Abstract
The use of plasma citrulline as a biomarker for acute and prolonged gastrointestinal injury via exposure to total- and partial-body irradiation (6 MV LINAC-derived photons; 0.80 Gy min−1) in nonhuman primate models was investigated. The irradiation exposure covered gastrointestinal injuries spanning lethal, mid-lethal, and sub-lethal doses. The acute gastrointestinal injury was assessed via measurement of plasma citrulline and small intestinal histopathology over the first 15 days following radiation exposure and included total-body irradiation at 13.0 Gy, 10.5 Gy, and 7.5 Gy and partial-body irradiation at 11.0 Gy with 5% bone marrow sparing. The dosing schemes of 7.5 Gy total-body irradiation and 11.0 Gy partial-body irradiation included time points out to day 60 and day 180, respectively, which allowed for correlation of plasma citrulline to prolonged gastrointestinal injury and survival. Plasma citrulline values were radiation-dependent for all radiation doses under consideration with nadir values ranging from 63–80 % lower than radiation-naïve NHP plasma. The nadir values were observed at day 5 to 7 post irradiation. Longitudinal plasma citrulline profiles demonstrated prolonged gastrointestinal injury resulting from acute high-dose irradiation had long lasting effects on enterocyte function. Moreover, plasma citrulline did not discriminate between total-body or partial-body irradiation over the first 15 days following irradiation and was not predictive of survival based on the radiation models considered herein.
Keywords: biological indicators, radiation damage, gastrointestinal tract, plasma
INTRODUCTION
The Medical Countermeasures Against Radiological Threats (MCART) consortium is charged with developing medical countermeasures (MCMs) to treat the key sequelae of the acute radiation syndrome (ARS) and the delayed effects of acute radiation exposure (DEARE) (MacVittie 2012c). The foundation by which MCART has established a pipeline for investigating MCMs is development of well-characterized radiation animal models that accurately mimic the key organ-specific sequelae observed in humans and involving hematopoietic-ARS (H-ARS), acute and prolonged gastrointestinal-ARS (GI-ARS), and delayed lung injury (lung-DEARE). Furthermore, the MCART models have been progressively refined to encompass an understanding of the dose- and time-dependent organ-specific and multi-organ injury, morbidity, and mortality in a consensus paradigm (MacVittie 2014). That is, the MCART animal models are designed to evaluate latency, incidence, severity, and duration of organ-specific injuries and how these injuries are linked to potential multi-organ injuries and outcome.
An important aspect of evaluating the MCART animal models is the identification and validation of biomarkers that inform not only on organ-specific sequelae but also the consensus paradigm where the link between acute, prolonged, and delayed injuries can be investigated (Jones et al. 2014a). Biomarkers, defined as any molecule that can be detected, quantified and display a dependent relationship with a particular insult (e.g., radiation), are highly desirable for their potential prediction of injury outcome (prognostic biomarker) or potential use in injury characterization (diagnostic biomarker). An ideal radiation biomarker would originate from a readily accessible biological fluid (e.g., saliva, urine, blood), afford robust, efficient quantification, and exhibit dose- and time-dependence to the radiation event (Ossetrova et al. 2010, Pandey et al. 2010, Coy et al. 2011). Circulating citrulline fits the aforementioned criteria; most notably, it is readily quantified in plasma and displays a dose and temporal dependence in radiation animal models (Lutgens et al. 2003, Gupta et al. 2011, Elliott et al. 2014, Jones et al. 2014a, Jones et al. 2014b, Moroni et al. 2014).
Circulating citrulline originates almost exclusively from small bowel enterocytes, as such, disruption in small intestinal homeostasis (e.g., GI-ARS) triggers concomitant disruption in circulating citrulline concentration (Wakabayashi et al. 1991, Wakabayashi et al. 1995). By extension, measurement of circulating citrulline reflects the present status of functional enterocyte mass (Lutgens et al. 2004, Lutgens and Lambin 2007). As it relates to GI-ARS, the rapidly proliferating small intestine epithelium is acutely prone to high-dose irradiation with onset of GI injury occurring within days of the irradiation insult (Potten 1990). It is within this context that circulating citrulline has been identified as a biomarker for GI-ARS.
We report the evaluation and characterization of plasma citrulline from nonhuman primate(s) (NHP; rhesus macaques) as a biomarker for acute and prolonged GI injuries sustained via high-dose irradiation. In particular, total-body irradiation (TBI) and partial-body irradiation (PBI) NHP models covering radiological doses spanning GI lethal to long term survival out to day 180 were used to correlate circulating citrulline concentration to acute and prolonged GI injuries. To the best of our knowledge, this is the first report to comprehensively evaluate the extent to which circulating citrulline can be reliably classified as a prognostic and/or diagnostic biomarker of GI injuries sustained via high-dose irradiation using a large animal radiation model.
MATERIALS AND METHODS
Radiation Animal Model
Male rhesus macaques (Macaca mulatta) plasma and small intestine samples were provided by the laboratory of Dr. Thomas J. MacVittie, University of Maryland, School of Medicine, Department of Radiation Oncology (Baltimore, MD). Descriptions of the animal models including radiation exposure and dosimetry, medical management (supportive care and health status monitoring), and generation of plasma and tissue have been previously described (MacVittie et al. 2012a, MacVittie et al. 2012b). NHP were exposed to TBI (7.5 Gy, 10.5 Gy, and 13.0 Gy) and PBI/BM5 (11.0 Gy/bone marrow sparing 5 %) with 6 MV LINAC-derived photons at an approximate rate of 0.80 Gy min−1. The NHP receiving PBI/BM5 were positioned such that their tibiae were outside the beam field.
Quantification of Plasma Citrulline
Plasma citrulline was quantified via liquid chromatography tandem mass spectrometry (LC-MS/MS) as reported previously (Jones et al. 2014b). Briefly, 50 uL of plasma spiked with a stable-label internal standard (4,4,5,5-d4-L-citrulline (d4-Cit)) was protein precipitated with acetonitrile and was analyzed by LC-MS/MS.
Intestinal Histopathology
Samples of small intestine (duodenum, jejunum, and ileum) were collected at necropsy, fixed in formalin, embedded in paraffin, and cut, mounted, and stained with hematoxylin and eosin (H&E). H&E stained sections were scanned into an Aperio ScanScope CS (Leica Biosystems, Buffalo Grove, IL) and processed via Aperio ImageScope (v12.1.0.5029; Leica Biosystems).
Statistical Analysis
Statistical analyses were performed using GraphPad Prism (v 6.03). Linear regression analysis, Mann Whitney test, and Pearson correlation were used to calculate the fit and significance. All tests were performed using two-sided tests at the 0.05 significance level.
RESULTS
Quantification of plasma citrulline from TBI and PBI NHP models
Plasma citrulline from NHP radiation models was analyzed in order to assess the use of citrullline as a biomarker for acute and prolonged GI-ARS. Two different radiation models, TBI and PBI, were characterized. The TBI doses included 13.0 Gy, 10.5 Gy, and 7.5 Gy. The PBI dose was 11.0 Gy with 5 % bone marrow sparing (PBI/BM5).
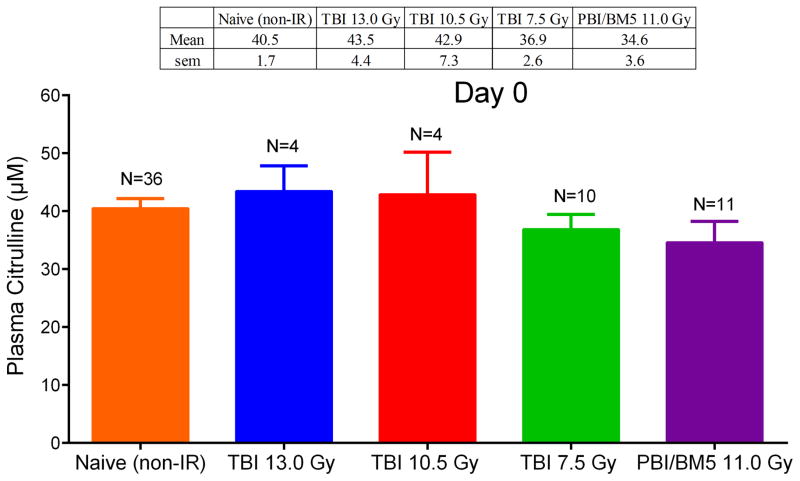
Recently, plasma citrulline from 36 naïve (non-irradiated) NHP was reported (Jones et al. 2014b). The mean ± standard error of the mean (sem) was 40.5 ± 1.7 μM. This number was in good agreement with day 0 time points for all NHP radiation models considered herein (Fig. 1). A comparison of naïve (non-irradiated) NHP plasma citrulline to day 0 time points for the TBI and PBI/BM5 models yielded no statistical difference across all comparable combinations with plasma citrulline mean values statistical equivalent from all day 0 time points and naïve plasma (p > 0.09 for all combinations). Day 0 corresponded to a blood draw from the NHP prior to the irradiation event and given its statistical similarity to naïve plasma citrulline was considered to be the non-irradiated control for each radiation model.
Fig. 1.
Plasma citrulline concentration in μM for radiation naïve NHP and at day 0 for NHP exposed to TBI 13.0 Gy, TBI 10.5 Gy, TBI 7.5 Gy, and PBI/BM5 11.0 Gy. Day 0 samples correspond to a blood drawn the day of and prior to the IR event. The mean and sem were displayed in the table above the graph. The number of individual samples was displayed above the bar for each category. There were no statistical differences for any of the corresponding categories (p > 0.09).
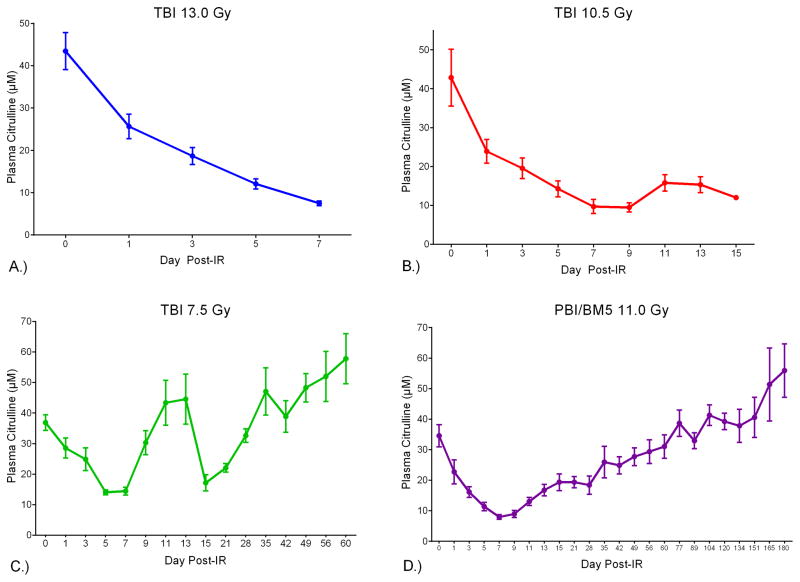
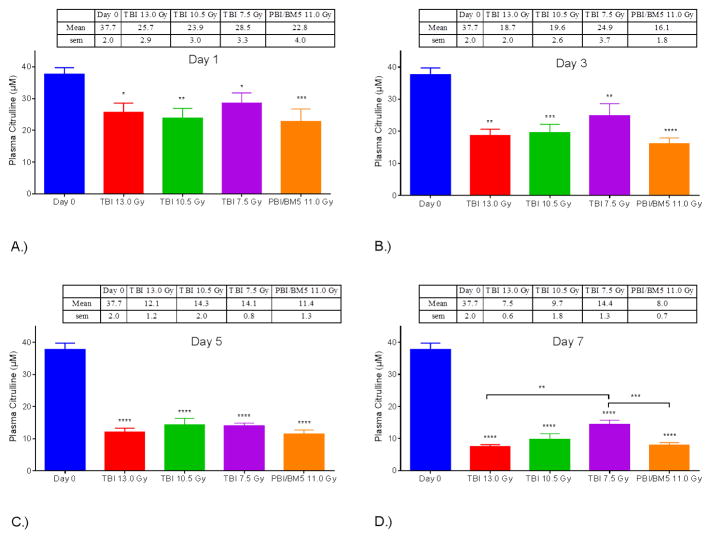
Plasma citrulline concentration versus day post-IR for each radiological dose revealed the temporal relationship between plasma citrulline and high-dose irradiation (Fig. 2). Refer to Table 1 for the specific time points and numbers of individual samples per IR dose. Linear regression analysis for plasma citrulline versus day post-IR out to the longitudinal nadir (day 5 for TBI 7.5 Gy and day 7 for TBI 13.0 Gy, 10.5 Gy and PBI/BM5 11.0 Gy) demonstrated significant associations between plasma citrulline concentration and the day post-IR for all four doses (p < 0.0001 for all doses). Lower plasma citrulline concentration was associated with increasing number of days post IR out to day 7 (day 5 for TBI 7.5 Gy) with each successive odd day being progressively lower in plasma citrullline (Fig. 3 and Fig. 4A). All four dosing schedules had plasma citrulline concentrations that were significantly lower than day 0 (and naïve plasma) starting 24-hours from the IR event. On the other hand, there were no significant associations between differing IR doses for plasma citrulline within a specific day out to the longitudinal nadir. For example, at day 1 post-IR all dosing schedules were significantly associated with lower plasma citrulline compared to day 0 yet the plasma citrulline per IR dose were statistically indistinguishable. Refer to Fig. 3 for graphical representation of plasma citrulline versus IR dose per day. Of note, starting at day 7 TBI 7.5 Gy plasma citrulline concentrations started to statistically deviate from the TBI 13.0 Gy (p = 0.004) and PBI/BM5 11.0 Gy (p = 0.0006).
Fig. 2.
Plasma citrulline versus the day post-IR for NHP exposed to A.) TBI 13.0 Gy. B.) TBI 10.5 Gy. C.) TBI 7.5 Gy. D.) PBI/BM5 11.0 Gy. Citrulline concentrations are expressed in μM as mean ± sem. Refer to Table 1 for numbers of individual samples per time point.
Table 1.
Specific time-points and numbers of individual samples per the dosing schedule.
|
|
|||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day Post-IR
|
|||||||||||||||||||||||||
| 0 | 1 | 3 | 5 | 7 | 9 | 11 | 13 | 15 | 21 | 28 | 35 | 42 | 49 | 56 | 60 | 77 | 89 | 104 | 120 | 134 | 151 | 165 | 180 | ||
|
|
|||||||||||||||||||||||||
| Radiological Dose (Gy) | TBI 13.0 | 4 | 4 | 4 | 4 | 4 | |||||||||||||||||||
| TBI 10.5 | 4 | 4 | 4 | 4 | 4 | 4 | 3 | 3 | 1 | ||||||||||||||||
| TBI 7.5 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 7 | 5 | 5 | 5 | 5 | 5 | 5 | |||||||||
| PBI/BM5 11.0 | 11 | 11 | 11 | 11 | 11 | 11 | 10 | 10 | 10 | 10 | 8 | 8 | 8 | 8 | 8 | 8 | 7 | 7 | 6 | 4 | 4 | 3 | 3 | 3 | |
Fig. 3.
NHP plasma citrulline after IR and grouped by irradiation dose. Mean ± sem plasma citrulline at day 1 (A), day 3 (B), day 5 (C) and day 7 (D) for NHP in the TBI 13.0 Gy, TBI 10.5 Gy, TBI 7.5 Gy, and PBI/BM5 11.0 Gy groups shown with day 0 values. All plasma citrulline values for each day and each dose group were significantly less than the concentration at day 0. Furthermore, the plasma citrulline on day 7 in the TBI 7.5 Gy group was significantly greater than that measured in day 7 samples from the TBI 13.0 Gy and PBI/BM5 11.0 Gy groups. *p < 0.05, ** p < 0.01, *** p < 0.005, **** p < 0.0001.
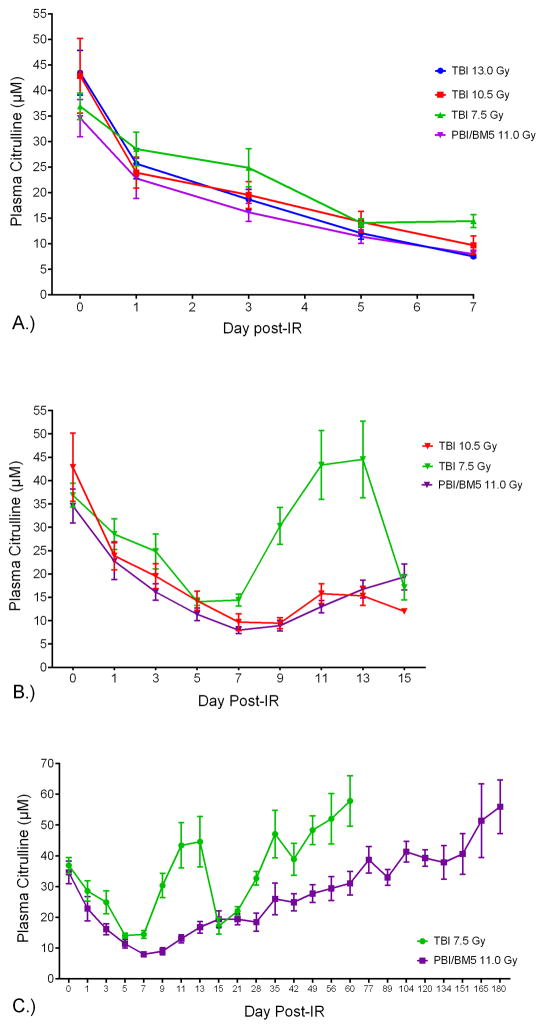
Fig. 4.
NHP plasma citrulline versus day post-IR for specific time points. A). NHP plasma citrulline versus day post-IR out to day 7 for TBI 13.0 Gy, TBI 10.5 Gy, TBI 7.5 Gy, and PBI/BM5 11.0 Gy. B.) NHP plasma citrulline versus day post-IR out to day 15 for TBI 10.5 Gy, TBI 7.5 Gy, and PBI/BM5 11.0 Gy. C.) NHP plasma citrulline versus day post-IR out to day 60 for TBI 7.5 Gy, and day 180 for PBI/BM5 11.0 Gy. The concentrations were expressed in μM as mean ± sem. Refer to Table 1 for numbers of individual samples per time point.
Next, plasma citrulline was evaluated for TBI 10.5 Gy, 7.5 Gy, and PBI/BM5 11.0 Gy out to day 15 (Fig. 4B). Since TBI 13.0 Gy resulted in lethality due to GI-ARS, plasma samples beyond day 7 were not obtained. The temporal profiles for TBI 10.5 Gy and PBI 11.0 Gy were statistically indistinguishable (p = 0.84) ending at day 13. The comparison for day 15 between TBI 10.5 Gy and PBI/BM5 11.0 Gy was not valid given there was only one sample at day 15 for TBI 10.5 Gy. These results demonstrated that plasma citrulline did not distinguish between TBI and PBI/BM5 at similar doses. Similar for TBI 7.5 Gy, TBI 10.5 Gy, and PBI/BM5 11.0 Gy dosing schedules was a plateau effect for the next consecutive odd day following the longitudinal nadir. For example, TBI 7.5 Gy where the nadir was reached at day 5 (14.1 ± 0.8 μM) the next consecutive odd day (day 7) recorded a plasma citrulline concentration of 14.4 ± 1.3 μM. The nadir for TBI 10.5 Gy (day 7; 9.6 ± 1.8 μM) and PBI/BM5 11.0 Gy (day 7; 8.0 ± 0.8 μM) was followed by plasma citrulline concentrations at day 9 of 9.6 ± 1.2 μM and 8.9 ± 1.2 μM, respectively. Whereas TBI 10.5 Gy and PBI/BM5 11.0 Gy displayed modest increases in plasma citrulline concentrations following the nadir levels, plasma citrulline concentrations from TBI 7.5 Gy displayed a rebound effect returning to and exceeding day 0 mean values at day 11 and day 13. There was considerable variability among the individual samples for those days with coefficient of variation (CV) greater than 50 %. The elevated mean values at day 13 (44.6 ± 8.2 μM) for TBI 7.5 Gy was immediately followed by sharp decreases in plasma citrulline at day 15 (17.1 ± 2.7 μM).
Samples from TBI 7.5 Gy and PBI/BM5 11.0 Gy were collected through day 60 and day 180, respectively. This provided an opportunity to evaluate the role plasma citrulline played as surviving NHP lived through the acute phase and entered the prolonged GI-ARS. Refer to Fig. 4C for the comparative temporal profiles for TBI 7.5 Gy and PBI/BM5 11.0 Gy out to their respective end points. The general temporal profile for TBI 7.5 Gy plasma citrulline was characterized by sharp decreases and increases over the first 15 days following IR and then subsequent steady increases in plasma citrulline reaching mean values greater than day 0 for NHP that survived past day 35. In contrast, the general temporal profile for PBI/BM5 11.0 Gy followed a steeper, prolonged slope to its nadir value and subsequent steady increase in plasma citrulline with mean values exceeding day 0 values for NHP that survived past day 77.
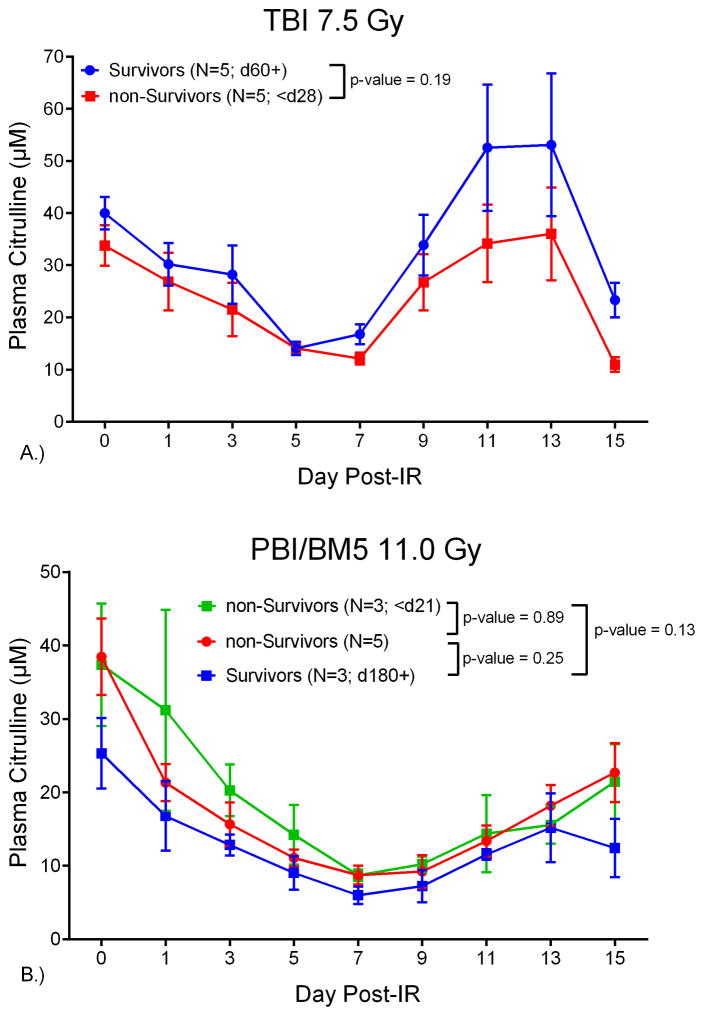
Survivors of the TBI 7.5 Gy dosing schedule were defined as individual NHP that survived the course of the study (day 60+). The TBI 7.5 Gy NHP were divided into survivors (n=5) and non-survivors (n=5). All non-survivors met euthanasia criteria by day 28 and all survivors lived to day 60. Refer to Fig. 5A for the plot of plasma citrulline concentration versus day post-IR for survivors and non-survivors from the TBI 7.5 Gy dosing schedule. The temporal profile for survivors and non-survivors for plasma citrulline concentration up to day 15 were not significantly different (p = 0.19). A similar analysis was performed for NHP exposed to PBI/BM5 11.0 Gy. These NHP were divided into three groups designated survivors (n=3, day 180+), non-survivors (n=3; < day 21), and non-survivors (n=5; > day 21 and < day 180) (Fig. 5B). All non-survivors were removed from the study once euthanasia criteria were satisfied and all survivors were defined as NHP who were still alive at the endpoint of the study (day 180). The plasma citrulline temporal profiles for the 3 groups out to day 15 post-IR were not significantly different (p = 0.89, 0.13, 0.25 comparing the three groups).
Fig. 5.
NHP plasma citrulline versus day post-IR for survivors and non-survivors of A.) TBI 7.5 Gy; n = 5 survivors at day 60 and n = 5 non-survivors that met euthanasia criteria by day 28 and B.) PBI/BM5 11.0 Gy; n = 3 survivors at day 180, n = 3 non-survivors that met euthanasia criteria by day 21, and n = 5 non-surivovrs that met euthanasia criteria between day 21 and day 180. The concentrations were expressed in μM as mean ± sem.
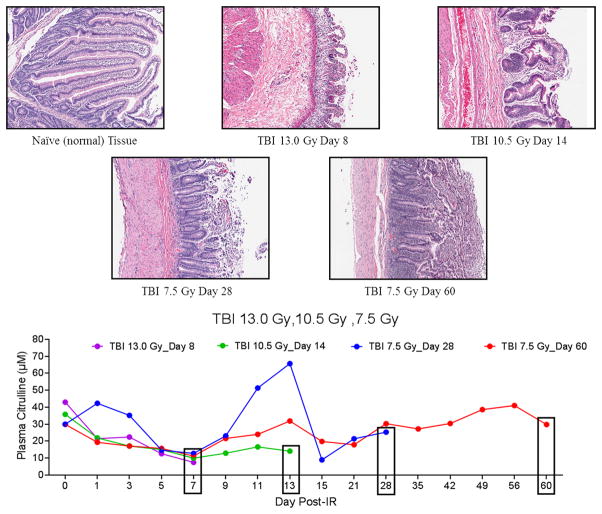
Small intestine histopathology
Sections of the small intestine (jejunum) from matching NHP whose plasma citrulline was analyzed were collected and investigated via H&E staining (Fig. 6 and 7). The jejunum tissue was specifically analyzed as it represented small intestine absorptive function (high abundance of enterocytes) and well-defined crypt-to-villus structure. Naïve NHP jejunum tissue displayed normal crypt-to-villus architecture as indicated by distinct crypt “pockets” and extended villi. Representative H&E images of jejunum tissue from time points corresponding to the euthanasia date from NHP exposed to TBI 13.0 Gy, 10.5 Gy, and 7.5 Gy highlighted radiation-induced damage to the small intestine (Fig. 6). The H&E images from the TBI doses 13.0 Gy (day 8) and 10.5 Gy (day 14) were marked by extensive damage to the crypts, severe villus blunting, and loss of epithelial surface layer which was characteristic of severe morbidity and mortality. Not surprising, GI exposed to the higher dose (TBI 13.0 Gy) displayed more systematic tissue damage than GI exposed to TBI 10.5 Gy. Jejunum tissue from TBI 7.5 Gy from two different time points (a non-survivor at day 28 and an end of study survivor at day 60) were also presented in Fig. 6. The TBI 7.5 Gy time points corresponded to prolonged GI injury. Day 28 was marked by crypt regeneration in terms of depth and cellular output while day 60 was characterized by continued regeneration of crypt structure and increased presence of villi. The plasma citrulline temporal profiles for the corresponding NHP from the H&E images were included in Fig. 6. The individual plasma citrulline traces highlighted both variability and consistency among individual NHP for both inter- and intra-dosing schedules. For example, plasma citrulline profiles for TBI 13.0 Gy (day 8), TBI 10.5 Gy (day 14), and TBI 7.5 Gy (day 60) all displayed analogous temporal profiles out to day 7. Conversely, the plasma citrulline profiles for TBI 7.5 Gy day 28 and day 60 varied considerably between days 1–3 and 11–13 but had very similar values for day 0 and convergent values for days 5–9 and 15–28.
Fig. 6.
Representative H&E stained sections of NHP jejunum and corresponding plasma citrulline profile for NHP exposed to TBI 13.0, 10.5, or 7.5 Gy. Naïve tissue represented normal (non-irradiated) jejunum. The day under each H&E image corresponded to the day of euthanasia. Images were displayed at 10× magnification. The graph represented the plasma citrulline profiles of the same NHP for which the histology was presented. The boxed day corresponded to the last plasma citrulline data point for each NHP.
Fig. 7.
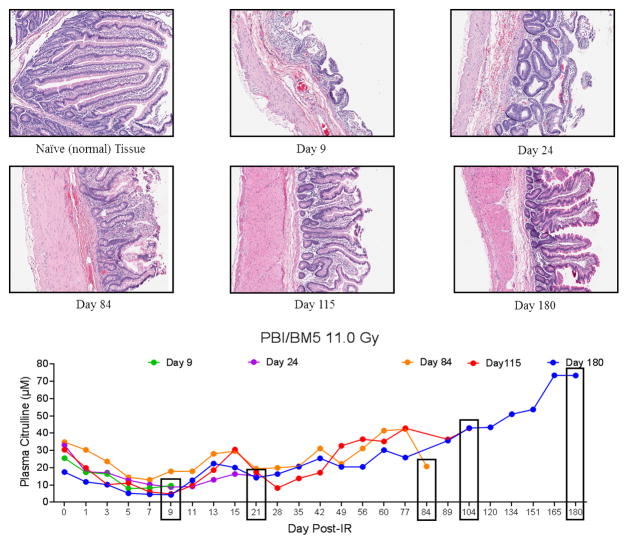
Representative H&E stained sections of NHP jejunum and corresponding plasma citrulline profile for NHP exposed to PBI/BM5 11.0 Gy. Naïve tissue representd normal (non-irradiated) jejunum. The day under each H&E image corresponded to the day of euthanasia. Images were displayed at 10× magnification. The graph represented the plasma citrulline profiles of the same NHP for which the histology was presented. The boxed day corresponded to the last plasma citrulline data point for each NHP.
Similar comparative analysis of the jejunum at specific time points and the corresponding plasma citrulline temporal profile was carried out for PBI/BM5 11.0 Gy (Fig. 7). Unique to the PBI/BM5 11.0 Gy dosing schedule was the end date of the study (day 180). Consequently, surviving NHP were monitored for acute (days 0–15) and “extended” prolonged (day 15–180) GI radiation-induced injuries. The day 9 time point which represented the acute GI phase was characteristic of lethal GI-ARS (extensive villus blunting, near complete loss of crypts, and massive reduction in epithelial surface). The later time points were characterized by continued regeneration and cellular output from surviving crypts leading to villus reconstitution. Noticeable depth to crypt “pockets” and extended protrusion of villi were observed for the day 180 survivor yet continued presence of inflammation and incomplete mucosal reconstitution indicated the prolonged GI injury involved persistent and on-going recovery.
DISCUSSION
Plasma citrulline from NHP exposed to TBI and PBI/BM5 were investigated in order to evaluate the use of circulating citrulline as a biomarker for acute and prolonged GI-ARS. The dosing schedules corresponded to approximate LD90/15 (TBI 13.0 Gy), LD10/15 (TBI 10.5 Gy and PBI/BM5 11.0 Gy), and LD50/60 (TBI 7.5 Gy and PBI/BM5 11.0 Gy) where LDXX/YY corresponded accordingly: LD=lethal dose, XX=percent mortality, and YY=day post-IR (MacVittie et al. 2012a, MacVittie et al. 2012b). The 13.0 Gy and 10.5 Gy TBI dosing schedules covered time points out to day 7 and day 15 (consecutive odd days), respectively, and corresponded to acute GI-ARS. The lower TBI dosing schedule of 7.5 Gy and the PBI/BM5 11.0 Gy consisted of time points not only covering the acute GI phase but also allowed for blood collection from the surviving NHP out to day 60 (TBI 7.5 Gy) and day 180 (PBI/BM5 11.0 Gy). The latter time points reflected the transition of acute to prolonged GI-ARS as surviving NHP pushed past the acute GI injury and into H-ARS and varying degrees of multi-organ injury.
A unique aspect of the NHP radiation model is the nature by which longitudinal studies allow for routine blood sampling from the same individual NHP over the course of the study (or lifetime of NHP during the study). Each study started at day 0, the time point just prior to the IR event, and ended at the pre-determined end point for the specific study or at the time euthanasia criteria were satisfied. All day 0 time points regardless of the IR dosing schedule yielded plasma citrulline concentrations that were statistically equivalent to naïve NHP plasma. Naïve NHP plasma represented blood draws from NHP who were healthy and had never been enrolled in a radiation study. The day 0 time point differed from naïve NHP in that at the time of day 0 sample collection, the NHP were fasted for 18 hours, anesthetized, and administered an antiemetic. The fasting, administration of medication, and pre-IR handling did not affect the NHP’s plasma citrulline. Also of note, considering the day 0 and naïve plasma citrulline were statistically indistinguishable if day 0 (n = 29) and naïve (n = 36) plasma citrulline values were collated, the number of individual baseline NHP plasma samples reached 65 with a plasma citrulline concentration of 39.3 ± 1.3 μM (mean ± sem). This further established baseline NHP plasma citrulline to be highly conserved and nearly identical to human plasma citrulline baseline levels (40 ± 10 μM) (Rabier and Kamoun 1995, Crenn et al. 2008) which indicated the NHP radiation model was an ideal human surrogate especially in regard to the use of circulating citrulline as a potential biomarker of radiation injury.
The use of plasma citrulline as a diagnostic and/or prognostic biomarker of GI-ARS was statistically evaluated in relation to both irradiation dose and time post-IR. There were significant associations between non-irradiated (day 0) plasma citrulline and plasma citrulline sampled 24-hours post-IR for all four dosing schedules (p < 0.05). However, plasma citrulline sampled 24-hours post-IR did not discriminate between the individual IR dosing schedules. Consequently, NHP plasma citrulline at the 24-hour time point following the IR event was diagnostic of high-dose irradiation yet was not informative for discerning radiological dose. The radiation dose to plasma citrulline relationship was consistent for all four dosing schedules out to day 7 with the exception being the plasma citrulline levels for the low TBI dose of 7.5 Gy started to deviate at day 7 when compared to TBI 13.0 Gy and PBI/BM5 11.0 Gy. Taken as a whole, the data established a near linear relationship between day post-IR and decreasing plasma citrulline values for exposure to high-dose irradiation. This relationship was consistent for each dosing schedule out to the longitudinal nadir at which time plasma citrulline concentration plateaued. Of note, the longitudinal nadir was observed at day 5 for TBI 7.5 Gy and at day 7 for the other dosing schemes. When compared to the mean day 0 value, plasma citrulline at nadir decreased 63 % (TBI 7.5 Gy), 74 % (TBI 10.5 Gy), 79 % (PBI/BM5 11.0 Gy), and 80 % (TBI 13.0 Gy). This data was consistent with the link between small intestine enterocyte mass and circulating citrulline and the observation that rapidly proliferating small intestine epithelium was acutely prone to injury from high-dose irradiation. The time points, day 5 and 7, for plasma citrulline to reach its nadir value agreed well with the reported NHP transit time of approximately 7 days for epithelial cells to migrate from the proliferating crypts to the villus tip (MacVittie et al. 2012b). Furthermore, the nadir values and time points were dose dependent with the lowest IR dose (TBI 7.5 Gy) having the highest mean nadir value at day 5 of 14.1 ± 0.8 μM and the highest IR dose (TBI 13.0 Gy) registered the lowest mean nadir value at day 7 of 7.5 ± 0.6 μM.
The acute phase of GI-ARS continued out to day 15 following the IR event. In regards to plasma citrulline, the first 7 days following high-dose irradiation were marked by a near linear decrease in plasma citrulline reaching a nadir value at day 5 or 7 as detailed above. The TBI 13.0 Gy dosing schedule was considered GI lethal (LD90/15); accordingly, all four NHP assayed in this group succumbed to euthanasia criteria and were therefore not available for time points beyond day 7. The histopathology of jejunum tissue at day 8 from a representative NHP in the lethal GI dose study clearly revealed signs of morbidity and mortality by near complete evisceration of the mucosal fine structure of the GI similar to the GI-injury noted in previous reports of NHP, mouse, and minipig radiation models (Wilson 1959, Vigneulle et al. 2002, Booth et al. 2012, MacVittie et al. 2012b, Elliott et al. 2014).
The other three dosing schedules were also evaluated between days 7 and 15. This period was marked by divergence of TBI 7.5 Gy plasma citrulline from the statistically similar traces of TBI 10.5 Gy and PBI/BM5 11.0 Gy models. The nearly identical plasma citrulline profiles during the acute phase of GI-ARS for TBI 10.5 Gy and PBI/BM5 11.0 Gy tracked very well to the similar GI lethality of the two dosing schemes (both reflect approximate LD10/15). Conversely, TBI 10.5 Gy and PBI/BM5 11.0 Gy corresponded to substantially different outcomes for H-ARS. The TBI 10.5 Gy model was H-ARS lethal and PBI/BM5 11.0 Gy was an approximate LD50/60 for the hematopoietic subsyndrome. These dosing schedules in addition to the lethal GI-ARS dose of TBI 13.0 Gy highlighted the limited role plasma citrulline has as a predictive biomarker for survival outcome. The PBI/BM5 dosing schedule spared approximately 5 % bone marrow (tibiae, ankles, and feet) which enhanced survival and off-set life-threatening subsyndromes (e.g., acute H-ARS) by effectively preserving a portion of regenerative hematopoietic stem and progenitor cells (Maillie et al. 1966, Bond and Robinson 1967, Bond et al. 1991, Rauchwerger 1972, Monroy et al. 1988, Baltschukat and Nothdurft 1990, Bertho et al. 2005). GI histology for similar doses of TBI and PBI/BM5 models demonstrated nearly identical loss of crypts at day 7 post-IR and represented approximately 28 % of the non-irradiated mean crypt value indicating GI injury in both TBI and PBI/BM5 models were similar in severity and duration throughout the acute GI-ARS (MacVittie et al. 2012a). Consequently, it was of no surprise that the plasma citrulline data reflected near identical plasma citrulline profiles for the TBI 10.5 Gy and PBI/BM5 11.0 Gy over the acute GI phase. In effect, there was limited if any correlation solely based on plasma citrulline levels that would differentiate similar TBI and PBI/BM5 dosing events.
The lower TBI 7.5 Gy dose highlighted the varying degree to which plasma citrulline rebounded back to pre-IR levels following the longitudinal nadir. The plasma citrulline profile for TBI 7.5 Gy reached its nadir value at day 5, plateaued until day 7, and then demonstrated highly variable (CV > 50 %) for days 11 and 13. The highly variable rebound effect was followed by a drop in plasma citrulline at day 15 (17.1 ± 2.7 μM) near to the day 5 nadir value (14.1 ± 0.8 μM) and was statistical similar to the day 15 PBI/BM5 value (19.3 ± 2.7 μM). The plasma citrulline profile for TBI 7.5 Gy was also consistent with the NHP small intestine epithelial cell transit time (~7 days). The lower dose of 7.5 Gy resulted in less overall small intestine tissue damage compared to the higher TBI and PBI doses (Farese et al. 2012, MacVittie et al. 2012b). Therefore, at TBI 7.5 Gy, concomitant disruption to the small intestine enterocyte mass was less pronounced resulting in less impact on plasma citrulline when compared to higher doses. This translated into an increased nadir both in terms of plasma citrulline concentration and day post-IR. The striking rebound to near day 0 values for plasma citrulline after day 7 and return to near-nadir values at day 15 for the TBI 7.5 Gy model suggested TBI doses in the HARS regime resulted in less uniform enterocyte mass disruption which led to high variability in plasma citrulline profile for individual NHP. The drastic drop in plasma citrulline at day 15 corresponded to the second small intestine epithelial cell turnover which had a consolidating effect for all plasma citrulline recoveries.
NHP exposed to TBI 7.5 Gy or PBI/BM5 11.0 Gy also experienced the H-ARS with a lethality of LD50/60. NHP that survived the initial H- and GI-ARS often experience the intertwined subsyndromes of prolonged GI and multi-organ injury. The plasma citrulline profile for TBI 7.5 Gy beyond day 15 was characterized by steady increases of mean plasma citrulline that, starting on day 35, were greater than the mean value on day 0. In regards to the prolonged GI injury, the rising plasma citrulline levels, viewed as a surrogate for enterocyte mass, indicated that enterocyte mass returned to levels exceeding pre-IR values. This suggested enterocyte mass as a function of plasma citrulline production was recovering and may have reached a level of over-production. The fluctuation in plasma citrulline levels (day 35+) and excessive plasma citrulline concentration emphasized small intestine epithelial homeostasis was not yet achieved during the course of the study. The histopathological measurements at day 28 and day 60 for NHP subjected to TBI 7.5 Gy provided visual evidence of the continued GI injury and on-going recovery of the small intestine crypt-to-villus fine structure. In addition, plasma citrulline concentrations over the first 15 days post-IR did not discriminate surviving and non-surviving NHP in the TBI 7.5 Gy; this emphasized the limited role plasma citrulline played as a prognostic biomarker for survival of NHP exposed to IR doses in the H-ARS range. Of note, plasma citrulline in the TBI 7.5 Gy model was indicative of the radiation insult and informative for revealing the extensive damage to enterocyte function. Yet, as the GI injury transitioned out of the acute and into the prolonged phase of the syndrome, plasma citrulline displayed no correlation to the concomitant H-ARS co-morbidities such as neutropenia, thrombocytopenia, and subsequent infection and hemorrhage which tend to dominate survival outcome for this dosing schedule (Farese et al. 2012, Plett et al. 2012).
The PBI/BM5 11.0 Gy model yielded acute GI injury comparable with TBI 10.5 Gy (both models have an expected LD10/15) yet provided an opportunity to establish the link between acute and delayed radiation effects as the surviving NHP encountered prolonged GI injuries and other delayed subsyndromes. The PBI/BM5 11.0 Gy plasma citrulline profile for the acute GI phase was discussed above and mirrored that of the TBI 10.5 Gy model. Beyond day 15, the PBI/BM5 11.0 Gy plasma citrulline profile followed steady albeit modest increase in plasma citrulline. By day 77 the plasma citrulline concentration returned to the day 0 value and was followed by consistent levels above the day 0 value. Similar to the TBI 7.5 Gy dosing schedule, enterocyte mass as a function of plasma citrulline production recovered to day 0 values and then proceeded to stay increasingly elevated throughout the remainder of the study. This suggested that small intestine enterocyte functional homeostasis was not achieved during the course of the study and the prolonged GI injury was still persistent out to day 180. Further evidence for persistent prolonged GI injury in the PBI/BM5 11.0 Gy model was seen in the longitudinal histopathology of the small intestine where GI recovery was erratic and still evolving at day 180. NHP exposed to PBI/BM5 11.0 Gy were able to be systematically parsed into survivors and non-survivors. Within the non-surviving cohort, further distinction was made between early non-survivors and late non-survivors (those NHP that met euthanasia criteria early versus late in the 180-day study). Plasma citrulline did not discriminate any of the three groups over the first 15 days post-IR. The data clearly indicated plasma citrulline was not a robust predictor of survival in the PBI/BM5 11.0 Gy dosing schedule even though the model was characterized by extensive acute GI damage followed by prolonged GI injury and recovery. Survival for the NHP exposed to PBI/BM5 11.0 Gy was affected by the combined effects of GI injury, variable myelosuppression (and recovery by active hematopoiesis from spared bone marrow), multi-organ injury, and delayed lung injury of which plasma citrulline played an minor role.
The role plasma citrulline plays as a biomarker for acute and prolonged GI injury following high-dose irradiation should be understood in the context of the complexity of radiation-induced injuries. The notion that a specific radiation injury (e.g., acute GI injury) can be thought of as a discrete, isolated event belies the well-documented understanding that radiation affects the biological “whole”. Furthermore, the concept that one single biomarker linked to a specific biological role (e.g., enterocyte function) might inform categorically on specific or general radiation injuries underestimates the interconnected role injury and recovery play in a complex biological unit. Towards developing a panel of biomarkers that inform on radiation injury and repair, plasma citrulline demonstrated important features of a radiation biomarker especially in regards to providing insight into the present state of the acute and prolonged GI injury such as its well documented link to enterocyte mass and its inverse response to high-dose irradiation. Although it was not evaluated here, plasma citrulline’s ability to inform on enterocyte mass may be useful in assessing the efficacy of MCMs that mitigate GI-ARS. However, plasma citrulline as a single biomarker was limited in its ability to discern radiological dose, latency/severity/duration of injury, and ultimately outcome. The full utility of plasma citrulline as a biomarker for acute and prolonged GI-ARS may be realized as “one of many” on a panel of biomarkers/bioindicators. The recommendation that a panel of biomarkers/bioindicators that encompasses a wide variety of parameters has been described previously (Gorin et al. 2006, Blakely et al. 2010, Riecke et al. 2010, Moroni et al. 2014) and has been postulated to potentially provide a more comprehensive understanding of the radiation insult, subsequent injuries and outcomes, and the role that MCMs have in treatment and mitigation from IR exposure.
CONCLUSION
The role plasma citrulline may have as a biomarker for acute and prolonged GI injury was examined in various NHP radiation models that covered the sublethal, mid-lethal, and lethal GI syndrome. Plasma citrulline concentration was determined longitudinally over the course of acute GI-ARS (days 0–15) and throughout prolonged GI-ARS (days 15–180). The baseline plasma citrulline concentration for 65 NHP was reported and represented the most comprehensive comparison to human plasma citrulline to date. The values confirmed plasma citrulline from naïve NHP was identical to normal human plasma citrulline further validating the NHP’s role of human surrogate for studies of radiation-induced injury. Plasma citrulline values markedly decreased after high-dose irradiation with statistical significance starting 24-hours after the IR event and reaching nadir values by days 5–7. However, plasma citrulline did not discriminate between radiological doses covering lethal to surviving GI-ARS. Furthermore, plasma citrulline was not predictive of NHP survival. The longitudinal plasma citrulline profiles suggested acute and prolonged GI-ARS were highly disruptive to small intestine enterocyte function and recovery of enterocytes was variable and persistent. Matching histological evidence over the same time course further substantiated the extensive impact high-dose irradiation has on the acute and prolonged GI injury.
Acknowledgments
Funding Source:
This project has been funded in whole or in part with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN272201000046C
This work was funded with Federal funds from the National Institute of Allergy and Infectious Diseases (Contract number HHSN272201000046C) and, in part, by the University of Maryland School of Pharmacy Mass Spectrometry Center (SOP1841-IQB2014). The authors would like to thank all members of the Medical Countermeasures Against Radiological Threats (MCART) consortium for their dedication, support, and guidance in establishing biomarker identification and validation as a priority in the radiation medical counter measure field. Additionally, we would like to acknowledge and thank all members of the Kane laboratory.
References
- Baltschukat K, Nothdurft W. Hematological effects of unilateral and bilateral exposures of dogs to 300-kVp X rays. Radiat Res. 1990;123:7–16. [PubMed] [Google Scholar]
- Bertho J-M, Prat M, Frick J, Demarquay C, Gaugler M-H, Dudoignon N, Clairand I, Chapel A, Gorin N-C, Thierry D, Gourmelon P. Application of autologous hematopoietic cell therapy to a nonhuman primate model of heterogeneous high-dose irradiation. Radiat Res. 2005;163:557–570. doi: 10.1667/rr3352. [DOI] [PubMed] [Google Scholar]
- Blakely WF, Ossetrova NI, Whitnall MH, Sandgren DJ, Krivokrysenko VI, Shakhov A, Feinstein E. Multiple parameter radiation injury assessment using a nonhuman primate radiation model-biodosimetry applications. Health Phys. 2010;98:153–159. doi: 10.1097/HP.0b013e3181b0306d. [DOI] [PubMed] [Google Scholar]
- Bond VP, Carsten AL, Bullis J, Roth SP. Severity of organ injury as a predictor of acute mortality for disparate patterns of absorbed dose distribution. Radiat Res. 1991;128:S9–11. [PubMed] [Google Scholar]
- Bond VP, Robinson CV. A mortality determinant in nonuniform exposures of the mammal. Radiat Res. 1967;7:265–275. [PubMed] [Google Scholar]
- Booth C, Tudor G, Tudor J, Katz BP, MacVittie TJ. Acute Gastrointestinal Syndrome in High-Dose Irradiated Mice. Health Phys. 2012;103:383–399. doi: 10.1097/hp.0b013e318266ee13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coy SL, Cheema AK, Tyburski JB, Laiakis EC, Collins SP, Fornace A. Radiation metabolomics and its potential in biodosimetry. Int J Radiat Biol. 2011;87:802–823. doi: 10.3109/09553002.2011.556177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crenn P, Messing B, Cynober L. Citrulline as a biomarker of intestinal failure due to enterocyte mass reduction. Clin Nutr. 2008;27:328–339. doi: 10.1016/j.clnu.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Elliott TB, Deutz NE, Gulani J, Koch A, Olsen CH, Christensen C, Chappell M, Whitnall MH, Moroni M. Gastrointestinal acute radiation syndrome in Göttingen minipigs (Sus scrofa domestica) Comp Med. 2014;64:456–463. [PMC free article] [PubMed] [Google Scholar]
- Farese AM, Cohen MV, Katz BP, Smith CP, Jackson W, Cohen DM, Macvittie TJ. A Nonhuman Primate Model of the Hematopoietic Acute Radiation Syndrome Plus Medical Management. Health Phys. 2012;103:367–382. doi: 10.1097/HP.0b013e31827a307e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorin N-CNC, Fliedner TM, Gourmelon P, Ganser a, Meineke V, Sirohi B, Powles R, Apperley J. Consensus conference on European preparedness for haematological and other medical management of mass radiation accidents. Ann Hematol. 2006;85:671–679. doi: 10.1007/s00277-006-0153-x. [DOI] [PubMed] [Google Scholar]
- Gupta PK, Brown J, Biju PG, Thaden J, Deutz NE, Kumar S, Hauer-Jensen M, Hendrickson HP. Development of high-throughput HILIC-MS/MS methodology for plasma citrulline determination in multiple species. Anal Methods. 2011;3:1759–1768. doi: 10.1039/c1ay05213f. [DOI] [Google Scholar]
- Jones JW, Scott AJ, Tudor G, Xu P-T, Jackson IL, Vujaskovic Z, Booth C, MacVittie TJ, Ernst RK, Kane MA. Identification and Quantitation of Biomarkers for Radiation-induced Injury via Mass Spectrometry. Health Phys. 2014;106:106–119. doi: 10.1097/HP.0b013e3182a4ed3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JW, Tudor G, Bennett A, Farese AM, Moroni M, Booth C, MacVittie TJ, Kane MA. Development and validation of a LC-MS/MS assay for quantitation of plasma citrulline for application to animal models of the acute radiation syndrome across multiple species. Anal Bioanal Chem. 2014;406:4663–4675. doi: 10.1007/s00216-014-7870-0. [DOI] [PubMed] [Google Scholar]
- Jones JW, Tudor G, Li F, Tong Y, Katz B, Farese AM, MacVittie TJ, Booth C, Kane MA. Citrulline as a biomarker in the murine total-body irradiation model: correlation of circulating and tissue citrulline to small intestine epithelial histopathology. Health Phys. 2015 doi: 10.1097/HP.0000000000000346. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutgens LC, Lambin P. Biomarkers for radiation-induced small bowel epithelial damage: an emerging role for plasma Citrulline. World J Gastroenterol. 2007;13:3033–3042. doi: 10.3748/wjg.v13.i22.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutgens LC, Deutz NE, Gueulette J, Cleutjens JP, Berger MP, Wouters BG, von Meyenfeldt MF, Lambin P. Citrulline: A physiologic marker enabling quantitation and monitoring of epithelial radiation-induced small bowel damage. Int J Radiat Oncol. 2003;57:1067–1074. doi: 10.1016/S0360-3016(03)00781-8. [DOI] [PubMed] [Google Scholar]
- Lutgens LC, Deutz N, Granzier-Peeters M, Beets-Tan R, De Ruysscher D, Gueulette J, Cleutjens J, Berger M, Wouters B, von Meyenfeldt M, Lambin P. Plasma citrulline concentration: a surrogate end point for radiation-induced mucosal atrophy of the small bowel. A feasibility study in 23 patients. Int J Radiat Oncol Biol Phys. 2004;60:275–285. doi: 10.1016/j.ijrobp.2004.02.052. [DOI] [PubMed] [Google Scholar]
- MacVittie TJ, Bennett A, Booth C, Garofalo M, Tudor G, Ward A, Shea-Donohue T, Gelfond D, McFarland E, Jackson W, Lu W, Farese AM. The prolonged gastrointestinal syndrome in rhesus macaques: the relationship between gastrointestinal, hematopoietic, and delayed multi-organ sequelae following acute, potentially lethal, partial-body irradiation. Health Phys. 2012;103:427–453. doi: 10.1097/HP.0b013e318266eb4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacVittie TJ, Farese AM, Bennett A, Gelfond D, Shea-Donohue T, Tudor G, Booth C, McFarland E, Jackson W. The acute gastrointestinal subsyndrome of the acute radiation syndrome: a rhesus macaque model. Health Phys. 2012;103:411–426. doi: 10.1097/HP.0b013e31826525f0. [DOI] [PubMed] [Google Scholar]
- MacVittie TJ. The MCART Consortium animal models series. Health Phys. 2012;103:340–342. doi: 10.1097/HP.0b013e318261175a. [DOI] [PubMed] [Google Scholar]
- MacVittie TJ. The MCART consortium animal models series: an evolving MCART. Health Phys. 2014;106:1–6. doi: 10.1097/HP.0b013e3182a03a2b. [DOI] [PubMed] [Google Scholar]
- Maillie HD, Krasavage W, Mermagen H. On the partial-body irradiation of the dog. Health Phys. 1966;12:883–887. doi: 10.1097/00004032-196607000-00001. [DOI] [PubMed] [Google Scholar]
- Monroy RL, Skelly RR, Taylor P, Dubois A, Donahue RE, MacVittie TJ. Recovery from severe hematopoietic suppression using recombinant human granulocyte-macrophage colony-stimulating factor. Exp Hematol. 1988;16:344–348. [PubMed] [Google Scholar]
- Moroni M, Port M, Koch A, Gulani J, Meineke V, Abend M. Significance of bioindicators to predict survival in irradiated minipigs. Health Phys. 2014;106:727–733. doi: 10.1097/HP.0000000000000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossetrova NI, Sandgren DJ, Gallego S, Blakely WF. Combined approach of hematological biomarkers and plasma protein SAA for improvement of radiation dose assessment triage in biodosimetry applications. Health Phys. 2010;98:204–208. doi: 10.1097/HP.0b013e3181abaabf. [DOI] [PubMed] [Google Scholar]
- Pandey BN, Kumar A, Tiwari P, Mishra KP. Radiobiological basis in management of accidental radiation exposure. Int J Radiat Biol. 2010;86:613–635. doi: 10.3109/09553001003746059. [DOI] [PubMed] [Google Scholar]
- Plett PA, Sampson CH, Chua HL, Joshi M, Booth C, Gough A, Johnson CS, Katz BP, Farese AM, Parker J, MacVittie TJ, Orschell CM. Establishing a murine model of the hematopoietic syndrome of the acute radiation syndrome. Health Phys. 2012;103:343–355. doi: 10.1097/HP.0b013e3182667309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten CS. A comprehensive study of the radiobiological response of the murine (BDF1) small intestine. Int J Radiat Biol. 1990;58:925–73. doi: 10.1080/09553009014552281. [DOI] [PubMed] [Google Scholar]
- Rabier D, Kamoun P. Metabolism of citrulline in man. Amino Acids. 1995;9:299–316. doi: 10.1007/BF00807268. [DOI] [PubMed] [Google Scholar]
- Rauchwerger JM. Radiation protection by tibia-shielding in adult, weanling and suckling mice. I. Comparative protection studies. Int J Radiat Biol Relat Stud Phys Chem Med. 1972;22:269–278. doi: 10.1080/09553007214551041. [DOI] [PubMed] [Google Scholar]
- Riecke A, Ruf CG, Meineke V. Assessment of radiation damage-the need for a multiparametric and integrative approach with the help of both clinical and biological dosimetry. Health Phys. 2010;98:160–167. doi: 10.1097/HP.0b013e3181b97306. [DOI] [PubMed] [Google Scholar]
- Vigneulle RM, Rao S, Fasano A, MacVittie TJ. Structural and functional alterations of the gastrointestinal tract following radiation-induced injury in the rhesus monkey. Dig Dis Sci. 2002;47:1480–1491. doi: 10.1023/a:1015846514471. [DOI] [PubMed] [Google Scholar]
- Wakabayashi Y, Yamada E, Hasegawa T, Yamada R. Enzymological evidence for the indispensability of small intestine in the synthesis of arginine from glutamate. Arch Biochem Biophys. 1991;291:1–8. doi: 10.1016/0003-9861(91)90097-3. [DOI] [PubMed] [Google Scholar]
- Wakabayashi Y, Yamada E, Yoshida T, Takahashi N. Effect of intestinal resection and arginine-free diet on rat physiology Effect of intestinal on rat physiology resection and arginine-free diet. Am J Physiol Gastrointest Liver Physiol. 1995;269:G313–G318. doi: 10.1152/ajpgi.1995.269.2.G313. [DOI] [PubMed] [Google Scholar]
- Wilson SG. Radiation-induced gastrointestinal death in the monkey. Am J Pathol. 1959;35:1233–1251. [PMC free article] [PubMed] [Google Scholar]