SUMMARY
Purpose
We conducted a phase II multicenter study evaluating Caphosol in patients receiving head and neck radiation (H/N RT) +/− chemotherapy or biologic sensitizer.
Materials/Methods
The primary endpoint of the study tested the rate of functional mucositis (WHO grade > or equal to 2) with the hypothesis that <75% of patients would develop > or equal to 2 mucositis with Caphosol compared with a historical rate of >90%. New methods were applied with higher than historic rigor. 5 Institutions were included in this study: Moffitt Cancer Center (MCC), MD Anderson Cancer Center (MDACC), Duke University Cancer Center (DUCC), University of Florida (UF) and Temple University Cancer Center (TUCC). Caphosol was taken by patients at least 4 times a day and up to 10 times per day commencing with day 1 of RT and for a total duration of 8 weeks after completion of RT. Detailed questionnaires were completed weekly by patients and a unique algorithm was used to generate the WHO grade of mucositis.
Results
98 Patients were enrolled in the study. 59/98 (60%) patients were evaluable for the primary endpoint giving us 80% power. All evaluable patients experienced WHO grade > or equal to 2 mucositis and the trial failed to reject the null hypothesis. > or equal to 2 mucositis rates at weeks 2, 4, 6, 11 and 15 were as follows: 45%, 90%, 98%, 71%, 50%.
Conclusion
We were unable to demonstrate that Caphosol significantly reduced WHO grade 2 or higher mucositis below a 90% historic rate. We are not surprised with this finding given our rigorous methodology in grading.
Keywords: Head and neck cancer, Caphosol, Mucositis, Radiotherapy, Oral
Introduction
Radiation therapy with systemic therapy is the standard of care in the treatment of locally advanced squamous cell carcinoma of the head and neck [1,2]. While clinical outcomes may be favorable, acute treatment related toxicity may be considerable [3].
Mucositis is a debilitating complication of head and neck cancer radiation therapy and chemotherapy that is characterized by inflammation of the mucous membranes, erythema, ulceration, and pseudomembrane formation [4]. Mucositis can cause pain and dysphagia which is further complicated by the frequent association of xerostomia and altered taste. Mucositis may lead to anorexia, weight loss, weakness and contribute to depression. Furthermore severe inflammation and ulceration of the mucosa predisposes patients to both oral and systemic infections. Difficulties of pain management and nutrition can be exacerbated by side effects of opioid use and the need for parenteral nutrition. The incidence of mucositis is estimated at approximately 400,000 patients per year with about 97% of patients receiving head and neck radiation therapy experiencing this side effect [5,6].
Caphosol is an electrolyte solution, designed in part to replace the normal ionic pH balance in the oral cavity and may be useful in the prevention and treatment of mucositis in cancer patients [7,8]. When mixed together the calcium solution and the phosphate solution form a stable supersaturated solution with a composition resembling that of natural saliva. It has been postulated that Caphosol’s high ionic content plays a role in mediating the inflammatory process, coagulation cascade, and in assisting with tissue repair by diffusing ions into the intercellular spaces in the epithelium thus permeating the mucosal lesion [9]. Calcium is known to play a crucial role in several aspects of the inflammatory process including effecting leukocyte chemotaxis, modulation of adhesion molecules, and the elaboration of arachiodonic metabolites, and phosphate is a central compound involved in cellular and tissue repair.
While its effectiveness has been documented in patients with hematological malignancies undergoing stem cell transplant, there have been no prospective evaluations in radiotherapy-related mucositis of the head and neck region [9]. The purpose of this study was to estimate the effect of Caphosol on the incidence of oral mucositis in patients receiving radiation therapy with or without systemic therapy for the treatment of head and neck cancer and to correlate the extent of mucosal injury and components of WHO mucositis data with clinical outcomes (including oral intake, swallowing function, and pain), and patient preference.
Materials and methods
The study was conducted at 5 centers in North America including Moffitt Cancer Center (MCC) (serving as the coordinating data center), MD Anderson Cancer Center (MDACC), Duke University Cancer Center (DUCC), University of Florida (UF) and Temple University Cancer Center (TUCC). After institutional review board approval at each participating center, patients provided informed written consent. This process took place in accordance with the Health Insurance Portability and Accountability Act, Good Clinical Practices, and with local and legal requirements. Data monitoring at the sites was performed by MCC.
Patients with newly diagnosed squamous cell carcinoma of the head and neck including the oral cavity, oropharynx, nasopharynx, hypopharynx and larynx were eligible including postoperative cases. Eligible patients were planned to receive at least 60 Gy or greater to at least one of 9 pre-defined anatomic mucosal subsites seen on direct view of the oral cavity and oropharynx (Fig. 1). Conventional fractionation, accelerated fraction and hyperfractionation radiation therapy schedules were permissible. 2D, 3D- conformal and intensity modulated radiotherapy (IMRT) techniques were allowed. Major radiation protocol violations included treatment breaks greater than 2 weeks and receiving less than 80% of the prescribed dose. Concurrent platinum based chemotherapy or cetuximab was permissible. Systemic therapy consisted of cisplatin 100 mg/m2 every 3 weeks, cisplatin 30–40 mg/m2 weekly, Carboplatin 2 AUC/week or cetuximab 400 mg/m2 loading dose 7 days before radiation plus 250 mg/m2/week for 6–7 weeks. Patients were not eligible if they demonstrated mucosal ulceration at baseline unless the surgical site was at least 95% healed, if they demonstrated active infections of the oral cavity or oropharynx other than candidiasis, if they had received induction chemotherapy, or if they had significant comorbidities precluding adequate compliance.
Fig. 1.
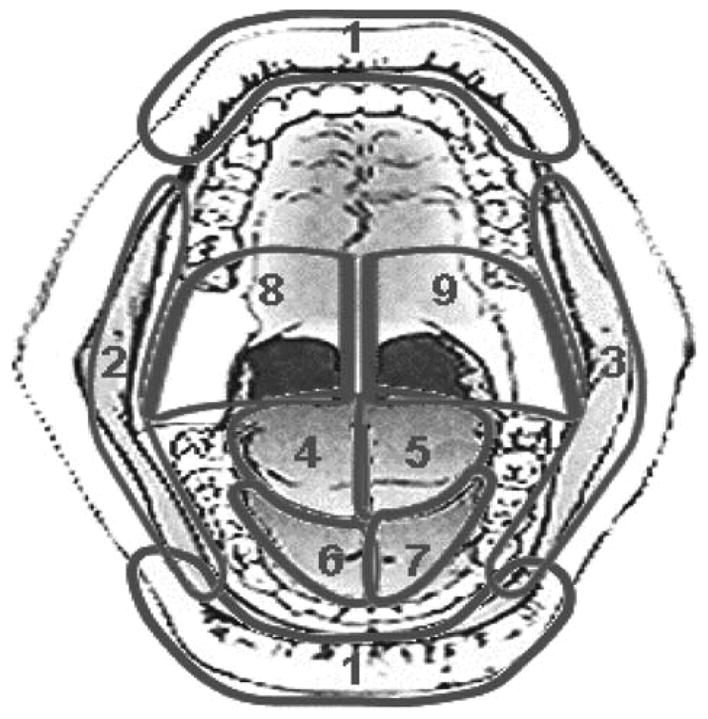
9 Zones evaluated by investigators for ulceration.
The study was an open label single arm trial. Caphosol use with radiation therapy with or without cisplatin or cetuximab commenced day #1 of radiation and continued for 8 weeks after the completion of therapy. Patients took Caphosol by mixing the calcium and phosphate solutions together, rinsing for 1 min with a half portion, spitting and then repeating with the remainder at least 4 times per day and up to 10 times per day. Patients were assessed weekly during radiation therapy, 4 weeks and 8 weeks after therapy. Standard of care topical anesthetics including “Magic Mouthwash” consisting of combinations of lidocaine, diphenhydramine, Maalox, and/or nystatin was permissible and the management of candidiasis was left to the discretion of the treating physician. Other systemic or topical agents for the treatment of mucositis were not allowed.
Investigators developed new structured patient evaluation and data capture methods for this trial. These included mandatory completion of a web-based study specific mucositis training module for each investigator, specific guidance on assignment of food type, oral feedings, and a unique automated algorithm for assigning details. The algorithm standardized WHO grade I to IV mucositis based on the presence of pain, mode of nutrition, form of nutrition and presence of ulceration.
Patients completed a patient satisfaction tool (PST) that included mouth and throat pain, swallowing, eating and overall symptoms. Grading was by improvement, no change or worsening symptoms. Adverse events monitoring took place as per Common Terminology Criteria version 3.0. Severe adverse events (SAEs) attributable to Caphosol were defined as those resulting in death, life threatening, requiring hospitalization, requiring intervention to prevent permanent impairment, or as determined by the principal investigator.
Statistical methods
The evaluable patients for the primary analysis were defined as those who used at least 80% of the study agent at a minimum of 4 doses per day without major radiation protocol violations. The primary end point of this study was the development of functional mucositis WHO grade ≥2. We hypothesized that less than 75% of patients would develop grade 2 or greater mucositis with Caphosol compared with a historical rate of greater than 90% [3]. Using 80% power and a two-sided significance level of 0.05 it was determined that 48 valuable patients would be needed. The comparison was performed using an exact test for the binomial distribution. The WHO grade was summarized at each time point.
Results
Patients
The study was opened on April 9th 2009 and the last patient was enrolled on April 9th 2010. A total of 98 patients participated.
Enrollment by institution was as follows: MCC 30/98 (31%), MDACC 17/98 (17%), DUCC 20/98 (20%), UF 20/98 (20%), TUCC 11/98 (11%). Patient demographic information, disease characteristics and treatment details are seen in Table 1. 10/98 Patients (10%) had diabetes. The median age of the study population was 58 (range 38–90). The median number of fractions, total dose (Gy) and radiation duration (days) were as follows: 35 (range 25–65), 70 (range 50–74.4), 47 (range 33–60). The lower extreme of total dose of radiation received and the higher extreme of radiation duration were due to toxicity and noncompliance. There was concordance amongst institutions regarding scored grade of mucositis over time with the exception of higher rates of grade 2 and lower rates of grade 4 mucositis seen at DUCC during weeks 7 and 11 (p < 0.004).
Table 1.
Demographics, disease characteristics and treatment details.
| Characteristic | Caphosol (n = 98)
|
|
|---|---|---|
| No | % | |
| Sex | ||
| Male | 83 | 85 |
| Female | 15 | 15 |
| Tobacco use (Current or within 6 months) | 33 | 34 |
| Alcohol use | 50 | 51 |
| Primary tumor location | ||
| Oral cavity | 14 | 14 |
| Nasopharynx | 5 | 5 |
| Oropharynx | 74 | 76 |
| Hypopharynx | 1 | 1 |
| Supraglottic larynx | 3 | 3 |
| Unknown Primary | 1 | 1 |
| Histology | ||
| Squamous cell carcinoma | 95 | 97 |
| Squamous cell carcinoma variant | 3 | 3 |
| Dentition | ||
| Edentulous | 13 | 13 |
| Excellent | 45 | 46 |
| Mild-good | 13 | 13 |
| Moderate-fair | 20 | 20 |
| Poor | 6 | 6 |
| Missing | 1 | 1 |
| T stage | ||
| Tx | 1 | 1 |
| T0 | 3 | 3 |
| T1 | 28 | 29 |
| T2 | 29 | 29 |
| T3 | 25 | 26 |
| T4 | 12 | 12 |
| N stage | ||
| N0 | 17 | 17.3 |
| N1 | 13 | 13.3 |
| N2 | 63 | 64.3 |
| N3 | 5 | 5.1 |
| KPS | ||
| 100 | 15 | 15 |
| 90 | 60 | 61 |
| ≤80 | 23 | 24 |
| Radiation fractionation | ||
| Conventional definitive (70 Gy/35 fx/7 weeks) | 52 | 53 |
| Accelerated definitive (70 Gy/35fx/6 weeks) | 6 | 6 |
| Concomitant boost definitive (70–72 Gy/6 weeks with bid boost weeks 5–6) | 15 | 15 |
| Hyperfractionation definitive (74.4–81.6 Gy/1.2 Gy per fx bid/7–8 weeks) | 7 | 7 |
| Post operative (60–66 Gy/30–33fx/6–6.5 weeks) | 18 | 18 |
| Chemotherapy/sensitizer | ||
| Cisplatin | 63 | 64 |
| Carboplatin | 7 | 7 |
| Cetuximab | 10 | 10 |
KPS = Karnofsky Performance Status; fx = fractions; Gy = gray; bid = twice per day.
Efficacy/safety
79/98 (81%) of the patients completed the study per protocol. 8 patients discontinued use of Caphosol based on their preference to do so, 4 patients had nausea and vomiting secondary to chemotherapy and could not tolerate it, 2 were lost to follow up, one patient felt that is was worsening xerostomia, one developed pneumonia, one had severe mucositis precluding its use, one patient went for surgery and one died from pneumonia and sepsis. Of these 79 patients, 59/98 (60%) patients were evaluable for primary analysis.
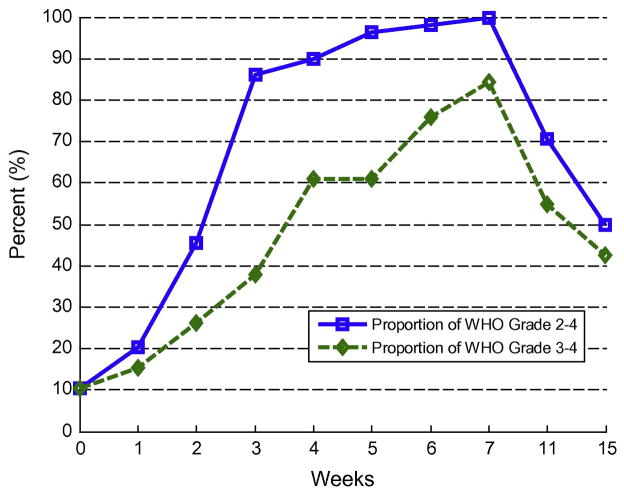
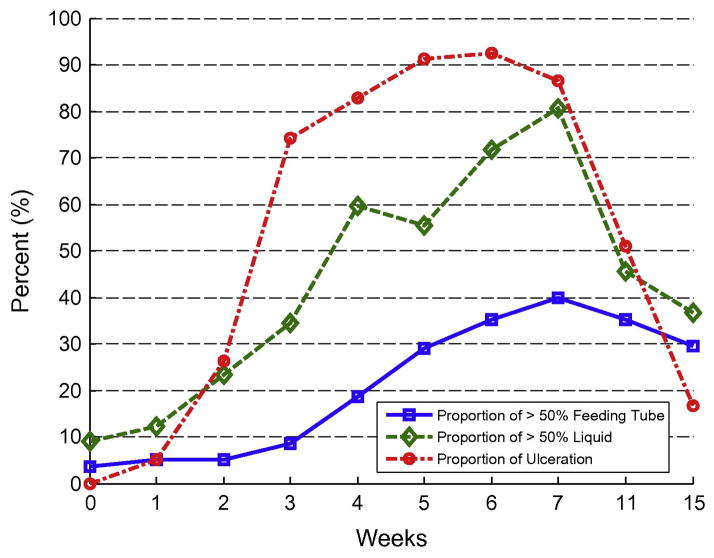
All of the evaluable patients experienced grade 2 or higher WHO mucositis; consequently, the trial failed to reject the null hypothesis. Table 2 shows the incidences of mucositis over time for evaluable patients. Figs. 2 and 3 show the proportions of grade 2 or higher mucositis with time and show the components of liquid use, feeding tube use and ulceration. At baseline 2 patients (4%) were using the feeding tube for more than 50% of nutrition and 5 patients (9%) were on a diet consisting of greater than 50% liquids. Table 3 shows mean Caphosol use per week.
Table 2.
WHO mucositis grade over time.
| WHO grade | Week
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 11 | 15 | ||
| Grade 0 | n | 51 | 43 | 27 | 8 | 4 | 0 | 0 | 0 | 10 | 26 |
| % | 89.5 | 72.9 | 47.4 | 13.8 | 6.8 | 0.0 | 0.0 | 0.0 | 19.6 | 48.1 | |
| Grade 1 | n | 0 | 4 | 4 | 0 | 2 | 2 | 1 | 0 | 5 | 1 |
| % | 0.0 | 6.8 | 7.0 | 0.0 | 3.4 | 3.7 | 1.9 | 0.0 | 9.8 | 1.9 | |
| Grade 2 | n | 0 | 3 | 11 | 28 | 17 | 19 | 12 | 7 | 8 | 4 |
| % | 0.0 | 5.1 | 19.3 | 48.3 | 28.8 | 35.2 | 22.2 | 15.6 | 15.7 | 7.4 | |
| Grade 3 | n | 4 | 6 | 12 | 17 | 25 | 17 | 22 | 20 | 10 | 7 |
| % | 7.0 | 10.2 | 21.1 | 29.3 | 42.4 | 31.5 | 40.7 | 44.4 | 19.6 | 13.0 | |
| Grade 4 | n | 2 | 3 | 3 | 5 | 11 | 16 | 19 | 18 | 18 | 16 |
| % | 3.5 | 5.1 | 5.3 | 8.6 | 18.6 | 29.6 | 35.2 | 40.0 | 35.3 | 29.6 | |
| Total | n | 57 | 59 | 57 | 58 | 59 | 54 | 54 | 45 | 51 | 54 |
Fig. 2.
Percentage of WHO grades ≥2 over time.
Fig. 3.
Percentage of significant feeding tube use, liquid intake and ulceration over time.
Table 3.
Mean use of Caphosol per week.
| Variable | N | Freq. of missing subjects | Mean |
|---|---|---|---|
| Week 1 | 98 | 0 | 27.73 |
| Week 2 | 95 | 3 | 28.36 |
| Week 3 | 93 | 5 | 31.22 |
| Week 4 | 94 | 4 | 30.83 |
| Week 5 | 90 | 8 | 30.72 |
| Week 6 | 87 | 11 | 31.63 |
| Week 7 | 86 | 12 | 30.2 |
| Week 8 | 81 | 17 | 27.32 |
| Week 9 | 76 | 22 | 26.57 |
| Week 10 | 70 | 28 | 26.59 |
| Week 11 | 71 | 27 | 25.2 |
| Week 12 | 68 | 30 | 24.9 |
| Week 13 | 61 | 37 | 25.18 |
| Week 14 | 59 | 39 | 23.24 |
| Week 15 | 48 | 50 | 20.81 |
| Week 16 | 30 | 68 | 15.4 |
| Week 17 | 14 | 84 | 13.64 |
| Week 18 | 5 | 93 | 14 |
| Week 19 | 1 | 97 | 3 |
| Total dose | 98 | 0 | 342.18 |
Fig. 4 demonstrates overall results of the PST. Between weeks 4 and 11 roughly 50% of patients reported that Caphosol had improvement of symptoms which highly correlated with pain, swallowing and eating scores.
Fig. 4.
Overall results of PST.
No patients developed severe adverse events that were attributable to Caphosol.
Discussion
All of the evaluable patients in our study experienced grade 2 or higher WHO mucositis despite using Caphosol, and we were thus unable to reject the null hypothesis. Between weeks 4 and 11 roughly 50% of patients reported through the PST that Caphosol had improved their symptoms which highly correlated with pain, swallowing and eating scores. Patients reported that they liked using the product even at week 7. However, we found that by week 7, all of the evaluable patients had developed WHO grade ≥2 mucositis and 84% had developed WHO grade ≥3 mucositis. Peak ulceration was seen at week 6 in 93% of our patients while at week 7 we observed peaks in significant feeding tube use and liquid diet. These data are instructive as a baseline in an era where the majority of patients are treated with IMRT.
Regarding the non-evaluable patients, this was secondary to difficulty with compliance. As other studies have shown there may be decreased compliance with self-administered agents for mucositis prevention and treatment. We believe that lack of compliance may be due to a variety of factors including possible taste of the product, healing mucositis after radiotherapy is completed, and the frequency of use of the agent required.
Mucositis remains a significant source of morbidity seen during head and neck radiation therapy and chemoradiation [3]. While a large number of mucositis agents have been tested, only palifermin (recombinant keratinocyte growth factor) has been consistently shown to significantly alter the severity and duration of mucositis [10]. Palifermin was approved by the FDA in 2004 for treatment of mucositis seen in hematological malignancies in the transplant setting. Recently 2 randomized trials have been reported on the use of palifermin for head and neck cancer radiation induced mucositis [11,12]. These trials generally have shown a benefit in physician assessment of mucositis but improvements were not seen in the patient reported outcome (PRO) data. Additionally there remain some lingering concerns about the safety of its use in this setting including possible stimulation of tumor cells by growth factors [13].
Initial experience with Caphosol reported prevention of mucositis [7]. Subsequently a randomized, double-blind, placebo-controlled trial of 95 patients demonstrated a reduction in the frequency, intensity, and duration of oral mucositis associated with hematopoietic stem cell transplant with no adverse events reported [9].
While the effectiveness of Caphosol has been formally tested in the stem cell transplant setting there have been no prospective evaluations of head and neck radiation therapy induced oral mucositis [8]. Therefore we sought to estimate the effect of Caphosol on the incidence of oral mucositis in patients receiving radiation therapy with or without chemotherapy or sensitizer for head and neck cancer and to correlate the extent of mucosal injury and WHO mucositis data with clinical outcomes.
We used newly developed patient evaluations and data capture methods not previously used in mucositis intervention trials. These included a web-based study specific mucositis training module for the investigators, specific guidance on assignments of food type, oral feedings, and an automated algorithm for assigning mucositis grade. One may argue over the assigned priorities for our algorithm, however, we feel that the WHO mucositis grading scale is a functional one with oral nutrition taking priority over ulceration and pain.
We acknowledge that our study has several limitations. 5–10% of our patients were considered to have grade 3 or 4 mucositis at baseline due to oral liquid diet or significant use of the feeding tube and around 10% of the patients were un-evaluable for the primary endpoint at baseline. In future studies these patients will need to be considered for exclusion. The effect on the ability to eat and swallow could be missed because areas of mucosa involved in swallowing ability are not treated or assessed and this is a limitation of all oral mucositis rinse products. The use of IMRT compared with other techniques and the administration of analgesics could have affected our outcomes as well.
As an open label, non-placebo controlled trial, there was no blinding of the mucositis raters and we enrolled a fairly non-homogenous patient and treatment cohort.
Also, this study was powered to evaluate a reduction in WHO grade 3 or higher mucositis and was not designed to formally assess patient preference or the ability for this agent to abate specific symptoms associated with mucositis even in patients with high-grade mucositis.
Possible reasons for inhomogeneity among differing centers in rates of mucositis could be due to pain management, nutrition and hydration. We would like to stress that it is very important to have homogeneous ways of assessing mucositis and to employ a similar supportive therapy in future trials.
Most of our patients had a diagnosis of oropharyngeal carcinoma and were treated with chemoradiation using cisplatin with standard fractionation. The majority of these patients are likely to be human papilloma virus (HPV) positive and have a relatively good prognosis. While it is unlikely that HPV status affects the rate of mucositis or response to Caphosol this possibility exists and was not explored.
Its few weaknesses notwithstanding, our system worked across multiple institutions and we were able to develop a system to more reliably grade mucositis. Patient acceptance, tolerance and compliance were excellent with the successful completion of enrollment of the study within 1 year with good participation by all centers. Rates of mucositis were similar with the exception of lower rates of grade 4 mucositis seen at DUCC during weeks 7 and 11. While the exact reason for this is unclear, it is possibly explained by more aggressive nutritional support, hydration and pain management at that center.
In conclusion, although patients reported modest improvements in symptoms, our study did not show a significant decrease in WHO grade ≥2 mucositis compared with the historic rate of 90% nor did it suggest any added toxicity from use of Caphosol. The new methods of investigator training, structured data collection, automated grading and patient satisfaction tools used in this trial may improve the reliability and consistency of data amongst investigators in future mucositis studies.
Acknowledgments
Funding sources
EUSA Pharma provided financial support for this trial including data management and biostatistical analysis.
Footnotes
Conflict of Interest
David I. Rosenthal, MD was a consultant to EUSA Pharma in 2010. The authors have no other disclosures or conflicts of interest.
Drugs and devices
Caphosol (supersaturated calcium phosphate rinse), EUSA Pharma, Oxford, England.
References
- 1.Bernier J, Cooper JS, Pajak TF, van Glabbeke M, Bourhis J, Forastiere A, et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501) Head Neck. 2005;27(10):843–50. doi: 10.1002/hed.20279. [DOI] [PubMed] [Google Scholar]
- 2.Pignon JP, le Maitre A, Maillard E, Bourhis J. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92(1):4–14. doi: 10.1016/j.radonc.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 3.Trotti A, Bellm LA, Epstein JB, Frame D, Fuchs HJ, Gwede CK, et al. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiother Oncol. 2003;66(3):253–62. doi: 10.1016/s0167-8140(02)00404-8. [DOI] [PubMed] [Google Scholar]
- 4.Sonis ST, Elting LS, Keefe D, Peterson DE, Schubert M, Hauer-Jensen M, et al. Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer. 2004;100(9 Suppl):1995–2025. doi: 10.1002/cncr.20162. [DOI] [PubMed] [Google Scholar]
- 5.Sonis ST. Oral mucositis in cancer therapy. J Support Oncol. 2004;2(6 Suppl 3):3–8. [PubMed] [Google Scholar]
- 6.Kostler WJ, Hejna M, Wenzel C, Zielinski CC. Oral mucositis complicating chemotherapy and/or radiotherapy: options for prevention and treatment. CA Cancer J Clin. 2001;51(5):290–315. doi: 10.3322/canjclin.51.5.290. [DOI] [PubMed] [Google Scholar]
- 7.Papas AJE, Sobel S, Olsen TO. Effects of preventive regimen on oral mucositis. J Dent Res IADR. 1981;544:940. [Google Scholar]
- 8.Wasko-Grabowska A, Rzepecki P, Oborska S, Barzal J, Gawronski K, Mlot B, et al. Efficiency of supersaturated calcium phosphate mouth rinse treatment in patients receiving high-dose melphalan or BEAM prior to autologous blood stem cell transplantation: a single-center experience. Transplant Proc. 2011;43(8):3111–3. doi: 10.1016/j.transproceed.2011.08.053. [DOI] [PubMed] [Google Scholar]
- 9.Papas AS, Clark RE, Martuscelli G, O’Loughlin KT, Johansen E, Miller KB. A prospective, randomized trial for the prevention of mucositis in patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant Apr. 2003;31(8):705–12. doi: 10.1038/sj.bmt.1703870. [DOI] [PubMed] [Google Scholar]
- 10.Meropol NJ, Somer RA, Gutheil J, Pelley RJ, Modiano MR, Rowinsky EK, et al. Randomized phase I trial of recombinant human keratinocyte growth factor plus chemotherapy: potential role as mucosal protectant. J Clin Oncol. 2003;21(8):1452–8. doi: 10.1200/JCO.2003.10.079. [DOI] [PubMed] [Google Scholar]
- 11.Le QT, Kim HE, Schneider CJ, Murakozy G, Skladowski K, Reinisch S, et al. Palifermin reduces severe mucositis in definitive chemoradiotherapy of locally advanced head and neck cancer: a randomized, placebo-controlled study. J Clin Oncol. 2011;29(20):2808–14. doi: 10.1200/JCO.2010.32.4095. [DOI] [PubMed] [Google Scholar]
- 12.Henke M, Alfonsi M, Foa P, Giralt J, Bardet E, Cerezo L, et al. Palifermin decreases severe oral mucositis of patients undergoing postoperative radiochemotherapy for head and neck cancer: a randomized, placebo-controlled trial. J Clin Oncol. 2011;29(20):2815–20. doi: 10.1200/JCO.2010.32.4103. [DOI] [PubMed] [Google Scholar]
- 13.Bossi P, Locati LD, Licitra L. Palifermin in prevention of head and neck cancer radiation-induced mucositis: not yet a definitive word on safety and efficacy profile. J Clin Oncol. 2012 Jan 3; doi: 10.1200/JCO.2011.39.1136. [DOI] [PubMed] [Google Scholar]