Abstract
The objective of this analysis was to evaluate the effect of 12 months of treatment with lurasidone on weight in patients with schizophrenia. Post-hoc, observed-case analysis included pooled data from six studies on 40–160 mg/day lurasidone; two studies included active comparators (2–6 mg/day risperidone or 200–800 mg/day quetiapine XR). Overall, 593 patients completed 12 months of treatment (N=471 lurasidone, N=89 risperidone, N=33 quetiapine XR). The mean baseline weight was 72.8, 80.8, and 72.4 kg in the lurasidone, risperidone, and quetiapine XR groups, respectively. The mean weight change at month 12 was −0.4 kg with lurasidone, +2.6 kg with risperidone, and +1.2 kg with quetiapine XR. Weight gain of at least 7% from study baseline was observed in 16.0, 25.8, and 15.2% of patients, and weight loss of at least 7% was seen in 18.5, 6.7, and 9.1% of patients treated with lurasidone, risperidone, and quetiapine XR, respectively. A shift from normal/underweight baseline BMI status to overweight/obese at month 12 occurred in 10.2, 27.6, and 15.0% of patients in the lurasidone, risperidone, and quetiapine XR groups, respectively. Conversely, 14.3, 1.7, and 7.7% of patients, respectively, shifted from overweight/obese to normal/underweight. In summary, a low potential for clinically significant weight gain was observed in patients with schizophrenia treated continuously with lurasidone for 12 months.
Keywords: antipsychotic agents, drug therapy, lurasidone, obesity, schizophrenia, weight
Introduction
According to recent estimates, more than one-third of adults in the USA are obese (BMI≥30 kg/m2; Flegal et al., 2012; Ogden et al., 2012). Obesity and other cardiovascular disease risk factors are more prevalent in individuals with schizophrenia than in the general population (McEvoy et al., 2005; Goff et al., 2005a, 2005b; McElroy, 2009; De Hert et al., 2009b; Correll et al., 2010; Casey et al., 2011). Increased body weight is associated with elevated risk of developing hypertension, dyslipidemia, metabolic syndrome, type 2 diabetes mellitus, and coronary heart disease (Must et al., 1999; Nguyen et al., 2008, 2010). Two recent meta-analyses demonstrated that longer duration of illness was associated with the development of metabolic syndrome and increased cardiovascular risk in patients with schizophrenia (Mitchell et al., 2013a, 2013b).
Atypical antipsychotic medications vary in their propensity for causing weight gain and metabolic disturbances (American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity, 2004; Newcomer, 2005; Henderson, 2007; Newcomer, 2007). A recent meta-analysis of 212 short-term trials comprising more than 40 000 patients with schizophrenia or related disorders found that the mean weight gain was generally higher for olanzapine, clozapine, and iloperidone; intermediate for quetiapine, risperidone, and paliperidone; and lower for lurasidone, asenapine, amisulpride, aripiprazole, and ziprasidone (Leucht et al., 2013). A similar pattern has been observed in controlled studies of longer-term treatment (12 months or more; Csernansky et al., 2002; Kasper et al., 2003; Meltzer et al., 2010; Pappadopulos et al., 2012) and large, ‘real-world’ effectiveness studies of long-term therapy (up to 36 months; Lieberman et al., 2005; Bushe et al., 2012).
Lurasidone is an atypical antipsychotic agent that acts as an antagonist with high affinity for dopamine D2, 5-hydroxytryptamine (5-HT)2A, and 5-HT7 receptors, and as a partial agonist with moderate to high affinity for 5-HT1A receptors (Ishibashi et al., 2010). Notably, lurasidone demonstrates weak affinity for 5-HT2C receptors and no appreciable affinity for muscarinic M1 and histamine H1 (Ishibashi et al., 2010). The negligible activity at 5-HT2C and H1 receptors suggests that lurasidone may have a low propensity for causing weight gain (Kroeze et al., 2003).
In five double-blind, placebo-controlled, 6-week studies, lurasidone has demonstrated efficacy at fixed daily doses of 40, 80, 120, and 160 mg in the treatment of schizophrenia (Nakamura et al., 2009; Meltzer et al., 2011; Nasrallah et al., 2013; Ogasa et al., 2013; Loebel et al., 2013a). The mean change in weight from baseline to study endpoint in short-term studies was +0.43 kg for lurasidone-treated patients (20–160 mg/day, N=1486) and −0.02 kg for patients receiving placebo (N=696); the proportion of patients with at least 7% weight gain was 4.8% for lurasidone and 3.3% for placebo (Sunovion Pharmaceuticals Inc., 2013). The present analysis pooled data from six long-term clinical studies to evaluate change in weight and BMI in patients with schizophrenia who received 12 months of treatment with lurasidone at doses of 20–160 mg/day.
Methods
Study design
The lurasidone clinical trial database includes six studies of at least 12 months in duration in patients with schizophrenia or schizoaffective disorder (Table 1). In this post-hoc analysis, individual patient data from all six of these long-term studies were pooled for lurasidone; data for risperidone and extended-release quetiapine (quetiapine XR) were from one study each. The dose range of lurasidone in these studies was 20–160 mg/day, whereas risperidone was flexibly dosed between 2 and 6 mg/day, and quetiapine XR was flexibly dosed between 200 and 800 mg/day. Study medication was taken once daily with a meal or within 30 min of eating. Patients were instructed to take the study medication in the morning in three of the studies and in the evening in one study; timing of administration was not specified in the other two studies (Table 1).
Table 1.
Summary of studies included in this pooled analysis
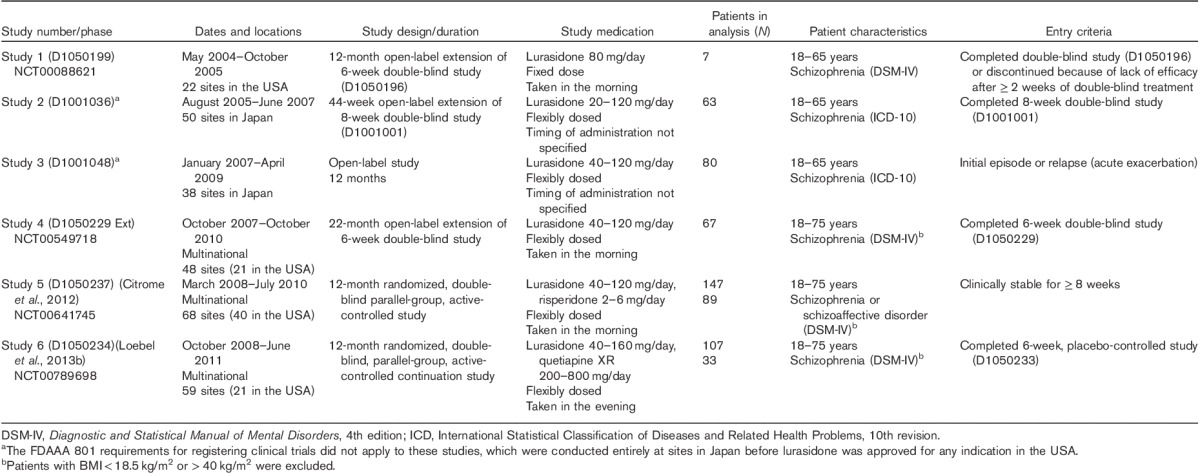
All study protocols were approved by an independent ethics committee or institutional review board, and written informed consent was provided by all patients before initiation of study procedures. All studies were conducted in accordance with the International Conference on Harmonization Good Clinical Practice guidelines and with the ethical principles of the Declaration of Helsinki.
Patients
Eligible patients were adults aged between 18 and 75 years with a diagnosis of schizophrenia (all studies) or schizoaffective disorder (Citrome et al., 2012). Other, more specific inclusion criteria, such as duration of illness and baseline disease severity, varied among studies. Patients were excluded if they presented with an acute or unstable medical condition, a recent history of substance abuse, evidence of tardive dyskinesia or another severe, chronic movement disorder, or were judged to be at imminent risk of injury to self or others. Patients with BMI less than 18.5 kg/m2 or greater than 40 kg/m2 were excluded from three of the studies (Table 1).
Statistical analysis
The analysis population included all patients who completed 12 months of study treatment in any of the included studies. Duration of exposure was calculated from the first dose of active study medication. Intermediate visits were assigned to the closest assessment time point (3, 6, 9, or 12 months). Month 3 was operationally defined as study days 61–151, month 6 as days 152–242, month 9 as days 243–333, and month 12 as days 334–424. These assessment windows were selected to accommodate differences in the timing of assessments across studies.
Standard criteria for BMI categories were applied: underweight was defined as BMI less than 18.5 kg/m2, normal weight was defined as BMI of at least 18.5 kg/m2 and less than 25 kg/m2, overweight defined as BMI of at least 25 kg/m2 and less than 30 kg/m2, and obese was defined as BMI of at least 30 kg/m2 (Centers for Disease Control and Prevention, 2015).
Changes from baseline in weight, BMI, and waist circumference were summarized by treatment group at each time point, and comparisons of lurasidone (pooled) with risperidone, as well as with quetiapine XR, were conducted using t-tests with P-values unadjusted for multiple comparisons. The proportion of patients with an increase or decrease in weight of at least 7% from baseline was compared for lurasidone (pooled) versus risperidone and for lurasidone (pooled) versus quetiapine XR using Fisher’s exact tests. Comparisons of risperidone versus quetiapine XR were not performed. The proportion of patients with a shift in BMI category (normal/underweight, overweight/obese) was calculated from baseline (start of active study medication) to month 12 for each treatment group.
Classification and regression tree (CART) analysis and stepwise multiple regression analysis were used to investigate patient age, sex, race, and duration of illness as potential predictors of change in body weight during treatment with lurasidone. CART analysis assessed the impact of the input variables (age, sex, race, and duration of illness) on the dichotomous outcome of at least 7% weight gain (yes vs. no). In the stepwise regression analysis, these variables were examined as predictors of weight change measured as a continuous variable.
Results
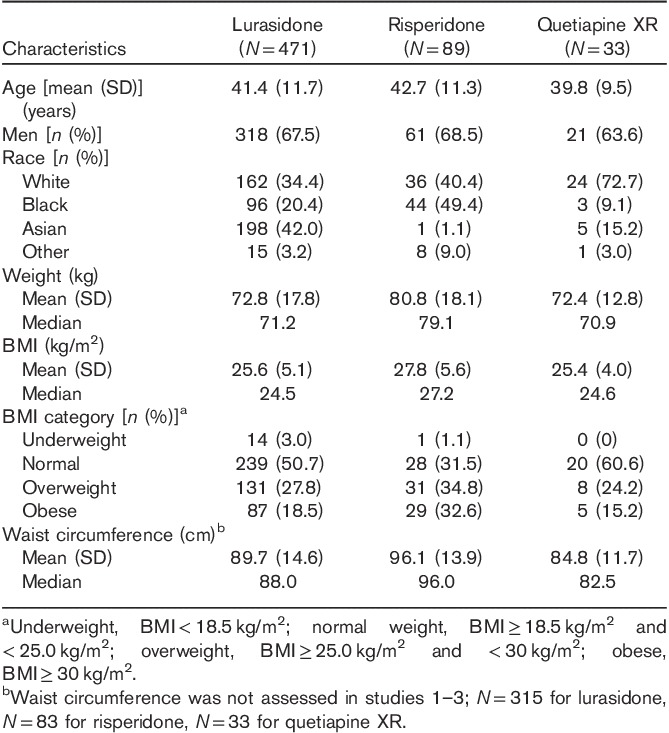
This analysis included 593 patients who completed 12 months of treatment (N=471 lurasidone, N=89 risperidone, N=33 quetiapine XR; Fig. 1). Study completion rates were 38.2% for the lurasidone group, 44.1% for the risperidone group, and 38.8% for the quetiapine XR group. The average age and sex were similar across the lurasidone, risperidone, and quetiapine XR groups; however, differences were observed in patients’ racial backgrounds (Table 2). The mean weight and BMI at baseline were higher in the risperidone group (80.8 kg and 27.8 kg/m2, respectively) compared with the lurasidone group (72.8 kg and 25.6 kg/m2, respectively) and the quetiapine XR group (72.4 kg and 25.4 kg/m2, respectively).
Fig. 1.
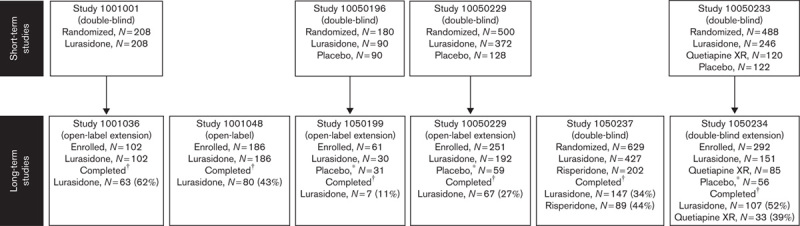
Patient disposition. *Patients who received placebo during Studies 196, 229, and 233 were switched to lurasidone during Studies 199, 229ext, and 234, respectively. †Patients who completed 12 months of study treatment.
Table 2.
Demographics and baseline clinical characteristics
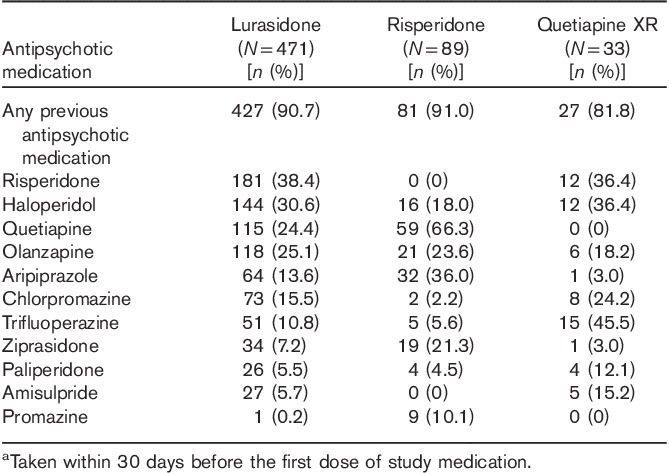
High rates of prior antipsychotic use (within 30 days of starting study medication) were observed: 90.7% for patients in the lurasidone group (pooled), 91.0% for patients in the risperidone group, and 81.8% for patients in the quetiapine XR group (Table 3). No patients had taken clozapine for at least 30 days before study entry. The distribution of patients who had taken other antipsychotic agents associated with increased risk of weight gain (e.g. olanzapine, quetiapine) was similar across the pooled treatment groups, except for a lower rate of prior quetiapine use in the lurasidone and quetiapine groups compared with the risperidone group. Across the 12-month study period, the mean modal daily doses of study medication were 90.6 mg lurasidone, 4.4 mg risperidone, and 660.6 mg quetiapine XR.
Table 3.
Prior antipsychotic medications (≥10% of patients in any group)a
Change in weight, BMI, and waist circumference during 12 months of treatment
Decreases in the mean weight were observed for patients treated with lurasidone; the mean change in weight was −0.5 kg at month 3, which then remained generally stable through 12 months of treatment (−0.4 kg at month 12; Fig. 2). In contrast, increases in mean weight were observed for risperidone (+2.1 kg at month 3 and +2.6 kg at month 12) and quetiapine XR (+1.6 kg at month 3 and +1.2 kg at month 12) at all assessments. Change in mean weight with lurasidone was significantly different from that with risperidone at all postbaseline assessments (all P’s<0.001); comparisons of lurasidone with quetiapine XR were not statistically significant, despite numerical differences. Similarly, the change in mean BMI at month 12 was significantly different for lurasidone (−0.1 kg/m2) compared with risperidone (+0.9 kg/m2, P<0.001) but not for lurasidone versus quetiapine XR (+0.5 kg/m2). There was an increase in the mean waist circumference after 12 months of treatment in all three groups (+0.4 cm with lurasidone, +2.7 cm with risperidone, and +0.6 cm with quetiapine XR), with a significantly larger increase for risperidone compared with lurasidone (P<0.001).
Fig. 2.
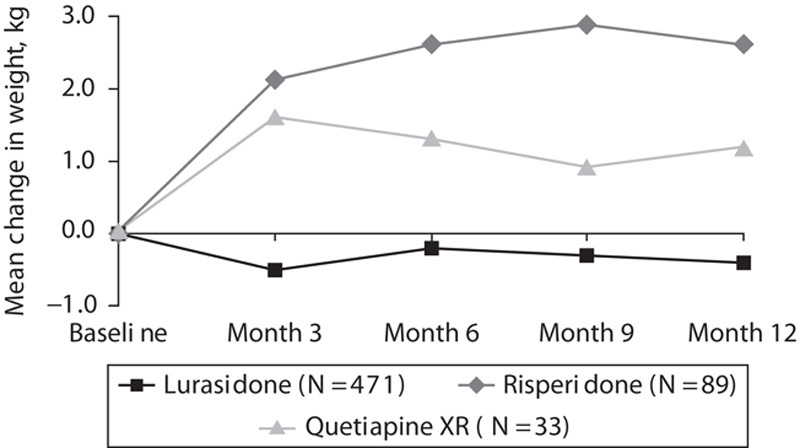
Mean change from baseline weight (kg) after 3, 6, 9, and 12 months of treatment with lurasidone (40–160 mg/day), risperidone (2–6 mg/day), or quetiapine XR (200–800 mg/day). *P<0.0001 versus risperidone.
The proportion of patients with weight gain of at least 7% was 9.3% at month 3 and 16.0% at month 12 with lurasidone, 22.5% at month 3 and 25.8% at month 12 with risperidone, and 12.1% at month 3 and 15.2% at month 12 with quetiapine XR (Fig. 3). Comparisons at month 12 were not statistically significant among the treatment groups. Weight loss of at least 7% at month 12 was observed in a greater proportion of patients receiving lurasidone (18.5%) compared with risperidone (6.7%, P<0.001) or quetiapine (9.1%, P=NS; Fig. 3). After 12 months of treatment with lurasidone, 10.2% of patients who were in the normal/underweight BMI category at baseline had shifted to overweight/obese compared with 27.6% of risperidone patients and 15.0% of quetiapine XR patients. Conversely, 14.3% of patients taking lurasidone had shifted from overweight/obese at baseline to normal/underweight at month 12, compared with 1.7% of patients taking risperidone and 7.7% of patients taking quetiapine XR.
Fig. 3.
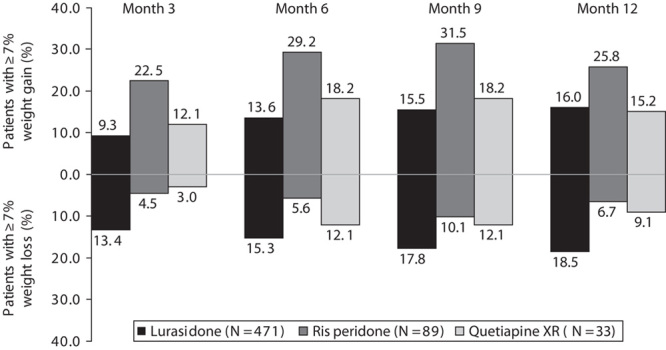
Proportion of patients with at least 7% increase in weight and at least 7% decrease in weight from baseline after 3, 6, 9, and 12 months of treatment with lurasidone (40–160 mg/day), risperidone (2–6 mg/day), or quetiapine XR (200–800 mg/day). *P<0.0001 versus risperidone.
Among lurasidone-treated patients, the CART analysis found no significant effect of age, sex, or duration of illness on the proportion of patients with at least 7% weight gain at month 12, but Asian patients were significantly more likely to experience weight gain than patients of other races (Fig. 4). After 12 months of treatment with lurasidone, weight gain of at least 7% was observed in 12.4% of whites, 12.5% of blacks, and 21.2% of Asians (28.8% of Asian women and 16.8% of Asian men). In the stepwise regression analysis, age and baseline weight were found to be significant predictors of weight gain at month 12; younger age (P=0.040) and lower baseline body weight (P=0.002) were associated with greater weight gain.
Fig. 4.
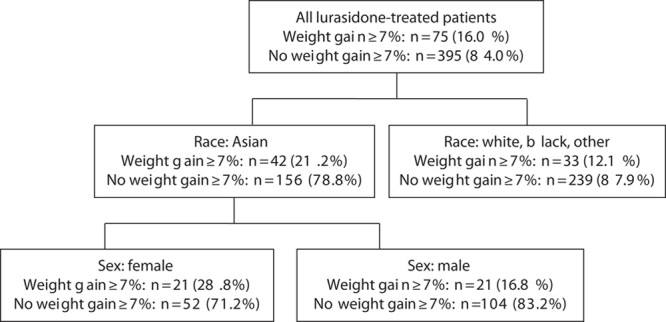
Classification and regression tree analysis of weight gain of at least 7% at month 12 in patients treated with lurasidone (40–160 mg/day).
Discussion
Treatment with lurasidone was associated with a low potential for clinically significant weight gain over a 12-month period in this observed-case analysis of pooled data from six longer-term clinical trials in patients with schizophrenia. Treatment with risperidone and quetiapine XR was associated with modest weight gain (a mean change at month 12 of +2.6 kg and +1.2 kg, respectively). At month 12, a similar proportion of patients treated with lurasidone (16.0%) and quetiapine XR (15.2%) experienced weight gain of at least 7%; however, weight loss of at least 7% was observed in twice as many patients receiving lurasidone (18.5%) as those receiving quetiapine XR (9.1%). In comparison, the risperidone group had the largest proportion of patients with weight gain of at least 7% at month 12 (25.8%) and the smallest proportion of patients with weight loss of at least 7% (6.7%). We note that the baseline weight was, on average, 8 kg greater in the risperidone group compared with the lurasidone (pooled) and quetiapine XR groups; this may have reduced estimates of weight change associated with risperidone in comparison with lurasidone and quetiapine XR treatment. Patients treated with lurasidone for 12 months were more likely to experience a shift from a higher BMI category (overweight/obese) to a lower BMI category (normal/underweight) compared with patients treated with risperidone or quetiapine XR.
Results of this analysis suggest that lurasidone has a lower liability for long-term weight gain than several other atypical antipsychotic agents, which is consistent with previous pooled analyses of short-term studies on lurasidone in patients with schizophrenia (Citrome, 2012; Sunovion Pharmaceuticals Inc., 2013). Reflecting these findings, a recent comprehensive meta-analysis of short-term clinical studies on 15 antipsychotic agents in patients with schizophrenia found that lurasidone was one of only three agents (along with haloperidol and ziprasidone) that did not produce more weight gain than placebo (Leucht et al., 2013).
Overweight and obesity not only increase the risk for metabolic syndrome, diabetes, and cardiovascular disease (Nguyen et al., 2008, 2010; Mitchell et al., 2013b) but may have additional negative consequences in patients with schizophrenia, including decreased quality of life, increased stigma and social discrimination, and reduced adherence to antipsychotic treatment (Weiden et al., 2004b; Kolotkin et al., 2008; Monteleone et al., 2009). Weight gain has been shown to be a predictor of medication nonadherence in patients with schizophrenia (Weiden et al., 2004b; Velligan et al., 2009; Wong et al., 2011), despite the increased risk of relapse associated with discontinuing treatment (Perkins, 2002; Weiden et al., 2004a).
Younger, treatment-naive patients and patients belonging to certain racial and ethnic groups (e.g. black and Hispanic patients) appear to be at greater risk for antipsychotic-related weight gain (Meyer et al., 2009; Salimi et al., 2009; Maayan and Correll, 2011). In the present analysis, younger age was significantly associated with weight gain during lurasidone treatment; however, weight gain of at least 7% was observed in a similar proportion of white patients and black patients taking lurasidone for 12 months. Asian patients (particularly Asian women) were significantly more likely than patients of other races to experience at least 7% weight gain, which is consistent with previous reports that Asian patients may be more likely to experience adverse effects of antipsychotic medications (Bond, 1991). Genetic factors may account for differences in medication-related weight gain based on race or ethnicity (Lett et al., 2012). A recent meta-analysis found a significant association between the leptin −2548A allele and the risk for antipsychotic-induced weight gain in Asian patients with schizophrenia (Shen et al., 2014).
The development of antipsychotic agents with lower metabolic risk, an increased clinical awareness of antipsychotic-related metabolic problems, and the incorporation of physical health management concerns into treatment guidelines (American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity, 2004; De Hert et al., 2009a) have provided greater impetus for clinicians to consider switching medication to reduce the likelihood of metabolic adverse effects. Studies investigating the effects of a medication switch have shown that switching patients to atypical antipsychotic agents with lower metabolic liability (e.g. aripiprazole, lurasidone, or ziprasidone) generally resulted in weight loss and improvement in metabolic indices, without compromising treatment efficacy (Casey et al., 2003; Weiden et al., 2003a, 2003b, 2008; Newcomer et al., 2008; Alptekin et al., 2009; Barak and Aizenberg, 2011; Ganguli et al., 2011; Stroup et al., 2011; Stahl et al., 2013). In an open-label study of patients with schizophrenia or schizoaffective disorder who were transitioned to lurasidone from other antipsychotic agents, improvement was observed in weight and metabolic parameters after 6 weeks (McEvoy et al., 2013) and 6 months (Citrome et al., 2014) of treatment. Similarly, patients with schizophrenia who were treated with open-label lurasidone for up to 6 months after completing 6 weeks of double-blind treatment with olanzapine experienced a mean weight loss of 1.9 kg after switching to lurasidone, with sustained therapeutic efficacy (Stahl et al., 2013).
This analysis has several limitations, including its post-hoc nature and the lack of a placebo control group. This observed-case analysis included only patients who completed 12 months of treatment. The number of patients treated with quetiapine XR for 12 months (N=33) was relatively small compared with the other treatment groups. Between-group differences in dropout rates could have influenced the magnitude of weight change observed at month 12, to the extent that treatment discontinuation was associated with weight change; however, the proportion of patients in the underlying studies who discontinued study medication was similar for lurasidone, risperidone, and quetiapine XR. In addition, detailed information was not collected with regard to prior antipsychotic treatment (e.g. dose, duration of treatment, impact on weight). Thus, the magnitude of antipsychotic-induced weight gain that may have occurred before the patients’ enrollment in the studies included in this analysis is unknown. The effect of antipsychotic medication dose on weight gain was not evaluated in this analysis because five of six longer-term studies used flexible dosing.
In summary, the results of this post-hoc, pooled analysis of 593 patients with schizophrenia who completed 12 months of study treatment suggest that lurasidone, in the dose range of 40–160 mg/day, is associated with a low potential for long-term weight gain. Consistent with prior reports, Asian patients appeared to be more susceptible to weight gain during antipsychotic therapy. The findings of this analysis suggest that lurasidone may be an important treatment option for patients judged to be at higher risk for weight gain, those who present with cardiovascular risk factors, or those who have experienced weight gain or metabolic disturbances on their current treatment regimen. Regular monitoring of metabolic parameters (including weight, lipid levels, and glycemic control) is now considered the standard of care for patients with schizophrenia and may help limit the effects of long-term antipsychotic treatment on weight and metabolic parameters.
Acknowledgements
Nancy Holland, PhD, Synchrony Medical Communications, LLC, provided medical writing and editorial assistance for this manuscript under the direction of the authors. Financial support for this writing and editorial assistance from Synchrony Medical Communications, LLC, were provided by Sunovion Pharmaceuticals Inc., Marlborough, Massachusetts, USA. Clinical research was sponsored by Sunovion Pharmaceuticals Inc. The sponsor was involved in the study design, data collection, and analysis of data.
Conflicts of interest
The studies and this analysis were sponsored by Sumitomo Dainippon Pharma Co. Ltd., Osaka, Japan, and Sunovion Pharmaceuticals Inc., Marlborough, Massachusetts, USA, a wholly-owned US subsidiary of Sumitomo Dainippon Pharma Co Ltd. Dr Meyer reports having received research support from Bristol-Myers Squibb, National Institutes of Health (as General Clinical Research Center support), National Institute of Mental Health, and Pfizer Inc., and speaking or advising fees from Alkermes, Arbor Scientia, Bristol-Myers Squibb Company, Forum Pharmaceuticals Inc., Genentech, Neuroscience Education Institute, Otsuka America Inc., and Sunovion Pharmaceuticals Inc. Drs Mao, Pikalov, Cucchiaro, and Loebel are employees of Sunovion Pharmaceuticals Inc.
Footnotes
ClinicalTrials.gov identifiers: NCT00088621 (Study D1050199); NCT00549718 (Study D1050229); NCT00641745 (Study D1050237); NCT00789698 (Study D1050234). FDAAA 801 registration requirements did not apply for Study D1001036 and Study D1001048.
Portions of this manuscript were presented at the annual meeting of the American Psychiatric Association, 18–22 May 2013, San Francisco, California, USA; the annual meeting of the New Clinical Drug Evaluation Unit, 28–31 May 2013, Hollywood, Florida, USA; the World Congress of Biological Psychiatry, 23–27 June 2013, Kyoto, Japan; the annual meeting of the European Congress of Psychiatry, 1–4 March 2014, Munich, Germany; and the biennial conference of the Schizophrenia International Research Society, 5–9 April 2014, Florence, Italy.
References
- Alptekin K, Hafez J, Brook S, Akkaya C, Tzebelikos E, Ucok A, et al. (2009). Efficacy and tolerability of switching to ziprasidone from olanzapine, risperidone or haloperidol: an international, multicenter study. Int Clin Psychopharmacol 24:229–238. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity (2004). Consensus development conference on antipsychotic drugs and obesity and diabetes. J Clin Psychiatry 65:267–272. [DOI] [PubMed] [Google Scholar]
- Barak Y, Aizenberg D. (2011). Switching to aripiprazole as a strategy for weight reduction: a meta-analysis in patients suffering from schizophrenia. J Obes 2011:pii: 898013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond WS. (1991). Ethnicity and psychotropic drugs. Clin Pharm 10:467–470. [PubMed] [Google Scholar]
- Bushe CJ, Slooff CJ, Haddad PM, Karagianis JL. (2012). Weight change from 3-year observational data: findings from the worldwide schizophrenia outpatient health outcomes database. J Clin Psychiatry 73:e749–e755. [DOI] [PubMed] [Google Scholar]
- Casey DE, Carson WH, Saha AR, Liebeskind A, Ali MW, Jody D, Ingenito GG, Aripiprazole Study Group (2003). Switching patients to aripiprazole from other antipsychotic agents: a multicenter randomized study. Psychopharmacology (Berl) 166:391–399. [DOI] [PubMed] [Google Scholar]
- Casey DA, Rodriguez M, Northcott C, Vickar G, Shihabuddin L. (2011). Schizophrenia: medical illness, mortality, and aging. Int J Psychiatry Med 41:245–251. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2015). About BMI for adults (last updated 23 February 2015). Available at: http://www.cdc.gov/healthyweight/assessing/bmi/adult_bmi/index.html. [Accessed 10 June 2015].
- Citrome L. (2012). Lurasidone for the acute treatment of adults with schizophrenia: what is the number needed to treat, number needed to harm, and likelihood to be helped or harmed? Clin Schizophr Relat Psychoses 6:76–85. [DOI] [PubMed] [Google Scholar]
- Citrome L, Cucchiaro J, Sarma K, Phillips D, Silva R, Tsuchiya S, Loebel A. (2012). Long-term safety and tolerability of lurasidone in schizophrenia: a 12-month, double-blind, active-controlled study. Int Clin Psychopharmacol 27:165–176. [DOI] [PubMed] [Google Scholar]
- Citrome L, Weiden PJ, McEvoy JP, Correll CU, Cucchiaro J, Hsu J, et al. (2014). Effectiveness of lurasidone in schizophrenia or schizoaffective patients switched from other antipsychotics: a 6-month, open-label, extension study. CNS Spectr 19:330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll CU, Druss BG, Lombardo I, O’Gorman C, Harnett JP, Sanders KN, et al. (2010). Findings of a U.S. national cardiometabolic screening program among 10,084 psychiatric outpatients. Psychiatr Serv 61:892–898. [DOI] [PubMed] [Google Scholar]
- Csernansky JG, Mahmoud R, Brenner R, Risperidone-USA-79 Study Group (2002). A comparison of risperidone and haloperidol for the prevention of relapse in patients with schizophrenia. N Engl J Med 346:16–22. [DOI] [PubMed] [Google Scholar]
- De Hert M, Dekker JM, Wood D, Kahl KG, Holt RI, Möller HJ. (2009a). Cardiovascular disease and diabetes in people with severe mental illness position statement from the European Psychiatric Association (EPA), supported by the European Association for the Study of Diabetes (EASD) and the European Society of Cardiology (ESC). Eur Psychiatry 24:412–424. [DOI] [PubMed] [Google Scholar]
- De Hert M, Schreurs V, Vancampfort D, Van Winkel R. (2009b). Metabolic syndrome in people with schizophrenia: a review. World Psychiatry 8:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Kit BK, Ogden CL. (2012). Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 307:491–497. [DOI] [PubMed] [Google Scholar]
- Ganguli R, Brar JS, Garbut R, Chang CC, Basu R. (2011). Changes in weight and other metabolic indicators in persons with schizophrenia following a switch to aripiprazole. Clin Schizophr Relat Psychoses 5:75–79. [DOI] [PubMed] [Google Scholar]
- Goff DC, Cather C, Evins AE, Henderson DC, Freudenreich O, Copeland PM, et al. (2005a). Medical morbidity and mortality in schizophrenia: guidelines for psychiatrists. J Clin Psychiatry 66:183–194. Quiz 147, 273–4. [DOI] [PubMed] [Google Scholar]
- Goff DC, Sullivan LM, McEvoy JP, Meyer JM, Nasrallah HA, Daumit GL, et al. (2005b). A comparison of ten-year cardiac risk estimates in schizophrenia patients from the CATIE study and matched controls. Schizophr Res 80:45–53. [DOI] [PubMed] [Google Scholar]
- Henderson DC. (2007). Weight gain with atypical antipsychotics: evidence and insights. J Clin Psychiatry 68 (Suppl 12):18–26. [PubMed] [Google Scholar]
- Ishibashi T, Horisawa T, Tokuda K, Ishiyama T, Ogasa M, Tagashira R, et al. (2010). Pharmacological profile of lurasidone, a novel antipsychotic agent with potent 5-hydroxytryptamine 7 (5-HT7) and 5-HT1A receptor activity. J Pharmacol Exp Ther 334:171–181. [DOI] [PubMed] [Google Scholar]
- Kasper S, Lerman MN, McQuade RD, Saha A, Carson WH, Ali M, et al. (2003). Efficacy and safety of aripiprazole vs. haloperidol for long-term maintenance treatment following acute relapse of schizophrenia. Int J Neuropsychopharmacol 6:325–337. [DOI] [PubMed] [Google Scholar]
- Kolotkin RL, Corey-Lisle PK, Crosby RD, Swanson JM, Tuomari AV, L’italien GJ, Mitchell JE. (2008). Impact of obesity on health-related quality of life in schizophrenia and bipolar disorder. Obesity (Silver Spring) 16:749–754. [DOI] [PubMed] [Google Scholar]
- Kroeze WK, Hufeisen SJ, Popadak BA, Renock SM, Steinberg S, Ernsberger P, et al. (2003). H1-histamine receptor affinity predicts short-term weight gain for typical and atypical antipsychotic drugs. Neuropsychopharmacology 28:519–526. [DOI] [PubMed] [Google Scholar]
- Lett TA, Wallace TJ, Chowdhury NI, Tiwari AK, Kennedy JL, Müller DJ. (2012). Pharmacogenetics of antipsychotic-induced weight gain: review and clinical implications. Mol Psychiatry 17:242–266. [DOI] [PubMed] [Google Scholar]
- Leucht S, Cipriani A, Spineli L, Mavridis D, Orey D, Richter F, et al. (2013). Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet 382:951–962. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, et al. , Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators (2005). Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 353:1209–1223. [DOI] [PubMed] [Google Scholar]
- Loebel A, Cucchiaro J, Sarma K, Xu L, Hsu C, Kalali AH, et al. (2013a). Efficacy and safety of lurasidone 80 mg/day and 160 mg/day in the treatment of schizophrenia: a randomized, double-blind, placebo- and active-controlled trial. Schizophr Res 145:101–109. [DOI] [PubMed] [Google Scholar]
- Loebel A, Cucchiaro J, Xu J, Sarma K, Pikalov A, Kane JM. (2013b). Effectiveness of lurasidone vs. quetiapine XR for relapse prevention in schizophrenia: a 12-month, double-blind, noninferiority study. Schizophr Res 147:95–102. [DOI] [PubMed] [Google Scholar]
- Maayan L, Correll CU. (2011). Weight gain and metabolic risks associated with antipsychotic medications in children and adolescents. J Child Adolesc Psychopharmacol 21:517–535. [DOI] [PubMed] [Google Scholar]
- McElroy SL. (2009). Obesity in patients with severe mental illness: overview and management. J Clin Psychiatry 70 Suppl 3:12–21. [DOI] [PubMed] [Google Scholar]
- McEvoy JP, Meyer JM, Goff DC, Nasrallah HA, Davis SM, Sullivan L, et al. (2005). Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res 80:19–32. [DOI] [PubMed] [Google Scholar]
- McEvoy JP, Citrome L, Hernandez D, Cucchiaro J, Hsu J, Pikalov A, Loebel A. (2013). Effectiveness of lurasidone in patients with schizophrenia or schizoaffective disorder switched from other antipsychotics: a randomized, 6-week, open-label study. J Clin Psychiatry 74:170–179. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Bobo WV, Lee MA, Cola P, Jayathilake K. (2010). A randomized trial comparing clozapine and typical neuroleptic drugs in non-treatment-resistant schizophrenia. Psychiatry Res 177:286–293. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Cucchiaro J, Silva R, Ogasa M, Phillips D, Xu J, et al. (2011). Lurasidone in the treatment of schizophrenia: a randomized, double-blind, placebo- and olanzapine-controlled study. Am J Psychiatry 168:957–967. [DOI] [PubMed] [Google Scholar]
- Meyer JM, Rosenblatt LC, Kim E, Baker RA, Whitehead R. (2009). The moderating impact of ethnicity on metabolic outcomes during treatment with olanzapine and aripiprazole in patients with schizophrenia. J Clin Psychiatry 70:318–325. [DOI] [PubMed] [Google Scholar]
- Mitchell AJ, Vancampfort D, De Herdt A, Yu W, De Hert M. (2013a). Is the prevalence of metabolic syndrome and metabolic abnormalities increased in early schizophrenia? A comparative meta-analysis of first episode, untreated and treated patients. Schizophr Bull 39:295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AJ, Vancampfort D, Sweers K, van Winkel R, Yu W, De Hert M. (2013b). Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders – a systematic review and meta-analysis. Schizophr Bull 39:306–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteleone P, Martiadis V, Maj M. (2009). Management of schizophrenia with obesity, metabolic, and endocrinological disorders. Psychiatr Clin North Am 32:775–794. [DOI] [PubMed] [Google Scholar]
- Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. (1999). The disease burden associated with overweight and obesity. JAMA 282:1523–1529. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Ogasa M, Guarino J, Phillips D, Severs J, Cucchiaro J, Loebel A. (2009). Lurasidone in the treatment of acute schizophrenia: a double-blind, placebo-controlled trial. J Clin Psychiatry 70:829–836. [DOI] [PubMed] [Google Scholar]
- Nasrallah HA, Silva R, Phillips D, Cucchiaro J, Hsu J, Xu J, Loebel A. (2013). Lurasidone for the treatment of acutely psychotic patients with schizophrenia: a 6-week, randomized, placebo-controlled study. J Psychiatr Res 47:670–677. [DOI] [PubMed] [Google Scholar]
- Newcomer JW. (2005). Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs 19 (Suppl 1):1–93. [DOI] [PubMed] [Google Scholar]
- Newcomer JW. (2007). Metabolic considerations in the use of antipsychotic medications: a review of recent evidence. J Clin Psychiatry 68 (Suppl 1):20–27. [PubMed] [Google Scholar]
- Newcomer JW, Campos JA, Marcus RN, Breder C, Berman RM, Kerselaers W, et al. (2008). A multicenter, randomized, double-blind study of the effects of aripiprazole in overweight subjects with schizophrenia or schizoaffective disorder switched from olanzapine. J Clin Psychiatry 69:1046–1056. [DOI] [PubMed] [Google Scholar]
- Nguyen NT, Magno CP, Lane KT, Hinojosa MW, Lane JS. (2008). Association of hypertension, diabetes, dyslipidemia, and metabolic syndrome with obesity: findings from the National Health and Nutrition Examination Survey, 1999 to 2004. J Am Coll Surg 207:928–934. [DOI] [PubMed] [Google Scholar]
- Nguyen NT, Nguyen XM, Wooldridge JB, Slone JA, Lane JS. (2010). Association of obesity with risk of coronary heart disease: findings from the National Health and Nutrition Examination Survey, 1999–2006. Surg Obes Relat Dis 6:465–469. [DOI] [PubMed] [Google Scholar]
- Ogasa M, Kimura T, Nakamura M, Guarino J. (2013). Lurasidone in the treatment of schizophrenia: a 6-week, placebo-controlled study. Psychopharmacology (Berl) 225:519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. (2012). Prevalence of obesity in the United States, 2009–2010. NCHS Data Brief 1–8. [PubMed] [Google Scholar]
- Pappadopulos E, Newcomer JW, Kolluri S. (2012). Changes in weight, plasma lipids, and glucose in adults treated with ziprasidone: a comprehensive analysis of Pfizer-initiated clinical trials. J Clin Psychiatry 73:e742–e748. [DOI] [PubMed] [Google Scholar]
- Perkins DO. (2002). Predictors of noncompliance in patients with schizophrenia. J Clin Psychiatry 63:1121–1128. [DOI] [PubMed] [Google Scholar]
- Salimi K, Jarskog LF, Lieberman JA. (2009). Antipsychotic drugs for first-episode schizophrenia: a comparative review. CNS Drugs 23:837–855. [DOI] [PubMed] [Google Scholar]
- Shen J, Ge W, Zhang J, Zhu HJ, Fang Y. (2014). Leptin -2548g/a gene polymorphism in association with antipsychotic-induced weight gain: a meta-analysis study. Psychiatr Danub 26:145–151. [PubMed] [Google Scholar]
- Stahl SM, Cucchiaro J, Simonelli D, Hsu J, Pikalov A, Loebel A. (2013). Effectiveness of lurasidone for patients with schizophrenia following 6 weeks of acute treatment with lurasidone, olanzapine, or placebo: a 6-month, open-label, extension study. J Clin Psychiatry 74:507–515. [DOI] [PubMed] [Google Scholar]
- Stroup TS, McEvoy JP, Ring KD, Hamer RH, LaVange LM, Swartz MS, et al. (2011). A randomized trial examining the effectiveness of switching from olanzapine, quetiapine, or risperidone to aripiprazole to reduce metabolic risk: comparison of antipsychotics for metabolic problems (CAMP). Am J Psychiatry 168:947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunovion Pharmaceuticals Inc. (2013). Latuda® (lurasidone hydrochloride) tablets, for oral use [package insert]. Marlborough, MA: Sunovion Pharmaceuticals Inc. [Google Scholar]
- Velligan DI, Weiden PJ, Sajatovic M, Scott J, Carpenter D, Ross R, Docherty JP, Expert Consensus Panel on Adherence Problems in Serious and Persistent Mental Illness (2009). The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry 70 (Suppl 4):1–46. [PubMed] [Google Scholar]
- Weiden PJ, Daniel DG, Simpson G, Romano SJ. (2003a). Improvement in indices of health status in outpatients with schizophrenia switched to ziprasidone. J Clin Psychopharmacol 23:595–600. [DOI] [PubMed] [Google Scholar]
- Weiden PJ, Simpson GM, Potkin SG, O’Sullivan RL. (2003b). Effectiveness of switching to ziprasidone for stable but symptomatic outpatients with schizophrenia. J Clin Psychiatry 64:580–588. [DOI] [PubMed] [Google Scholar]
- Weiden PJ, Kozma C, Grogg A, Locklear J. (2004a). Partial compliance and risk of rehospitalization among California Medicaid patients with schizophrenia. Psychiatr Serv 55:886–891. [DOI] [PubMed] [Google Scholar]
- Weiden PJ, Mackell JA, McDonnell DD. (2004b). Obesity as a risk factor for antipsychotic noncompliance. Schizophr Res 66:51–57. [DOI] [PubMed] [Google Scholar]
- Weiden PJ, Newcomer JW, Loebel AD, Yang R, Lebovitz HE. (2008). Long-term changes in weight and plasma lipids during maintenance treatment with ziprasidone. Neuropsychopharmacology 33:985–994. [DOI] [PubMed] [Google Scholar]
- Wong MM, Chen EY, Lui SS, Tso S. (2011). Medication adherence and subjective weight perception in patients with first-episode psychotic disorder. Clin Schizophr Relat Psychoses 5:135–141. [DOI] [PubMed] [Google Scholar]


