Abstract
Ribosome-inactivating proteins (RIPs) belong to a family of enzymes that attack eukaryotic ribosomes and potently inhibit cellular protein synthesis. RIPs possess several biomedical properties, including anti-viral and anti-tumor activities. Multiple RIPs are known to inhibit tumor cell proliferation through inducing apoptosis in a variety of cancers, such as breast cancer, leukemia/lymphoma, and hepatoma. This review focuses on the anti-tumor activities of RIPs and their apoptotic effects through three closely related pathways: mitochondrial, death receptor, and endoplasmic reticulum pathways.
Keywords: Ribosome-inactivating protein (RIP), Anti-tumor, Apoptosis, Cancer
Introduction
Ribosome-inactivating proteins (RIPs) are a family of enzymes that inhibit the eukaryotic ribosome via N-glycosidase activity, by which they cleave a specific adenine residue from the 28S RNA within the 60S ribosomal subunit, therefore inhibiting protein synthesis [1, 2]. In addition to their effect on ribosomal RNA (rRNA), some RIPs display a variety of anti-microbial activities in vitro, including anti-fungal, anti-bacterial, and broad-spectrum anti-viral properties against both human and animal pathogens.
Ribosome-inactivating proteins were initially discovered in the castor oil plant Ricinus communis, from which ricin was isolated. RIPs are widely distributed among higher plants, and a few have been found in several fungi and bacteria. Plant RIPs are classified into three main categories based on their physical properties. Type I RIPs are single-chain proteins of approximately 30 kDa with N-glycosidase activity, including trichosanthin (TCS) and cucurmosin [3]. Type II RIPs, such as ricin and abrin, comprise two different domains: a 30-kDa enzymatic A-chain (similar to type I RIPs) linked to a slightly larger B-chain with lectin properties and specificity for sugars possessing galactose-like structures [3]. Thus far, type III RIPs, also considered atypical type I RIPs, have only been described in maize and barley, and the function of their extra domains remains unknown [4]. Therefore, the division of RIPs into types I and II RIP is now favored.
Over the past decade, RIPs appeared to be a great research interest due to their potential use in cancer therapy. Some RIPs exhibit strong toxicity towards cancer cells and low toxicity towards normal cells; they impede or inhibit tumor growth mostly via apoptosis, but the exact mechanism remains poorly understood. The aim of this study was to summarize the anti-tumor activities of RIPs and their possible apoptotic mechanisms to hopefully provide new insights for cancer research and treatment.
Anti-tumor activity
Effects of RIPs on breast cancer
The type I RIPs TCS, momordica anti-human immunodeficiency virus (HIV) protein of 30 kDa (MAP30), gelonium anti-HIV protein of 31 kDa (GAP31), gelonin, marmorin, and α-momorcharin (α-MMC) have been shown to negatively affect the growth of breast tumor cells in vitro and in vivo [5–7]. TCS inhibits cell viability, causes cell cycle arrest, and significantly reduces tumor volume and weight by inducing apoptosis through caspase-8 and caspase-9 in breast tumor cells [5]. MAP30, which shares 59% sequence similarity with TCS, effectively inhibits human breast cancer MDA-MB-231 cells through down-regulating the expression of human epidermal growth factor receptor-2 (HER2), similarly to GAP31 [6]. HER2 overexpression is observed in approximately 30% of all human breast cancers, and HER2-overexpressing tumor cells may be less sensitive to chemotherapy; in this case, the combination of MAP30 and GAP31 could represent a therapeutic strategy [7]. HER2 and fibroblast growth factor-inducible 14-kDa protein (Fn14) are frequently co-expressed in human breast tumors, and HER2 directly induces increase in Fn14 expression, therefore sensitizing tumor cells to an immunotoxin generated by fusing Fn14 antibodies to recombinant gelonin (designated hSGZ) [8]. Indeed, hSGZ can rapidly internalize and deliver recombinant gelonin (rGel) to the cytosol of tumor cells, where it enzymatically blocks protein synthesis. Because Fn14 enhances breast cancer cell migration and invasion, a question of whether there is a way to damage tumor cells while reducing Fn14 expression was raised. We assume that MAP30 and hSGZ used together might achieve a better outcome, and breast tumor cells can be sequentially treated with MAP30 and hSGZ; MAP30 would decrease HER2 expression and lead to reduced Fn14 expression, then hSGZ would target Fn14-positive cells and exert its function without increasing the invasive capacity of tumor cells. However, this hypothesis remains to be verified by appropriate experiments.
Many cell membrane receptors are expressed at low levels in normal cells but are highly expressed in tumor cells. Estrogen receptor α (ERα) is expressed in approximately 75% of breast cancer tissues at higher levels compared with those in normal breast tissues (P = 0.001) [9]. In ERα-positive breast cancer cells, the ERα-mediated signaling pathway is involved in the inhibitory action of marmorin on proliferation; many drugs target ERα. Marmorin inhibits angiogenesis by lowering the viability of human umbilical vein endothelial cells in vitro; therefore, it was suggested that marmorin might starve tumors to death by reducing the amount of blood vessels in vivo [10]. Marmorin also induces DNA damage and endoplasmic reticulum stress, resulting in the induction of apoptosis in mice bearing MDA-MB-231 tumor xenografts [10].
Ribosome-inactivating proteins have the potential to become innovative anti-tumor agents, but they also possess toxic adverse effects, including severe systemic anaphylaxis, immunogenicity, and toxicity. To reduce the undesirable effects and achieve better therapeutic efficacy, Deng et al. [11] modified α-MMC with polyethylene glycol (PEG) to explore the anti-tumor efficacy on breast carcinoma; they demonstrated that α-MMC PEGylation extends the half-life of α-MMC and mitigates non-specific toxicity. Indeed, α-MMC-PEG exhibited improved anti-tumor efficacy with tolerable toxic reactions.
Effects of RIPs on leukemia and lymphoma
Trichosanthin significantly inhibits the proliferation of various leukemia and lymphoma cell lines [12]. Notably, TCS can damage leukemia and lymphoma cells through different mechanisms according to the cell type. TCS induces apoptosis in T-lymphocyte cell lines, but inhibits growth of B-lymphocyte cell lines via S-phase cell cycle arrest [12]. It has been suggested that cucurmosin is more potent than TCS in killing the chronic myelogenous leukemia K562 cells; both cucurmosin and TCS down-regulate P210Bcr-Ab1 and inhibit tyrosine kinase, resulting in cell growth suppression [13]. Cucurmosin also inhibits proliferation and induces apoptosis in tumor cells; interestingly, cucurmosin combined with trans-retinoic acid or arsenic trioxide was shown to synergistically increase these effects on the human acute promyelocytic leukemia NB4 cell line [14].
Articulatin-D, the first cytotoxic RIP with a B-chain lacking sugar-binding activity, has been shown to highly inhibit leukemia and lymphoma cells in vitro; the highest toxicity was obtained with Jurkat cells, followed by Molt-4, U-937, HL-60, and Raji cells [15]. With its special physical properties, articulatin-D is a good candidate for the synthesis of immunotoxins capable of efficiently and specifically killing tumor cells.
Immunotoxins are emerging targeted agents composed of a toxin fragment and an antibody/cytokine. Saporin and rGel have been widely used to construct immunotoxins, which have been reported to be useful in cancer treatment by multiple studies [16–18]; several such molecules have been evaluated clinically [19, 20]. It is feasible to locate cancer cells through membrane proteins CD22, CD7, CD19, and CD38, and corresponding antibody HB22.7, HB2, BU12, and OKT10 are used to construct immunotoxins. HB22.7-saporin was cytotoxic against a panel of non-Hodgkin’s lymphoma (NHL) cell lines and was shown to significantly prevent tumor development in a xenograft model of NHL [21]. HB2-saporin, BU12-saporin, and OKT10-saporin were shown to be selectively cytotoxic toward human acute lymphoblastic leukemia in vitro and in vivo [22–24].
Luster et al. [25] have reported that treatment with rGel-BLyS, rGel fused to a B-lymphocyte stimulator, rapidly reduced the tumor burden and markedly prolonged survival in xenograft mouse models of spread lymphoma or leukemia; in this setting, cell death was not induced by caspase activation but rather was partially mediated by the ribotoxic stress response. Furthermore, the rGel-BLyS fusion toxin combined with the proteasome inhibitor bortezomib restrained lymphoma growth and down-regulated nuclear factor kappa B (NF-κB) activity, which is critical for cellular proliferation and survival [26].
Effects of RIPs on hepatoma and other cancers
MAP30 was shown to display anti-tumor activity in cell cultures and mice. In HepG2 cells, for example, cell viability was inhibited by MAP30 in time- and dose-dependent manners, with S-phase arrest; moreover, apoptosis and necrosis induced by MAP30 resulted in tumor volume reduction in HepG2-bearing mice [27]. Cucurmosin induced G0/G1 arrest and apoptosis in HepG2 cells; these effects also translated into potent anti-tumor activities in vivo [28]. Abrus agglutinin not only activates the caspase cascade but also suppresses Akt phosphorylation and NF-κB expression in HepG2 cells [29]. The effects of RIPs on other cancers are summarized in Table 1.
Table 1.
Anti-tumor activities of various ribosome-inactivating proteins (RIPs)
| RIP | Tumor type | Tested cell line(s) |
|---|---|---|
| Type I | ||
| Trichosanthin | Breast cancer | MDA-MB-231a and MCF-7 [5] |
| Lymphoma | CEM, Hut-78, Raji, and Daudi [12] | |
| Cervical cancer | HeLa [37]; Caski [38] | |
| Choriocarcinoma | JAR and BeWo [39] | |
| Colon cancer | CT-26 [40]; LoVo [41] | |
| Hepatoma | HepG2 [42] | |
| Leukemia | Molt-4 and Jurkat [12]; K562 [43] | |
| Lung cancer | 3LLa [44] | |
| Melanoma | B16 [45] | |
| Nasopharyngeal cancer | CNE1a and CNE2a [46]; CNE2 [47] | |
| Prostate cancer | RM-1 [48] | |
| Gastric cancer | MCG803 [49] | |
| α-Momorcharin | Breast cancer | MCF-7, EMT-6a, and MDA-MB-231a [11] |
| Colon cancer | SW480 and SW620 [50] | |
| Epidermoid | A431 and Hep-2 [50] | |
| Hepatoma | Hep G2 and SMMC-7721 [50] | |
| Lung cancer | NCI-H460 and A549 [50] | |
| Melanoma | B16, M14, SK-MEL-28, and A2058 [50] | |
| Nasopharyngeal cancer | CNE2 and HONE1 [51] | |
| Momordica anti-HIV protein of 30 kDa | Bladder cancer | 5637 [52] |
| Breast cancer | MDA-MB-231a [6]; BT20 [53]; MCF-7 [54] | |
| Epidermoid | A431 [53] | |
| Glioma | U87MG [53] | |
| Hepatoma | Hep G2a [27]; Hep-3B [53] | |
| Melanoma | Malme-3M [53] | |
| Myeloma | U266 [53] | |
| Neuroblastoma | SK-N-SH [53] | |
| Prostate cancer | DU145 [53] | |
| Lung cancer | A549 [55] | |
| Cucurmosin | Lung cancer | A549 [13] |
| Melanoma | B16 [13] | |
| Hepatoma | HepG2a [28] | |
| Leukemia | NB4 [14]; K562a [56] | |
| Myeloma | RPM18226 [57] | |
| Pancreatic cancer | BxPC-3 [58]; SW-1990 [59]; PANC-1a [60]; CFPAC-1 [61] | |
| Saporin | Leukemia | NALM-6a [22]; HSB-2a [23]; CCRF CEMa [24] |
| Glioma | U87MG [62] | |
| Lymphoma | Ramos, Rajia, Daudi, DOHH-2, and Granta 519, SUDHL-4 [21]; HDLM2, KM/H2, and L428 [63] | |
| Neuroblastoma | SK-N-MCa [64] | |
| Ovarian cancer | PA-1a [64] | |
| Melanoma | SK-Mel-1a [64], SK-Mel-28 [65] | |
| Pancreatic cancer | BxPC-3a [66] | |
| Prostate cancer | LNCaPa, CWR22Rv1, and DU145 [67]; PC-3a [68] | |
| Gelonin | Breast cancer | MDA-MB-231a, BT-474, SKBR3, MCF-7, and Eb1 [8] |
| Melanoma | MDA-MB-435a, WM35, WM46, WM3211, WM1346, WM1361A, WM1366, WM793, WM983A, WM983B, MeWo, SB2, A375, A375M, SK-MEL-1, SK-MEL-3, SK-MEL-5, SK-MEL-24, SK-MEL-28, SK-MEL-32, WM35P2N1, AAB-527, and Sbcl2 [18] | |
| Cervical cancer | ME-180 [69] | |
| Ovarian cancer | SKOV3 [69] | |
| Pancreatic cancer | Capan-1, Capan-2, MIA-PaCa-2, AsPC-1, BxPC-3, and L3.6P1 [69] | |
| Sarcoma | HT-1080 [69] | |
| Gastric cancer | NCI N-87 [69] | |
| Bladder cancer | T-24a [69]; RT112a [70] | |
| Epidermoid | A431 [71] | |
| Glioma | U87 MG [69]; 9L [72] | |
| Prostate cancer | PC-3 [72] | |
| Colon cancer | HT-29a [71]; CT26a and LS174T [72] | |
| Leukemia | NALM-6a [25]; HL-60 [73] | |
| Lung cancer | Calu-3 [69]; A549a, H1975, and HCC827 [74] | |
| Lymphoma | Rec-1a and NUDHL-1a [25]; Minoa, JeKo-1, SP53 [26]; OCI-Ly3, OCI-Ly10a, SUDHL-4, and SUDHL-6 [75] | |
| Marmorin | Breast cancer | MCF-7a and MDA-MB-231a [10] |
| α-Sarcin | Astrocytoma | 251-MG [76] |
| Breast cancer | MCF-7 [76] | |
| Glioma | RuGli [76] | |
| Pancreatic cancer | Patu II [76] | |
| Bladder cancer | EJ [77] | |
| Colon cancer | HT29 and BCS-TC2 [76]; SW1222 [78] | |
| Sarcoma | HT-1080 [76]; S-180 [79]; RD [80] | |
| Curcin | Lung cancer | NCL-H446 [81] |
| Gastric cancer | SGC-7901 [81] | |
| Sarcoma | S-180 [82] | |
| α-Luffin | Breast cancer | MCF-7 [83] |
| Choriocarcinoma | JEG-3 [83] | |
| Hepatoma | HepG2 [83] | |
| MCP30 | Prostate cancer | LNCaP, PC-3, and PIN [84] |
| Gelonium anti-HIV protein of 31 kDa | Breast cancer | MDA-MB-231a [6] |
| Type II | ||
| Riproximin | Breast cancer | MCF-7 and MDA-MB-231 [62] |
| Larynx cancer | Hep2 [62] | |
| Leukemia | AR230, CML-T1, HL-60, LAMA84, SKW-3, K562, and BV173 [62] | |
| Lung cancer | NCI-H460 and Lewisa [62] | |
| Pancreatic cancer | ASMLb [62] | |
| Prostate cancer | PC-3 [62] | |
| Sarcoma | Saos-2 [62] | |
| Cervical cancer | KB-3-1a [62]; HeLa [85] | |
| Colon cancer | HT-29, CC531b, and CT-26a [62]; HCT116 [86] | |
| Abrus agglutinin | Hepatoma | HepG2a [29] |
| Momordica charantia lectin | Nasopharyngeal cancer | CNE1 and CNE2 [35] |
| Articulatin-D | Leukemia | Jurkat, Molt-4, and HL-60 [15] |
| Lymphoma | U937 and Raji [15] | |
| Mistletoe lectin I | Leukemia | NALM-6 [87] |
| Foetidissimin II | Cervical cancer | HeLa [88] |
| Leukemia | TF-1a [88] | |
| Ebulin I & Nigrin b | Cervical cancer | HeLa [89] |
aCell lines that have been studied in mouse.
bCell lines that have been studied in rat.
Cellular mechanism of RIPs
Entry mechanism
Ribosome-inactivating proteins must enter cells to inactivate the eukaryotic ribosome via their RNA N-glycosidase activity. First, type II RIPs bind to glycoproteins and/or glycolipids on the cell membrane and enter the cell via endocytosis; then, RIPs undergo retrograde transport from the Golgi apparatus to the endoplasmic reticulum via an intracellular pathway [30]. The enzymatic moieties will not be released to cytosol and reach the ribosomes to exert their function until they exploit the endoplasmic reticulum-associated degradation pathway [3].
It is difficult for type I RIPs to enter cells because of their sugar-binding activity deficiency. They can enter cells to some extent, probably due to their interaction with phospholipids in the cell membrane; however, the exact entry mechanism remains unclear. To facilitate the entry of type I RIPs into cells, they can be linked to proper carriers such as monoclonal antibodies and other molecules. The resulting conjugates can be specifically toxic to target cells. Several immunotoxins have been well studied in experiment therapies against hematologic and solid tumors. The entry pathways of type I RIPs, type II RIPs, and immunotoxins are shown in Figure 1.
Figure 1.
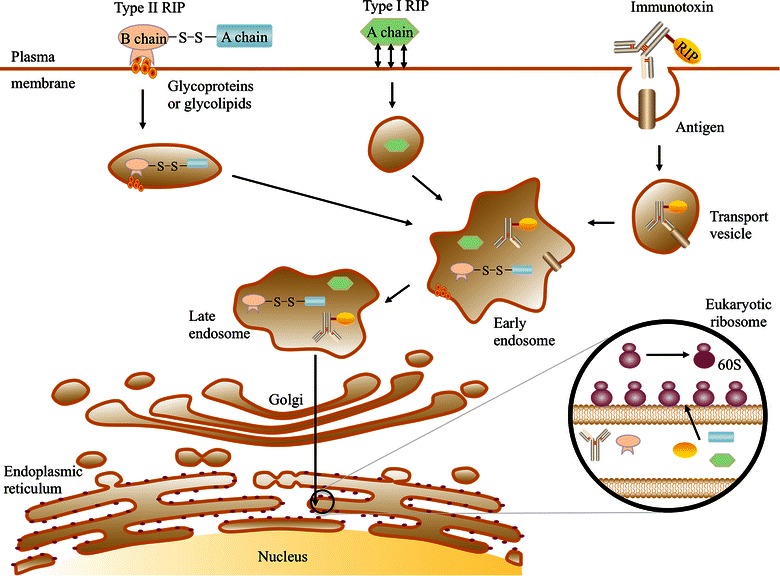
Cell entry mechanism of ribosome-inactivating proteins (RIPs). Different types of RIPs enter the cell through endocytosis and are subsequently degraded in the endoplasmic reticulum. They inactivate ribosomes through cleavage of the A4324 N-glycosidic bond, resulting in protein synthesis blockade.
Induction of apoptosis in tumor cells
Caspases play an important role in apoptosis. They are classified into three types: initiator, executioner, and cytokine processor caspases. Great progress has been made in studying the three signaling pathways related to caspase activation, including mitochondrial, death receptor, and endoplasmic reticulum stress signaling pathways. The connections among these pathways are shown in Figure 2 [31]. Apoptosis also occurs through apoptosis-inducing factor (AIF), which is caspase-independent [32].
Figure 2.
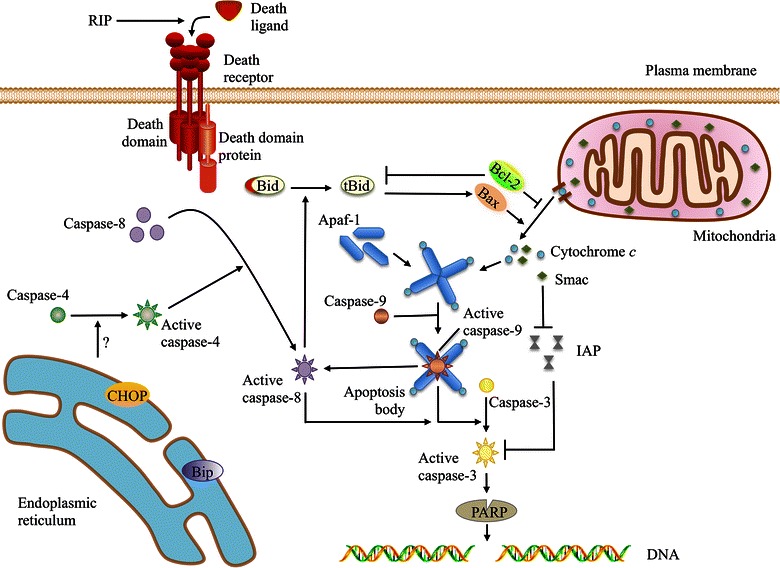
The apoptotic mechanism of RIPs. RIPs may trigger the death receptor pathway by facilitating the combination of the death ligand and its receptor. Caspase-8 is recruited and activated by death domain proteins such as Fas-associated protein with death domain (FADD). C/EBP homologous protein (CHOP) and immunoglobulin-binding protein (Bip) are increased under RIP-induced endoplasmic reticulum stress, in which activated caspase-4 contributes to capase-8 activation. The release of second mitochondria-derived activator of caspases (Smac) and cytochrome c, which can be increased by Bax or decreased by Bcl-2, is promoted by RIP. Cytochrome c aggregates with apoptotic protease-activating factor 1 (Apaf-1) and becomes an apoptotic body that activates caspase-9, which in turn activates caspase-3 and caspase-8. Activated caspase-3 cleaves poly(ADP-ribose) polymerase (PARP), resulting in DNA fragmentation and apoptosis. Smac protects caspase-3 from inhibitor of apoptosis protein (IAP) inhibition. Caspase-8 cuts Bid into tBid, which is necessary for Bax oligomerization in the mitochondrial outer membrane. The inhibition of tBid insertion into the mitochondrial membrane by Bcl-2 prevents cytochrome c release [31].
Mitochondria-mediated apoptosis
Recent studies have indicated that apoptosis-inducing substances can lead to excessive reactive oxygen species production, intracellular Ca2+ imbalance, and a series of pathologic changes, resulting in mitochondrial membrane potential and permeability changes. Then, the pro-apoptotic factors cytochrome c, AIF, second mitochondria-derived activator of caspases (Smac), and apoptotic protease-activating factor 1 (Apaf-1) are released from the mitochondria to participate in the process of apoptosis.
Mitochondrial membrane potential depolarization and caspase-9 activation were detected in MCF-7 cells and to a lesser extent in MDA-MB-231 cells after marmorin treatment [10]. Li et al. [33] reported the loss of mitochondrial membrane potential (the point of no return in apoptotic cascades) in HL-60 cells after apoptosis was induced by TCS. In addition, Orrenius et al. [34] noted that cytochrome c release is dominated by the Bcl-2 family of proteins. Furthermore, simultaneous Bax up-regulation, Bcl-2 down-regulation, and poly(ADP-ribose) polymerase (PARP) cleavage were noted in Abrus agglutinin-treated HepG2 cells, caspase-3/7 activity levels failed to increase after Bax knockout, and Bcl-2-overexpressing hepatocellular carcinoma cells were found to be ricin-resistant [29].
Several pumps, such as the Na+–K+ pump and the Ca2+ pump, maintain concentration gradients of various ions to achieve appropriate membrane potential. Alterations in the mitochondrial membrane potential after the induction of apoptosis lead to changes in membrane permeability. The results mentioned above suggest that changes in mitochondrial membrane permeability could cause apoptosis, which is induced by RIPs through decreasing the Bcl-2/Bax ratio (modifying the outer mitochondrial membrane permeability); this in turn enhances cytochrome c and Smac translocation into the cytoplasm and activates caspase-9 and the downstream executioner caspase-3, thereby increasing the production of cleaved PARP and resulting in DNA fragmentation and apoptosis [34–36].
Death receptor-mediated apoptosis
Death receptors, such as Fas, deliver apoptotic signals into the cytoplasm by binding to Fas ligand (FasL); the signals are then passed to downstream procaspase-8, the activation of which demands the cytoplasmic adaptor molecule, which is indispensable to the binding and proteolysis of procaspase-8 for activation. Once activated, the initiator caspase-8 can activate caspase-3, eventually leading to cell apoptosis.
Marmorin was found to trigger the death receptor apoptotic pathway in MCF7 cells; this pathway is also preferentially activated in MDA-MB-231 cells [10]. Due to caspase-3 deficiency in MCF7 cells, caspase-8 amplifies the apoptotic signal through cleavage of the protein Bid, which punctures the mitochondria and causes mitochondrial collapse, thereby generating sufficient effector caspase levels. Conversely, TCS does not affect Fas or FasL levels, indicating that the Fas/FasL pathway is not involved in TCS-induced apoptosis [32].
Endoplasmic reticulum stress-mediated apoptosis
Endoplasmic reticulum stress is found in cells exposed to environmental toxins, hypoxia, viruses, ultraviolet light, and other stimuli. Its manifestations include misfolded and/or unfolded protein aggregation in the endoplasmic reticulum lumen as well as Ca2+ balance disorders. Endoplasmic reticulum stress can promote a series of physiologic changes in the endoplasmic reticulum. Accumulated misfolded and/or unfolded proteins are processed, allowing cells to maintain their normal functions and remain alive. However, excessive endoplasmic reticulum stress can cause apoptosis.
Trichosanthin treatment was shown to up-regulate the endoplasmic reticulum stress-related proteins Bip (immunoglobulin-binding protein) and CHOP (C/EBP homologous protein) in HL-60 cells, thereby activating caspase-4, which is involved in caspase-3 activation [89]. Endoplasmic reticulum stress was also described in marmorin-treated MCF7 and MDA-MB-231 cells, as evidenced by CHOP up-regulation and caspase-12 cleavage [10].
Horrix et al. [86] identified activation of the unfolded protein response (UPR) in response to endoplasmic reticulum stress; the UPR is induced in MDA-MB-231 cells exposed to low concentrations of the type II RIP riproximin. As many cancer cells activate the UPR to cope with stressors, α-MMC was shown to down-regulate the UPR in NPC cells; however, substantial apoptosis was not observed until the α-MMC dosage reached a certain threshold, indicating that α-MMC at low concentrations probably inhibit increased cell generation via down-regulation of the UPR [51]. There are two conceivable strategies to initiate apoptosis through endoplasmic reticulum stress: (1) prolonging the UPR to induce apoptosis, which likely occurs in riproximin-induced apoptosis; and (2) blocking the UPR so that tumors are vulnerable to stressors, as with α-MMC.
Future research emphasis
Conventional cancer drugs that are currently in use often lack tumor specificity, which greatly limits the therapeutic dose and curative effect. A feasible way to overcome this issue is the use of targeted therapy, as follows: (1) suitable targeted delivery such as with the immunotoxins mentioned above or with bi-specific antibodies (containing two different specific antigen recognition Fab fragments); (2) a tumor-specific expression strategy, in which the cDNA of RIP is synthesized and cloned into a plasmid vector controlled by a cancer-specific promoter, eventually producing RIP in the cell cytoplasm. These strategies must be investigated in a series of preclinical studies before assays can be conducted in human subjects. Several saporin-containing immunotoxins in clinical trials have exhibited promising results [20], whereas other RIP-containing immunotoxins have barely been studied. A few factors must be considered when translating preclinical data into the clinic: the risk of immunogenicity and toxicity in patients should be minimized; the minimum effect dose and maximum tolerated dose should be determined; and possible adverse effects during treatment should be predicted. Tumor-specific expression strategies are rarely reported; therefore, this idea remains to be explored.
Conclusions
Abundant evidence indicates that RIPs exert their cell-killing abilities through a variety of mechanisms, many of which are caspase-dependent. Although several mechanisms involved in RIP-induced apoptosis have been elucidated, more studies are required to reveal the precise mechanism. Considering the potential use of RIPs in important diseases and their effectiveness as immunotoxins for targeted therapy, RIPs are worthy of further exploration.
Authors’ contribution
MZ conceived the topic and drafted the manuscript. MZ helped in the manuscript preparation. DL and JW participated in the data collection. WJ and OS helped to conceive the topic and contributed significantly to the manuscript revision. All authors read and approved the final manuscript.
Acknowledgements
We thank the Basic Research Program of Shenzhen (JCYJ20120613113228732) and the University Innovation Program of Guangdong Province (201410590040).
Compliance with ethical guidelines
Competing interests The authors declare that they have no competing interests.
Contributor Information
Meiqi Zeng, 2140220106@email.szu.edu.cn.
Manyin Zheng, Email: 83484191@qq.com.
Desheng Lu, Email: delu@szu.edu.cn.
Jun Wang, Email: yxywj@szu.edu.cn.
Wenqi Jiang, Email: jiangwq@sysucc.org.cn.
Ou Sha, Email: shaou@szu.edu.cn.
References
- 1.Endo Y, Tsurugi K. RNA, N-glycosidase activity of ricin A-chain. Mechanism of action of the toxic lectin ricin on eukaryotic ribosomes. J Biol Chem. 1987;262:8128–8130. [PubMed] [Google Scholar]
- 2.Perentesis JP, Miller SP, Bodley JW. Protein toxin inhibitors of protein synthesis. BioFactors. 1992;3:173–184. [PubMed] [Google Scholar]
- 3.Puri M, Kaur I, Perugini MA, Gupta RC. Ribosome-inactivating proteins: current status and biomedical applications. Drug Discov Today. 2012;17:774–783. doi: 10.1016/j.drudis.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Nielsen K, Boston RS. Ribosome-inactivating proteins: a plant perspective. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:785–816. doi: 10.1146/annurev.arplant.52.1.785. [DOI] [PubMed] [Google Scholar]
- 5.Fang EF, Zhang CZ, Zhang L, Wong JH, Chan YS, Pan WL, et al. Trichosanthin inhibits breast cancer cell proliferation in both cell lines and nude mice by promotion of apoptosis. PLoS One. 2012;7:e41592. doi: 10.1371/journal.pone.0041592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee-Huang S, Huang PL, Sun Y, Chen HC, Kung HF, Huang PL, et al. Inhibition of MDA-MB-231 human breast tumor xenografts and HER2 expression by anti-tumor agents GAP31 and MAP30. Anticancer Res. 2000;20:653–659. [PubMed] [Google Scholar]
- 7.Roh H, Pippin JA, Green DW, Boswell CB, Hirose CT, Mokadam N, et al. HER2/neu antisense targeting of human breast carcinoma. Oncogene. 2000;19:6138–6143. doi: 10.1038/sj.onc.1204001. [DOI] [PubMed] [Google Scholar]
- 8.Zhou H, Hittelman WN, Yagita H, Cheung LH, Martin SS, Winkles JA, et al. Antitumor activity of a humanized, bivalent immunotoxin targeting fn14-positive solid tumors. Cancer Res. 2013;73:4439–4450. doi: 10.1158/0008-5472.CAN-13-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wan J, Zhou L. Expression of ERα and ERβ and its diagnostic value for breast cancer. Guangxi Yixue. 2011;33:1449–1451. [Google Scholar]
- 10.Pan WL, Wong JH, Fang EF, Chan YS, Ye XJ, Ng TB. Differential inhibitory potencies and mechanisms of the type I ribosome inactivating protein marmorin on estrogen receptor (ER)-positive and ER-negative breast cancer cells. Biochim Biophys Acta. 2013;1833:987–996. doi: 10.1016/j.bbamcr.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 11.Deng NH, Wang L, He QC, Zheng JC, Meng Y, Meng YF et al. PEGylation alleviates the non-specific toxicities of alpha-Momorcharin and preserves its antitumor efficacy in vivo. Drug Deliv. 2014 [Epub ahead of print]. [DOI] [PubMed]
- 12.Wang YY, Ouyang DY, Zheng YT. Mechanism of trichosanthin against human leukemia/lymphoma cells in vitro. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2007;15:729–732. [PubMed] [Google Scholar]
- 13.Hou X, Meehan EJ, Xie J, Huang M, Chen M, Chen L. Atomic resolution structure of cucurmosin, a novel type 1 ribosome-inactivating protein from the sarcocarp of Cucurbita moschata. J Struct Biol. 2008;164:81–87. doi: 10.1016/j.jsb.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 14.Xie JM, Liu M, Liu TB, Chen MH, Yang AQ, Yang P. Effects of cucurmosin combined with common chemotherapeutics on proliferation and apoptosis of NB4 cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2012;20:1327–1331. [PubMed] [Google Scholar]
- 15.Das MK, Sharma RS, Mishra V. A cytotoxic type-2 ribosome inactivating protein (from leafless mistletoe) lacking sugar binding activity. Int J Biol Macromol. 2011;49:1096–1103. doi: 10.1016/j.ijbiomac.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Cao Y, Marks JD, Marks JW, Cheung LH, Kim S, Rosenblum MG. Construction and characterization of novel, recombinant immunotoxins targeting the Her2/neu oncogene product: in vitro and in vivo studies. Cancer Res. 2009;69:8987–8995. doi: 10.1158/0008-5472.CAN-09-2693. [DOI] [PubMed] [Google Scholar]
- 17.Polito L, Bortolotti M, Mercatelli D, Battelli MG, Bolognesi A. Saporin-S6: a useful tool in cancer therapy. Toxins. 2013;5:1698–1722. doi: 10.3390/toxins5101698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou H, Ekmekcioglu S, Marks JW, Mohamedali KA, Asrani K, Phillips KK, et al. The TWEAK receptor Fn14 is a therapeutic target in melanoma: immunotoxins targeting Fn14 receptor for malignant melanoma treatment. J Invest Dermatol. 2013;133:1052–1062. doi: 10.1038/jid.2012.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borthakur G, Rosenblum MG, Talpaz M, Daver N, Ravandi F, Faderl S, et al. Phase 1 study of an anti-CD33 immunotoxin, humanized monoclonal antibody M195 conjugated to recombinant gelonin (HUM-195/rGEL), in patients with advanced myeloid malignancies. Haematologica. 2013;98:217–221. doi: 10.3324/haematol.2012.071092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polito L, Bortolotti M, Pedrazzi M, Bolognesi A. Immunotoxins and other conjugates containing saporin-s6 for cancer therapy. Toxins. 2011;3:697–720. doi: 10.3390/toxins3060697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kato J, O’Donnell RT, Abuhay M, Tuscano JM. Efficacy and toxicity of a CD22-targeted antibody-saporin conjugate in a xenograft model of non-Hodgkin’s lymphoma. Oncoimmunology. 2012;1:1469–1475. doi: 10.4161/onci.21815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flavell DJ, Flavell SU, Boehm DA, Emery L, Noss A, Ling NR, et al. Preclinical studies with the anti-CD19-saporin immunotoxin BU12-SAPORIN for the treatment of human-B-cell tumours. Br J Cancer. 1995;72:1373–1379. doi: 10.1038/bjc.1995.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morland BJ, Barley J, Boehm D, Flavell SU, Ghaleb N, Kohler JA, et al. Effectiveness of HB2 (anti-CD7)—saporin immunotoxin in an in vivo model of human T-cell leukaemia developed in severe combined immunodeficient mice. Br J Cancer. 1994;69:279–285. doi: 10.1038/bjc.1994.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flavell DJ, Boehm DA, Noss A, Warnes SL, Flavell SU. Therapy of human T-cell acute lymphoblastic leukaemia with a combination of anti-CD7 and anti-CD38-SAPORIN immunotoxins is significantly better than therapy with each individual immunotoxin. Br J Cancer. 2001;84:571–578. doi: 10.1054/bjoc.2000.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luster TA, Mukherjee I, Carrell JA, Cho YH, Gill J, Kelly L, et al. Fusion toxin BLyS-gelonin inhibits growth of malignant human B cell lines in vitro and in vivo. PLoS One. 2012;7:e47361. doi: 10.1371/journal.pone.0047361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lyu MA, Pham LV, Sung B, Tamayo AT, Ahn KS, Hittelman WN, et al. The therapeutic effects of rGel/BLyS fusion toxin in in vitro and in vivo models of mantle cell lymphoma. Biochem Pharmacol. 2012;84:451–458. doi: 10.1016/j.bcp.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 27.Fang EF, Zhang CZ, Wong JH, Shen JY, Li CH, Ng TB. The MAP30 protein from bitter gourd (Momordica charantia) seeds promotes apoptosis in liver cancer cells in vitro and in vivo. Cancer Lett. 2012;324:66–74. doi: 10.1016/j.canlet.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 28.Xie J, Que W, Liu H, Liu M, Yang A, Chen M. Anti-proliferative effects of cucurmosin on human hepatoma HepG2 cells. Mol Med Rep. 2012;5:196–201. doi: 10.3892/mmr.2011.605. [DOI] [PubMed] [Google Scholar]
- 29.Mukhopadhyay S, Panda PK, Das DN, Sinha N, Behera B, Maiti TK, et al. Abrus agglutinin suppresses human hepatocellular carcinoma in vitro and in vivo by inducing caspase-mediated cell death. Acta Pharmacol Sin. 2014;35:814–824. doi: 10.1038/aps.2014.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stirpe F. Ribosome-inactivating proteins. Toxicon. 2004;44:371–383. doi: 10.1016/j.toxicon.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 31.Yi X, Yin XM, Dong Z. Inhibition of Bid-induced apoptosis by Bcl-2. tBid insertion, Bax translocation, and Bax/Bak oligomerization suppressed. J Biol Chem. 2003;278:16992–16999. doi: 10.1074/jbc.M300039200. [DOI] [PubMed] [Google Scholar]
- 32.Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- 33.Li J, Xia X, Ke Y, Nie H, Smith MA, Zhu X. Trichosanthin induced apoptosis in HL-60 cells via mitochondrial and endoplasmic reticulum stress signaling pathways. Biochim Biophys Acta. 2007;1770:1169–1180. doi: 10.1016/j.bbagen.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 34.Orrenius S. Mitochondrial regulation of apoptotic cell death. Toxicol Lett. 2004;149:19–23. doi: 10.1016/j.toxlet.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 35.Fang EF, Zhang CZ, Ng TB, Wong JH, Pan WL, Ye XJ, et al. Momordica Charantia lectin, a type II ribosome inactivating protein, exhibits antitumor activity toward human nasopharyngeal carcinoma cells in vitro and in vivo. Cancer Prev Res (Phila) 2012;5:109–121. doi: 10.1158/1940-6207.CAPR-11-0203. [DOI] [PubMed] [Google Scholar]
- 36.Sha O, Niu J, Ng TB, Cho EY, Fu X, Jiang W. Anti-tumor action of trichosanthin, a type 1 ribosome-inactivating protein, employed in traditional Chinese medicine: a mini review. Cancer Chemother Pharmacol. 2013;71:1387–1393. doi: 10.1007/s00280-013-2096-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang YL, Huang LM, Hui Y, Lu H. Experimental study of trichosanthin’s effect on Hela cells proliferation. Lishizhen Med Materia Medica Res. 2007;18:280–281. [Google Scholar]
- 38.Peng P, Huang L, Wang Y, You C, Cao W, Song H, et al. Effect of recombinant trichosanthin on proliferation of human cevical cancer Caski cells. Zhongguo Zhong Yao Za Zhi. 2011;36:2539–2542. [PubMed] [Google Scholar]
- 39.Jiao Y, Liu W. Low-density lipoprotein receptor-related protein 1 is an essential receptor for trichosanthin in 2 choriocarcinoma cell lines. Biochem Biophys Res Commun. 2010;391:1579–1584. doi: 10.1016/j.bbrc.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 40.Huang CX, Zhao JG, Li ZY, Li D, Xia DJ, Wang QQ, et al. Multi-chaperone-peptide-rich mixture from colo-carcinoma cells elicits potent anticancer immunity. Cancer Epidemiol. 2010;34:494–500. doi: 10.1016/j.canep.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 41.Gao DF, Wang BQ, Cao GM, Zhang XL. Cloning of trichosanthin gene and its induction effects on the apoptpsis of colorectal carcinoma LoVo cell. Fudan Xuebao. 2010;37:157–161. [Google Scholar]
- 42.Mondal A. A novel extraction of trichosanthin from Trichosanthes kirilowii roots using three-phase partitioning and its in vitro anticancer activity. Pharm Biol. 2014;52:677–680. doi: 10.3109/13880209.2013.864684. [DOI] [PubMed] [Google Scholar]
- 43.Kong M, Ke YB, Zhou MY, Ke XY, Lu B, Nie HL. Study on trichosanthin induced apoptosis of leukemia K562 cells. Shi Yan Sheng Wu Xue Bao. 1998;31:233–243. [PubMed] [Google Scholar]
- 44.Cai Y, Xiong S, Zheng Y, Luo F, Jiang P, Chu Y. Trichosanthin enhances anti-tumor immune response in a murine Lewis lung cancer model by boosting the interaction between TSLC1 and CRTAM. Cell Mol Immunol. 2011;8:359–367. doi: 10.1038/cmi.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bi L, Li H, Zhang Y. Effect of trichosanthin of cell cycle and apoptosis of murine melanoma cells. Zhongguo Zhong Xi Yi Jie He Za Zhi. 1998;18:35–37. [PubMed] [Google Scholar]
- 46.Kang M, Ou H, Wang R, Liu W, Mao Y, Tang A. Effect of trichosanthin on apoptosis and telomerase activity of nasopharyngeal carcinomas in nude mice. J BUON. 2013;18:675–682. [PubMed] [Google Scholar]
- 47.Liu F, Wang B, Wang Z, Yu S. Trichosanthin down-regulates Notch signaling and inhibits proliferation of the nasopharyngeal carcinoma cell line CNE2 in vitro. Fitoterapia. 2012;83:838–842. doi: 10.1016/j.fitote.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 48.Shi Z, Shan SD, Yuan T, Gui YP, Cao CH, Zhang JF. Mechanism of trichosanthin inducing apoptosis of mouse prostatic cancer RM-1 cells in vitro. Zhong Yao Cai. 2009;32:239–242. [PubMed] [Google Scholar]
- 49.Xu J, Gao DF, Yan GL, Fan JM. Induced apoptotic action of recombinant trichosanthin in human stomach adenocarcinoma MCG803 cells. Mol Biol Rep. 2009;36:1559–1564. doi: 10.1007/s11033-008-9352-y. [DOI] [PubMed] [Google Scholar]
- 50.Bian X, Shen F, Chen Y, Wang B, Deng M, Meng Y. PEGylation of alpha-momorcharin: synthesis and characterization of novel anti-tumor conjugates with therapeutic potential. Biotechnol Lett. 2010;32:883–890. doi: 10.1007/s10529-010-0242-8. [DOI] [PubMed] [Google Scholar]
- 51.Pan WL, Wong JH, Fang EF, Chan YS, Ng TB, Cheung RC. Preferential cytotoxicity of the type I ribosome inactivating protein alpha-momorcharin on human nasopharyngeal carcinoma cells under normoxia and hypoxia. Biochem Pharmacol. 2014;89:329–339. doi: 10.1016/j.bcp.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hao L, Zhang ZG, Han CH, Zhao Y, Liang Q, Jiang B et al. Expression of Momordica charantia MAP30 and its anti-tumor effect on bladder cancer cells. Minerva Urol Nefrol. 2014. [Epub ahead of print] [PubMed]
- 53.Lee-Huang S, Huang PL, Chen HC, Huang PL, Bourinbaiar A, Huang HI, et al. Anti-HIV and anti-tumor activities of recombinant MAP30 from bitter melon. Gene. 1995;161:151–156. doi: 10.1016/0378-1119(95)00186-A. [DOI] [PubMed] [Google Scholar]
- 54.Qiu HI, Rang J, Ding XZ, Hu SB, Zhang YM, Zhu DQ, et al. Prokaryotic expression of MAP30 from Momordica charantia and its biological activity. China Biotechnol. 2014;34:40–46. [Google Scholar]
- 55.Meng Y, Lin S, Liu S, Fan X, Li G, Meng Y. A novel method for simultaneous production of two ribosome-inactivating proteins, alpha-MMC and MAP30, from Momordica charantia L. PLoS One. 2014;9:e101998. doi: 10.1371/journal.pone.0101998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu T, Liu H, Xie J, Hu J. Effect of cucurmosin on chronic myeloid leukemia K562 cell line. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2013;21:891–894. doi: 10.7534/j.issn.1009-2137.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 57.Liu T, Yang P, Xie Jie M, Hu J. Inhibitory effect of pumpkin protein on expression of Notch signal in RPMI8226 myeloma cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2014;22:1012–1015. doi: 10.7534/j.issn.1009-2137.2014.04.023. [DOI] [PubMed] [Google Scholar]
- 58.Zhang B, Huang H, Xie J, Xu C, Chen M, Wang C, et al. Cucurmosin induces apoptosis of BxPC-3 human pancreatic cancer cells via inactivation of the EGFR signaling pathway. Oncol Rep. 2012;27:891–897. doi: 10.3892/or.2011.1573. [DOI] [PubMed] [Google Scholar]
- 59.Xie J, Wang C, Yang A, Zhang B, Yin Q, Huang H, et al. Cucurmosin kills human pancreatic cancer SW-1990 cells in vitro and in vivo. Anticancer Agents Med Chem. 2013;13:952–956. doi: 10.2174/18715206113139990109. [DOI] [PubMed] [Google Scholar]
- 60.Wang C, Yang A, Zhang B, Yin Q, Huang H, Chen M, et al. PANC-1 pancreatic cancer cell growth inhibited by cucurmosin alone and in combination with an epidermal growth factor receptor-targeted drug. Pancreas. 2014;43:291–297. doi: 10.1097/MPA.0000000000000087. [DOI] [PubMed] [Google Scholar]
- 61.Xie J, Wang C, Zhang B, Yang A, Yin Q, Huang H, et al. Cucurmosin induces the apoptosis of human pancreatic cancer CFPAC-1 cells by inactivating the PDGFR-beta signalling pathway. Pharmacol Rep. 2013;65:682–688. doi: 10.1016/S1734-1140(13)71046-6. [DOI] [PubMed] [Google Scholar]
- 62.Adwan H, Bayer H, Pervaiz A, Sagini M, Berger MR. Riproximin is a recently discovered type II ribosome inactivating protein with potential for treating cancer. Biotechnol Adv. 2014;32:1077–1090. doi: 10.1016/j.biotechadv.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 63.Vooijs WC, Otten HG, van Vliet M, van Dijk AJ, de Weger RA, de Boer M, et al. B7-1 (CD80) as target for immunotoxin therapy for Hodgkin’s disease. Br J Cancer. 1997;76:1163–1169. doi: 10.1038/bjc.1997.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Beitz JG, Davol P, Clark JW, Kato J, Medina M, Frackelton AR, Jr, et al. Antitumor activity of basic fibroblast growth factor-saporin mitotoxin in vitro and in vivo. Cancer Res. 1992;52:227–230. [PubMed] [Google Scholar]
- 65.Torres Demichelis V, Vilcaes AA, Iglesias-Bartolome R, Ruggiero FM, Daniotti JL. Targeted delivery of immunotoxin by antibody to ganglioside GD3: a novel drug delivery route for tumor cells. PLoS One. 2013;8:e55304. doi: 10.1371/journal.pone.0055304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Duxbury MS, Ito H, Ashley SW, Whang EE. CEACAM6 as a novel target for indirect type 1 immunotoxin-based therapy in pancreatic adenocarcinoma. Biochem Biophys Res Commun. 2004;317:837–843. doi: 10.1016/j.bbrc.2004.03.128. [DOI] [PubMed] [Google Scholar]
- 67.Kuroda K, Liu H, Kim S, Guo M, Navarro V, Bander NH. Saporin toxin-conjugated monoclonal antibody targeting prostate-specific membrane antigen has potent anticancer activity. Prostate. 2010;70:1286–1294. doi: 10.1002/pros.21164. [DOI] [PubMed] [Google Scholar]
- 68.Siva AC, Wild MA, Kirkland RE, Nolan MJ, Lin B, Maruyama T, et al. Targeting CUB domain-containing protein 1 with a monoclonal antibody inhibits metastasis in a prostate cancer model. Cancer Res. 2008;68:3759–3766. doi: 10.1158/0008-5472.CAN-07-1657. [DOI] [PubMed] [Google Scholar]
- 69.Zhou H, Marks JW, Hittelman WN, Yagita H, Cheung LH, Rosenblum MG, et al. Development and characterization of a potent immunoconjugate targeting the Fn14 receptor on solid tumor cells. Mol Cancer Ther. 2011;10:1276–1288. doi: 10.1158/1535-7163.MCT-11-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Martinez-Torrecuadrada JL, Cheung LH, Lopez-Serra P, Barderas R, Canamero M, Ferreiro S, et al. Antitumor activity of fibroblast growth factor receptor 3-specific immunotoxins in a xenograft mouse model of bladder carcinoma is mediated by apoptosis. Mol Cancer Ther. 2008;7:862–873. doi: 10.1158/1535-7163.MCT-07-0394. [DOI] [PubMed] [Google Scholar]
- 71.Pirie CM, Liu DV, Wittrup KD. Targeted cytolysins synergistically potentiate cytoplasmic delivery of gelonin immunotoxin. Mol Cancer Ther. 2013;12:1774–1782. doi: 10.1158/1535-7163.MCT-12-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shin MC, Zhang J, David AE, Trommer WE, Kwon YM, Min KA, et al. Chemically and biologically synthesized CPP-modified gelonin for enhanced anti-tumor activity. J Control Release. 2013;172:169–178. doi: 10.1016/j.jconrel.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Duzkale H, Pagliaro LC, Rosenblum MG, Varan A, Liu B, Reuben J, et al. Bone marrow purging studies in acute myelogenous leukemia using the recombinant anti-CD33 immunotoxin HuM195/rGel. Biol Blood Marrow Transplant. 2003;9:364–372. doi: 10.1016/S1083-8791(03)00129-0. [DOI] [PubMed] [Google Scholar]
- 74.Zhou X, Qiu J, Wang Z, Huang N, Li X, Li Q, et al. In vitro and in vivo anti-tumor activities of anti-EGFR single-chain variable fragment fused with recombinant gelonin toxin. J Cancer Res Clin Oncol. 2012;138:1081–1090. doi: 10.1007/s00432-012-1181-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lyu M-A, Rai D, Ahn KS, Sung B, Cheung LH, Marks JW, et al. The rGel/BLyS fusion toxin inhibits diffuse large B-cell lymphoma growth in vitro and in vivo. Neoplasia. 2010;12:366–375. doi: 10.1593/neo.91960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Turnay J, Olmo N, Jimenez A, Lizarbe MA, Gavilanes JG. Kinetic study of the cytotoxic effect of alpha-sarcin, a ribosome inactivating protein from Aspergillus giganteus, on tumour cell lines: protein biosynthesis inhibition and cell binding. Mol Cell Biochem. 1993;122:39–47. doi: 10.1007/BF00925735. [DOI] [PubMed] [Google Scholar]
- 77.Wawrzynczak EJ, Henry RV, Cumber AJ, Parnell GD, Derbyshire EJ, Ulbrich N. Biochemical, cytotoxic and pharmacokinetic properties of an immunotoxin composed of a mouse monoclonal antibody Fib75 and the ribosome-inactivating protein alpha-sarcin from Aspergillus giganteus. Eur J Biochem. 1991;196:203–209. doi: 10.1111/j.1432-1033.1991.tb15805.x. [DOI] [PubMed] [Google Scholar]
- 78.Carreras-Sangra N, Tome-Amat J, Garcia-Ortega L, Batt CA, Onaderra M, Martinez-del-Pozo A, et al. Production and characterization of a colon cancer-specific immunotoxin based on the fungal ribotoxin alpha-sarcin. Protein Eng Des Sel. 2012;25:425–435. doi: 10.1093/protein/gzs032. [DOI] [PubMed] [Google Scholar]
- 79.Olson BH, Jennings JC, Roga V, Junek AJ, Schuurmans DM. Alpha sarcin, a new antitumor agent II. Fermentation and antitumor spectrum. Appl Microbiol. 1965;13:322–326. doi: 10.1128/am.13.3.322-326.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Olmo N, Turnay J, Gonzalez de Buitrago G, Lopez de Silanes I, Gavilanes JG, Lizarbe MA. Cytotoxic mechanism of the ribotoxin alpha-sarcin. Induction of cell death via apoptosis. Eur J Biochem. 2001;268:2113–2123. doi: 10.1046/j.1432-1327.2001.02086.x. [DOI] [PubMed] [Google Scholar]
- 81.Luo MJ, Yang XY, Liu WX, Xu Y, Huang P, Yan F, et al. Expression, purification and anti-tumor activity of curcin. Acta Biochim Biophys Sin (Shanghai) 2006;38:663–668. doi: 10.1111/j.1745-7270.2006.00208.x. [DOI] [PubMed] [Google Scholar]
- 82.Zhao Q, Wang W, Wang Y, Xu Y, Chen F. The effect of curcin from Jatropha curcas on apoptosis of mouse sarcoma-180 cells. Fitoterapia. 2012;83:849–852. doi: 10.1016/j.fitote.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 83.Liu L, Wang R, He W, He F, Huang G. Cloning and soluble expression of mature alpha-luffin from Luffa cylindrica and its antitumor activities in vitro. Acta Biochim Biophys Sin (Shanghai) 2010;42:585–592. doi: 10.1093/abbs/gmq056. [DOI] [PubMed] [Google Scholar]
- 84.Xiong SD, Yu K, Liu XH, Yin LH, Kirschenbaum A, Yao S, et al. Ribosome-inactivating proteins isolated from dietary bitter melon induce apoptosis and inhibit histone deacetylase-1 selectively in premalignant and malignant prostate cancer cells. Int J Cancer. 2009;125:774–782. doi: 10.1002/ijc.24325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Voss C, Eyol E, Frank M, von der Lieth CW, Berger MR. Identification and characterization of riproximin, a new type II ribosome-inactivating protein with antineoplastic activity from Ximenia americana. FASEB J. 2006;20:1194–1196. doi: 10.1096/fj.05-5231fje. [DOI] [PubMed] [Google Scholar]
- 86.Horrix C, Raviv Z, Flescher E, Voss C, Berger MR. Plant ribosome-inactivating proteins type II induce the unfolded protein response. Cell Mol Life Sci. 2011;68:1269–1281. doi: 10.1007/s00018-010-0524-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Seifert G, Jesse P, Laengler A, Reindl T, Luth M, Lobitz S, et al. Molecular mechanisms of mistletoe plant extract-induced apoptosis in acute lymphoblastic leukemia in vivo and in vitro. Cancer Lett. 2008;264:218–228. doi: 10.1016/j.canlet.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 88.Zhang D, Halaweish FT. Isolation and characterization of ribosome-inactivating proteins from Cucurbitaceae. Chem Biodivers. 2007;4:431–442. doi: 10.1002/cbdv.200790035. [DOI] [PubMed] [Google Scholar]
- 89.Citores L, Ferreras JM, Munoz R, Benitez J, Jimenez P, Girbes T. Targeting cancer cells with transferrin conjugates containing the non-toxic type 2 ribosome-inactivating proteins nigrin b or ebulin I. Cancer Lett. 2002;184:29–35. doi: 10.1016/S0304-3835(02)00169-6. [DOI] [PubMed] [Google Scholar]


