Abstract
Objective
The Advisory Committee on Immunization Practices (ACIP) has recommended routine human papillomavirus (HPV) vaccination at age 11 or 12 years for girls since 2006 and for boys since 2011. We sought to describe adolescent HPV vaccination coverage, doses administered from 2009 to 2012, and age at first vaccination by sex.
Methods
Aggregate data were analyzed from 8 Immunization Information System sentinel sites on HPV vaccinations in children and adolescents aged 11 to 12 years, 13 to 15 years, and 16 to 18 years. Vaccination coverage by age group was reported for 2009 to 2012, and weekly doses administered were determined. Age at first HPV vaccination was calculated for girls in 2007 and 2011 and for boys in 2011.
Results
This analysis included data on 2.9 million adolescents aged 11 to 18 years. There were small increases in coverage for girls, with receipt of ≥1 dose of HPV vaccine reaching 27.1% of ages 11 to 12, 47.9% of ages 13 to 15, and 57.1% of ages 16 to 18 by December 31, 2012. Uptake of ≥1 dose in boys reached ~18% for all age groups. Doses administered showed seasonal variation, with highest uptake before back to school among girls and steady increases in boys after the 2009 ACIP recommendation for permissive use. Doses administered to boys surpassed those administered to girls by September 2012. Among vaccinated girls, more received vaccine at the recommended age of 11 to 12 years in 2011 (74.2%) compared to 2007 (9.9%). In 2011, 27.3% of vaccinated boys received their first dose at age 11 to 12 years.
Conclusions
HPV vaccination coverage increased among adolescents between 2009 and 2012. However, increases among girls were small, and coverage for boys and girls remained below target levels.
Keywords: adolescent, human papillomavirus vaccine, immunization
Quadrivalent and bivalent human papillomavirus (HPV) vaccines were licensed for use in girls in 2006 and 2009, respectively.1 In 2009, the quadrivalent HPV vaccine was licensed for use in boys.2 The Advisory Committee on Immunization Practices (ACIP) recommended routine vaccination, involving a series of 3 doses, for 11- or 12-year-old girls in 20063 and for 11- or 12-year-old boys in 2011.2 For male subjects, the recommendation for routine use followed guidance by ACIP stating that quadrivalent HPV vaccine may be provided to male subjects aged 9 to 26 years since October 2009.2 In 2012, data from the National Immunization Survey–Teen (NIS-Teen) showed that 53.8% of female adolescents 13 to 17 years of age in the United States had received ≥1 HPV vaccine dose, and 33.4% had received 3 doses.4 Data from 2012 NIS-Teen also indicated that 20.8% of male adolescents 13 to 17 years of age had received ≥1 HPV vaccine dose, and 8.3% had received 3 doses.4
The NIS is the main source of vaccine coverage data for children and adolescents in the United States and is used to monitor progress toward the Healthy People 2020 targets for vaccination coverage. The target is 80% 3-dose coverage among adolescent girls by the age of 13 to 15 years. In addition to the NIS, data from Immunization Information System (IIS) can be used to obtain coverage data. The NIS-Teen collects vaccination information for adolescents aged 13 to 17 years in the United States using a random-digit-dialed sample of landline and, starting in 2011, cellular telephone numbers. Parents/guardians provide vaccination and sociodemographic information on the adolescent and are asked for permission to contact their child’s vaccination provider. The provider is mailed a questionnaire to obtain a vaccination history from the medical record.5,6 Data from IIS can serve as useful complements to NIS-Teen because IIS typically covers wider age ranges and may be able to provide local estimates; in addition, IIS data have been used to provide more timely information regarding vaccine use.7–9
The objectives of this study were to use IIS to examine vaccination coverage and doses administered in male and female adolescents from October 2009 through December 2012 to assess trends in vaccine administration during this time period and to compare age at first vaccination by sex.
Methods
Study Population
An IIS is a confidential, computerized, population-based information system that collects and consolidates vaccination data from vaccination providers. It also provides important tools for designing and sustaining effective immunization strategies at the provider and immunization program levels. These tools include clinical decision support, vaccination coverage reports, interoperability with electronic health record systems, and vaccine inventory management, as well as the ability to generate reminder and recall messages.10 The IIS Sentinel Site Project is a collaborative project between the US Centers for Disease Control and Prevention (CDC) and selected state/city-based IIS to implement procedures to increase data completeness, timeliness, and accuracy. Data are also used for immunization program evaluation and vaccination coverage estimates. Although IIS sentinel site results are not intended to be representative of and generalizable to vaccination practices in the United States, rates have compared favorably to those from NIS (eg, influenza vaccination in children).11 For the 2008 to 2012 IIS sentinel site project period, 4 sentinel site areas (Arizona, Colorado, Oregon, and Wisconsin) consisted of subsets of each state; the other 4 sentinel sites consisted of all of Michigan, Minnesota, North Dakota and New York City (Table 1). All sites are composed of geographically contiguous counties or zip code areas in which ≥85% of area vaccine provider sites that serve children and adolescents aged <19 years are registered (enrolled) with the IIS. Sites must also have a minimum of 20,000 children and at least 85% of children aged <19 years in their respective geographic areas who have at least 2 immunizations recorded in the IIS.12
Table 1.
Characteristics of Immunization Information Systems Sentinel Sites, 2012
| Site | Geographic Area | Provider Participation in IIS Sentinel Site area* |
No. of Adolescents (11–18 Years) Participating in the IIS sentinel site area† |
Adolescent Participation* |
Year IIS Expanded to Include Adolescents |
Opt In or Opt Out?‡ | Middle School Vaccination Requirements |
|---|---|---|---|---|---|---|---|
| Arizona | 7 counties in northern Arizona (includes Flagstaff) |
93% | 116,259 | 139% | Since it began in 1998 | Opt out | MenACWY, Tdap |
| Colorado | 14 counties in southwestern Colorado |
98% | 15,357 | 99% | Since it began in 2002 | Opt out | Tdap |
| Michigan | Entire state | 88% | 1,270,692 | 105% | Since it began in 1998 | Opt out | MenACWY, Tdap |
| Minnesota | Entire state | 93% | 639,203 | 100% | Since it began in 2002 | Opt out | Tdap |
| New York City | Manhattan, Bronx, Brooklyn, Staten Island, Queens |
93% | 1,290,197 | 80% | 2005 | NA; mandate to report immunizations for all<19 y |
Tdap |
| North Dakota | Entire state | 98% | 81,119 | 113% | 1996: in 2008, mandate to report immunizations for all <19 y |
Opt out | MenACWY, Tdap |
| Oregon | Washington and Multnomah counties (greater Portland) |
93% | 158,190 | 91% | Since it began in 1996; began actively soliciting adolescent immunization data in 2000 |
Neither; voluntary IIS but cannot have data removed once it has been entered |
Tdap |
| Wisconsin | 5 counties in southern Wisconsin (includes Madison) |
90% | 148,970 | 106% | Since it began in 1999 | Opt out | Tdap |
IIS = Immunization Information Systems; MenACWY = meningococcal conjugate vaccine; Tdap = tetanus toxoid, diphtheria toxoid, acellular pertussis vaccine.
Provider participation and percentage adolescent participation numbers are as of July 2012. Provider participation is defined as having submitted data to the IIS within the past 12 months. An adolescent participating in the IIS is defined as having a demographic record in the IIS and having 2 or more immunizations recorded there. The number of adolescents participating in the IIS may include those who moved out of the sentinel site area but providers have not updated the IIS to reflect that; thus, some sites have adolescent participation rates that exceed 100% when using a census-based denominator.
Number of participating adolescents as of December 31, 2012.
“Opt in” is defined as “voluntary inclusion and requires parents or individuals to actively indicate a willingness to participate in an IIS.” “Opt out” is defined as “voluntary exclusion in which consent is automatic or assumed unless a parent or individual chooses to exclude themselves or their children from participation” in an IIS. From: Boom JA, Sahni LC, Nelson CS, Dragsbaek AC, Franzini L. Immunization Information System opt-in consent: at what cost? J Public Health Manag Pract. 2010; 16:E18–E25.
Data
Using IIS sentinel site data, vaccination records were assessed by quarter according to calendar year (quarter 1, January–March; quarter 2, April–June; quarter 3, July–September; quarter 4, October–December) for all adolescents aged 11 to 12 years, 13 to 15 years, and 16 to 18 years during quarter 4, 2009, through quarter 4, 2012. Birth cohorts were defined for each quarter to include all adolescents in the age groups. Age and vaccination status were determined as of the last day of the quarter; vaccinations given at any point until the end of the quarter were included in the analysis. Valid vaccinations were identified by CDC vaccine codes (CVX codes)13 and included bivalent HPV vaccine for girls and quadrivalent HPV vaccine for boys and girls. Each site processed individual vaccination data in accordance with business rules established by the American Immunization Registry Association Modeling of Immunization Registry Operations Workgroup.14 Within each age group, sites reported counts of the number of vaccine recipients by quarter, sex, and the number of HPV vaccine doses received to the CDC. Doses administered to 11 to 18-year-olds were reported for October 17, 2009, through December 31, 2012, in monthly and weekly intervals. For male and female adolescents aged 13 to 15 years old at the end of the specified quarters (quarter 3, 2007, and quarter 4, 2011, for girls; quarter 4, 2011, for boys), sites reported the number of adolescents by year of age at which they received their first HPV vaccination, regardless of whether it was in that quarter or at an earlier time. This study was exempt from institutional review board review because it involved examination of secondary, deidentified data.
Analysis
Analyses were conducted using Microsoft Excel (2010) and SAS (SAS, Cary, NC) software. Vaccination coverage was calculated using denominators from the US Census intercensal population estimates.15 Use of census-based denominators to calculate adolescent participation in IIS and vaccination coverage levels has been described elsewhere.8,16 The unweighted average for the 8 sites (ie, average site-specific coverage) was calculated by summing the percentage of adolescents vaccinated at each site and dividing by 8, the total number of sites. Unweighted averages were used to allow for each site to be represented equally when calculating combined vaccination coverage. HPV series completion was calculated as the number of adolescents who received 3 doses of HPV vaccine out of the total number who received at least 1 dose. Unlike in the NIS-Teen,4 series completion calculations included all adolescents who received ≥1 HPV doses in the denominator, regardless of the amount of time that had elapsed since the first HPV vaccination. Trend analyses were conducted for coverage in boys and girls using linear regression analyses (SAS, version 9.3 for Windows). Vaccination coverage estimates are expected to be lower among 11- to 12-year-olds because they are not older than the recommended vaccination age range and still have time to be vaccinated before their 13th birthday; however, the data provide useful information on patterns of uptake.
Weekly doses administered were calculated on the basis of the doses administered for the varying time periods. Intervals ranged from a single week up to month-long increments. In order to analyze the data in a consistent manner, a weekly average was calculated on the basis of the total number of doses administered for that month.
Results
Vaccine coverage data were available on an average of 2.9 million adolescents 11 to 18 years of age per quarter. Overall among girls, 27.1% of 11- to 12-year-olds, 47.9% of 13- to 15-year-olds, and 57.1% of 16- to 18-year-olds received ≥1 dose of HPV vaccine by December 31, 2012 (Table 2). Only 6.5% of 11- to 12-year-olds, 25.2% of 13- to 15-year-olds, and 37.4% of 16- to 18-year-olds received all 3 doses of HPV vaccine. Of girls who initiated the series, 22.9% of 11- to 12-year-olds, 52.2% of 13- to 15-year-olds, and 65.5% of 16- to 18-year-olds received all 3 doses of HPV vaccine. Approximately 18% of male participants in all age groups received ≥1 dose of HPV vaccine by December 31, 2012. Of boys who initiated the series, 13.5% of 11- to 12-year-olds, 23.2% of 13- to 15-year-olds, and 24.7% of 16- to 18-year-olds received all 3 doses of HPV vaccine.
Table 2.
Average Percentage of Adolescents With Documented Receipt of HPV Vaccine as of Quarter 4, 2012, Immunization Information System Sentinel Sites, United States
| Age Group | No. of Doses of HPV Vaccine Received |
Vaccination Coverage, % |
|
|---|---|---|---|
| Male | Female | ||
| 11–12 y | ≥1 | 16.4 | 27.1 |
| ≥2 | 7.2 | 14.9 | |
| 3 | 2.4 | 6.5 | |
| 13–15 y | ≥1 | 20.7 | 47.9 |
| ≥2 | 11.3 | 36.5 | |
| 3 | 5.2 | 25.2 | |
| 16–18 y | ≥1 | 18.0 | 57.1 |
| ≥2 | 9.8 | 47.6 | |
| 3 | 4.6 | 37.4 | |
| 11–12 y | 3-dose series completion* | 13.5 | 22.9 |
| 13–15 y | 3-dose series completion* | 23.2 | 52.2 |
| 16–18 y | 3-dose series completion* | 24.7 | 65.5 |
HPV = human papillomavirus.
Percentage of male and female adolescents who received 3 doses among those who had at least 1 HPV dose as of quarter 4, 2012, regardless of time elapsed between first HPV vaccination and end of quarter.
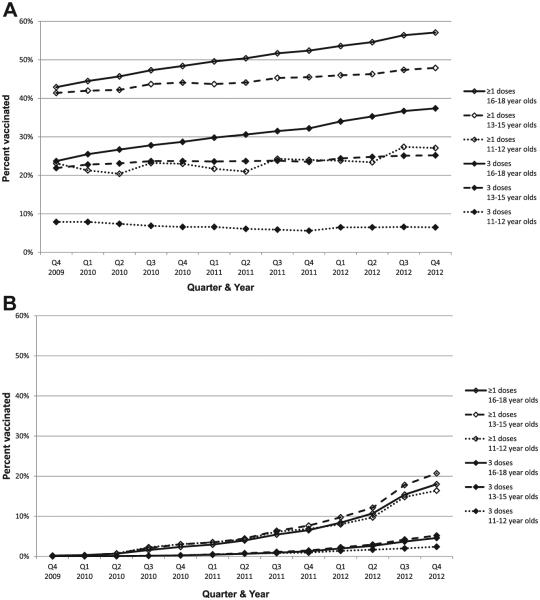
Figure 1 illustrates the trends in vaccination coverage from October 2009 to December 2012 for female and male adolescents. Using linear regression techniques, the percentage of girls who received ≥1 HPV vaccine dose increased 4.8 percentage points for 11- to 12-year-olds, 6.1 percentage points for 13- to 15-year-olds, and 14.1 percentage points for 16- to 18-year-olds from quarter 4, 2009, to quarter 4, 2012. There was no significant change in the rate of increase in coverage among girls (P = .74) after the ACIP recommendation for routine vaccination of boys was released. Receipt of ≥1 dose of HPV vaccine in boys started to increase after licensure of quadrivalent vaccine for boys and ACIP guidance that vaccine could be used in boys in late 2009; ≥1 dose coverage increased to approximately 18% in male participants (16.4% for 11- to 12-year-olds, 20.7% for 13- to 15-year-olds, and 18.0% of 16- to 18-year-olds) a year after the ACIP recommendation for routine vaccination was released. For boys, there was no significant difference in coverage by age group (P = .43), but there was a significant increase in the rate of increase in coverage immediately after the ACIP recommendation for routine use in male adolescents (P = .003).
Figure 1.
(A) Trends in HPV vaccination coverage for girls by age group, IIS sentinel sites, United States, quarter 4, 2009, to quarter 4, 2012. (B) Trends in HPV vaccination coverage for boys by age group, IIS sentinel sites, United States, quarter 4, 2009, to quarter 4, 2012.
Among vaccinated 13- to 15-year-old girls, the percentage vaccinated at the recommended age of 11 to 12 years increased over time (Table 3). By the end of quarter 3, 2007, only 9.9% of vaccinated 13- to 15-year-old girls received the first HPV vaccine dose before their 13th birthday, whereas 74.2% of vaccinated girls received the first HPV vaccine dose by their 13th birthday by the end of quarter 4, 2011. By the end of quarter 4, 2011, 27.3% of vaccinated 13- to 15-year-old boys received the first HPV vaccine dose before their 13th birthday. Quarter 3, 2007, and quarter 4, 2011, reflect time periods shortly after recommendations were made for routine use for girls and boys, respectively. Compared to the percentage of girls vaccinated at the recommended age by the end of October 2007, a larger percentage of boys were vaccinated at the recommended age by the end of December 2011.
Table 3.
Average Percentage of Vaccinated 13- to 15-Year-Old Adolescents at First HPV Vaccination, Quarter 3, 2007, and Quarter 4, 2011, Immunization Information System Sentinel Sites, United States
| Female |
Male |
|||
|---|---|---|---|---|
| Age at First HPV Vaccination | Quarter 3, 2007 | Quarter 4, 2011 | Quarter 3, 2007 | Quarter 4, 2011 |
| <11 y | 0.1 | 8.7 | NA | 0.4 |
| 11 y | 0.1 | 36.5 | NA | 5.4 |
| 12 y | 9.7 | 29.0 | NA | 21.5 |
| 13 y | 31.0 | 14.7 | NA | 30.8 |
| 14 y | 35.6 | 8.7 | NA | 29.6 |
| 15 y | 23.5 | 2.4 | NA | 12.3 |
NA = not applicable.
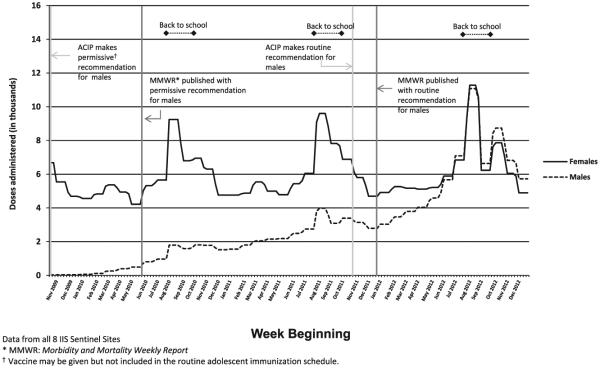
Figure 2 illustrates the doses administered to adolescents of both sexes from October 18, 2009, to December 31, 2012, by week. For girls, vaccination peaked during July and August each year, coinciding with back-to-school time periods. Additionally, doses administered appear to have decreased at the end of November and did not increase until around March. The number of total doses administered increased approximately 3.5% each year from 2010 to 2012. For boys, there was a clear increase in the number of doses administered, starting after the ACIP permissive recommendation and publication of guidance published in Morbidity and Mortality Weekly Report2 and smaller increases around the back-to-school period until summer 2012, when the doses administered to boys was similar to that for girls. The weekly number of doses administered to boys for all 8 sites combined surpassed the number of doses administered to girls by mid-September 2012 and remained higher through the end of 2012. Examination of individual sites revealed that the number of doses administered to boys only surpassed the number administered to girls in 6 of the 8 sites (January 2012 in New York City, May 2012 in Oregon, June 2012 in Arizona, North Dakota, and Wisconsin, and October 2012 in Michigan) (data not shown).
Figure 2.
HPV vaccine doses administered to 11- to 18-year-olds by week and sex, IIS sentinel sites, United States, October 18, 2009, to December 31, 2012.
Discussion
This study used provider-verified vaccination data from IIS to examine HPV vaccine uptake in adolescents of both sexes from 2009 to 2012. These data provide information for girls from 3 to 5 years after ACIP recommended routine vaccination, and for boys from the time a permissive recommendation was made through 14 months after the ACIP recommended routine vaccination. Although some data have been published that examine vaccination of adolescent boys since the ACIP recommendation was made,4,17 this is to our knowledge the first study to present doses administered to adolescent boys compared to adolescent girls during the time period surrounding the ACIP recommendation for routine vaccination of boys. We found that doses administered to boys increased soon after the ACIP recommendation for routine use, whereas doses administered to girls showed seasonal variation and only small increases across the years studied. By the end of December 2012, the weekly number of doses administered to boys for all 8 sites combined surpassed the number of doses administered to girls.
Coverage in female adolescents increased during this time period, but the increases were small. The highest coverage was observed in 16- to 18-year-olds, and the percentage who received at least 1 dose in this age group increased from 42.9% to 57.1%. Our data on coverage among female adolescents are consistent with other studies.18–21 Also similar to other studies was the finding that older female adolescents were more likely to have initiated and completed vaccination.18–21 Provider recommendation is a major factor associated with HPV vaccine initiation.22–25 However, providers have a preference to vaccinate older female adolescents.26,27 Although overall coverage in girls remains below target levels and older adolescents have higher coverage than those aged 11 or 12 years, our data demonstrate that among those vaccinated, a larger percentage are getting vaccinated at the recommended age than were immediately after ACIP’s recommendation was released. In the fourth quarter of 2011, of 13- to 15-year-old girls who had received at least 1 dose of the vaccine, 74.2% had received their first dose before age 13. This represents a 7-fold increase over the third quarter of 2007. A larger percentage of vaccinated 13- to 15-year-old boys were vaccinated at the recommended age (27.3%) compared to 13- to 15-year-old girls (9.9%) at a similar time period after the recommendation for routine vaccination.
Soon after the vaccine was licensed for boys and a permissive recommendation was made by ACIP in 2009, the number of doses administered to boys started to increase. After ACIP made a routine recommendation, there were further increases. Nevertheless, 1-dose vaccine coverage in boys reached only 16% by the end of December 2012. Prelicensure studies indicated that acceptability would be high for vaccination of boys, but that parents and providers were more likely to vaccinate girls than boys.28 Similar to what was reported for girls, one survey found that providers would be less likely to strongly recommend vaccination to younger compared to older adolescent boys.28 However, we found that in contrast to girls, vaccination initiation and series completion was similar in all 3 age groups of boys. The lack of coverage difference by age among boys could be due to more provider familiarity with HPV vaccine when it was licensed for use in boys, or less discomfort discussing sexuality with male adolescents or their parents. However, boys vaccinated in this time period and their families are early adopters who may have different attitudes and behaviors than those who have not yet been vaccinated; they may be more likely to request vaccination, and the strength of the provider recommendation may have been less important. Further data will be needed to see if this trend in vaccine uptake among boys continues.
Examination of the number of doses administered to girls by week showed variations in vaccine administration during each year. The trends we found suggest the importance of back-to-school health care visits for administration of immunizations, even for those not required for school entry. None of the IIS sentinel sites had middle school entry requirements for HPV vaccination; nevertheless, the number of doses administered increased each summer before school started.29–31 All of the states in which the IIS sentinel sites are located had middle school entry requirements for a least 1 other vaccine recommended for adolescents.29,31 Of note, trends over each year were similar, and we did not observe any declines in uptake during specific years that would suggest an adverse impact of specific reports of safety concerns in the media or high visibility statements about the vaccine.32 Nevertheless, such publicity could have prevented increases in vaccine uptake. Doses administered to boys by week increased continuously during this time period and equaled or surpassed doses administered to girls in some areas.
A variety of interventions have been previously identified that could increase HPV vaccination coverage in adolescents. These include improving parental education, increasing the strength and consistency of provider recommendations, and preventing missed opportunities.33 Although the proportion of adolescents who make preventive health care visits is low,34 the overall number of provider visits made by adolescents has increased over the past 2 decades,35 likely supported by the recommendation that adolescents get screened annually for a variety of behaviors and risk factors.36 However, the number of visits made by adolescents decreases with age. Most visits are to pediatricians and family practice physicians, with an increasing percentage of visits to gynecologists for girls and to subspecialists for boys in older adolescence.35 A recent analysis demonstrated that 84% of unvaccinated adolescent girls had at least 1 missed opportunity for the HPV vaccine.33 As adolescents age, the opportunities for vaccination decrease; thus, it is important for providers to minimize the number of missed opportunities for vaccination by providing vaccinations during both preventive- and acute-care visits.37
Results from this study were similar to results from NIS-Teen despite differences in the age groups examined and the geographic areas covered.6,18 However, there are some disparities in HPV coverage estimates (CDC, unpublished data) that might reflect differences in geographic assessment areas because not all sentinel site areas are comparable with the NIS-Teen geographic areas, underreporting in the IIS, bias in NIS-Teen estimates, or a combination of these and other unidentified factors. Even if data on 13- to 17-year-olds were collected in this study, IIS and NIS-Teen data may not be directly comparable. Bias may remain in NIS-Teen estimates after weighting adjustments, nonresponse bias, and possible incomplete vaccination histories reported by providers.38–40
This study’s findings are subject to several important limitations. First, although provider enrollment in the IIS sentinel sites is high, the reporting completeness is unknown, and some provider sites do not contribute any immunization data to the IIS. This lack of reporting could have resulted in lower or higher HPV vaccination coverage in the IIS sentinel sites. Second, because study results are from 8 project areas, results are not generalizable to the overall US population (eg, the southeastern United States is not represented). However, the IIS sentinel sites are population based and include 2.9 million adolescents aged 11 to 18 years residing across the United States, and the vaccination histories are provider verified. The data presented here are not weighted; however, the Sentinel Site Project’s goal is not to produce national estimates of HPV vaccination in adolescents. Results using weighted data would be skewed toward sites with larger populations. IIS may underestimate adolescent vaccination coverage because of information lacking from unenrolled providers, incomplete reporting by enrolled IIS providers, failure to include vaccinations administered at nontraditional locations, and disproportionate outmigration. However, IIS sentinel sites have >85% provider enrollment and strive to enroll all providers licensed to vaccinate in their jurisdiction, including traditional and complementary vaccinators. Although the denominators used in this analysis used consistent methodology from the US Census, use of those denominators may have led to artificially lower HPV vaccination coverage for sites with lower adolescent participation rates. Conversely, the use of US Census–determined denominators could result in overestimates of vaccination coverage if adolescents moved out of the state/city after vaccination and the IIS was unaware of the migration.
Despite these limitations, this is to our knowledge the first study to report on HPV vaccination using IIS and to examine trends in doses administered to boys and girls by week since 2009. Although HPV vaccination coverage is increasing in both boys and girls, it is still below Healthy People 2020 target levels. It is important to continue to monitor uptake of HPV vaccine in both boys and girls, at both the national and local levels. The IIS can be useful for identifying trends in administration and coverage, especially when recommendations have changed recently, and can be used to target activities at the local level to improve vaccination.
What’s New.
We report on human papillomavirus vaccination in adolescent boys using the Immunization Information System since the October 2011 Advisory Committee on Immunization Practices recommendation for routine use and to uniquely examine trends in doses administered to adolescent boys and girls by week since 2009.
Acknowledgments
The authors gratefully acknowledge the contributions of the Sentinel Site Project. The following are members of the project staff: Lisa Rasmussen, Patty Gast, Arizona; Diana Herrero, Paul Gillenwater, Colorado; Rachel Potter, Beatrice Salada, Michigan; Karen White, Emily Emerson, Minnesota; Molly Howell, Mary Woinarowicz, North Dakota; Vikki Papadouka, Alexandra Ternier, New York City; Andrew Osborn, Mary Beth Kurilo, Oregon; Stephanie Schauer, Thomas Maerz, Wisconsin.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
The authors declare that they have no conflict of interest.
References
- 1.Centers for Disease Control and Prevention FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2010;59:626–629. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention FDA licensure of quadrivalent human papillomavirus vaccine (HPV4, Gardasil) for use in males and guidance from the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2010;59:630–632. [PubMed] [Google Scholar]
- 3.Markowitz LE, Dunne EF, Saraiya M, et al. Quadrivalent human papillomavirus vaccine. Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2007;56:1–24. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention National and state vaccination coverage among adolescents aged 13–17 years—United States, 2012. MMWR Morb Mortal Wkly Rep. 2013;62:685–693. [PMC free article] [PubMed] [Google Scholar]
- 5.Jain N, Singleton JA, Montgomery M, Skalland B. Determining accurate vaccination coverage rates for adolescents: the National Immunization Survey–Teen 2006. Public Health Reports. 2009;124:642–651. doi: 10.1177/003335490912400506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention National, state, and local area vaccination coverage among adolescents aged 13 through 17 years—United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61:671–677. [PubMed] [Google Scholar]
- 7.White KE, Pabst LJ, Cullen KA. Up-to-date haemophilus influenzae type b vaccination coverage during a vaccine shortage. Pediatrics. 2011;127:e707–e712. doi: 10.1542/peds.2010-2129. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention Seasonal influenza vaccination coverage among children aged 6 months–18 years—eight immunization information system sentinel sites, United States, 2009–10 influenza season. MMWR Morb Mortal Wkly Rep. 2010;59:1266–1269. [PubMed] [Google Scholar]
- 9.Boom J, Dragsbaek A, Nelson C. The success of an immunization information system in the wake of Hurricane Katrina. Pediatrics. 2007;119:1213–1217. doi: 10.1542/peds.2006-3251. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention Progress in immunization information systems—United States, 2009. MMWR Morb Mortal Wkly Rep. 2011;60:10–12. [PubMed] [Google Scholar]
- 11.Williams L, Fiore A, White KE. Influenza vaccination coverage among children aged 6–59 months—eight immunization information system sentinel sites, United States, 2007–08 influenza season. MMWR Morb Mortal Wkly Rep. 2008;57:1043–1046. [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention Q&A about sentinel sites. Available at: http://www.cdc.gov/vaccines/programs/iis/activities/sentinel-sites.htm. Accessed February 9, 2011.
- 13.Immunization Information System HL7 standard code set. CVX—vaccines administered. Available at: http://www2a.cdc.gov/nip/IIS/IISStandards/vaccines.asp?rpt=cvx. Accessed February 10, 2011.
- 14.Williams W, Lowery E, Lyalin D, et al. Development and utilization of best practice operational guidelines for immunization information systems. J Public Health Manag Pract. 2011;17:449–456. doi: 10.1097/PHH.0b013e31821138fe. [DOI] [PubMed] [Google Scholar]
- 15.US Census Bureau Population and housing unit estimates. Available at: http://www.census.gov/popest/. Accessed July 10, 2012.
- 16.Pabst LJ, Papadouka V, Cullen KA, Bartlett DL. Methodological considerations: denominators for Immunization Information System–based vaccine coverage assessments; Paper presented at: 43rd National Immunization Conference; Dallas, Texas. Apr 1, 2009. [Google Scholar]
- 17.Reiter PL, Gilkey MB, Brewer NT. HPV vaccination among adolescent males: results from the National Immunization Survey–Teen. Vaccine. 2013;31:2816–2821. doi: 10.1016/j.vaccine.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dorell CG, Yankey D, Santibanez TA, Markowitz LE. Human papillomavirus vaccination series initiation and completion, 2008–2009. Pediatrics. 2011;128:830–839. doi: 10.1542/peds.2011-0950. [DOI] [PubMed] [Google Scholar]
- 19.Reiter PL, Brewer NT, Gottlieb SL, et al. Parents’ health beliefs and HPV vaccination of their adolescent daughters. Social Sci Med. 2009;69:475–480. doi: 10.1016/j.socscimed.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 20.Chao C, Velicer C, Slezak JM, Jacobsen SJ. Correlates for human papillomavirus vaccination of adolescent girls and young women in a managed care organization. Am J Epidemiol. 2010;171:357–367. doi: 10.1093/aje/kwp365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pruitt SL, Schootman M. Geographic disparity, area poverty, and human papillomavirus vaccination. Am J Prev Med. 2010;38:525–533. doi: 10.1016/j.amepre.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gust DA, Darling N, Kennedy A, Schwartz B. Parents with doubts about vaccines: which vaccines and reasons why. Pediatrics. 2008;122:718–725. doi: 10.1542/peds.2007-0538. [DOI] [PubMed] [Google Scholar]
- 23.Gerend MA, Weibley E, Bland H. Parental response to human papillomavirus vaccine availability: uptake and intentions. J Adolesc Health. 2009;45:528–531. doi: 10.1016/j.jadohealth.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 24.Dempsey AF, Abraham LM, Dalton V, Ruffin M. Understanding the reasons why mothers do or do not have their adolescent daughters vaccinated against human papillomavirus. Ann Epidemiol. 2009;19:531–538. doi: 10.1016/j.annepidem.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dempsey AF, Zimet GD, Davis RL, Koutsky L. Factors that are associated with parental acceptance of human papillomavirus vaccines: a randomized intervention study of written information about HPV. Pediatrics. 2006;117:1486–1493. doi: 10.1542/peds.2005-1381. [DOI] [PubMed] [Google Scholar]
- 26.Kahn JA, Rosenthal SL, Tissot AM, et al. Factors influencing pediatricians’ intention to recommend human papillomavirus vaccines. Ambul Pediatr. 2007;7:367–373. doi: 10.1016/j.ambp.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 27.Daley MF, Crane LA, Markowitz LE, et al. Human papillomavirus vaccination practices: a survey of US physicians 18 months after licensure. Pediatrics. 2010;126:425–433. doi: 10.1542/peds.2009-3500. [DOI] [PubMed] [Google Scholar]
- 28.Liddon N, Hood J, Wynn BA, Markowitz LE. Acceptability of human papillomavirus vaccine for males: a review of the literature. J Adolesc Health. 2010;46:113–123. doi: 10.1016/j.jadohealth.2009.11.199. [DOI] [PubMed] [Google Scholar]
- 29.Knighton C, Thomas E, Stanwyck C. School-entry vaccination laws in the United States, 2009–2010: a brief overview; Paper presented at: 44th National Immunization Conference; Atlanta, Ga. Apr 19–22, 2010. [Google Scholar]
- 30.Immunization Action Coalition State mandates on immunization and vaccine-preventable diseases. Available at: http://www.immunize.org/laws/. Accessed October 11, 2012.
- 31.School vaccination requirements, exemptions, and Web links. Available at: http://www2a.cdc.gov/nip/schoolsurv/schImmRqmt.asp. Accessed January 24, 2014.
- 32.Grady D. Remark on HPV vaccine could ripple for years. New York Times; New York City: Sep 20, 2011. [Google Scholar]
- 33.Centers for Disease Control and Prevention Human papillomavirus vaccination coverage among adolescent girls, 2007–2012, and postlicensure vaccine safety monitoring, 2006–2013—United States. MMWR Morb Mortal Wkly Rep. 2013;62:591–595. [PMC free article] [PubMed] [Google Scholar]
- 34.Tsai Y, Zhou F, Wortley P, et al. Trends and characteristics of preventive care visits among commercially insured adolescents, 2003–2010. J Pediatr. 2014;164:625–630. doi: 10.1016/j.jpeds.2013.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rand CM, Shone LP, Albertin C, et al. National health care visit patterns of adolescents: implications for delivery of new adolescent vaccines. Arch Pediatr Adolesc Med. 2007;161:252–259. doi: 10.1001/archpedi.161.3.252. [DOI] [PubMed] [Google Scholar]
- 36.Elster A, Kuznets N. AMA Guidelines for Adolescent Preventive Services (GAPS): Recommendations and Rationale. Williams & Wilkins; Baltimore, Md: 1994. [Google Scholar]
- 37.Dempsey A, Cohn L, Dalton V, Ruffin M. Patient and clinic factors associated with adolescent human papillomavirus vaccine utilization within a university-based health system. Vaccine. 2010;28:989–995. doi: 10.1016/j.vaccine.2009.10.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blumberg SJ, Luke JV, Davidson G, et al. Wireless substitution: state-level estimates from the National Health Interview Survey, January–December 2007. Natl Health Stat Rep. 2009;1–13:16. [PubMed] [Google Scholar]
- 39.Copeland KR, Dorell C, Khare M, et al. Assessment of Bias in the National Immunization Survey–Teen: An Updated Benchmark to the National Health Interview Survey. American Association for Public Opinion Research 65th Annual Conference; Chicago, Ill: May 16, 2010. [Google Scholar]
- 40.Montgomery M, Jain N, Singleton JA, Khare M. Assessment of Bias in the National Immunization Survey–Teen: Benchmarking to the National Health Interview Survey. American Association for Public Opinion Research 63rd Annual Conference; New Orleans, La: May 16, 2008. [Google Scholar]