To the Editor: Ignatzschineria is a recently described genus of bacteria that have been rarely implicated in human disease (1–3). We report a patient in France with septicemia caused by I. ureiclastica.
In October 2013, a 69-year-old man was found unconscious in a forest close to Tours in the Loire Valley, France. The patient had hypotension with auricular fibrillation complicated by cardiorespiratory arrest and was admitted to the general intensive care unit of Tours University Hospital. He also had cyanosis of the extremities, a necrotic skin lesion on the right shoulder, and a large number of maggots around the genital organs. Empiric treatment with ceftriaxone was initiated. Blood cultures on admission revealed several microbes: Enterococcus faecalis, Enterobacter cloacae, Providencia stuartii, Corynebacterium spp., and a gram-negative bacillus resembling Pseudomonas. This bacillus was sensitive to all β-lactams, aminosides, fluoroquinolones, colistin, and trimethoprim/sulfamethoxazole but was resistant to fosfomycin. Ten days after admission to the hospital, the patient was found dead in his bed from no evident cause, despite recent improvement of his clinical state. No autopsy was conducted.
The unidentified gram-negative bacillus was an oxidase-positive strict aerobe. The 16S rRNA and gyrB genes were amplified and sequenced (4,5). The 897-bp 16S rRNA sequence obtained for the bacterium was 99% identical to sequences from I. larvae type strain L1/68T (GenBank accession no. AJ252143) and I. ureiclastica type strain FFA3T (GenBank accession no. EU008089). The 973-bp gyrB sequence of the isolate was 96% similar to the sequence of I. ureiclastica type strain FFA3T (GenBank accession no. FJ966120) and 92% with I. larvae type strain L1/68T (GenBank accession no. FJ966121). The 16S rRNA and gyrB sequences (GenBank accession nos. KR184134 and KR184135) were compared with those of all members of the genus Ignatzschineria and with those of several species belonging to the class Gammaproteobacteria. Two phylogenetic trees were deduced by the neighbor-joining method (Figure).
Figure.
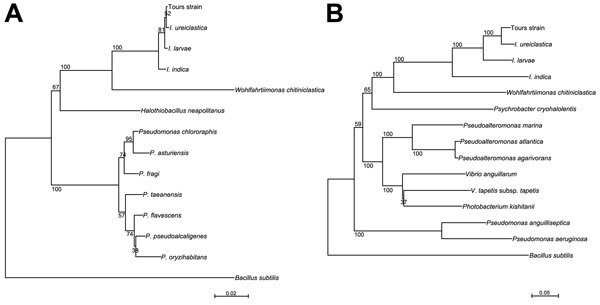
Phylogenetic trees showing relationships between the clinical isolate identified in this study (“Tours strain”) and type strains of members of the genus Ignatzschineria. A) Relationships among 16S rRNA sequences of “Tours strain” (GenBank accession no. KR184134) and Ignatzschineria strains; scale bar represents 2% differences in nucleotide sequence. B) Relationships among gyrB sequences of “Tours strain” (GenBank accession no. KR184135) and Ignatzschineria strains; scale bars represent 5% differences in nucleotide sequence. Bacillus subtilis was included as an outgroup organism. Numbers at branch nodes are bootstrap values.
The genus Ignatzschineria, which is the revised nomenclature for Schineria, was first described in 2001. It comprises 3 species: I. larvae, I. indica, and I. ureiclastica (6–8), and belongs to the family Xanthomonadaceae, class Gammaproteobacteria,. All 3 species have been isolated from larvae Wohlfahrtia magnifica flies (9), which are found in Europe, Asia, and North Africa and cause myiasis in several animal species but rarely in humans. Ignatzschineria spp. is the dominant species in the anterior portion of the digestive tract in larvae, together with Providencia (9). Providencia was also found in blood cultures from this patient. Cases of I. larvae and Ignatzschineria sp. bacteremia were reported in France: 1 in a homeless patient (2) and the other in a patient with type 2 diabetes (1), both with a foot wound invaded by maggots. Three cases of I. indica infection were recently described in the United States: 2 cases of bacteremia and 1 urinary tract infection (3). These 3 cases were clearly associated with fly larvae infestations and myiasis.
The presence of I. ureiclastica in the blood cultures of the patient reported here and the presence of bacteria from the same genus in 4 other cases of bacteremia suggest an association between Ignatzschineria bacteremia and wounds infected by maggots in patients with poor hygiene. Systematic blood cultures should therefore be conducted for such patients. The epidemiologic importance of Ignatzschineria spp. might have been underestimated because of the presence of other microbes in samples and identification difficulties, which in some cases might have led to a conclusion of simple contamination.
The species of fly larvae found in wounds and the bacteria transmitted appear to differ among geographic regions. In France, I. larvae and I. ureiclastica are the species associated with the W. magnifica fly, which is present in Europe, Asia, and North America. In the United States, the 3 human infections reported were all caused by I. indica and seemed to be associated with larvae of the Phaenicia sericata fly, found throughout the world. A geographic specificity of Ignatzschineria spp. linked to the geographic distribution of fly larvae is therefore remarkable.
The larvae used in maggot therapy are “sterile” larvae of the P. sericata fly. A possible risk for infection with Ignatzschineria exists with larval therapy, especially with I. indica.
The pathogenic power of Ignatzschineria spp. remains to be demonstrated. However, a wound invaded by maggots seems to be strongly associated with the presence of Ignatzschineria spp. in clinical samples, with the possibility of a specific geographic distribution of the species implicated. Clinicians and microbiologists should be aware of the possibility of invasive Ignatzschineria infections in presence of maggots in patients with poor hygiene and should check specifically for this bacterium.
Footnotes
Suggested citation for this article: Le Brun C, Gombert M, Robert S, Mercier E, Lanotte P. Association of necrotizing wounds colonized by maggots with Ignatzschineria–associated septicemia [letter]. Emerg Infect Dis. 2015 Oct [date cited]. http://dx.doi.org/10.3201/eid2110.150748
References
- 1.Maurin M, Delbano JN, Mackaya L, Colomb H, Guier C, Mandjee A, et al. Human infection with Schineria larvae. Emerg Infect Dis. 2007;13:671–3. 10.3201/eid1304.061151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roudiere L, Jean-Pierre H, Comte C, Zorgniotti I, Marchandin H, Jumas-Bilak E. Isolation of Schineria sp. from a man. Emerg Infect Dis. 2007;13:659–61. 10.3201/eid1304.061255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barker HS, Snyder JW, Hicks AB, Yanoviak SP, Southern P, Dhakal BK, et al. First case reports of Ignatzschineria (Schineria) indica associated with myiasis. J Clin Microbiol. 2014;52:4432–4. 10.1128/JCM.02183-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rådström P, Bäckman A, Qian N, Kragsbjerg P, Påhlson C, Olcén P. Detection of bacterial DNA in cerebrospinal fluid by an assay for simultaneous detection of Neisseria meningitidis, Haemophilus influenzae, and streptococci using a seminested PCR Strategy. J Clin Microbiol. 1994;32:2738–44 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamamoto S, Harayama S. PCR amplification and direct sequencing of gyrB genes with universal primers and their application to the detection and taxonomic analysis of Pseudomonas putida strains. Appl Environ Microbiol. 1995;61:1104–9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tóth E, Kovács G, Schumann P, Kovács AL, Steiner U, Halbritter A, et al. Schineria larvae gen. nov., sp. nov., isolated from the 1st and 2nd larval stage of Wohlfahrtia magnifica (Diptera: Sarcophagidae). Int J Syst Evol Microbiol. 2001;51:401–7 . 10.1099/00207713-51-2-401 [DOI] [PubMed] [Google Scholar]
- 7.Tóth EM, Borsodi AK, Euzéby JP, Tindall BJ, Márialigeti K. Proposal to replace the illegitimate genus name Schineria Tóth et al. 2001 with the genus name Ignatzschineria gen. nov. and to replace the illegitimate combination Schineria larvae Tóth et al. 2001 with Ignatzschineria larvae comb. nov. Int J Syst Evol Microbiol. 2007;57:179–80. [DOI] [PubMed]
- 8.Gupta AK, Dharne MS, Rangrez AY, Verma P, Ghate HV, Rohde M, et al. Ignatzschineria indica sp. nov. and Ignatzschineria ureiclastica sp. nov., isolated from adult flesh flies (Diptera: Sarcophagidae). Int J Syst Evol Microbiol. 2011;61:1360–9. 10.1099/ijs.0.018622-0 [DOI] [PubMed] [Google Scholar]
- 9.Tóth EM, Hell É, Kovács G, Borsodi AK, Márialigeti K. Bacteria isolated from the different developmental stages and larval organs of the obligate parasitic fly, Wohlfahrtia magnifica (Diptera: Sarcophagidae). Microb Ecol. 2006;51:13–21. 10.1007/s00248-005-0090-6 [DOI] [PubMed] [Google Scholar]


