Abstract
OBJECTIVES
A case of drug-induced hepatitis mediated by troxis necrosis, a form of autoimmune hepatitis, is described.
METHODS
Clinical data, light and electron microscopy of an ultrasound-guided core needle liver biopsy specimen, were examined to investigate the cause of transaminitis in a 26 year old male patient on Cellcept and Plaquenil for the treatment of lupus erythematosus. A systematic PUBMED review of troxis necrosis as the underlying mechanism for drug-induced hepatitis was performed.
RESULTS
Liver function tests (LFT) were significant for elevated AST (305) and ALT (174); autoimmune workup was significant for anti-ANA positivity and α-SMA negativity. On light microscopy, the liver biopsy shows focal areas of lymphocytic infiltrate surrounding and forming immunologic synapses with lobular hepatocytes, indicating lobular hepatitis of autoimmune nature. Electron microscopy confirmed the presence of immunologic synapses. Upon cessation of the offending medications, the LFT returned to baseline with no further intervention. Literature search yielded 7 previously reported cases of drug-induced hepatitis mediated by troxis necrosis.
CONCLUSION
Troxis necrosis is a novel mechanism for drug-induced hepatitis, including immunomodulatory medications including monoclonal anti-TWEAK antibody, Cellcept and Plaquenil, two widely used immunosuppression/anti-rejection medications.
INTRODUCTION
The liver is a unique multifunctional organ that performs a variety of metabolic and homeostatic functions. An important and under-recognized function of the hepatocytes is antigen presentation. Indeed, healthy hepatocytes normally do not express MHC class II molecules; however, in clinical hepatitis, viral or autoimmune, hepatocytes often exhibit aberrant MHC class II expression, a key component in conferring cellular immunity and lymphocyte-induced targeted cell injury (Kobayashi et al., 1997; Herkel et al., 2003).
Lymphocyte-induced target cell injury, defined by sensitized lymphocytes forming direct attachments to antigen-presenting cells to induce cellular injury, has been described previously (Sigal, 2005; Wang et al., 2001; French & Enbom, 2014). Specifically, T cells, via T cell receptor (TCR) and CD28, bind to B7 and LFA-1 and ICAM-1 on the antigen presenting cell plasma membrane to trigger downstream T cell sensitization and activation (Sigal, 2005; Dustin & Shaw, 1999). The amalgam interaction binding between T cells and the target cell is termed immunologic synapse formation, which has previously been demonstrated in tissue culture by electron microscopy (Dustin & Shaw, 1999; Huang et al, 2002). By this mechanism the target cell is slowly devoured by the lymphocyte in a piecemeal manner, known as troxis necrosis, generating nubbins of cytoplasm and anuclear cytoplasmic residues (Wang et al., 2001; French & Enbom, 2014).
In this case report, we present a patient with systemic lupus erythematous with lupus nephritis, who was treated with immunomodulatory medications that led to the development of acute drug-induced hepatitis. Microscopic and ultrastructural studies revealed the underlying mechanism is primarily mediated by lymphocyte-induced targeted hepatocyte injury.
CASE REPORT
A 26 year old man was diagnosed with systemic lupus erythematous (SLE) on 5/2014 based on malar rash, alopecia, arthritis, serositis, nephrotic range proteinuria, and class III nephritis on kidney biopsy. He was placed on prednisone, hydroxychloroquine 200mg bid, and mycophenolate Mofetil (Cellecept) 1000mg bid; he was simultaneously enrolled in a randomized clinical trial employing an anti-TWEAK monoclonal antibody in addition to standard therapy for SLE nephritis, and received the first dose of experimental agent at 20mg/kg on 11/5/14. Despite an initial transient response, he was withdrawn from the clinical trial on 1/6/15 (last date of study drug administration) due to recrudescent nephritis activity (proteinuria 2.9 gr/24 hours), skin vasculitis, autoimmune bone marrow exhaustion, and arthritis. Prednisone was increased to 60 mg on 1/15/15, and he also received blood transfusions with the intention to start iv. cyclophosphamide. Patient continued to perform poorly, reported new onset extreme fatigue and malaise, and found to have a spike in his liver function tests transaminases (AST 305, ALT 174).
He was admitted on 2/21/15 with a concern of viral hepatitis, namely CMV or EBV. Both Cellcept and PLQ (at stable doses since May 2014) were held. Autoimmune hepatitis was considered unlikely, given negative prior serologies (a-SMA, AAA, a-LKM1, ANCA), and the unorthodox response to steroids. NFALD was entertained, however a RUQ ultrasound failed to disclose it. Infectious workup showed no reactivity for hepatitis A, B, and C antibodies, and the patient had negative CMV, EBV, and HSV serum PCR tests. Liver biopsy was obtained on 2/3/2015. On light microscopy, the liver biopsy showed focal areas of lymphocytic infiltrates surrounding and forming immunologic synapses with lobular hepatocytes, indicating lobular hepatitis of an autoimmune nature (FIGURE 1). Immunohistochemistry showed that the predominant lymphocyte population was that of CD4 (FIGURE 2, 3). Electron microscopy confirmed the presence of immunologic synapse, where the plasma membrane of a lymphocyte binds to the plasma membrane of a hepatocyte (FIGURE 4).
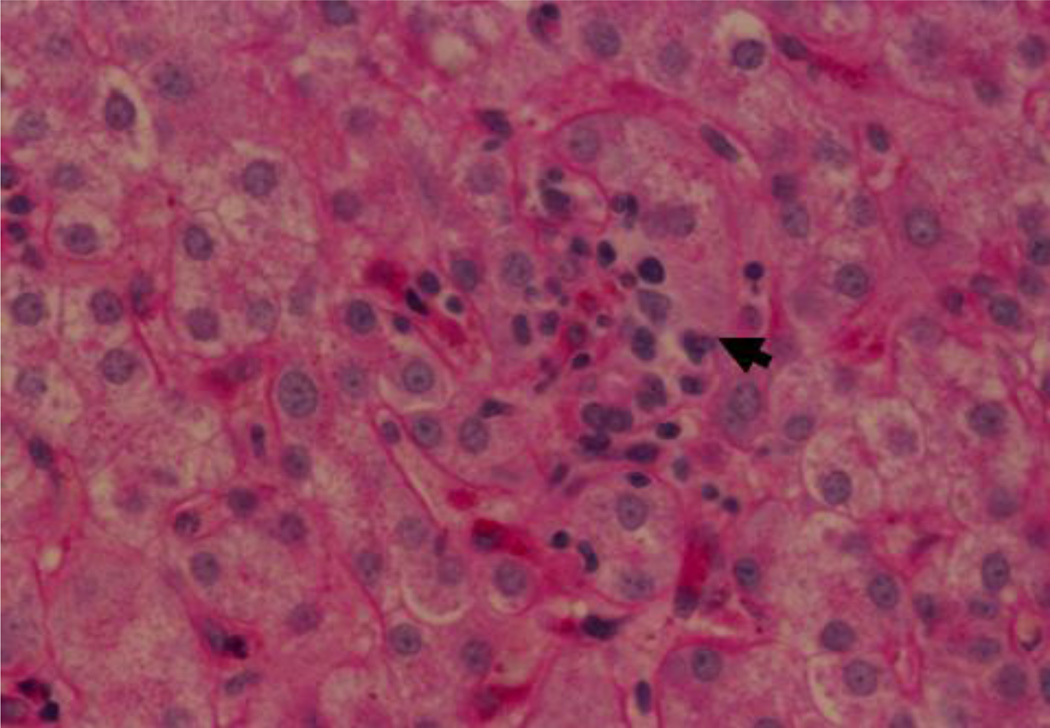
Fig 1.
Hematoxylin eosin stain of the liver biopsy showing a cluster of lymphocytes (arrow) removing cytoplasm from adjacent hepatocytes. X1040.
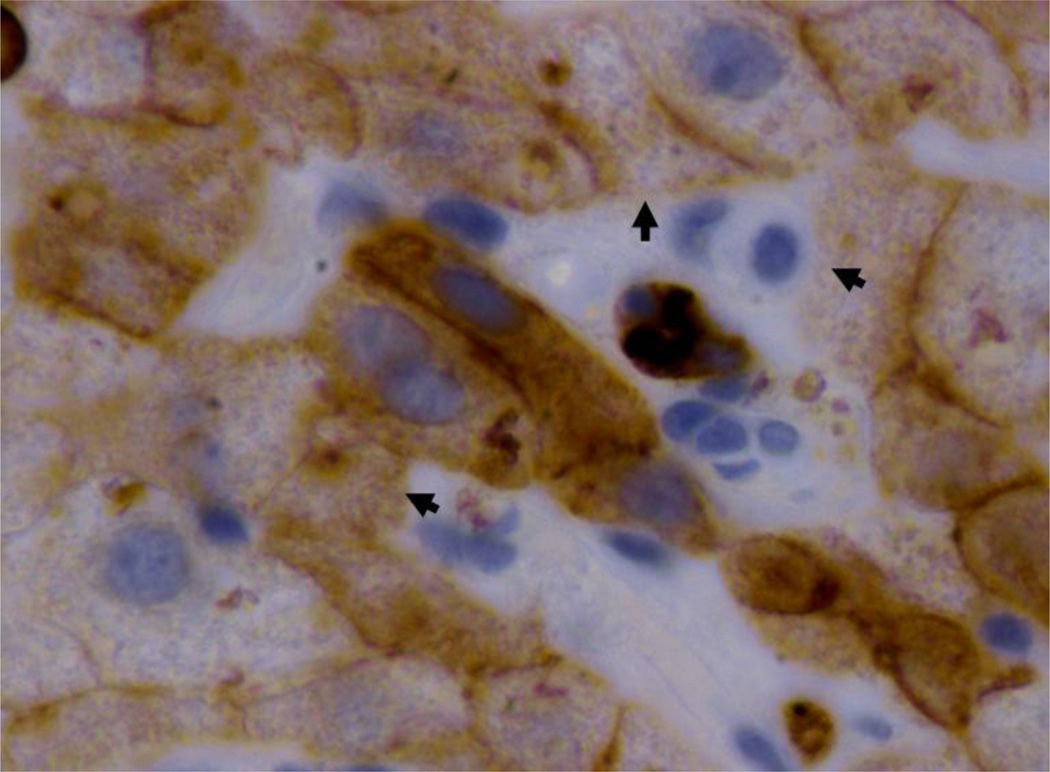
Fig 2.
Immunohistochemistry stain brown for CAM5.2 (CK8 and 18) showing the loss of liver cell cytoplasm where lymphocytes have removed it (arrows). Some hepatocytes are already partial or almost completely removed by the lymphocytes coming from the sinusoids. Brown fragments coming from half laten hepatocytes are seen in the sinusoidals lumens. X1560.
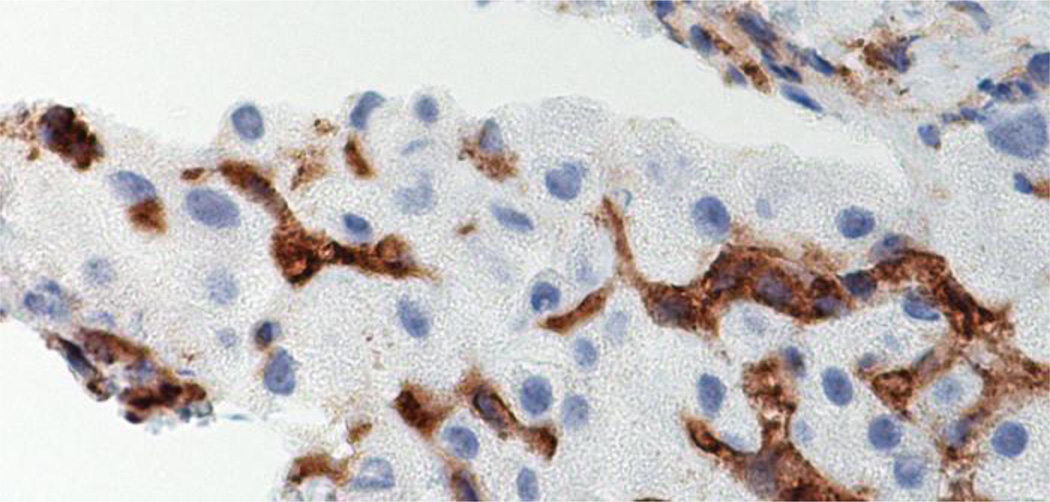
Fig 3.
Immunohistochemistry stain brown for CD4 lymphocytes fill the sinusoids between the hepatocytes. X1560
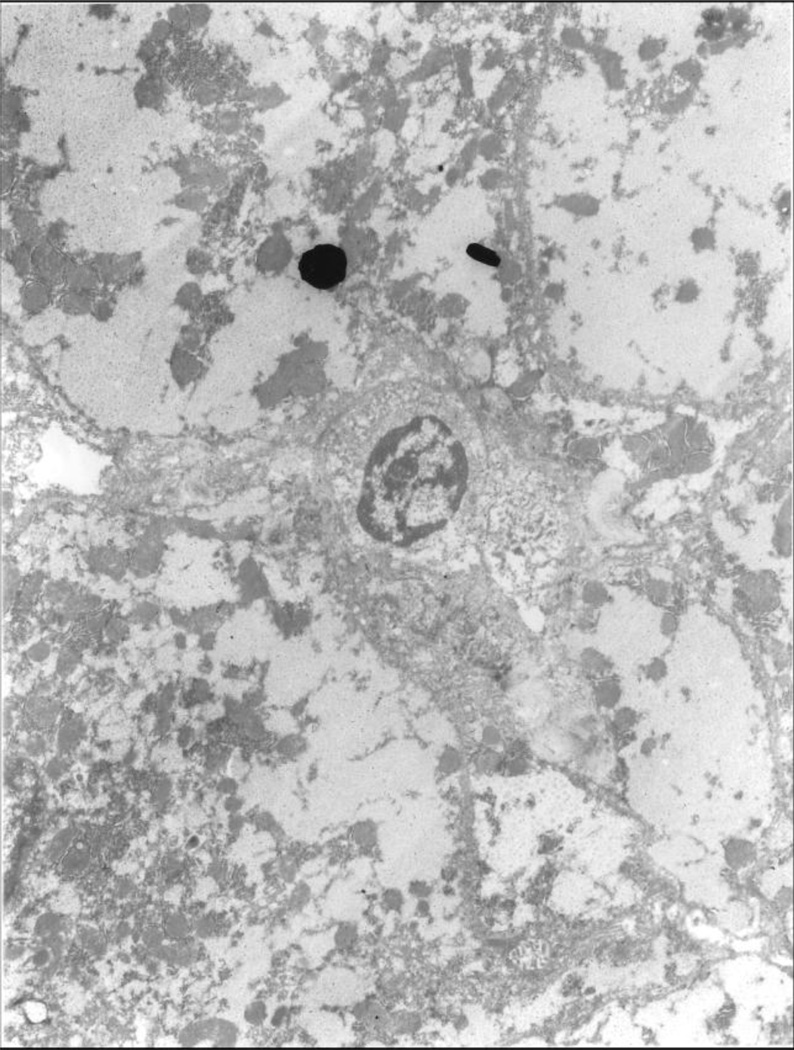
Fig 4.
Electron microscopic photo showing a lymphocyte in the center binding to a hepatocyte surface above it (black dot). X4,500.
Over the subsequent 3–4 weeks the LFT gradually improved (AST = 74; ALT = 71; Alkaline phosphatase = 97) with no further intervention. A week later, and while on 50 mg of prednisone daily, the patient experienced acute shortness of breath, with a significant Hgb drop to 6, and new lung infiltrates verified on bronchoscopy to represent diffuse alveolar hemorrhage. He was managed with high dose pulse methylprednisolone (1000mg daily ×3), Plasma Exchange (daily ×3, and qod afterwards for a total of 5 sessions), and cyclophosphamide (750mg/m2), and his clinical condition improved.
DISCUSSION
Troxis necrosis (Troxis = nibbling in Greek) is a type of liver injury involving destructive immunologic signaling between the hepatocytes and T cells (Wang et al., 2001; French & Enbom, 2014). Through immunohistochemistry and electron microscopy, we have previously demonstrated that there is an infiltration of lymphocytes in the liver parenchyma simulating lobular hepatitis (Wang et al., 2001; French & Enbom, 2014; Enbom et al., 2014; Chen et al., 2010). Ultra structurally, the lymphocytes and hepatocytes formed focal synaptic connections on the cell membrane. There was a loss of liver cell cytoplasmic volume. In the sinusoidal area, traversed by the lymphocytes, there were irregular bits of liver cytoplasm either connected to neighboring hepatocytes or as discohesive nubbins without nuclear structure remaining. The composite findings are features of troxis necrosis, previously known as piecemeal necrosis. The piecemeal necrosis is a novel mechanism of liver injury, hypothesized to be mediated by the MHC class II antigen presenting function of hepatocytes. Indeed, healthy hepatocytes normally do not express MHC class II molecules; however, in clinical hepatitis, viral or autoimmune, hepatocytes often exhibit aberrant MHC class II expression (Kobayashi et al., 1997; Herkel et al., 2003). Herein, the hepatocyte internalizes, metabolizes, and presents the antigenic derivative from the medication on MHC class II molecule on its membrane surface. The circulating lymphocytes advance through the endothelium-lined hepatic sinusoids, recognize the foreign antigen, bind to the antigen-presenting hepatocytes in an MHC class II restricted manner, and become sensitized. The lymphocytes remove the liver cell cytoplasm at the MHC location, resulting in nibbling of the hepatocytes by the sensitized T cell. The phagocytized liver membrane residual is transported via an endosome to the lysosome within the T cell for digestion. As a result the liver is gradually diminished to a nubbins and finally disappear.
Cases of acute liver injury mediated by troxis necrosis have been reported in patients with Hepatitis B and C, non-alcoholic steatohepatitis (NASH), primary biliary cirrhosis (PBC), and autoimmune hepatitis (Wang et al., 2001; French & Enbom, 2014; Enbom et al., 2014; Chen et al., 2010). In drug-implicated troxis necrosis, some of the reported cases to date have been linked to the consumption of herbal supplement such as Hydroxycut and Herbalife products (Enbom et al., 2014; Chen et al., 2010). Literature search yielded 7 previously reported cases of drug-induced hepatitis mediated by troxis necrosis (Wang et al., 2001; Enbom et al., 2014; Chen et al., 2010). In this report, the implicated drugs are three immune modulatory medications used to treat systemic lupus erythematosus, including the experimental drug anti-TWEAK monoclonal antibody. The temporal relation between cessation of the offending medications and improvement in LFT indicates that our patient had drug-induced hepatitis.
Mycofenolate mofetil (MMF) is an immunosuppressive drug that works by interfering with de novo purine synthesis in B and T cells (Villarroel et al., 2009). It is used clinically to achieve immunomodulation in autoimmune and rheumatologic diseases, and transplantation (Villarroel et al., 2009). An association between biochemical hepatitis and MMF has been reported in a woman with severe atopic dermatitis and contact dermatitis (Nguyen & Cruz, 2014). In this case, there was a rapid resolution of the hepatitis after stopping MMF.
Hydroxychloroquine, an alpha-hydroxlyated derivative of cholorquine, is commonly used in the treatment of rheumatologic diseases. It is rarely associated with hepatotoxicity, with less than 1% of subjects showing elevated liver enzymes, which has been largely attributed to idiosyncratic toxic effect (Giner Galvañ et al., 2007). Three cases of acute liver failure associated with hydroxychloroquine have been reported in patients without baseline liver disease (Giner Galvañ et al., 2007).
The tumor necrosis factor TNF-like weak inducer of apoptosis (TWEAK) signaling pathway is often dysregulated in inflammatory diseases and cancer (Wajant, 2013; Bertin et al., 2013). The safety of monoclonal antibody targeting TWEAK signaling has been assessed in several phase I dose-ranging clinical trials in patients with various disease processes, including rheumatoid arthritis and advance solid tumors (Wisniacki et al., 2013; Lassen et al., 2014). The results from these studies have generally shown good tolerability with no hepatotoxicity reported. To our knowledge, this case report is the first to observe an association between LFT abnormalities, pathologic changes on liver biopsy, and anti-TWEAK monocloncal antibodies. The toxicity effect appears to be idiosyncractic, and underscores the need for surveillance for this potential side effect in future anti-TWEAK clinical trials.
In summary, we report drug-induced hepatitis caused by immunomodulatory medications commonly used in the treatment of a rheumatologic disease. We also report a potential association between the study drug monoclonal anti-TWEAK antibody and hepatotoxicity. In this case, troxis necrosis was observed in our patient’s liver biopsy, implicating a novel mechanism for drug-induced hepatitis secondary to immunomodulatory therapy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Bertin D, Stephan D, Khrestchatisky M, Desplat-Jégo S. Is TWEAK a Biomarker for Autoimmune/Chronic Inflammatory Diseases? Front Immunol. 2013 Dec 27;4:489. doi: 10.3389/fimmu.2013.00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen GC, Ramanathan VS, Law D, Funchain P, Chen GC, French S, Shlopov B, Eysselein V, Chung D, Reicher S, Pham BV. Acute liver injury induced by weight-loss herbal supplements. World J Hepatol. 2010 Nov 27;2(11):410–415. doi: 10.4254/wjh.v2.i11.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dustin ML, Shaw AS. Costimulation: building an immunological synapse. Science. 1999 Jan 29;283(5402):649–650. doi: 10.1126/science.283.5402.649. [DOI] [PubMed] [Google Scholar]
- 4.Enbom ET, Le MD, Oesterich L, Rutgers J, French SW. Mechanism of hepatotoxicity due to black cohosh (Cimicifuga racemosa): histological, immunohistochemical and electron microscopy analysis of two liver biopsies with clinical correlation. Exp Mol Pathol. 2014 Jun;96(3):279–283. doi: 10.1016/j.yexmp.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 5.French SW, Enbom ET. Piecemeal necrosis of the liver revisited a review. Exp Mol Pathol. 2014 Jun;96(3):307–309. doi: 10.1016/j.yexmp.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 6.Giner Galvañ V, Oltra MR, Rueda D, Esteban MJ, Redón J. Severe acute hepatitis related to hydroxychloroquine in a woman with mixed connective tissue disease. Clin Rheumatol. 2007 Jun;26(6):971–972. doi: 10.1007/s10067-006-0218-1. [DOI] [PubMed] [Google Scholar]
- 7.Herkel J, Jagemann B, Wiegard C, Lazaro JF, Lueth S, Kanzler S, Blessing M, Schmitt E, Lohse AW. MHC class II-expressing hepatocytes function as antigen-presenting cells and activate specific CD4 T lymphocyutes. Hepatology. 2003 May;37(5):1079–1085. doi: 10.1053/jhep.2003.50191. [DOI] [PubMed] [Google Scholar]
- 8.Huang J, Lo P, Zal T, Gascoigne N, Smith B, Levin SD, Grey HM. CD28 plays a critical role in the segregation of PKCθ within the immunological synapse. Proc Natl Acad Sci. 2002 Jul;99(14):9369–9373. doi: 10.1073/pnas.142298399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kobayashi H, Puri P, O'Briain DS, Surana R, Miyano T. Hepatic overexpression of MHC class II antigens and macrophage-associated antigens (CD68) in patients with biliary atresia of poor prognosis. J Pediatr Surg. 1997 Apr;32(4):590–593. doi: 10.1016/s0022-3468(97)90714-4. [DOI] [PubMed] [Google Scholar]
- 10.Lassen UN, Meulendijks D, Siu LL, Karanikas V, Mau-Sorensen M, Schellens JH, Jonker DJ, Hansen AR, Simcox ME, Schostack KJ, Bottino D, Zhong H, Roessler M, Vega-Harring SM, Jarutat T, Geho D, Wang K, DeMario M, Goss GD. A phase I monotherapy study of RG7212, a first-in-class monoclonal antibody targeting TWEAK signaling in patients with advanced cancers. Clin Cancer Res. 2015 Jan 15;21(2):258–266. doi: 10.1158/1078-0432.CCR-14-1334. Epub 2014 Nov 11. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen RH, Cruz PD., Jr Hepatitis due to mycophenolate mofetil used to treat atopic dermatitis and allergic contact dermatitis. Dermatitis. 2014 Sep-Oct;25(5):284–285. doi: 10.1097/DER.0000000000000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sigal LH. Basic science for the clinician 30: The immunologic synapse. J Clin Rheumatol. 2005 Aug;11(4):234–239. doi: 10.1097/01.rhu.0000173619.23349.09. [DOI] [PubMed] [Google Scholar]
- 13.Villarroel MC, Hidalgo M, Jimeno A. Mycophenolate mofetil: An update. Drugs Today (Barc) 2009 Jul;45(7):521–532. doi: 10.1358/dot.2009.45.7.1384878. [DOI] [PubMed] [Google Scholar]
- 14.Wajant H. The TWEAK-Fn14 system as a potential drug target. Br J Pharmacol. 2013 Oct;170(4):748–764. doi: 10.1111/bph.12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang MX, Morgan T, Lungo W, Wang L, Sze GZ, French SW. "Piecemeal" necrosis:renamed troxis necrosis. Exp Mol Pathol. 2001 Oct;71(2):137–146. doi: 10.1006/exmp.2001.2397. [DOI] [PubMed] [Google Scholar]
- 16.Wisniacki N, Amaravadi L, Galluppi GR, Zheng TS, Zhang R, Kong J, Burkly LC. Safety, tolerability, pharmacokinetics, and pharmacodynamics of anti-TWEAK monoclonal antibody in patients with rheumatoid arthritis. Clin Ther. 2013 Aug;35(8):1137–1149. doi: 10.1016/j.clinthera.2013.06.008. [DOI] [PubMed] [Google Scholar]