Abstract
Exposure to sufficiently high doses of ionizing radiation is known to cause fibrosis in many different organs and tissues. Connective tissue growth factor (CTGF/CCN2), a member of the CCN family of matricellular proteins, plays an important role in the development of fibrosis in multiple organs. The aim of the present study was to quantify the gene and protein expression of CTGF in a variety of organs from non-human primates (NHP) that were previously exposed to potentially lethal doses of radiation. Tissues from non-irradiated NHP, and NHP exposed to whole thoracic lung irradiation (WTLI) or partial-body irradiation with 5% bone marrow sparing (PBI/BM5) were examined by real-time quantitative reverse transcription PCR, western blot, and immunohistochemistry. Expression of CTGF was elevated in the lung tissues of NHP exposed to WTLI relative to the lung tissues of the non-irradiated NHP. Increased expression of CTGF was also observed in multiple organs from NHP exposed to PBI/BM5 compared to non-irradiated NHP; these included the lung, kidney, spleen, thymus and liver. These irradiated organs also exhibited histological evidence of increased collagen deposition compared to the control tissues. There was significant correlation of CTGF expression with collagen deposition in the lung and spleen of NHP exposed to PBI/BM5. Significant correlations were observed between spleen and multiple organs on CTGF expression and collagen deposition respectively, suggesting possible crosstalk between spleen and other organs. Our data suggest that CTGF levels are increased in multiple organs after radiation exposure and that inflammatory cell infiltration may contribute to the elevated levels of CTGF in multiple organs.
Keywords: CTGF, radiation-induced fibrosis, non-human primate, multiple organs
INTRODUCTION
The September 11, 2001 terrorist attack has stimulated much interest in the study of potential health risks from acute radiation exposure due to a nuclear radiation event (Poston JW 2005; DiCarlo et al. 2011). Much attention has been focused on the immediate effects of radiation, the so-called acute radiation syndrome (ARS), that involves the hematopoietic (H) and gastrointestinal (GI) systems (Hall and Giaccia 2006). Recent studies have shown that the H-ARS can be successfully managed with hematopoietic growth factors (Farese et al. 1996; Farese et al. 2013; Hankey et al. 2015). Therefore, government guidelines and research focuses have started to shift towards linking the ARS and the delayed effect of acute radiation exposure (DEARE) on multiple organs (Williams and McBride 2011).
Fibrosis is marked by the excessive accumulation of fibrous connective tissue (such as collagen and fibronectin) at sites of injury, which causes irreversible damage to organ structure, function with increased morbidity and potential mortality (Wynn 2007; Wynn and Ramalingam 2012). The role of radiation in the induction of fibrosis has been well documented. Following radiotherapy to treat cancer, patients may develop fibrosis in different organs, such as lung (Ghafoori et al. 2008; Williams et al. 2010), heart (Finch et al. 2014), liver (Lawrence et al. 1995), gastrointestinal (GI) tract (Stacey and Green 2014) and skin (Bourgeois et al. 2003). Additionally, excessive cutaneous fibrosis was observed in survivors of the Chernobyl power plant disaster five to nine years after the accident (Peter et al. 1999).
It is important to understand and elucidate the mechanisms that underlie radiation-induced fibrosis (RIF). Many animal models have been established to study RIF in multiple organs, such as lung (Jackson et al. 2012), GI (Rieder et al. 2012) and liver (Du et al. 2010). Until recently, these RIF models were established primarily using rodents. Our laboratory has established a non-human primate (NHP) research platform of radiation injury that permits the study of both short- and long-term damage to multiple organ systems (including the hematopoietic organs, lung, heart, kidney, GI) in a dose- and time-dependent manner ( Farese et al. 2012; MacVittie et al. 2012a; MacVittie et al. 2012b; Garofalo et al. 2014a). NHP models and dose-response relationships of whole thorax lung irradiation (WTLI) and partial-body irradiation with approximate 5% bone marrow sparing (PBI/BM5) were established to investigate the DEARE as a characteristic of multi-organ injury. These models were used to study the efficacy of medical countermeasures against radiation to enhance survival and overall quality-of-life (Farese et al. 2013; Farese et al. 2014; Garofalo et al. 2014b). Because of the similarity of NHP to humans, the NHP platform is unique in providing a closely relevant model to study RIF in multiple organs.
Substantial progress has been made in understanding the molecular and cellular mechanism of fibrosis (Wynn 2008; Liu 2011; Todd et al. 2012; Duffield 2014). Among the many molecules that are important for fibrogenesis, connective tissue growth factor (CTGF or CCN2) has been suggested to play a central role in fibrosis and blocking CTGF may halt or even reverse the process of fibrosis (Rachfal and Brigstock 2003; Lipson et al. 2012). CTGF has been shown to be required for persistent fibrosis after injection of transforming growth factor-beta (TGF-β) into the subcutaneous tissue of newborn mice (Mori et al. 1999). Blockade of CTGF by a neutralizing antibody or siRNA prevented the development of fibrosis in different organs such as liver, skin, kidney and lung (Li et al. 2006; Ikawa et al. 2008; Wang et al. 2011). Importantly, increased expression of CTGF was observed in patients experiencing radiation enteritis (Vozenin-Brotons et al. 2003; Bourgier et al. 2005). Increased expression of CTGF was also observed in various organs such as lung (Kalash et al. 2014), kidney (Liu and Wang 2008; Kruse et al. 2009) and liver (Cheng et al. 2015) after radiation exposure limited to the targeted areas in rodent models. However, there is still a lack of knowledge about CTGF's roles in RIF in multiple organs (within each individual organ and possibly across different organs), particularly in relevant NHP models of radiation exposure.
In this study, the expression of CTGF was investigated in multiple organs after exposure to lethal doses of radiation in two established NHP models of RIF. In the WTLI model, CTGF levels were assessed using real-time quantitative reverse transcription PCR (qRT-PCR), western blot and immunohistochemistry (IHC). In the PBI/BM5 model, IHC and Masson's trichrome staining were performed in the lung, kidney, spleen, thymus and liver. The correlation of CTGF expression and collagen deposition in the same organ was studied. The correlation of CTGF expression and collagen deposition between different organs was also studied respectively. Our data provide important insights into the relevance of CTGF to RIF in multiple NHP organs.
MATERIALS AND METHODS
Study animals
Male Chinese rhesus macaques (Macaca mulatta, 4.0–11.5kg BW) from several contemporary pre-clinical studies were included in this investigation. Table 1 summarized the sources of NHP used in the current study. The major endpoint for the pre-clinical studies was survival after radiation and control/test article treatments. Therefore, NHP were euthanized not on pre-selected time points but rather when individual NHP met euthanasia criteria. In the current paper, only NHP received control articles were used. NHP received test articles were excluded.
Table 1.
Sources of NHP used in the current study.
| Original preclinical studies |
NHP used in the current study |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Radiation exposure | Study Duration (days) | Dose cohort (Gy) | # of NHP treated with |
Total # of NHP | Dose cohort (Gy) | # of NHP treated with |
Total # of NHP | Age (years) at irradiation (mean±s.e.m) | Figures in the manuscript | ||
| Control articlea | Test article | Control articlea | Test article | |||||||||
| Garofalo et al. 2014a | WTLI | 180 | 9.0 | 8 | N/A | 8 | 9.0 | 8 | N/A | 8 | 4.39 ± 0.10 | Fig.1a&b |
| 9.5 | 10 | N/A | 10 | 9.5 | 0 | N/A | 0 | |||||
| 10.0 | 8 | N/A | 8 | 10.0 | 8 | N/A | 8 | |||||
| 10.5 | 10 | N/A | 10 | 10.5 | 0 | N/A | 0 | |||||
| 11.0 | 10 | N/A | 10 | 11.0 | 10 | N/A | 10 | |||||
| 12.0 | 4 | N/A | 4 | 12.0 | 4 | N/A | 4 | |||||
| Garofalo et al. 2014b | WTLI | 180 | 11.5 | 6 | 7 | 13 | 11.5 | 6 | 0 | 6 | 4.82 ± 0.19 | Fig.1c&d&f |
| unpublished | WTLI | 180 | 11.74 | 20 | 60 | 80 | 11.74 | 1 | 0 | 1 | 5.30 ± 0.00 | Fig.1e |
| unpublished | PBI/BM5 | 180 | 10.0 | 5 | 15 | 20 | 10.0 | 5 | 0 | 5 | 4.97 ± 0.09 | Fig.2-Fig.9 |
| 11.0 | 7 | 21 | 28 | 11.0 | 6b | 0 | 6b | |||||
control article: no article, saline or 5% dextrose in water (D5W) injection.
One lone NHP in the PBI/BM5 study received control article was not used because it was euthanized at day 9, not fitting the 1 month (mo), 3 mo, or 6 mo time points.
Tissues from four to seven non-irradiated NHP were used in the current study as the non-irradiated controls. These NHP were extra animals from the pre-clinical studies and of similar condition as the irradiated NHP such as age and body weight. Numbers of the control samples in each experiment may vary due to tissue availability. A maximum number of available control NHP tissues was used in each experiment to reduce the variance. The mean age ± standard error of mean (s. e. m) of the control NHP was 4.68 ± 0.22 years. The age of the control NHP was not significantly different compared to any irradiated group.
NHP housing and care was performed in accordance with the Animal Welfare Act (7 U.S.C. 2131 et seq.) at the University of Maryland's AAALAC-accredited animal facility. NHP were provided certified primate chow (Harlan Laboratories Inc., 2826 Latham Drive, Madison, WI 53744) ad libitum that was supplemented with fresh fruit and vegetables (e.g., apples, bananas, pears, grapes, sweet potato, green beans) and primate treats. All NHP were in good health, sero-negative for simian immunodeficiency virus, simian T cell leukemia virus type 1, and herpes B virus, and negative for malaria and tuberculosis.
Irradiation and dosimetry
Detailed irradiation procedures and dosimetry methods were described previously (MacVittie et al. 2012a; Garofalo et al. 2014a; Garofalo et al. 2014b). Briefly, NHP were exposed to WTLI or PBI/BM5. Irradiations were performed at the University of Maryland Medical Center, Department of Radiation Oncology, utilizing a 6 MV photon beam derived from a clinical linear accelerator and delivered at a dose rate of approximately 0.80 Gy min−1 (2 MV average, Varian TrueBeam™). NHP were sedated with ketamine, secured in a supine restraint device and administered xylazine to ensure proper radiation field placement would be sustained. The radiation field for WTLI included both lungs in their entirety. For PBI/BM5, the NHP were positioned with their tibiae, ankles, and feet outside of the beam field. Radiation doses were prescribed to each animal's midline as measured by the separation at the xiphoid process. Exposures were bilateral and uniform. Half of each prescribed dose was delivered by an anteroposterior (AP) beam and half by a posteroanterior (PA) beam. In vivo dosimetry was performed using either silicon diodes or optically stimulated luminescence detectors (OSLDs, Landauer nanoDots™).
Medical management
All NHP received medical management during the study according to an Institutional Animal Care and Use Committee (IACUC)-approved protocol of triggers and treatments, as described previously (Farese et al. 2012; MacVittie et al. 2012a; Garofalo et al. 2014a; Garofalo et al. 2014b). Treatments were based on clinical signs and symptoms and included rehydration fluids, antibiotics, analgesics, antidiarrheals, antipyretics, antiemetics, cytoprotection for mucosal ulcers, corticosteroids, nutritional support, and blood transfusions. The animals were observed for 180 day (d) post-exposure, or until euthanasia per IACUC-stipulated clinical criteria. All medical management procedures were performed in accordance with the Animal Welfare Act.
Euthanasia
Euthanasia criteria and procedures were described previously (Farese et al. 2012; MacVittie et al. 2012a; Garofalo et al. 2014a). Briefly, NHP that met pre-established euthanasia criteria according to the IACUC protocol were euthanized with DEA Class III euthanasia solution (Euthasol®, Vibrac AH Inc., 3200 Meacham Blvd., Fort Worth, TX 76137). All surviving NHP were euthanized at the end of each study.
Tissue collection
Samples from major organs were collected at necropsy. Tissue samples were snap-frozen in liquid nitrogen, stored in RNAlater solution (QIAGEN Inc., 27220 Turnberry Lane, Suite 200, Valencia, CA 91335), or fixed in 10% buffered formalin and later processed for histology.
qRT-PCR
Total RNA was isolated from homogenized samples of lung tissues using Qiagen's RNeasy mini kit (QIAGEN Inc.) with DNase I treatment. Total RNA was reverse transcribed into cDNA using iScript™ Reverse Transcription Supermix for RT-qPCR (Bio-Rad Laboratories Inc., 4000 Alfred Nobel Drive, Hercules, CA 94547). The real-time PCR reaction included SsoFast Probes supermix (Bio-Rad Laboratories Inc.), Macaca mulatta specific Taqman® Gene probes and cDNA template. Macaca mulatta specific Taqman® Gene probes (CTGF (Rh02840320_m1) and Actin (Rh03043379_gH)) were purchased from Life Technologies (7335 Executive Way, Frederick, MD 21704). The assay was performed on the CFX96 Real-time PCR Detection System (Bio-Rad Laboratories Inc.). Each sample was run in duplicate. Individual results for CTGF were normalized against their respective endogenous control (Actin) results, and values for each sample were reported as fold change relative to the mean normalized CTGF of the controls.
Western blot
Snap frozen lung tissue samples were lysed in RIPA buffer (Cell Signaling Technology Inc., 3 Trask Lane, Danvers, MA 01923). Protein concentrations were determined using QuickStart Bradford Protein Assay (Bio-Rad Laboratories Inc.). Lysates containing 20–40 μg protein were electrophoresed on SDS-PAGE gels and electro-transferred onto polyvinylidene fluoride (PVDF) membranes. The blots were blocked with 5% non-fat dry milk and then incubated overnight at 4°C with goat polyclonal antibody specific for CTGF (1:500, Santa Cruz Biotechnology, 10410 Finnell Street, Dallas, TX 75220. Catalog number sc-14939). The reactions were visualized using SuperSignal® West Femto kit (ThermoFisher Scientific Inc., 81 Wyman Street, Waltham, MA 02451) and the chemilumescence signals were acquired using a GE ImageQuant LAS4000 Luminescent Image Analyzer (GE Healthcare Bio-Sciences, 800 Centennial Avenue, Piscataway, NJ 08855). Density of protein bands was quantified using NIH ImageJ (version 1.45S) image processing and analysis program (NIH Image, 9000 Rockville Pike, Bethesda, MD). The blots then were stripped and re-probed with glyceraldehyde 3-phosphate dehydrogenase (GAPDH, Santa Cruz Biotechnology) as loading controls. Data were reported as fold change of CTGF protein relative to the mean normalized CTGF of the controls.
IHC and trichrome staining and quantification
Tissues procured at necropsy were fixed in 10% formalin, embedded in paraffin and cut into 5 μm sections. For IHC staining, antigen retrieval was performed in a PT Module (ThermoFisher Scientific Inc.) using a programmable controlled heating and cooling cycle. BloxAll solution (Vector Laboratories, Inc., 30 Ingold Road, Burlingame, CA 94010) was used to quench the endogenous peroxidase activity. Specimens were blocked with 2.5% normal horse serum (Vector Laboratories, Inc.) and then incubated at 4°C overnight with goat polyclonal antibody against CTGF (1:1000 diluted in 2.5% normal horse serum, Santa Cruz Biotechnology. Catalog number sc-14939) or isotype control, normal goat IgG (1:1000, EMD Millipore, 28820 Single Oak Drive, Temecula, CA 92590. Catalog number NI02). Specimens were washed and then subsequently incubated with an ImmPRESS™ anti-goat Ig (Peroxidase) Polymer Detection system (Vector Laboratories, Inc.) following the manufacturer's instructions. ImmPACT DAB kit (Vector Laboratories, Inc.) was used to develop the substrate to the desired stain intensity. The slides were counterstained with hematoxylin and scanned by a ScanScope system (Aperio ePathology: Leica Biosystems, 1360 Park Center Drive, Vista, CA 92081) with an approximate resolution of 0.5 microns per pixel. A web based platform eSlide Manager (Aperio ePathology) was utilized to save, view and analyze the virtual slides. CTGF expression in the IHC images was calculated using the Positive Pixel Count (PPC) v8 algorithm of Aperio's annotation software (ImageScope v12, Aperio ePathology). The PPC algorithm calculates the percent of positivity (percent of positively stained pixels among all pixels (positive and negative pixels) excluding white areas (there is no pixels in white areas)) of virtual slides. Positive pixels are defined by the hue and saturation values of a designated color (brown color here, a product of DAB oxidation). Negative pixels are pixels that do not fall into the positive-color specification. The parameters of the PPC algorithm in this study were 0.1 for hue value, 0.4 for hue width, and 0.15 for Color Saturation Threshold. To assess renal glomerular expression of CTGF, 20 glomeruli were randomly selected from each virtual slide. CTGF positivity in each glomerulus was quantified and the mean of the 20 measurements was calculated for each organ. CTGF positivity in renal tubules and other organs was quantified as follows: 10 random fields of 0.5 mm × 0.5 mm were chosen from each virtual slide at lower magnification (to avoid large vessels), CTGF positivity in each of the 10 fields was quantified and the mean of the 10 measurements was calculated for each organ.
To visualize and quantify collagen deposition, Masson's trichrome staining was performed using a commercial kit (Sigma-Aldrich Corp., P.O.Box 14508, St. Louis, MO 63178. Catalog number HT15 kit) following the manufacturer's instructions. Stained slides were scanned and the images were managed using the Aperio system as described for the IHC stained slides. The blue staining of collagen was deconvoluted and the percent of positive blue staining was calculated using the Color Deconvolution v9 algorithm (Aperio ePathology). The algorithm accurately measures the area for each stain separately even when different stains are superimposed at the same location. In the algorithm window, the RGB values for the aniline blue staining were 0.83, 0.50, and 0.23; the RGB values for non-blue staining were 0.51, 0.71 and 0.50; the RGB values for the third channel were 0, 0, and 0. Percent of fibrotic area (collagen positivity) was calculated automatically by the algorithm and corresponded to the aniline blue stained area.
Both IHC and trichrome staining were performed at least twice and the mean of both experiments was calculated for each organ.
Statistical analysis
Statistical analysis was performed with GraphPad Prism 5 (GraphPad Software, Inc., 7825 Fay Avenue, Suite 230, La Jolla, CA 92037). Values were expressed as the mean ± s.e.m. Statistical analysis was performed using unpaired Student's t test to compare the difference between the control group and the irradiated group. Correlations were calculated by using Pearson's correlation analysis. Differences were considered statistically significant at p < 0.05.
RESULTS
1. WTLI: Increased expression of CTGF in the lung tissues
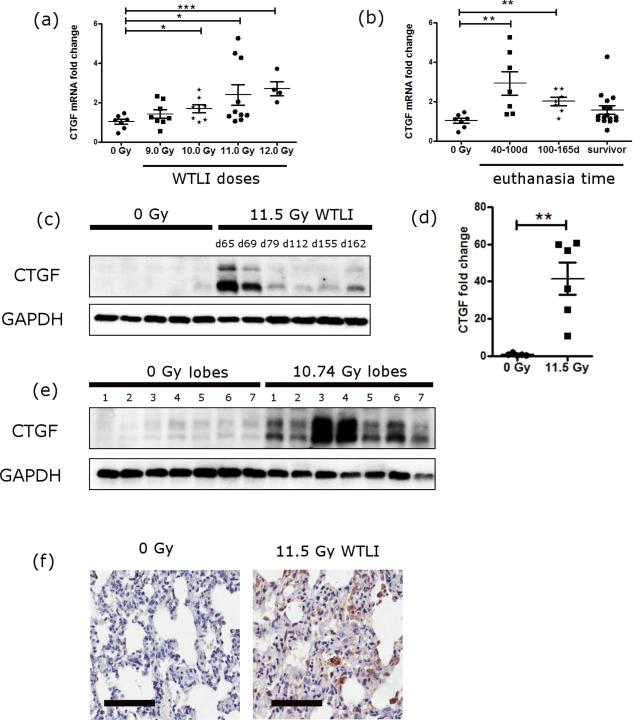
The mRNA levels of CTGF in the lung tissues of NHP exposed to different doses of WTLI were measured using qRT-PCR. Expression of CTGF mRNA increased in a radiation dose-dependent manner in the lungs of NHP exposed to 9.0-12.0 Gy WTLI (Fig. 1a). Except the 9.0 Gy cohort, all other dose cohorts had significantly higher CTGF levels compared to that of the non-irradiated controls. The relative fold changes of CTGF were 1.70 ± 0.20 (10.0 Gy), 2.40 ± 0.51 (11.0 Gy) and 2.73 ± 0.36 (12.0 Gy) vs. 1.04 ± 0.13 (controls), p<0.05, p<0.05 and p<0.001 respectively. Irradiated NHP were not euthanized at the same time point, which may contribute to the variation of CTGF expression within each dose cohort. The average survival time for each cohort was 180 d (9.0 Gy, all survivors), 147 ± 13 d (10.0 Gy), 133 ± 18 d (11.0 Gy) and 90 ± 14 d (12.0 Gy). The expression of CTGF mRNA also showed a time-dependent change after radiation exposure without considering radiation doses (Fig. 1b). NHP euthanized at early time points (d 40-100) after radiation exposure exhibited significantly increased CTGF mRNA expression compared to the control group (fold change of 2.93 ± 0.60 vs. 1.04 ± 0.13, p<0.01). NHP euthanized at later time points (d 100-165) also had a significantly increased level of CTGF (2.02 ± 0.21 vs. the control group 1.04 ± 0.13, p<0.01). NHP that survived to d 180 had increased but not significantly different levels of CTGF compared to the control group.
Fig. 1.
CTGF expression in the lungs of NHP exposed to WTLI. (a) mRNA levels of CTGF in the control group and each radiation dose (9.0-12.0 Gy WTLI) cohorts. CTGF mRNA level was determined using qRT-PCR. Two lung samples from each NHP were used for the assay (i.e. lung tissues distant to the hilum from two random lobes). The mean from both lobes for each NHP is shown here. Data are grouped based on the radiation doses irrespective the survival time after radiation. N = 7 in the control group, n = 8 in the 9.0 Gy group, n = 8 in the 10.0 Gy group, n = 10 in the 11.0 Gy group and n = 4 in the 12.0 Gy group (n is the number of NHP in each group). (b) NHP mRNA levels of CTGF based on the survival status irrespective of the radiation doses. Same data from (a) is plotted against the survival status of NHP after radiation (euthanized between d 40 to 100 after radiation, n=7; euthanized between d 100 to 165 after radiation, n=7; and survivors, n=16). (c) Western blot of CTGF in the lung tissues of control NHP and NHP exposed to 11.5 Gy WTLI. Survival time (in days) of each NHP in the 11.5 Gy WTLI group is indicated above the blot image. Each lane represents one NHP. GAPDH used as a loading control. N = 5 in the control group and n = 6 in the WTLI group. Lung samples from the left upper lobes and right upper lobes were used for western blot and the western blot of each lobe was repeated at least twice. Samples from the left upper lobes are shown here. (d) Quantification of CTGF band from (c). Values are the mean of results from both lobes. (e) Western blot of CTGF in lysate collected from each of the seven lung lobes of a non-irradiated NHP (5.7 years old) and a NHP exposed to 11.74 Gy WTLI (5.3 years old). Lobes numbered 1 through 7 represent the right upper lobe, right middle lobe, right lower lobe, accessory lobe, left lower lobe, left middle lobe, and left upper lobe, respectively. GAPDH was used as a loading control. (f) Representative IHC images of CTGF in lung tissues from control and 11.5 Gy WTLI-exposed NHP (image shown from right upper lobe). Black scale bar = 100 μm. *p < 0.05, **p<0.01, ***p<0.001.
The elevated expression of CTGF on protein level was shown by western blot. CTGF expression in the lung tissues from six NHP exposed to 11.5 Gy WTLI were compared to that of five non-irradiated NHP (Fig. 1c). The lung tissue lysates were prepared from tissues sampled at the same lung lobes in all NHP to minimize potential variation in CTGF expression due to anatomical location of sampling (tissues from both right and left upper lobes distant from hilum were assayed, with similar results). There was minimal expression of CTGF in all five non-irradiated NHP whereas all six NHP exposed to 11.5 Gy WTLI, an LD90/180 exposure level, had comparatively higher expression of CTGF. Consistent with the gene expression results, CTGF protein had higher expression at early time points (d 65 and 69) and then decreased at later time points in NHP exposed to WTLI. Mean protein levels of CTGF were significantly increased by 41.53 ± 8.51 fold in the irradiated NHP compared to the control group (p <0.01) (Fig. 1d).
Although lung tissues from the same lobe were used for CTGF expression by western blot (Fig. 1c), it is important to note that CTGF expression in different lobes of the same NHP may be different due to differential expression of fibrosis within the tissue volume over the study duration. To better characterize the distribution of CTGF protein expression throughout the whole organ, samples of the lung tissues taken from each of the seven lobes of the same NHP were used for a western blot (Fig. 1e). The expression of CTGF in all seven lobes of the non-irradiated animal was minimal. CTGF had various expression levels within the seven lobes of the NHP exposed to 10.74 Gy WTLI. Nonetheless, the CTGF level in each lobe from the irradiated NHP was much higher than that from the non-irradiated NHP (Fig. 1e).
To study the tissue distribution and localization of CTGF after exposure to radiation, formalin-fixed paraffin-embedded (FFPE) lung sections from the control and NHP exposed to 11.5 Gy WTLI-exposed NHP were subjected to IHC. In agreement with the gene expression and western blot results, there was minimal immunohistological evidence of CTGF expression in the lungs of control NHP. Conversely, CTGF was easily detectable in the WTLI lung and localized mainly to fibroblasts and inflammatory cells (based on cell morphology) (Fig. 1f).
2. PBI/BM5: Increased expression of CTGF and collagen in multiple organs
In the WTLI model, only the thoracic region was irradiated. The PBI/BM5 model provided an ideal opportunity to study whether CTGF was also induced in multiple organs and whether there was a time-dependent effect. The correlation of CTGF expression and collagen deposition was studied in multiple organs in this model. Tissues from multiple organs of NHP exposed to 10.0 Gy or 11.0 Gy using the PBI/BM5 exposure protocol were assayed using IHC for CTGF and Masson's trichrome staining. To better elucidate the possible time-dependent effect, NHP were grouped into three cohorts based on the time from irradiation to euthanasia: a) 1 mo (n=3), b) 3 mo (n=3) and c) 6 mo (n=4). Tissues from four or five non-irradiated NHP (based on tissue availability) were stained together for comparison.
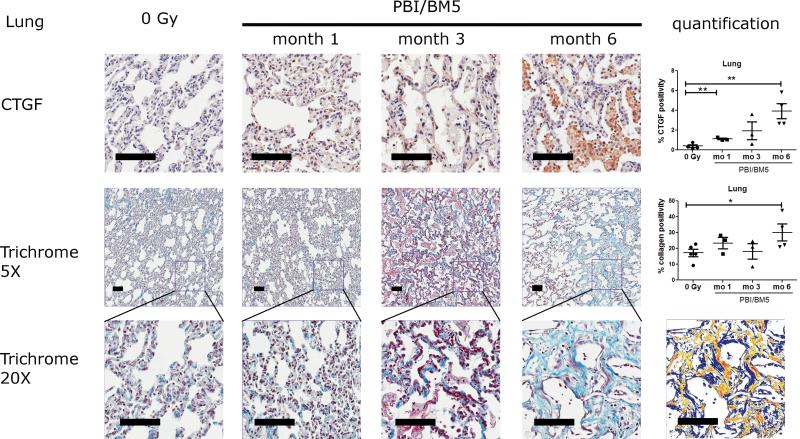
2.1. CTGF expression and collagen deposition in the lungs after PBI/BM5
The lungs of NHP exposed using the PBI/BM5 protocol exhibited an increased expression of CTGF when compared to the lungs from the control NHP (Fig. 2 top row). Fibroblast and inflammatory cells appeared to be the main source of CTGF expression. CTGF expression increased with time after radiation exposure; specifically, the percent positivity for CTGF staining equaled 0.39 ± 0.14 (control), 1.12 ± 0.08 (1 mo), 1.94 ± 0.89 (3 mo) and 3.92 ± 0.77 (6 mo). Moreover, the differences at 1 and 6 mo were statistically significant compared to the control (p <0.01 for both comparisons).
Fig. 2.
CTGF expression and collagen deposition in the lungs of NHP exposed to 10 or 11 Gy PBI/BM5. Lung tissues from the control NHP were included for comparison. All samples were from the right lower lobe. The top row shows representative images of CTGF IHC staining at different time points with the quantification results of CTGF positive pixel count in 10 random 0.5 mm × 0.5 mm fields (One NHP in the control group was not included due to appearance of black spots on the slides before IHC experiment, which would affect the quantification) . The middle row shows the trichrome staining of lung sections at each time points at 5x magnification and the quantification results of collagen staining. The values of CTGF and collagen positive staining are the mean of two repeated experiments. The bottom row shows the magnification of the square in the middle row at 20x magnification. The markup image at the bottom row shows the visualization of aniline blue staining of the 6 mo 20x trichrome image using the deconvolution algorithm. The black scale bar = 100 μm. Control group, n = 5 (4 in the CTGF IHC experiment); 1 mo, n = 3; 3 mo, n = 3; 6 mo, n=4. *p < 0.05, **p < 0.01.
To determine whether there was an increase in collagen deposition coincident with the observed increase in CTGF expression, FFPE lung sections were stained with Masson's trichrome to compare the percent of collagen positivity in lungs from irradiated NHP with lungs from the control NHP (Fig. 2 middle and bottom rows, both lower (5x) and higher magnification (20x) images were shown). The successful separation of aniline blue staining of collagen in the trichrome stained slides was evidenced by the pseudocolor markup images generated by the Imagescope software. The areas with weak, medium or strong positive aniline blue staining were marked by yellow, orange and red pseudocolors in the markup image. The area that was not stained by aniline blue (negative staining) was marked by a blue pseudocolor in the markup image. Relatively high basal levels of collagen were detected in the lungs of the control NHP where the percent collagen positivity was 17.18 ± 2.34. Similar amounts of collagen were observed in NHP euthanized at 1 and 3 mo post-irradiation: 23.41 ± 3.56 and 18.13 ± 4.86, respectively. Collagen positivity was significantly higher in the 6 mo post-irradiation group (30.16 ± 5.40, p <0.05).
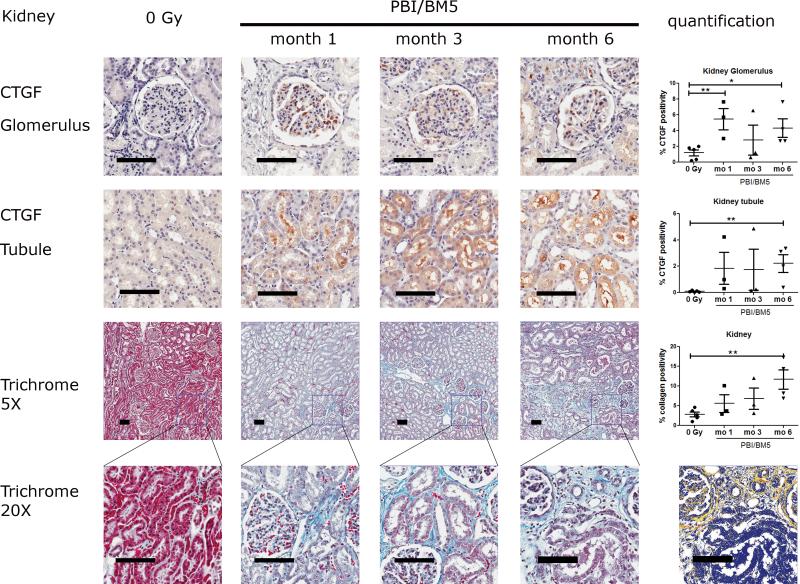
2.2. CTGF expression and collagen deposition in the renal cortex after PBI/BM5
Representative images of CTGF and trichrome staining in the renal cortex were shown in Fig. 3. There was some basal expression of CTGF in the control NHP, mainly limited to glomeruli (Fig. 3 top row). The irradiated kidneys had prominent expression of CTGF in both glomeruli and tubuli (Fig. 3 top and second rows). The percent CTGF positivity in the glomeruli were 1.20 ± 0.38 (control), 5.47 ± 1.35 (1 mo), 2.80 ± 1.89 (3 mo) and 4.32 ± 1.17 (6 mo). Glomerular CTGF expression was significantly elevated at early time points (1 mo, p <0.01), decreased at 3 mo and elevated again at 6 mo (p <0.05) after irradiation. The percent CTGF positivity in the tubuli were 0.09 ± 0.03 (control), 1.85 ± 1.21 (1 mo), 1.76 ± 1.56 (3 mo) and 2.22 ± 0.68 (6 mo). Significantly elevated CTGF expression in the renal tubuli was observed at 6 mo (p<0.01) after irradiation. The expression of CTGF in the glomerulus and tubuli showed a relatively large variation at 1 mo and 3 mo after irradiation.
Fig. 3.
CTGF expression and collagen deposition in the kidneys of NHP exposed to 10 or 11 Gy PBI/BM5. Kidney tissues from the control NHP were included for comparison. The top row shows the representative images of CTGF IHC staining in the glomeruli at different time points with the quantification results of CTGF positive pixel count in 20 random glomeruli at the right. The second row shows the representative images of CTGF IHC staining in the renal tubuli at different time points with the quantification results of CTGF positive pixel count in 10 random 0.5 mm × 0.5 mm fields at the right. The third row shows the trichrome staining of kidney sections at each time points at 5x magnification with the quantification results of collagen staining. The values of CTGF and collagen positive staining are the mean of two repeated experiments. The bottom row shows the magnification of the square in the third row at 20x magnification. The markup image at the bottom row shows the visualization of aniline blue staining of the 6 mo 20x trichrome image using the deconvolution algorithm. The black scale bar = 100 μm. Control group, n = 5; 1 mo, n = 3; 3 mo, n = 3; 6 mo, n=4. *p < 0.05, **p < 0.01.
Both lower (5x) and higher (20x) magnification of trichrome stained kidney sections were shown in Fig. 3 (3rd and the bottom rows). Tubulointerstitial renal fibrosis was observed as early as 1 mo and persisted to 6 mo after irradiation. The percent collagen positivity in the renal cortex were 2.81 ± 0.63 (control), 5.59 ± 2.25 (1 mo), 6.80 ± 2.68 (3 mo) and 11.68 ± 2.48 (6 mo), respectively. The collagen level at 6 mo was significantly higher compared to the control group (p <0.01). A markup image showing the successful separation of blue staining in a kidney slide was included in Fig. 3 bottom row.
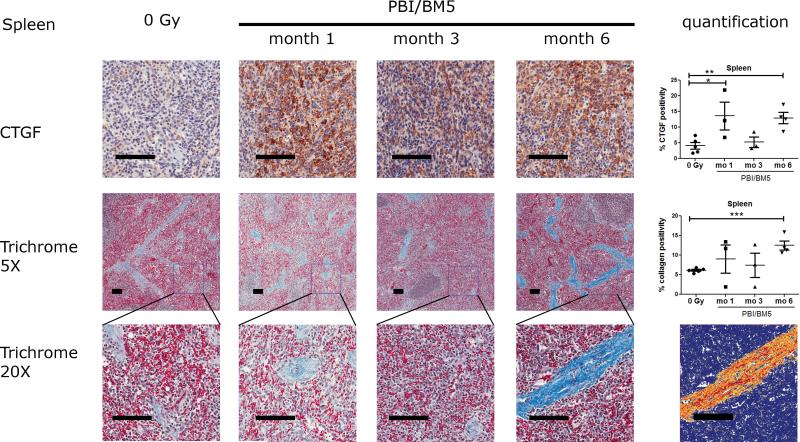
2.3. CTGF expression and collagen deposition in the spleens after PBI/BM5
Representative images of CTGF and collagen staining in the spleens were shown in Fig. 4. There was basal expression of CTGF in the spleen of the non-irradiated NHP that was mainly limited to the red pulp. A very high level of CTGF was observed in the red pulp as early as 1 mo post-irradiation. The expression of CTGF in the red pulp decreased at 3 mo post-irradiation, but returned to high levels at 6 mo post-irradiation. The percent CTGF positivity in the spleens were 4.10 ± 1.06 (control), 13.62 ± 4.41 (1 mo), 5.24 ± 1.66 (3 mo) and 12.95 ± 1.77 (6 mo). The splenic expression of CTGF was significantly elevated at 1 mo (p<0.05) and 6 mo (p<0.01) post-irradiation compared to the control NHP.
Fig. 4.
CTGF expression and collagen deposition in the spleens of NHP exposed to 10 or 11 Gy PBI/BM5. Spleen tissues from the control NHP were included for comparison. The top row shows the representative images of CTGF IHC staining in the spleens at different time points with the quantification results of CTGF positive pixel count in 10 random 0.5 mm × 0.5 mm fields at the right. The second row shows the trichrome staining of splenic tissues at each time points at 5x magnification with the quantification results of collagen staining. The values of CTGF and collagen positive staining are the mean of two repeated experiments. The bottom row shows the magnification of the square in the second row at 20x magnification. The markup image at the bottom row shows the visualization of aniline blue staining of the 6 mo 20x trichrome image using the deconvolution algorithm. The black scale bar = 100 μm. control group, n = 5; 1 mo, n = 3; 3 mo, n = 3; 6 mo, n=4. *p < 0.05, **p < 0.01.
Both lower (5x) and higher (20x) magnification of trichrome stained spleen sections were shown in Fig. 4 (middle and bottom rows). Collagen was mainly localized in the trabeculae. Collagen levels within the control group were very similar. Collagen levels showed a large variation at 1 and 3 mo post-irradiation. However, all NHP euthanized at 6 mo post-irradiation were observed with high levels of collagen staining. The percent collagen positivity in the spleens were 6.14 ± 0.25 (control), 9.01 ± 3.59 (1 mo), 7.43 ± 3.12 (3 mo) and 12.47 ± 1.19 (6 mo), respectively. The collagen level at 6 mo post exposure in the spleens was significantly higher than the control group (p<0.001). A markup image showing the successful separation of blue staining in a splenic slide was included in Fig. 4 bottom row.
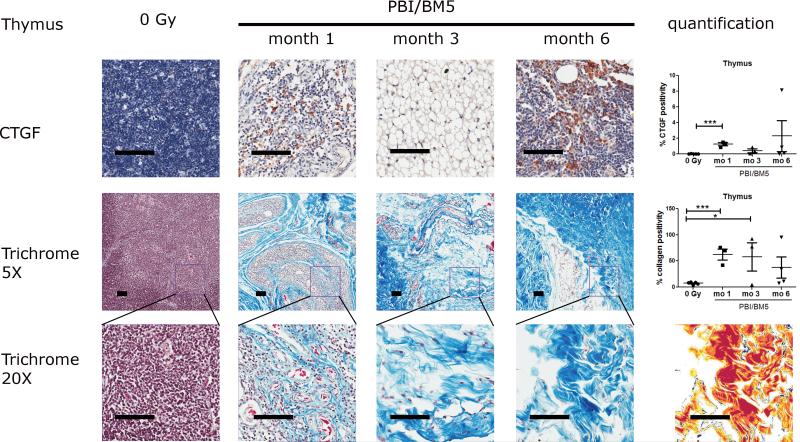
2.4. CTGF expression and collagen deposition in the thymus after PBI/BM5
Representative images of CTGF and collagen staining in the thymus were shown in Fig. 5. There was very minimal expression of CTGF in the thymus of the control NHP. Severe cases of thymic involution were observed in the irradiated NHP (3 out of 3 NHP at the 1 mo time point, 2 out of 3 NHP at the 3 mo time point, and 2 out of 4 NHP at the 6 mo time point). The severe reduction of cellularity in the irradiated thymus made the accurate quantification of CTGF expression difficult. However, strong expression of CTGF was observed in the remaining cortex of the irradiated thymus tissues. The percent CTGF positivity in the thymus was 0.03 ± 0.01 (control), 1.24 ± 0.20 (1 mo), 0.42 ± 0.25 (3 mo) and 2.29 ± 1.96 (6 mo), respectively. The expression of CTGF in the thymus was significantly elevated at 1 mo (p<0.001) post-irradiation compared to the control NHP.
Fig 5.
CTGF expression and collagen deposition in the thymus of NHP exposed to 10 or 11 Gy PBI/BM5. Thymic tissues from the control NHP were included for comparison. The top row shows the representative images of CTGF IHC staining in the thymus at different time points with the quantification results of CTGF positive pixel count in 10 random 0.5 mm × 0.5 mm fields at the right. The second row shows the trichrome staining of thymic tissues at each time points at 5x magnification with the quantification results of collagen staining at the right. The values of CTGF expression and collagen positive staining are the mean of two repeated experiments. The bottom row shows the magnification of the square in the second row at 20x magnification. The markup image at the bottom row shows the visualization of aniline blue staining of the 6 mo 20x trichrome image using the deconvolution algorithm. The black scale bar = 100 μm. control group, n = 5; 1 mo, n = 3; 3 mo, n = 3; 6 mo, n=4.
Both lower (5x) and higher (20x) magnification of trichrome stained thymus sections were shown in Fig. 5 (middle and bottom rows). There was very low expression of collagen in the thymus of the control NHP. However, abnormal amount of collagen was observed in the thymus as early as 1 mo after irradiation in all three NHP. Abnormal amount of collagen was also observed in thymus at later time points (2 out of 3 NHP at 3 mo and 2 out of 4 NHP at 6 mo post-irradiation). The percent collagen positivity in the thymus were 7.46 ± 1.03 (control), 62.70 ± 10.52 (1 mo), 57.97 ± 27.04 (3 mo) and 37.31 ± 20.25 (6 mo), respectively. The collagen levels at 1 mo and 3 mo were significantly higher than the control NHP (p<0.001 and p<0.05, respectively). A markup image showing the successful separation of blue staining in the thymus was included in Fig. 5 bottom row.
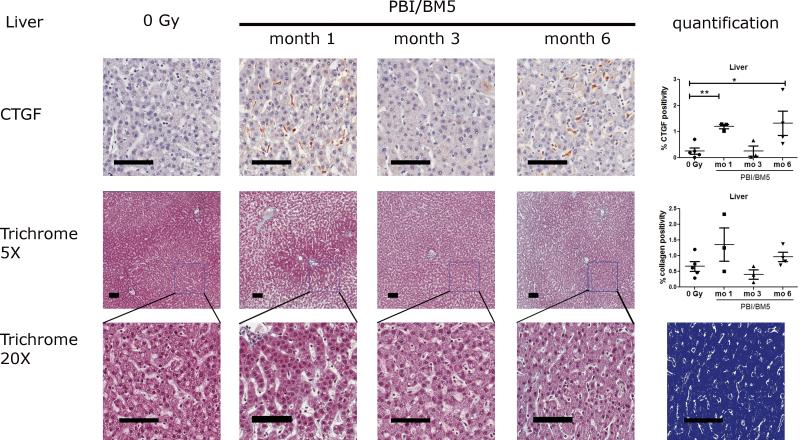
2.5. CTGF expression and collagen deposition in the livers after PBI/BM5
Representative images of CTGF and collagen staining in the liver tissues were shown in Fig. 6. There was minimal staining of CTGF in the livers of the control NHP. The expression of CTGF in the livers was highly elevated after radiation exposure at 1 mo and 6 mo time points (Fig. 6 top row). Hepatic stellate cells (HSCs) appeared to be the major cellular source of CTGF in the irradiated livers. Hepatocytes have little or no expression of CTGF in both the control and the irradiated liver tissues. The percent CTGF positivity in the livers was 0.26 ± 0.12 (control), 1.20 ± 0.09 (1 mo), 0.26 ± 0.20 (3 mo) and 1.32 ± 0.46 (6 mo), respectively. The expression of CTGF in the livers was significantly elevated at 1 mo and 6 mo post-irradiation compared to the control NHP (p<0.01 and p<0.05, respectively).
Fig. 6.
CTGF expression and collagen deposition in the livers of NHP exposed to 10 or 11 Gy PBI/BM5. Liver tissues from the control NHP were included for comparison. The top row shows the representative images of CTGF IHC staining in the liver at different time points with the quantification results of CTGF positive pixel count in 10 random 0.5 mm × 0.5 mm fields at the right. The second row shows the trichrome staining of liver sections at each time points at 5x magnification with the quantification results of collagen staining at the right. The values of CTGF expression and collagen deposition were the mean of two repeated experiments. The bottom row shows the magnification of the square in the second row at 20x magnification. The markup image at the bottom row shows the visualization of aniline blue staining of the 6 mo 20x trichrome image using the deconvolution algorithm. The black scale bar = 100 μm. control group, n = 5; 1 mo, n = 3; 3 mo, n = 3; 6 mo, n=4. *p < 0.05, **p < 0.01.
There were low levels of collagen staining in the hepatic parenchyma in the control and irradiated NHP (Fig. 6 middle and bottom rows). The percent collagen positivity in the liver was 0.66 ± 0.16 (control), 1.36 ± 0.53 (1 mo), 0.40 ± 0.15 (3 mo) and 0.96 ± 0.15 (6 mo), respectively. The differences were not significant at any time points.
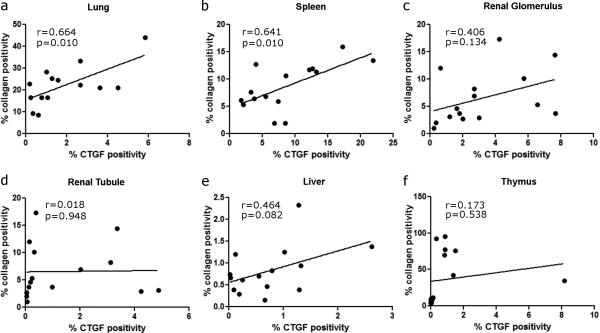
3. Correlation of CTGF expression and collagen deposition in individual organs
Because CTGF and collagen levels were increased in individual organs in NHP exposed to PBI/BM5 and the existing literature of CTGF's role in fibrosis, the correlation of CTGF expression and collagen deposition in individual organs were assessed (Fig. 7). CTGF expression significantly correlated to collagen deposition in the lung (n=14, r = 0.664, p = 0.010; Fig. 7a) and the spleen (n=15, r = 0.641, p = 0.010; Fig. 7b) of control NHP and NHP exposed to PBI/BM5. The correlations of CTGF and collagen in other organs were not significant (Fig. 7 c-f).
Fig. 7.
Correlation of CTGF expression and collagen deposition in individual organs from the control NHP and NHP exposed to 10 or 11 Gy PBI/BM5 (n= 14 for lung, and n=15 for other organs). Correlations of CTGF expression and collagen deposition in lung (a), spleen (b), renal glomerulus (c), renal tubule (d), liver (e) and thymus (f) are shown here.
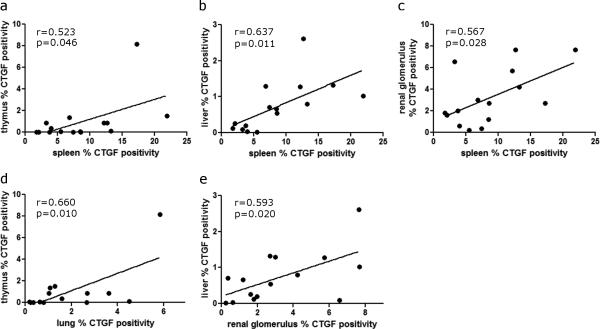
4. Correlation of CTGF expression between individual organs
The PBI/BM5 model provided an opportunity to study the concurrent expression of CTGF in multiple organs within the same NHP. The CTGF expression in each organ (lung, renal glomerulus/ tubule, spleen, thymus and liver) was correlated to other organs from the same NHP (both the control NHP and NHP exposed to PBI/BM5) (Fig. 8). Among the possible 15 comparisons for CTGF expression, five pairs of comparisons were significant: spleen and thymus (n=15, r=0.523, p=0.046; Fig. 8a), spleen and liver (n=15, r=0.637, p=0.011; Fig. 8b), spleen and renal glomerulus (n=15, r=0.567, p=0.028; Fig. 8c), lung and thymus (n=14, r=0.660, p=0.010, Fig. 8d), renal glomerulus and liver (n=15, r=0.593, p=0.020, Fig. 8e). Other comparisons were not shown as they were not significantly correlated. Among all the organs tested, spleen had the highest positivity of CTGF staining at any time points.
Fig. 8.
Correlation of CTGF expression between individual organs from the control NHP and NHP exposed to 10 or 11 Gy PBI/BM5 (n= 14 for lung, and n=15 for other organs). Only significantly correlated pairs are shown here: spleen and thymus (a), spleen and liver (b), spleen and renal glomerulus (c), lung and thymus (d) and renal glomerulus and liver (e).
5. Correlation of collagen deposition between individual organs
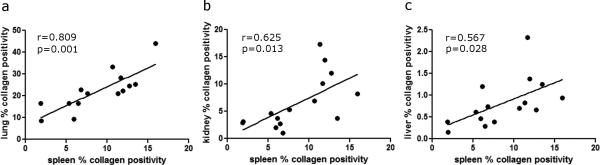
Similar correlation of collagen deposition was also performed in each organ (lung, kidney, spleen, thymus and liver) to other organs (Fig. 9). Among the 10 possible comparisons for collagen deposition, three pairs of comparisons were significant: spleen and lung (n=14, r=0.809, p=0.001, Fig. 9a), spleen and kidney (n=15, r=0.625, p=0.013, Fig. 9b), spleen and liver (n=15, r=0.567, p=0.028, Fig. 9c). Other comparisons were not shown as they were not significantly correlated.
Fig. 9.
Correlation of collagen deposition between individual organs from the control NHP and NHP exposed to 10 or 11 Gy PBI/BM5 (n=15). Only significantly correlated pairs are shown here: spleen and lung (a), spleen and kidney (b), and spleen and liver (c).
DISCUSSION
In this study, the presence and distribution of CTGF and collagen in multiple organs of NHP exposed to lethal doses of radiation was investigated for the first time. Our data suggest that increased expression of CTGF is a common feature in multiple organs after radiation exposure in the 10.0–12.0 Gy dose range. The LD50/180 for radiation-induced multi-organ injury plus medical management was estimated at 10.3 Gy and 9.7 Gy for the WTLI and PBI/BM5 exposure protocols respectively (MacVittie et al. 2012a; Garofalo et al. 2014a). NHP exposed to radiation using WTLI and PBI/BM5 protocols, at these dose ranges were associated with histopathological and radiographic evidence of fibrosis (MacVittie et al. 2012a; Garofalo et al. 2014a). The increased CTGF and collagen expression observed herein provided insight into the time course and mechanism of the fibrotic process in multiple organs after potentially lethal doses of radiation exposure.
Fibrotic diseases are a significant health burden because nearly 45% of all deaths in the developed world are attributed to fibrotic disorders (Wynn 2008). On the other hand, it is suggested that fibrosis is a common pathway causing organ injury and failure in many different organ systems including lung, kidney, liver, etc (Wynn 2007; Rockey et al. 2015). Therefore, there is great interest to study the mechanism of fibrotic disease. Radiation is known to induce fibrosis in multiple organs from both clinical and animal studies. Most RIF studies had been focused on a particular organ such as the lung, kidney, etc. However, significant knowledge gaps exist on the concurrent fibrotic process in multiple organs after radiation exposure in the same group of patients or animals. Our data showed that RIF affected multiple organs (lung, kidney, spleen, and thymus) over the time course of acute and delayed injury post PBI/BM5 exposure protocols. The time to onset of organ-dependent fibrosis after radiation exposure may vary considerably. The time to onset or latency of radiation-induced delayed effects in the heart, kidney and liver for instance have yet to be defined in the NHP model. Furthermore, the radiation dose threshold for overt organ damage and loss of function (with exception of the lung (Garofalo et al. 2014a)) is yet to be established. In the organs being studied here, fibrosis in the thymus and kidney developed relatively early (1 mo after radiation), while fibrosis in the lung developed at time points within 3 mo after radiation. Fibrosis in the spleen developed at 6 mo post-irradiation whereas the liver had a minimal level of fibrosis after radiation exposure at these doses and time frame. RIF was observed to affect multiple organs (including heart, skin, lungs, kidneys, gastro-intestinal tract and liver) in patients usually many years after receiving radiotherapy (Bentzen 2006; Yarnold and Brotons 2010). There are many factors that may affect RIF in patients, including radiation dose, radiation site, concurrent therapies, etc. Therefore, our model may provide a better understanding of the timelines for RIF in the affected organs.
Identifying common differentially expressed molecules in multiple organs helps to elucidate the key mechanism of fibrotic disease regardless of tissue specificity. CTGF is a known mediator of extracellular matrix production in wound healing and various fibrotic disorders. Although CTGF has been shown to be upregulated in multiple fibrotic diseases or models, there is a lack of knowledge about the time course of CTGF expression in multiple organs after radiation exposure. In the current study, CTGF expression had different time-dependent and radiation-exposure, model-dependent changes in various organs. All the organs tested in the PBI/BM5 model showed increased CTGF as early as 1 mo after radiation. After 1 mo, CTGF level (except lung tissues) was decreased at 3 mo and then increased to higher levels at 6 mo. The CTGF levels in the lungs steadily increased after PBI/BM5 exposure from 1 mo to 6 mo after irradiation. In the WTLI model, the lung CTGF level was elevated at early time points (before d 100 after radiation exposure) and decreased at later time points.
CTGF mRNA had a much higher expression in fibrosis-prone mice than in fibrosis-resistant mice from d 2 to d 200 after high dose thoracic irradiation (Kalash et al. 2014), suggesting that CTGF was important in RIF. The fibrosis-prone mice exhibited a peak of CTGF expression at early time points around d 14, reduced but still high level around d 100, and increased again at later time points (d 150 to d 200). CTGF level in our WTLI model showed a similar trend of early elevation and later decreases (Fig. 1b&c). But there was no second increase at the 6 mo time point (Fig. 1b). There are several possible explanations for the lack of second increase in our data: 1. In the NHP data, survivors at 6 months were those received lower doses of radiation, which may explain the little changes of CTGF at this time point. 2. The life span of mice and NHP is very different (2 years vs. 25 years). The 6 month means different things for mice and NHP.
The differential expression of CTGF in different lung lobes of NHP exposed to WTLI (Fig. 1e) may reflect the heterogeneous distribution of tissue damage as evidenced by CT scans and histological analysis (Garofalo et al. 2014a) or differences in regional pathophysiology that may favor development of pneumonitis rather than fibrosis, or vice versa.
Dexamethasone is a key part of medical management in the WTLI and PBI/BM5 NHP models. NHP were treated with dexamethasone on a subject-based, trigger-to-treat protocol based on clinical evidence of tachypnea (defined as non-sedated respiratory rate ≥ 80 breaths per minute) (MacVittie et al. 2012a; Garofalo et al. 2014a; Garofalo et al. 2014b). Dexamethasone has been shown to be a potent inducer of CTGF in murine models in a dose- and time-dependent manner (Dammeier et al. 1998; Okada et al. 2006). The effect of dexamethasone on the expression of CTGF in the current study is not clear. All the six NHP exposed to 11.5 Gy WTLI (Fig. 1c) received dexamethasone treatment at or within a week before euthanasia. Therefore, it is unknown whether the increased CTGF expression was partially because of the dexamethasone treatment in these NHP. NHP exposed to PBI/BM5 (Fig. 2-9) and euthanized at 1 mo post-irradiation did not receive dexamethasone treatment. Therefore, the increased expression of CTGF at 1 mo post PBI/BM5 was not due to dexamethasone treatment. There was no clear relationship of CTGF expression and dexamethasone treatment in the NHP euthanized at 3 mo and 6 mo post PBI/BM5. Because patients are most likely to receive steroid treatment when they develop radiation pneumonitis following thoracic irradiation (Berkey 2010), the possible contribution of steroid treatment on CTGF expression warrants further investigation.
The different responses of pulmonary CTGF expression in the WTLI and PBI/BM5 models supported the contention that the same organ may respond differently to radiation in different contexts, such as localized radiation versus systemic radiation. The systemic radiation exposure in the PBI/BM5 model may affect the individual response of each organ. An example of such dichotomous response was reported that the lung had different cytokine expression following total-body irradiation versus thoracic irradiation, even though the radiation dose was identical (Johnston et al. 2010). The differential response of the same organ under different radiation exposure protocols may reflect the interaction of different organs.
Based on literature and our data, we hypothesize that inflammation is an important determining factor for fibrosis after radiation exposure. Numerous reviews had discussed the link of inflammation to fibrosis (Kershenobich Stalnikowitz and Weissbrod 2003; Stramer et al. 2007; Lee and Kalluri 2010) and suggested macrophages as a master regulator of inflammation and fibrosis (Wynn and Barron 2010). Macrophages have been shown to produce TGF-β and platelet-derived growth factor (PDGF), both important for fibrosis (Wahl et al. 1990; Bonner et al. 1991). An elevated level of CTGF was observed in macrophages of human atherosclerotic plaques and CTGF induced mononuclear cell chemotaxis in a dose-dependent manner (Cicha et al. 2005). In vitro work showed that the elevated CTGF in macrophages was a result of endocytosis rather than CTGF production upon stimulation (Cicha et al. 2005). It has been shown that 50% of the body's monocytes (precursors of macrophages) are stored in the spleen red pulps in mice (Swirski et al. 2009) and similarly in human (van der Laan et al. 2014). Activated human monocytes express integrin αM/β2 that binds to CTGF and might mediate CTGF's internalization to monocytes (Schober et al. 2002). High levels of CTGF were observed in human bone marrow (Cicha et al. 2004). Considering the high levels of CTGF observed in the spleen red pulps (Fig. 4), the significant correlation of spleen with multiple organs on CTGF and collagen levels (Fig. 8 &9) and high expression of CTGF in pulmonary macrophages (Fig. 1f and Fig. 2), we postulate that inflammatory cells may bring CTGF to multiple irradiated organs during inflammation, thus contributing to fibrosis. The inflammatory cells may migrate to the injured organs directly from bone marrow or through spleen. Resident fibroblasts and endothelial cells also produce CTGF and contribute to fibrosis (Vozenin-Brotons et al. 2003; Yarnold and Brotons 2010). The relative contribution of bone-marrow derived cells versus the resident cells for CTGF expression and ultimately fibrosis may be organ-dependent.
The hypothesis of inflammation's contribution to fibrosis may help to explain several observations: 1.) CTGF expression peaked at the time of radiation pneumonitis in the WTLI model. This may be a result of bone marrow derived inflammatory cells transporting CTGF to the injured organs. This may also explain the observed intra-alveolar CTGF expression in the lungs and intra-tubular CTGF expression in the kidneys. High levels of CTGF in these inflammatory cells induce more mononuclear cells chemotaxis and fibrosis. 2.) The different kinetics of pulmonary CTGF expression in the WTLI and PBI/BM5 model. The expression peak of pulmonary CTGF was early in the WTLI model and late in the PBI/BM5 model. This may reflect the kinetics of inflammatory cell infiltration in different models. In the WTLI model, most bone marrow and spleen are not in the irradiation fields; therefore, the inflammatory infiltration to the lung may occur earlier and be more persistent. In the PBI/BM5 model, the spleen and approximately 95% of the bone marrow are myeloablated, resulting in severe cytopenia. Therefore, the infiltration of inflammatory cells is much slower and less persistent in the PBI/BM5 model than the WTLI model and pulmonary CTGF expression occurs later in this model. 3. This may also explain the very low level of fibrosis in the liver and high levels of fibrosis in the lung and kidney after radiation. It was shown that liver myofibroblasts were almost exclusively from the activation of resident hepatic stellate cells (Friedman et al. 1985), with very minimal contribution from bone-marrow derived mesenchymal cells or fibrocytes (Brenner et al. 2012). In contrast, bone-marrow derived cells constituted more than 80% of collagen I expressing cells in pulmonary fibrosis (Hashimoto et al. 2004). It has also been shown that 35% of myofibroblasts in renal fibrosis were from bone marrow-derived cells and the remaining myofibroblasts were derived from the expansion of resident cells or from sources of other than bone marrow (LeBleu et al. 2013). This suggests that bone-marrow derived cells (possibly through spleen) may contribute more to fibrosis than tissue resident cells in RIF.
The thymus is a primary lymphoid organ and it is known to be exquisitely sensitive to stress and toxic insults (Pearse 2006). The thymus has been shown to be the first major organ with prominent apoptosis as early as 6 hour after high-dose ionizing radiation (Johnson et al. 2013). Radiation-induced thymic injury was persistent for at least 6 months as shown by the lower counts of CD4+ and CD8+ subsets of naïve T cells and total CD4+ T cells in our model (MacVittie et al. 2014). In this study, high levels of collagen staining in the thymus were observed in NHP from 1 m to 6 m after irradiation. The strong expression of collagen was associated with severe lymphocyte depletion. Currently, it is unknown whether the high collagen expression was because of the de novo production of fibrous tissue or just tissue atrophy secondary to the massive loss of lymphocytes after radiation.
There was a lack of significant correlation of CTGF expression to collagen in multiple organs except lung and spleen (Fig. 7). There may be several explanations for the lack of correlation: 1.) this may merely reflect the different kinetics of CTGF expression and collagen deposition. For example, CTGF had a high expression level as early as 1 mo in the irradiated livers but hepatic parenchymal fibrosis may only happen after 6 mo. 2.) The pro-fibrotic property of CTGF may require other co-factors such as TGFβ, PDGF, fibronectin, etc (Brigstock 2010). Transgenic studies have shown that overexpression of CTGF alone in kidney or liver failed to cause any kind of fibrosis; on the other hand, when combined with fibrotic injuries, overexpression of CTGF in kidney and liver was associated with much exaggerated fibrotic responses compared to wild-type animals (Yokoi et al. 2008; Tong et al. 2009).
Currently there is no FDA approved anti-fibrotic therapy for radiation-induced injury and consequent morbidity and mortality for any organ system. Therapies targeting the molecules involved in initiating fibrosis and common to multiple organs have the potential to treat acute radiation-induced fibrosis in multiple organs. For example, an effective anti-CTGF treatment tested in the lung as a single target has the potential to translate to treatment for fibrosis of other tissue types.
Additional studies are warranted to define the dose- and time-dependent change of CTGF after radiation exposure, particularly at early time points. The tissues studied in the current study were from NHP that met euthanasia criteria, rather than at preselected time points. This may result in a potential selection bias to the more moribund NHP. The expression of CTGF and collagen should be studied in other organs that are susceptible to RIF such as small intestine, colon, heart, etc. Double staining of CTGF and cell specific markers will help to identify the exact cell types that express CTGF. Studying other growth factors or fibrogenic markers (such as TGFβ, PDGF, α-SMA, collagen, etc) together with CTGF will help to better elucidate CTGF's role in RIF. In situ hybridization and in vitro studies will help to determine whether the CTGF observed in inflammatory cells is produced or engulfed in these cells. The sample size in the current study is relatively small.
CONCLUSIONS
Collectively, these data demonstrate that expression of CTGF was increased in multiple organs after different modes of radiation exposure in well-established NHP models. These organs included the lung from NHP exposed to WTLI in addition to the lung, kidney, spleen, thymus and liver from NHP exposed using the PBI/BM5 protocol. Increased deposition of collagen was also observed in the lung tissues of NHP exposed to WTLI and multiple organs of NHP exposed to PBI/BM5. There was significant correlation of CTGF expression and collagen deposition in the lung and spleen of NHP exposed to PBI/BM5. The spleen had significant correlations on CTGF expression and collagen deposition to multiple other organs. Our data provides strong evidence suggesting CTGF's involvement in multi-organ RIF in clinically relevant models of human fibrosis.
Acknowledgements
This work was supported from Aeolus Inc. through BARDA contract HHSO100201100007C and National Institute of Allergy and Infectious Diseases (NIAID) contracts HHSN266200500043C and HHSN272201000046C. We acknowledge the tremendous support and expertise of the research staff of the Preclinical Radiobiology Lab at the University of Maryland, Baltimore.
Footnotes
Conflicts of interest:
The authors declare no conflict of interest.
REFERENCES
- Bentzen SM. Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nat Rev Cancer. 2006;6:702–713. doi: 10.1038/nrc1950. [DOI] [PubMed] [Google Scholar]
- Berkey FJ. Managing the adverse effects of radiation therapy. Am Fam Physician. 2010;82:381–8. 394. [PubMed] [Google Scholar]
- Bonner JC, Osornio-Vargas AR, Badgett A, Brody AR. Differential proliferation of rat lung fibroblasts induced by the platelet-derived growth factor-AA, -AB, and -BB isoforms secreted by rat alveolar macrophages. Am J Respir Cell Mol Biol. 1991;5:539–547. doi: 10.1165/ajrcmb/5.6.539. [DOI] [PubMed] [Google Scholar]
- Bourgeois JF, Gourgou S, Kramar A, Lagarde JM, Gall Y, Guillot B. Radiation-induced skin fibrosis after treatment of breast cancer: profilometric analysis. Skin Res Technol. 2003;9:39–42. doi: 10.1034/j.1600-0846.2003.00357.x. [DOI] [PubMed] [Google Scholar]
- Bourgier C, Haydont V, Milliat F, Francois A, Holler V, Lasser P, Bourhis J, Mathe D, Vozenin-Brotons MC. Inhibition of Rho kinase modulates radiation induced fibrogenic phenotype in intestinal smooth muscle cells through alteration of the cytoskeleton and connective tissue growth factor expression. Gut. 2005;54:336–343. doi: 10.1136/gut.2004.051169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner DA, Kisseleva T, Scholten D, Paik YH, Iwaisako K, Inokuchi S, Schnabl B, Seki E, De Minicis S, Oesterreicher C, Taura K. Origin of myofibroblasts in liver fibrosis. Fibrogenesis Tissue Repair. 2012;5:S17. doi: 10.1186/1755-1536-5-S1-S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigstock DR. Connective tissue growth factor (CCN2, CTGF) and organ fibrosis: lessons from transgenic animals. J Cell Commun Signal. 2010;4:1–4. doi: 10.1007/s12079-009-0071-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng W, Xiao L, Ainiwaer A, Wang Y, Wu G, Mao R, Yang Y, Bao Y. Molecular responses of radiation-induced liver damage in rats. Mol Med Rep. 2015;11:2592–2600. doi: 10.3892/mmr.2014.3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicha I, Yilmaz A, Klein M, Raithel D, Brigstock DR, Daniel WG, Goppelt-Struebe M, Garlichs CD. Connective tissue growth factor is overexpressed in complicated atherosclerotic plaques and induces mononuclear cell chemotaxis in vitro. Arterioscler Thromb Vasc Biol. 2005;25:1008–1013. doi: 10.1161/01.ATV.0000162173.27682.7b. [DOI] [PubMed] [Google Scholar]
- Cicha I, Garlichs CD, Daniel WG, Goppelt-Struebe M. Activated human platelets release connective tissue growth factor. Thromb Haemost. 2004;91:755–760. doi: 10.1160/TH03-09-0602. [DOI] [PubMed] [Google Scholar]
- Dammeier J, Beer HD, Brauchle M, Werner S. Dexamethasone is a novel potent inducer of connective tissue growth factor expression. Implications for glucocorticoid therapy. J Biol Chem. 1998;273:18185–18190. doi: 10.1074/jbc.273.29.18185. [DOI] [PubMed] [Google Scholar]
- DiCarlo AL, Maher C, Hick JL, Hanfling D, Dainiak N, Chao N, Bader JL, Coleman CN, Weinstock DM. Radiation injury after a nuclear detonation: medical consequences and the need for scarce resources allocation. Disaster Med Public Health Prep. 2011;5(Suppl 1):S32–44. doi: 10.1001/dmp.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du SS, Qiang M, Zeng ZC, Zhou J, Tan YS, Zhang ZY, Zeng HY, Liu ZS. Radiation-induced liver fibrosis is mitigated by gene therapy inhibiting transforming growth factor-beta signaling in the rat. Int J Radiat Oncol Biol Phys. 2010;78:1513–1523. doi: 10.1016/j.ijrobp.2010.06.046. [DOI] [PubMed] [Google Scholar]
- Duffield JS. Cellular and molecular mechanisms in kidney fibrosis. J Clin Invest. 2014;124:2299–2306. doi: 10.1172/JCI72267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farese AM, Brown CR, Smith CP, Gibbs AM, Katz BP, Johnson CS, Prado KL, MacVittie TJ. The ability of filgrastim to mitigate mortality following LD50/60 total-body irradiation is administration time-dependent. Health Phys. 2014;106:39–47. doi: 10.1097/HP.0b013e3182a4dd2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farese AM, Cohen MV, Katz BP, Smith CP, Gibbs A, Cohen DM, MacVittie TJ. Filgrastim improves survival in lethally irradiated nonhuman primates. Radiat Res. 2013;179:89–100. doi: 10.1667/RR3049.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farese AM, Cohen MV, Katz BP, Smith CP, Jackson W, 3rd, Cohen DM, MacVittie TJ. A nonhuman primate model of the hematopoietic acute radiation syndrome plus medical management. Health Phys. 2012;103:367–382. doi: 10.1097/HP.0b013e31825f75a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farese AM, Hunt P, Grab LB, MacVittie TJ. Combined administration of recombinant human megakaryocyte growth and development factor and granulocyte colony-stimulating factor enhances multilineage hematopoietic reconstitution in nonhuman primates after radiation-induced marrow aplasia. 1996;97:2145–2151. doi: 10.1172/JCI118652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch W, Shamsa K, Lee MS. Cardiovascular complications of radiation exposure. Rev Cardiovasc Med. 2014;15:232–244. doi: 10.3909/ricm0689. [DOI] [PubMed] [Google Scholar]
- Friedman SL, Roll FJ, Boyles J, Bissell DM. Hepatic lipocytes: the principal collagen-producing cells of normal rat liver. Proc Natl Acad Sci U S A. 1985;82:8681–8685. doi: 10.1073/pnas.82.24.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garofalo M, Bennett A, Farese AM, Harper J, Ward A, Taylor-Howell C, Cui W, Gibbs A, Lasio G, Jackson W, 3rd, MacVittie TJ. The delayed pulmonary syndrome following acute high-dose irradiation: a rhesus macaque model. Health Phys. 2014a;106:56–72. doi: 10.1097/HP.0b013e3182a32b3f. [DOI] [PubMed] [Google Scholar]
- Garofalo MC, Ward AA, Farese AM, Bennett A, Taylor-Howell C, Cui W, Gibbs A, Prado KL, MacVittie TJ. A pilot study in rhesus macaques to assess the treatment efficacy of a small molecular weight catalytic metalloporphyrin antioxidant (AEOL 10150) in mitigating radiation-induced lung damage. Health Phys. 2014b;106:73–83. doi: 10.1097/HP.0b013e3182a4d967. [DOI] [PubMed] [Google Scholar]
- Ghafoori P, Marks LB, Vujaskovic Z, Kelsey CR. Radiation-induced lung injury. Assessment, management, and prevention. Oncology (Williston Park) 2008;22:37–47. discussion 52-3. [PubMed] [Google Scholar]
- Hall E, Giaccia A. Radiobiology for the radiologist. 6th ed. Lippincott Williams & Wilkins; Philadelphia, PA: 2006. [Google Scholar]
- Hashimoto N, Jin H, Liu T, Chensue SW, Phan SH. Bone marrow-derived progenitor cells in pulmonary fibrosis. J Clin Invest. 2004;113:243–252. doi: 10.1172/JCI18847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankey KG, Farese AM, Blaauw EC, Gibbs AM, Smith CP, Katz BP, Tong Y, Prado KL, MacVittie TJ. Pegfilgrastim Improves Survival of Lethally Irradiated Nonhuman Primates. Radiat Res. doi: 10.1667/RR13940.1. (in press) [DOI] [PubMed] [Google Scholar]
- Ikawa Y, Ng PS, Endo K, Kondo M, Chujo S, Ishida W, Shirasaki F, Fujimoto M, Takehara K. Neutralizing monoclonal antibody to human connective tissue growth factor ameliorates transforming growth factor-beta-induced mouse fibrosis. J Cell Physiol. 2008;216:680–687. doi: 10.1002/jcp.21449. [DOI] [PubMed] [Google Scholar]
- Jackson IL, Xu P, Hadley C, Katz BP, McGurk R, Down JD, Vujaskovic Z. A preclinical rodent model of radiation-induced lung injury for medical countermeasure screening in accordance with the FDA animal rule. Health Phys. 2012;103:463–473. doi: 10.1097/HP.0b013e31826386ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SE, Li Z, Liu Y, Moulder JE, Zhao M. Whole-body imaging of high-dose ionizing irradiation-induced tissue injuries using 99mTc-duramycin. J Nucl Med. 2013;54:1397–1403. doi: 10.2967/jnumed.112.112490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston CJ, Hernady E, Reed C, Thurston SW, Finkelstein JN, Williams JP. Early alterations in cytokine expression in adult compared to developing lung in mice after radiation exposure. Radiat Res. 2010;173:522–535. doi: 10.1667/RR1882.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalash R, Berhane H, Au J, Rhieu BH, Epperly MW, Goff J, Dixon T, Wang H, Zhang X, Franicola D, Shinde A, Greenberger JS. Differences in irradiated lung gene transcription between fibrosis-prone C57BL/6NHsd and fibrosis-resistant C3H/HeNHsd mice. In Vivo. 2014;28:147–171. [PMC free article] [PubMed] [Google Scholar]
- Kershenobich Stalnikowitz D, Weissbrod AB. Liver fibrosis and inflammation. A review. Ann Hepatol. 2003;2:159–163. [PubMed] [Google Scholar]
- Kruse JJ, Floot BG, te Poele JA, Russell NS, Stewart FA. Radiation-induced activation of TGF-beta signaling pathways in relation to vascular damage in mouse kidneys. Radiat Res. 2009;171:188–197. doi: 10.1667/RR1526.1. [DOI] [PubMed] [Google Scholar]
- Lawrence TS, Robertson JM, Anscher MS, Jirtle RL, Ensminger WD, Fajardo LF. Hepatic toxicity resulting from cancer treatment. Int J Radiat Oncol Biol Phys. 1995;31:1237–1248. doi: 10.1016/0360-3016(94)00418-K. [DOI] [PubMed] [Google Scholar]
- LeBleu VS, Taduri G, O'Connell J, Teng Y, Cooke VG, Woda C, Sugimoto H, Kalluri R. Origin and function of myofibroblasts in kidney fibrosis. Nat Med. 2013;19:1047–1053. doi: 10.1038/nm.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SB, Kalluri R. Mechanistic connection between inflammation and fibrosis. Kidney Int Suppl. 2010;(119):S22–6. doi: 10.1038/ki.2010.418. doi:S22-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Xie Q, Shi Y, Li D, Zhang M, Jiang S, Zhou H, Lu H, Jin Y. Inhibition of connective tissue growth factor by siRNA prevents liver fibrosis in rats. J Gene Med. 2006;8:889–900. doi: 10.1002/jgm.894. [DOI] [PubMed] [Google Scholar]
- Lipson KE, Wong C, Teng Y, Spong S. CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis. Fibrogenesis Tissue Repair. 2012;5:S24. doi: 10.1186/1755-1536-5-S1-S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu DG, Wang TM. Role of connective tissue growth factor in experimental radiation nephropathy in rats. Chin Med J (Engl) 2008;121:1925–1931. [PubMed] [Google Scholar]
- Liu Y. Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol. 2011;7:684–696. doi: 10.1038/nrneph.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacVittie TJ, Bennett AW, V Cohen M, Farese AM, Higgins A, Hankey KG. Immune cell reconstitution after exposure to potentially lethal doses of radiation in the nonhuman primate. Health Phys. 2014;106:84–96. doi: 10.1097/HP.0b013e3182a2a9b2. [DOI] [PubMed] [Google Scholar]
- MacVittie TJ, Bennett A, Booth C, Garofalo M, Tudor G, Ward A, Shea-Donohue T, Gelfond D, McFarland E, Jackson W, 3rd, Lu W, Farese AM. The prolonged gastrointestinal syndrome in rhesus macaques: the relationship between gastrointestinal, hematopoietic, and delayed multi-organ sequelae following acute, potentially lethal, partial-body irradiation. Health Phys. 2012a;103:427–453. doi: 10.1097/HP.0b013e318266eb4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacVittie TJ, Farese AM, Bennett A, Gelfond D, Shea-Donohue T, Tudor G, Booth C, McFarland E, Jackson W., 3rd The acute gastrointestinal subsyndrome of the acute radiation syndrome: a rhesus macaque model. Health Phys. 2012b;103:411–426. doi: 10.1097/HP.0b013e31826525f0. [DOI] [PubMed] [Google Scholar]
- Mori T, Kawara S, Shinozaki M, Hayashi N, Kakinuma T, Igarashi A, Takigawa M, Nakanishi T, Takehara K. Role and interaction of connective tissue growth factor with transforming growth factor-beta in persistent fibrosis: A mouse fibrosis model. J Cell Physiol. 1999;181:153–159. doi: 10.1002/(SICI)1097-4652(199910)181:1<153::AID-JCP16>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Okada H, Kikuta T, Inoue T, Kanno Y, Ban S, Sugaya T, Takigawa M, Suzuki H. Dexamethasone induces connective tissue growth factor expression in renal tubular epithelial cells in a mouse strain-specific manner. Am J Pathol. 2006;168:737–747. doi: 10.2353/ajpath.2006.050656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse G. Histopathology of the thymus. Toxicol Pathol. 2006;34:515–547. doi: 10.1080/01926230600978458. [DOI] [PubMed] [Google Scholar]
- Peter RU, Gottlober P, Nadeshina N, Krahn G, Braun-Falco O, Plewig G. Interferon gamma in survivors of the Chernobyl power plant accident: new therapeutic option for radiation-induced fibrosis. Int J Radiat Oncol Biol Phys. 1999;45:147–152. doi: 10.1016/s0360-3016(99)00116-9. [DOI] [PubMed] [Google Scholar]
- Poston JWS, Warren K. Sinclair Keynote Address: Current challenges in countering radiological terrorism. Health Phys. 2005;89:450–456. doi: 10.1097/01.hp.0000172870.02790.6a. [DOI] [PubMed] [Google Scholar]
- Rachfal AW, Brigstock DR. Connective tissue growth factor (CTGF/CCN2) in hepatic fibrosis. Hepatol Res. 2003;26:1–9. doi: 10.1016/s1386-6346(03)00115-3. [DOI] [PubMed] [Google Scholar]
- Rieder F, Kessler S, Sans M, Fiocchi C. Animal models of intestinal fibrosis: new tools for the understanding of pathogenesis and therapy of human disease. Am J Physiol Gastrointest Liver Physiol. 2012;303:G786–801. doi: 10.1152/ajpgi.00059.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockey DC, Bell PD, Hill JA. Fibrosis--a common pathway to organ injury and failure. N Engl J Med. 2015;372:1138–1149. doi: 10.1056/NEJMra1300575. [DOI] [PubMed] [Google Scholar]
- Schober JM, Chen N, Grzeszkiewicz TM, Jovanovic I, Emeson EE, Ugarova TP, Ye RD, Lau LF, Lam SC. Identification of integrin alpha(M)beta(2) as an adhesion receptor on peripheral blood monocytes for Cyr61 (CCN1) and connective tissue growth factor (CCN2): immediate-early gene products expressed in atherosclerotic lesions. Blood. 2002;99:4457–4465. doi: 10.1182/blood.v99.12.4457. [DOI] [PubMed] [Google Scholar]
- Stacey R, Green JT. Radiation-induced small bowel disease: latest developments and clinical guidance. Ther Adv Chronic Dis. 2014;5:15–29. doi: 10.1177/2040622313510730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stramer BM, Mori R, Martin P. The inflammation-fibrosis link? A Jekyll and Hyde role for blood cells during wound repair. J Invest Dermatol. 2007;127:1009–1017. doi: 10.1038/sj.jid.5700811. [DOI] [PubMed] [Google Scholar]
- Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd NW, Luzina IG, Atamas SP. Molecular and cellular mechanisms of pulmonary fibrosis. Fibrogenesis Tissue Repair. 2012;5:11–1536-5-11. doi: 10.1186/1755-1536-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Z, Chen R, Alt DS, Kemper S, Perbal B, Brigstock DR. Susceptibility to liver fibrosis in mice expressing a connective tissue growth factor transgene in hepatocytes. Hepatology. 2009;50:939–947. doi: 10.1002/hep.23102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Laan AM, Ter Horst EN, Delewi R, Begieneman MP, Krijnen PA, Hirsch A, Lavaei M, Nahrendorf M, Horrevoets AJ, Niessen HW, Piek JJ. Monocyte subset accumulation in the human heart following acute myocardial infarction and the role of the spleen as monocyte reservoir. Eur Heart J. 2014;35:376–385. doi: 10.1093/eurheartj/eht331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vozenin-Brotons MC, Milliat F, Sabourin JC, de Gouville AC, Francois A, Lasser P, Morice P, Haie-Meder C, Lusinchi A, Antoun S, Bourhis J, Mathe D, Girinsky T, Aigueperse J. Fibrogenic signals in patients with radiation enteritis are associated with increased connective tissue growth factor expression. Int J Radiat Oncol Biol Phys. 2003;56:561–572. doi: 10.1016/s0360-3016(02)04601-1. [DOI] [PubMed] [Google Scholar]
- Wahl SM, McCartney-Francis N, Allen JB, Dougherty EB, Dougherty SF. Macrophage production of TGF-beta and regulation by TGF-beta. Ann N Y Acad Sci. 1990;593:188–196. doi: 10.1111/j.1749-6632.1990.tb16111.x. [DOI] [PubMed] [Google Scholar]
- Wang Q, Usinger W, Nichols B, Gray J, Xu L, Seeley TW, Brenner M, Guo G, Zhang W, Oliver N, Lin A, Yeowell D. Cooperative interaction of CTGF and TGF-beta in animal models of fibrotic disease. Fibrogenesis Tissue Repair. 2011;4:4–1536-4-4. doi: 10.1186/1755-1536-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JP, McBride WH. After the bomb drops: a new look at radiation-induced multiple organ dysfunction syndrome (MODS). Int J Radiat Biol. 2011;87:851–868. doi: 10.3109/09553002.2011.560996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JP, Johnston CJ, Finkelstein JN. Treatment for radiation-induced pulmonary late effects: spoiled for choice or looking in the wrong direction?. Curr Drug Targets. 2010;11:1386–1394. doi: 10.2174/1389450111009011386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18:1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA, Barron L. Macrophages: master regulators of inflammation and fibrosis. Semin Liver Dis. 2010;30:245–257. doi: 10.1055/s-0030-1255354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007;117:524–529. doi: 10.1172/JCI31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarnold J, Brotons MC. Pathogenetic mechanisms in radiation fibrosis. Radiother Oncol. 2010;97:149–161. doi: 10.1016/j.radonc.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Yokoi H, Mukoyama M, Mori K, Kasahara M, Suganami T, Sawai K, Yoshioka T, Saito Y, Ogawa Y, Kuwabara T, Sugawara A, Nakao K. Overexpression of connective tissue growth factor in podocytes worsens diabetic nephropathy in mice. Kidney Int. 2008;73:446–455. doi: 10.1038/sj.ki.5002722. [DOI] [PubMed] [Google Scholar]