can result in increased lethality, absent any blood sampling. It has been hypothesized by us (Plett et al. 2012) and others (Booth et al. 2012) that the extra fluid administered during vehicle administration may positively affect health and survival after radiation. Results presented herein show a significant shift to the left of the Admn 3 DRR compared to the other groups, suggesting that the stress of frequent handling over-shadowed any potential benefit of fluid support from the vehicle administrations. Further studies in similar models using similar radiation doses may be warranted to better understand the potential survival benefit of fluid administration balanced by the potential negative effects of repeated handling of the mice during fluid administration. Additionally, some Admn 2 protocols (study numbers 6, 7, 9, 10, 11, 13, and 14) required bleeding mice by tail snips twice during the study, but not more than once every 14 days, which appeared to not affect lethality in general. These results suggest that lethality is not negatively affected by intermittent handling of mice, such as blood sampling once every 14 days or dosing 3 times or fewer, and that care should be taken to limit the number of times that lethally-irradiated mice are handled during the acute phase.
Several measures can be taken to circumvent the demonstrated stress effects on lethality when repeat MCM administration is necessary for efficacy. Ideally, a complete DRR would be generated using groups of mice exposed to increasing doses of radiation and injected with vehicle using the same administration schedule required for the MCM. LDXX/30 values are then calculated from the DRR and radiation doses selected for the efficacy study. Absent construction of such a DRR, two or more doses of radiation would be selected for the efficacy study, taking care to select doses that may be lower than desired to allow for the possibility of increased lethality due to stress effects. Optimally, MCM can be engineered to require only a few injections, which has the added logistical benefit for ease of usage in the field.
Rigorous testing of new DRR curves is undertaken in the authors’ lab to ensure stability of LDXX/30 doses used in efficacy studies. Testing is carried out by exposing several groups of mice over time to the LD50/30 from the new DRR curve and documenting 30 day survival. These studies, comprising the five Admn 1 studies in the current paper with a total of 274 mice, gave an average LD of LD53/30, which is very close to the expected LD50/30. The range of actual LDXX/30 in individual studies was, however, LD39/30 to LD68/30, which exceeds the desirable ±20% variance. Drift in these studies is partly due to the use of inbred animals, which are inherently variable due to steep DRR curves compared to those generated in genetically diverse animals (Cerveny et al. 1989). To control for in-study drift as much as possible, 2 to 3 different doses of radiation (usually LD50/30, LD70/30 and/or LD90/30) are used in every efficacy study in the authors’ lab (Plett et al. 2012, Chua et al. 2014, Plett et al. 2014). Also important is sufficient group size. When two doses of radiation are used in efficacy studies, 20 mice/group provides 80% power with a two-tailed 5% significance level assuming a 30% reduction in lethality in treated mice (Chua et al. 2012, Plett et al. 2012, Chua et al. 2014, Plett et al. 2014).
The question remains as to what aspect of excessive handling/manipulation of the mice increases lethality? While this remains unanswered, a few hypotheses can be entertained. It is well known that common laboratory procedures such as handling, blood collection, restraining, and, in particular, oral gavage induce measureable stress in mice and other animals as shown by increases in corticosterone, glucose, growth hormone, heart rate, blood pressure, and behavior (Johnson et al. 2000, Balcombe et al. 2004, Hoggatt et al. 2010, Hurst and West 2010, Gouveia and Hurst 2013, Vandenberg et al. 2014). Moreover, C57BL/6 mice, the strain used in these studies, are one of the more anxiety-prone mouse strains (Kim et al. 2002, Michalikova et al. 2010). The body’s response to stress involves the sympathetic nervous system and the hypothalamic-pituitary-adrenal axis, resulting in the release of stress hormones from the adrenal cortex, including cortisol. After removal of the stressor, stress hormones return to basal levels, but if the stressful event continues (such as in Admn 3 mice), cortisol may be continually released. It has been shown in humans that prolonged exposure to stress hormones can have pathologic outcomes in several systems, including the immune system, thereby increasing morbidity and mortality (McEwen 1998, Vogelzangs et al. 2010).
The timing of laboratory manipulations may also play a role in inducing lethal stress when one considers that mice are nocturnal animals and frequent disruptions to their normal daytime sleep patterns for laboratory procedures may affect immunity (Trammell et al. 2014). Frequent handling may increase the chances of opportunistic infections, despite rigorous practices in the authors’ laboratory to ensure aseptic handling of the mice (cages are only opened in biosafety cabinets, gloved hands and cages are sprayed with disinfectant before opening/touching the mice, needles are not reused, tails are disinfected before snipping, and personnel wear full personal protective gear, including face masks).
CONCLUSIONS
These data illustrate the negative effect that stressful administration schedules of MCM can have on survival of lethally-irradiated mice in survival efficacy studies. Mice that underwent 29 consecutive daily SQ injections of vehicle, or six to nine every other day oral gavages, experienced significantly worse survival than mice undergoing one to three SQ or IM injections or no injections at all. Survival was most negatively affected by stressful administration schedules when higher doses of radiation were used (i.e., LD70/30) compared to lower doses (LD30/30). To circumvent the effect that administration schedules can have on study outcome, DRR can be constructed using the same administration schedule required for the MCM so that LDXX/30 values are reflective of the administration schedule. Absent construction of such a DRR, two or more doses of radiation can be selected for the efficacy study, taking care to select doses that may be lower than desired. Finally, engineering MCM to require fewer injections has the advantages of reducing stress to the animals and ease of utility in the field.
Acknowledgments
Funding:
This project has been funded in whole or in part with federal funds from the National Institute of Allergy and Infectious Diseases (NIAID) under contracts HHSN266200500043C and HHSN272201000046C, 1U01AI107340-01, 2R44 AI088288-03A1, and National Institute of Aging R01AG046246-01, National Institutes of Health, Department of Health and Human Services.
Literature cited
- Balcombe JP, Barnard ND, Sandusky C. Laboratory routines cause animal stress. Contemporary topics in laboratory animal science / American Association for Laboratory Animal Science. 2004;43:42–51. [PubMed] [Google Scholar]
- Baverstock K, Ash P. A review of radiation accidents involving whole body exposure and the relevance to the ld50/60for man. Br J Radiol. 1983;56:837. doi: 10.1259/0007-1285-56-671-837. [DOI] [PubMed] [Google Scholar]
- Booth C, Tudor G, Tudor J, Katz BP, MacVittie TJ. Acute gastrointestinal syndrome in high-dose irradiated mice. Health Phys. 2012;103:383–99. doi: 10.1097/hp.0b013e318266ee13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casarett A. Radiation biology. Englewood, New Jersey: Prentice-Hall Inc; 1968. [Google Scholar]
- Cerveny T, MacVittie T, Young R. Acute radiation syndrome in humans. Vol. 2. Falls church, va, Walker RI: Ttm publisher; 1989. pp. 17–36. [Google Scholar]
- Chua HL, Plett PA, Sampson CH, Joshi M, Tabbey R, Katz BP, MacVittie TJ, Orschell CM. Long-term hematopoietic stem cell damage in a murine model of the hematopoietic syndrome of the acute radiation syndrome. Health Physics. 2012;103:356–366. doi: 10.1097/HP.0b013e3182666d6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua HL, Plett PA, Sampson CH, Katz BP, Carnathan GW, MacVittie TJ, Lenden K, Orschell CM. Survival efficacy of the pegylated g-csf maxy-g34 and neulasta in a mouse model of lethal h-ars, and residual bone marrow damage in treated survivors. Health Physics. 2014;106:21–38. doi: 10.1097/HP.0b013e3182a4df10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farese AM, Cohen MV, Katz BP, Smith CP, Jackson WI, Cohen DM, MacVittie TJ. A nonhuman primate model of the hematopoietic acute radiation syndrome plus medical management. Health Physics. 2012;103:367–382. doi: 10.1097/HP.0b013e31825f75a7;10.1097/HP.0b013e31825f75a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett J, Orschell CM, Mendonca MS, Bigsby RM, Dynlacht JR. Subcutaneous wounding postirradiation reduces radiation lethality in mice. Radiat Res. 2014;181:578–83. doi: 10.1667/rr13267.1. [DOI] [PubMed] [Google Scholar]
- Gouveia K, Hurst JL. Reducing mouse anxiety during handling: Effect of experience with handling tunnels. PloS one. 2013;8:e66401. doi: 10.1371/journal.pone.0066401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahn D. Acute radiation response of mice from a cross between radiosensitive and radioresistant strains. Genetics. 1958;43:835–843. doi: 10.1093/genetics/43.5.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahn D, Hamilton K. Genetic variation in the acute lethal response of four inbred mouse strains to whole body x-irradiation. Genetics. 1957;42:189–198. doi: 10.1093/genetics/42.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoggatt AF, Hoggatt J, Honerlaw M, Pelus LM. A spoonful of sugar helps the medicine go down: A novel technique to improve oral gavage in mice. Journal of the American Association for Laboratory Animal Science : JAALAS. 2010;49:329–34. [PMC free article] [PubMed] [Google Scholar]
- Hoggatt J, Singh P, Stilger KN, Plett PA, Sampson CH, Chua HL, Orschell CM, Pelus LM. Recovery from hematopoietic injury by modulating prostaglandin e(2) signaling post-irradiation. Blood cells, molecules & diseases. 2013;50:147–53. doi: 10.1016/j.bcmd.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst JL, West RS. Taming anxiety in laboratory mice. Nat Meth. 2010;7:825–826. doi: 10.1038/nmeth.1500. DOI: http://www.nature.com/nmeth/journal/v7/n10/abs/nmeth.1500.html#supplementary-information. [DOI] [PubMed] [Google Scholar]
- Johnson EA, Sharp DS, Miller DB. Restraint as a stressor in mice:: Against the dopaminergic neurotoxicity of d-mdma, low body weight mitigates restraint-induced hypothermia and consequent neuroprotection. Brain Research. 2000;875:107–118. doi: 10.1016/s0006-8993(00)02601-9. DOI: http://dx.doi.org/10.1016/S0006-8993(00)02601-9. [DOI] [PubMed] [Google Scholar]
- Kim S, Lee S, Ryu S, Suk J-g, Park C. Comparative analysis of the anxiety-related behaviors in four inbred mice. Behavioural Processes. 2002;60:181–190. doi: 10.1016/s0376-6357(02)00085-2. DOI: http://dx.doi.org/10.1016/S0376-6357(02)00085-2. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Annals of the New York Academy of Sciences. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- Michalikova S, van Rensburg R, Chazot PL, Ennaceur A. Anxiety responses in balb/c, c57 and cd-1 mice exposed to a novel open space test. Behavioural Brain Research. 2010;207:402–417. doi: 10.1016/j.bbr.2009.10.028. DOI: http://dx.doi.org/10.1016/j.bbr.2009.10.028. [DOI] [PubMed] [Google Scholar]
- Plett PA, Chua HL, Sampson CH, Katz BP, Fam CM, Anderson LJ, Cox G, Orschell CM. Pegylated g-csf (bbt-015), gm-csf (bbt-007), and il-11 (bbt-059) analogs enhance survival and hematopoietic cell recovery in a mouse model of the hematopoietic syndrome of the acute radiation syndrome. Health Physics. 2014;106:7–20. doi: 10.1097/HP.0b013e3182a4dd4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plett PA, Sampson CH, Chua HL, Joshi M, Booth C, Gough A, Johnson CS, Katz BP, Farese AM, Parker J, MacVittie TJ, Orschell CM. Establishing a murine model of the hematopoietic syndrome of the acute radiation syndrome. Health Phys. 2012;103:343–55. doi: 10.1097/HP.0b013e3182667309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakhov AN, Singh VK, Bone F, Cheney A, Kononov Y, Krasnov P, Bratanova-Toshkova TK, Shakhova VV, Young J, Weil MM, Panoskaltsis-Mortari A, Orschell CM, Baker PS, Gudkov A, Feinstein E. Prevention and mitigation of acute radiation syndrome in mice by synthetic lipopeptide agonists of toll-like receptor 2 (tlr2) PloS one. 2012;7:e33044. doi: 10.1371/journal.pone.0033044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trammell RA, Verhulst S, Toth LA. Effects of sleep fragmentation on sleep and markers of inflammation in mice. Comparative medicine. 2014;64:13–24. [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Welshons WV, Vom Saal FS, Toutain PL, Myers JP. Should oral gavage be abandoned in toxicity testing of endocrine disruptors? Environmental health : a global access science source. 2014;13:46. doi: 10.1186/1476-069x-13-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelzangs N, Beekman ATF, Milaneschi Y, Bandinelli S, Ferrucci L, Penninx BWJH. Urinary cortisol and six-year risk of all-cause and cardiovascular mortality. The Journal of Clinical Endocrinology and Metabolism. 2010;95:4959–4964. doi: 10.1210/jc.2010-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuhas JM, Storer JB. The effect of age on two modes of radiation death and on hematopoietic cell survival in the mouse. Radiat Res. 1967;32:596–605. [PubMed] [Google Scholar]


