Abstract
Patient satisfaction with treatment is an important clinical index associated with the efficacy and adherence of treatment in schizophrenia. Although switching from oral antipsychotics to the long-acting injectable formulation may improve convenience, patient satisfaction has not been studied extensively. We carried out a 21-week, multicenter, randomized, open-label comparative study. A total of 154 patients with schizophrenia unsatisfied with current oral atypical antipsychotics were assigned randomly to either immediate or delayed switching to paliperidone palmitate, the long-acting injectable formulation of paliperidone. The Medication Satisfaction Questionnaire (MSQ) and the Treatment Satisfaction Questionnaire for Medication (TSQM) were used to evaluate patient satisfaction with treatment, whereas the Positive and Negative Syndrome Scale (PANSS) and the Personal and Social Performance (PSP) scale were used to evaluate efficacy. From baseline to the final assessment, the MSQ score increased significantly in both groups, and the increase was greatest after the first administration of paliperidone palmitate in the immediate switch group. The scores of TSQM effectiveness, convenience, and global satisfaction as well as the PSP total score increased significantly, whereas the PANSS total score decreased significantly in both groups. The immediate switch group showed a significant improvement in the TSQM convenience score compared with the delayed switch group on oral antipsychotics during the comparison period. Most adverse events were minor and tolerable. In short, switching from oral atypical antipsychotics to paliperidone palmitate because of poor satisfaction significantly improved patient satisfaction, with comparable efficacy and tolerability.
Keywords: atypical antipsychotics, long-acting injectable antipsychotics, Medication Satisfaction Questionnaire, paliperidone palmitate, schizophrenia, Treatment Satisfaction Questionnaire for Medication
Introduction
In recent years, both the research and the practice of management of schizophrenia have focused on the influence of patients’ subjective satisfaction with treatment on the clinical outcome. This is in line with the increased recognition of the patients’ right as a consumer of medical service to participate in the decision-making process of therapy (Awad and Voruganti, 2013) as well as reports on the association between patients’ subjective assessment and clinical outcomes of schizophrenia such as treatment adherence (Gharabawi et al., 2006). Medication adherence among patients using atypical antipsychotics may be higher than that in patients using typical antipsychotics in the short term, but this difference is no longer significant in the long term (Dolder et al., 2002). It was also found that about 95% of the patients could not keep up with the daily oral dosing schedule (Mahmoud et al., 1997). A recent study comparing medication satisfaction also found no significant difference in convenience between patients on typical antipsychotics and those on atypical antipsychotics (Fujikawa et al., 2008). These results indicate that the adherence to oral medications is still a challenge in the management of schizophrenia. Schizophrenic patients with a high adherence to treatment generally show symptom improvement, a lower rate of readmission, and a higher chance of finding a job. In contrast, nonadherent patients have a 3.7 times higher risk of recurrence than adherent patients (Fenton et al., 1997). It is noteworthy that high patient satisfaction with current oral atypical antipsychotics was reported to be associated with a lower rate of treatment discontinuation (Gharabawi et al., 2006). Moreover, the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study found that an antipsychotic medication was most frequently discontinued because of the patients’ own choice (29.9%) (Lieberman et al., 2005).
Patients’ subjective satisfaction with treatment is also associated with the efficacy of treatment and can be considered an indicator of the treatment’s success. Patient satisfaction with antipsychotic medication positively correlates with symptomatic improvement as assessed by the Positive and Negative Syndrome Scale (PANSS), improved community functioning (Mohamed et al., 2009), better quality of life (Hofer et al., 2004; Mohamed et al., 2009), as well as adherence. There are varying factors affecting patient satisfaction with antipsychotic medications, such as their expectations of efficacy, subjective experience, and side effects (Kalali, 1999; Rofail et al., 2005). Therefore, patient satisfaction with medication is a patient-centered indicator and an important clinical factor that influences the symptomatic effect, tolerance, efficacy, and convenience of treatment (Kane, 2001; Atkinson et al., 2004; Awad and Voruganti, 2013).
Meanwhile, the long-acting injection is expected to improve medication convenience with lower administration frequency as opposed to daily administered oral drugs. The long-acting injection also provides a more stable blood concentration and a better tolerability profile (Ereshefsky, 1999), ruling out gastrointestinal malabsorption or the first-pass effect through the liver. However, there are limited data on the association of long-acting injectable risperidone with improved medication satisfaction during the switch from oral antipsychotics (Lindenmayer et al., 2005) and the burden of receiving shots every other week may have worked as a limitation of the injectable formulation (Park et al., 2009). In contrast, paliperidone palmitate is an ester prodrug of paliperidone, an oral atypical antipsychotic with dopamine type 2 (D2) and serotonin (5-hydroxytryptamine 2A) receptor antagonism. Paliperidone palmitate was developed as a long-acting drug that can be administered intramuscularly once a month, without the need for an early oral supplementation (Gopal et al., 2011; Samtani et al., 2011). Randomized, double-blind clinical studies have reported the effectiveness, tolerability, and efficacy of paliperidone palmitate in patients with acute schizophrenia (Hough et al., 2010; Pandina et al., 2010; Hargarter et al., 2014).
In this light, paliperidone palmitate can be expected to overcome the limitation of existing oral atypical antipsychotics in terms of effectiveness, tolerance, efficacy, and convenience as well as to enhance patient satisfaction. So far, no well-designed studies have examined these aspects of paliperidone palmitate. In this study, schizophrenic patients unsatisfied with current oral atypical antipsychotics were randomized either to the immediate switch group or to the delayed switch group. Patients of the immediate switch group were switched immediately to paliperidone palmitate and those assigned to the delayed switch group maintained the current drug for 8 weeks before switching to paliperidone palmitate. In other words, patients of the delayed group maintained current atypical oral drugs while attempting dose adjustment or addition of another concomitant medication to enhance their satisfaction. Patients in the immediate switch group were switched to paliperidone palmitate upon enrollment. We compared the clinical outcomes between the two groups to determine the necessity of active, immediate intervention.
Materials and methods
This multicenter randomized open-label comparative study was carried out at 16 centers in Korea. The trial was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice. The study protocol was approved by the institutional review board and/or the independent ethics committee of each center. All participants provided informed consent after the study procedures had been fully explained.
Participants
Eligible participants were patients (men and women 20–65 years of age) with a clinical diagnosis of schizophrenia according to the Diagnostic and Statistical Manual of Mental Disorders Version IV (DSM-IV), who were unsatisfied with the current treatment of atypical antipsychotics. Enrolled patients fulfilled all three main inclusion criteria:
Had been on continuous oral medication with an identical atypical antipsychotic for the previous 4 weeks before screening.
Reported lack of satisfaction with current medication as measured by score 4 or less (neither dissatisfied nor satisfied) on the Medication Satisfaction Questionnaire (MSQ, a Likert seven-point scale with a single question, ‘Overall, how satisfied are you with your current medication?’).
Deemed by the investigator to potentially benefit from switching treatment in terms of symptom improvement or tolerability.
Patients were excluded from participating in the study if they satisfied at least one of the following exclusion criteria:
Diagnosis of a primary, active DSM-IV Axis I other than schizophrenia.
Known or suspected allergy, hypersensitivity, or intolerance to risperidone, paliperidone, or any of their excipients.
Risk of suicide.
Clozapine use within 60 days before screening.
Use of a long-acting injection, including paliperidone palmitate and risperidone, at least once within 90 days before screening.
History of neuroleptic malignant syndrome.
Pregnant or breast-feeding women.
History of congenital long QT syndrome or cardiac arrhythmia or currently taking drugs that may cause prolonged QT interval.
Interventions
Patients were randomized either to the immediate switch group or to the delayed switch group. Randomization was performed using randomly permuted blocks, with four patients per block, to ensure balanced treatment allocation. Patients with no history of oral paliperidone or oral or injectable risperidone were recommended to use oral paliperidone (3 mg/day) or risperidone (1 mg/day) for at least 3 days during the screening phase for tolerability. An overview of essential study procedures is presented in Fig. 1. Those assigned to the immediate switch group received paliperidone palmitate six times in 120 days. Paliperidone palmitate 150 and 100 mg eq. were administered on Day 1 (Visit 2) and Day 8 (Visit 3) as loading doses, respectively. Paliperidone palmitate 75 mg eq. was recommended for later visits, or 25, 50, 75, 100, or 150 mg eq. were administered at the investigator’s discretion. However, those assigned to the delayed switch group maintained the current oral atypical antipsychotics from Day 1 to Day 56 and received paliperidone palmitate four times from Day 57 to Day 120. Paliperidone palmitate 150 and 100 mg eq. were administered on Day 57 (Visit 5) and Day 64 (Visit 6) as loading doses, respectively. Paliperidone palmitate 75 mg eq. was recommended in later visits, or 25, 50, 75, 100, or 150 mg eq. were administered at the investigator’s discretion. The investigator or the attending physician at each study site administered paliperidone palmitate and recorded the dosing history of each patient. Drug supplies were inventoried and accounted for throughout the study period.
Fig. 1.
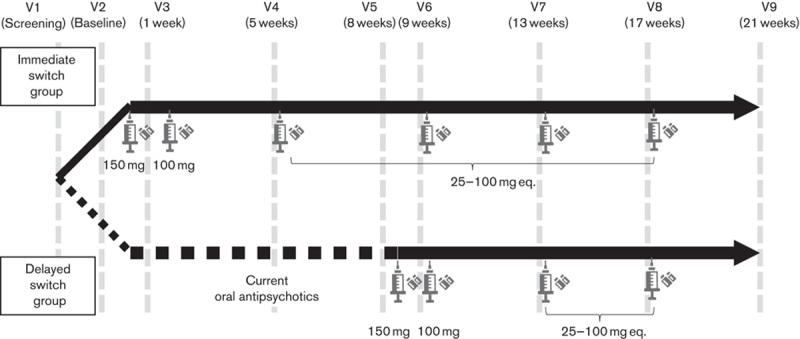
Summary of the study procedure.
The following medications could be used concomitantly with paliperidone palmitate: oral antipsychotics or paliperidone extended-release used as needed in less than 14 consecutive days or 30 days in total during the study; newly started benzodiazepines used as needed up to 6 mg/day; oral lorazepam, in less than 10 consecutive days or 20 days in total during the study; and newly started mood stabilizers, antidepressants (excluding monoamine oxidase inhibitors), β-blockers, sedative-hypnotics, and anticholinergic antiparkinsonian medications were used within allowed dose ranges, but were discouraged from regular prescription, if possible. A new psychotherapy could not be started during the study.
Outcomes
In this study, the MSQ was used to measure medication satisfaction (Potkin et al., 2006; Canuso et al., 2010) and was assessed at baseline and every visit.
The following were assessed at the same time points as the MSQ: Treatment Satisfaction Questionnaire for Medication (TSQM) (Atkinson et al., 2004), PANSS, and Personal and Social Performance (PSP) scale (Nasrallah et al., 2008). Safety evaluation included the regular reporting of adverse events and vital signs. Results of the laboratory tests, pregnancy test, physical examination, and ECG were recorded at screening and the final visit (or at premature discontinuation).
Statistical analysis
Efficacy was analyzed in the per-protocol analysis set and the full analysis set. The full analysis set included patients who were administered with the study drug or oral atypical drug at least once, did not violate the inclusion/exclusion criteria, and underwent MSQ assessment at baseline and at least once after that. The per-protocol analysis set included patients who did not violate the inclusion/exclusion criteria among those in the full analysis set and whose MSQ score was adjusted for missing data for the primary efficacy evaluation. In addition, the group of patients who completed the study without a protocol violation was defined as the strict per-protocol set. Safety analysis and demographic data were analyzed in the safety analysis set, which included all patients who received the study drug at least once. For efficacy variables with missing values after randomization, the last-observation-carried-forward imputation method was applied.
The primary efficacy endpoint was the change in the MSQ score from baseline to endpoint collected from all participants in two switch groups. Changes in the primary and secondary endpoints from baseline were analyzed for within-group and between-group comparisons. The paired t-test was used for within-group comparisons. Between-group comparisons including the change in scores were performed using analysis of covariance with separate terms in the model with baseline characteristics and treatment (i.e. MSQ, TSQM domain scores for effectiveness, side effects, convenience, and global satisfaction) included as covariates. In addition, the MSQ score per visit was divided into unsatisfied (score 1–4, including participants who were neither satisfied nor dissatisfied) and satisfied (score 5–7), and analyzed as a categorical variable using Fisher’s exact test. The statistical significance of differences between treatment groups in demographic and baseline characteristics was assessed using analysis of variance for continuous variables and the Cochran–Mantel–Haenszel test for association or the nonparametric Fisher’s exact test for discrete variables, as applicable. All statistical tests were performed at the two-sided 0.05. No adjustment was made for multiplicity.
Results
Baseline demographics, clinical characteristics, and patient disposition
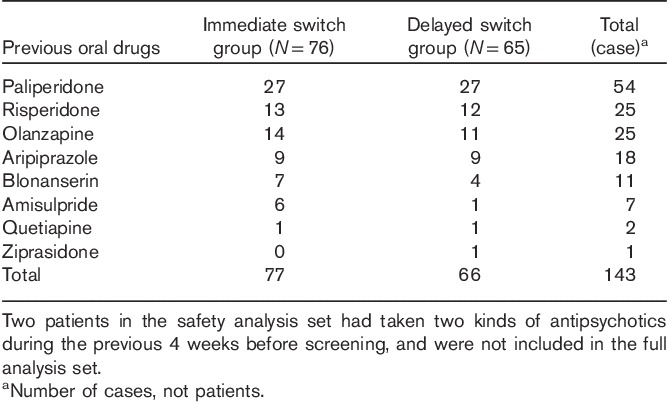
One hundred and seventy participants were screened, among whom 154 were randomized. A total of 134 patients were included in the full analysis set [immediate switch group (N=67), delayed switch group (N=67)]; 126 patients were included in the per-protocol set (eight were excluded for protocol violation) [immediate switch group (N=63), delayed switch group (N=63)]; and 85 patients were defined as the strict per-protocol set (23 patients were excluded for consent withdrawal, seven for adverse events, eight for lack of efficacy, and three were lost to follow-up) [immediate switch group (N=43), delayed switch group (N=42)] (Fig. 2). When all patients who received the study drug at least once (safety analysis set; N=141) were compared, there was no statistically significant difference between the immediate switch group (N=76) and the delayed switch group (N=65) in terms of sex, age at study participation, BMI, and distribution of the DSM-IV schizophrenia diagnosis subtype (Table 1). Contrary to our expectation, the duration of schizophrenia showed a statistically significant difference between the immediate and the delayed switch groups (P=0.0379), with the P-value in full analysis set being 0.0519. Previous oral atypical antipsychotics taken within 4 weeks before screening by 141 patients in safety analysis set were investigated (Table 2). Two patients of the safety analysis set had taken two kinds of antipsychotics during this period and were not included in the full analysis set. Among previous oral atypical antipsychotics, paliperidone was the most commonly used in 54 patients, followed by risperidone in 25 patients, olanzapine in 25 patients, aripiprazole in 18 patients, blonanserin in 11 patients, amisulpride in seven patients, and quetiapine in two patients and ziprasidone in one patient.
Fig. 2.
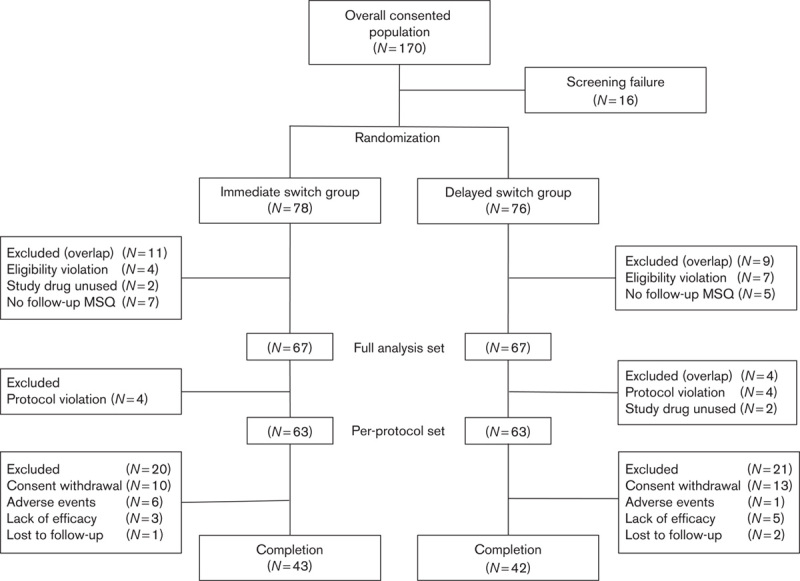
Patients’ disposition.
Table 1.
Baseline demographics and clinical characteristics (safety analysis set)
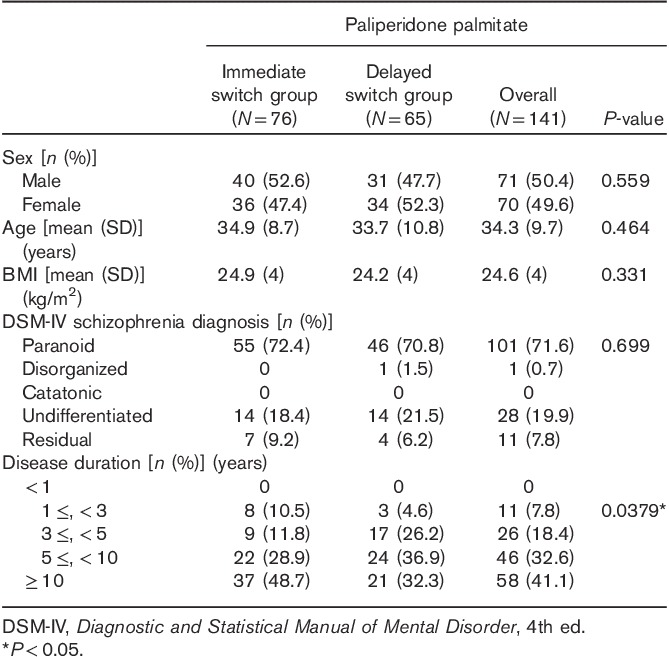
Table 2.
Previous oral drugs (safety analysis set)
Primary efficacy endpoint
In this study, the primary efficacy endpoint was the change in the MSQ score from baseline (Visit 2) to the final assessment (Visit 9). The MSQ score of the full analysis set (N=134) increased by 0.88 (±1.33) [mean (±SD)] points (P<0.0001), indicating a statistically significant change [0.99 (±1.38) in the immediate switch group vs. 0.78 (±1.29) in the delayed switch group, both P<0.0001]. The MSQ score of the per-protocol set (N=126) increased by 0.92 points (P<0.0001) and that of the strict per-protocol set (N=85) increased by 1.34 points (P<0.0001), both indicating a statistically significant change.
After switching to paliperidone palmitate, an improvement in the mean MSQ score was observed after the first administration of the study drug in the immediate switch group (from Visit 2 to Visit 3), whereas it was observed after the second administration in the delayed switch group (from Visit 6 to Visit 7) (Figs 3 and 4). The increase in the mean MSQ score was greatest after the first administration of paliperidone palmitate in the immediate switch group (from Visit 2 to Visit 3), whereas it was the greatest after the second administration in the delayed switch group (from Visit 6 to Visit 7). Specifically, the increase in the MSQ score was significantly greater from Visit 2 to Visit 5 (administration of paliperidone palmitate in the immediate switch group vs. current oral atypical antipsychotics in the delayed switch group) in the immediate switch group compared with the delayed switch group [0.78 (±1.32) vs. 0.37 (±1.01), respectively, P=0.05]. Interestingly, the mean MSQ score significantly increased both during the adjustment period of oral medication and after the switch to paliperidone palmitate in the delayed switch group [0.37 (±1.01), P=0.036 before switch (from Visit 2 to Visit 5) and 0.40 (±1.24), P=0.01 after switch (from Visit 5 to Visit 9)].
Fig. 3.
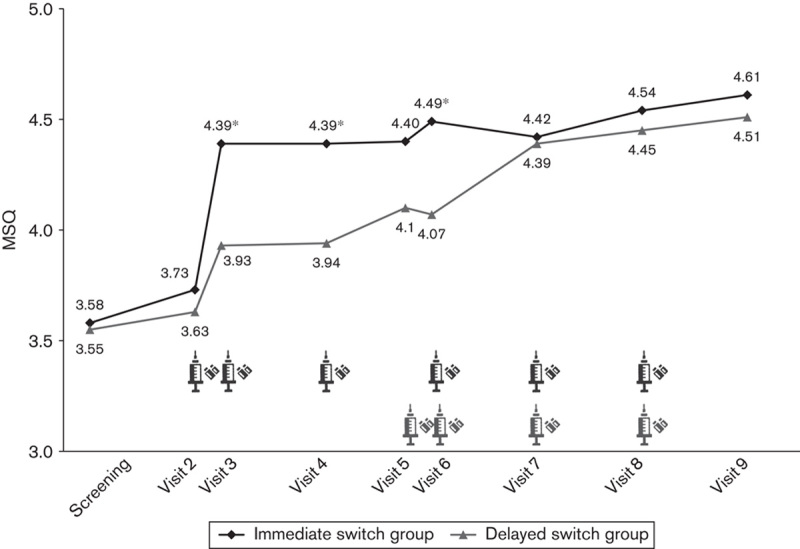
Change in the mean Medication Satisfaction Questionnaire (MSQ) score per visit in the immediate and delayed switch group (full analysis set).
Fig. 4.
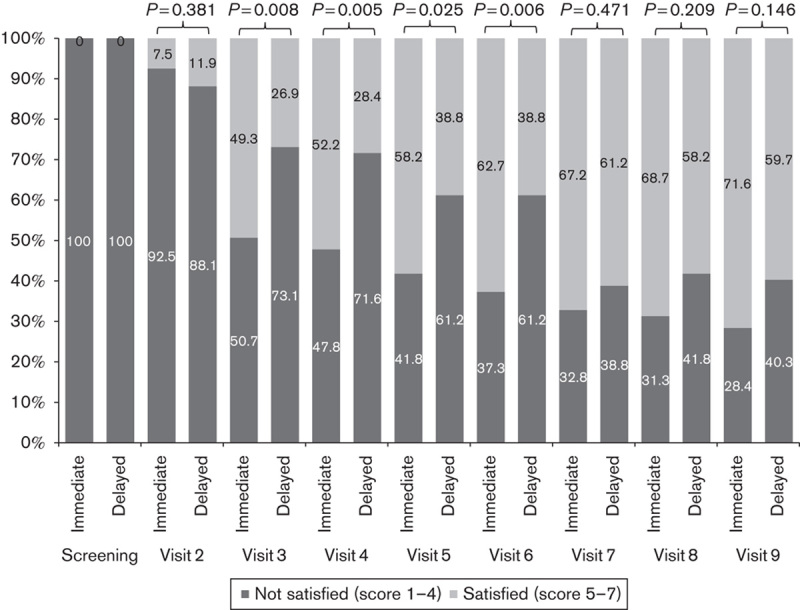
Proportion of unsatisfied group (score 1–4) and satisfied group (score 5–7) on the basis of the Medication Satisfaction Questionnaire (MSQ) score per visit (full analysis set).
Secondary efficacy endpoints
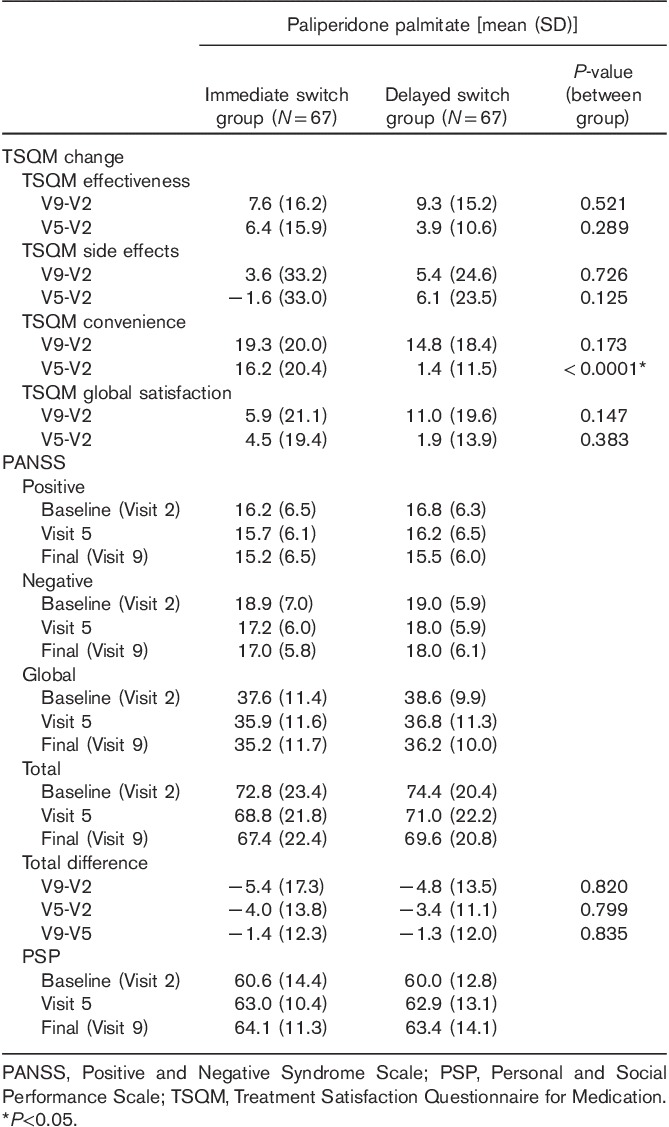
Table 3 shows the changes in the TSQM, PANSS, and PSP scores in the full analysis set. Compared with patients on current oral antipsychotics in the delayed switch group, patients on paliperidone palmitate in the immediate switch group showed a significant improvement in the TSQM convenience score (from Visit 2 to Visit 5). From baseline to endpoint, the scores of TSQM effectiveness, convenience, and global satisfaction increased significantly in both groups [mean change in the immediate switch group: 7.6 (P=0.0003); 19.3 (P<0.0001); 5.9 (P=0.026), mean change in the delayed switch group: 9.3 (P<0.0001); 14.76 (P<0.0001); 11.0 (P<0.0001)]. The scores of TSQM side effects did not change significantly in both groups [mean change in the immediate switch group: 3.6 (P=0.372), mean change in the delayed switch group: 5.4 (P=0.076)]. Compared with baseline, the total score of PANSS at the final assessment decreased by 5.37 (±17.32) points in the immediate switch group (P=0.0135) and by 4.76 (±13.5) in the delayed switch group (P=0.0053), both showing statistically significant changes. The total score of PSP increased by 3.49 (±12.71) points from baseline to the final assessment in the immediate switch group (P=0.0279) and by 3.36 (±10.85) in the delayed switch group (P=0.0137), both showing statistically significant changes. All scores of TSQM and PANSS at three time points (baseline, Visit 5, and endpoint) and the changes of those from baseline to the final assessment did not show any significant differences between the two groups.
Table 3.
Changes in PANSS, TSQM, and PSP scores (full analysis set)
Tolerability
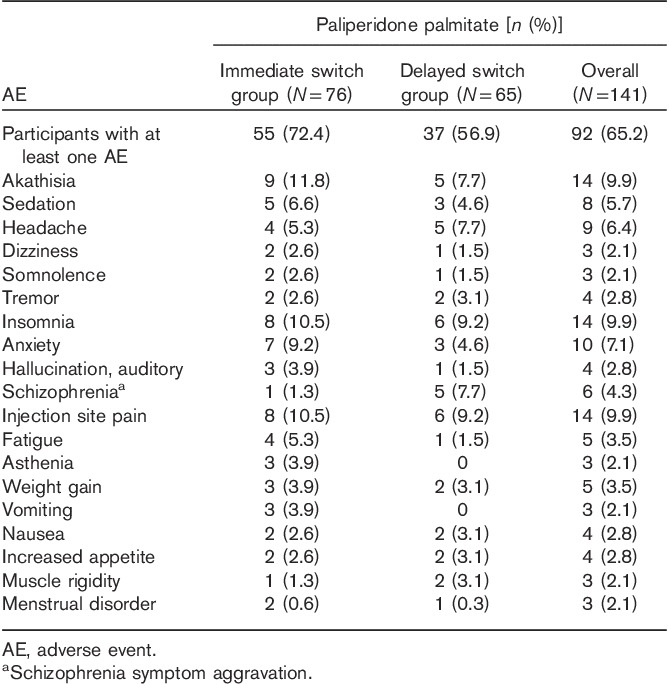
In the safety analysis set (N=141), 65.2% of the patients experienced at least one adverse event during the study (Table 4): 72.4% in the immediate switch group (N=76) versus 56.9% in the delayed switch group (N=65). The most common adverse events for the overall population were akathisia (N=14; 9.9%), insomnia (N=14; 9.9%), and injection site pain (N=9; 6.4%). The most common adverse events in the immediate group were akathisia (N=9; 11.8%), followed by insomnia and injection site pain (N=8; 10.5%, each), anxiety (N=7; 9.2%), sedation (N=5; 6.6%), and headache and fatigue (N=4; 5.3%, each). In the delayed group, insomnia and injection site pain each occurred in six patients (9.2%), and schizophrenia symptom aggravation, headache, and akathisia each occurred in five patients (7.7%). A total of five patients (3.5%) experienced five cases of serious adverse events, among whom three (N=3; 3.9%) were in the immediate switch group and two (N=2; 3.1%) were in the delayed switch group.
Table 4.
Treatment-emergent AEs reported at least 2% in any group (safety analysis set)
Discussion
To the best of our knowledge, this is the first well-designed study to measure change in patient satisfaction associated with switching from atypical oral antipsychotics to long-acting injectable atypical antipsychotics. We observed that switching from unsatisfactory oral atypical medication to paliperidone palmitate significantly increased patient satisfaction, mostly by improvement in medication convenience. The clinical efficacy and tolerability of paliperidone palmitate were comparable to those of oral medication. Taken together, these results suggest that paliperidone palmitate could provide a valuable treatment option for patients with schizophrenia who are not satisfied with their oral antipsychotic medication and in possible danger of nonadherence to treatment.
Recent studies have suggested that treatment satisfaction could be influenced by tolerability, symptomatic efficacy, effectiveness, and convenience (Hough et al., 2010; Gopal et al., 2011; Samtani et al., 2011). The route of administration is an important factor in the treatment of schizophrenia. In terms of medication convenience, long-acting oral antipsychotics are reported to be often favored to short-acting oral antipsychotics (Bitter et al., 2010). In one study comparing haloperidol depot and long-acting risperidone, patients treated with long-acting risperidone showed better results in terms of social activity and satisfaction with life (Mihajlovic et al., 2011). In addition, patients treated with long-acting risperidone showed improvement in the total PANSS score, clinical global impression, global assessment of functioning, and satisfaction (Parellada et al., 2005; Nick et al., 2006) compared with baseline. However, one limitation of long-acting risperidone is that injections are administered every other week. Paliperidone palmitate, in contrast, can be administered once a month. The results of this study showed a statistically significant improvement in the TSQM convenience score in both groups, which lends support to the expectation that paliperidone palmitate may increase patient satisfaction by enhancing the convenience of treatment.
It is noteworthy that noncompliance in schizophrenia is reported to occur in the early phase of treatment. Relapse occurs soon after treatment discontinuation (Emsley et al., 2013) and the most frequent reason for relapse is treatment discontinuation (Robinson et al., 1999). Our results show that paliperidone palmitate would improve treatment adherence as well as patient satisfaction in the early phase, although this study did not examine the timing of switch.
Compared with other studies on patients’ subjective satisfaction, we applied the MSQ and TSQM, which are validated and more sophisticated scales (Atkinson et al., 2004; Potkin et al., 2006; Canuso et al., 2010), and could more accurately evaluate the effects of paliperidone palmitate on patient satisfaction. Furthermore, significant improvements in PANSS and PSP scores from baseline to endpoint provided more support to the improvement in patient satisfaction with treatment.
This study has several limitations. As all patients were assigned to the treatment group without a placebo or a control group, a bias cannot be ruled out. Second, the reason for dissatisfaction of each patient was not taken into account. Further research is needed for a more accurate evaluation of the efficacy, tolerability of paliperidone palmitate and its effects on patient satisfaction.
Acknowledgements
Conflicts of interest
This research was supported by grants from the Janssen Pharmaceuticals, whose role was restricted to providing assistance to the investigators in the conception, conduct, and analysis of this study.
References
- Atkinson MJ, Sinha A, Hass SL, Colman SS, Kumar RN, Brod M, et al. (2004). Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes 2:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad AG, Voruganti LN. (2013). The impact of newer atypical antipsychotics on patient-reported outcomes in schizophrenia. CNS drugs 27:625–636. [DOI] [PubMed] [Google Scholar]
- Bitter I, Treuer T, Dilbaz N, Oyffe I, Ciorabai EM, Gonzalez SL, et al. (2010). Patients’ preference for olanzapine orodispersible tablet compared with conventional oral tablet in a multinational, randomized, crossover study. World J Biol Psychiatry 11:894–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canuso CM, Grinspan A, Kalali A, Damaraju CV, Merriman U, Alphs L, et al. (2010). Medication satisfaction in schizophrenia: a blinded-initiation study of paliperidone extended release in patients suboptimally responsive to risperidone. Int Clin Psychopharmacol 25:155–164. [DOI] [PubMed] [Google Scholar]
- Dolder CR, Lacro JP, Dunn LB, Jeste DV. (2002). Antipsychotic medication adherence: is there a difference between typical and atypical agents? Am J Psychiatry 159:103–108. [DOI] [PubMed] [Google Scholar]
- Emsley R, Chiliza B, Asmal L, Harvey BH. (2013). The nature of relapse in schizophrenia. BMC Psychiatry 13:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ereshefsky L. (1999). Pharmacologic and pharmacokinetic considerations in choosing an antipsychotic. J Clin Psychiatry 60 (Suppl 10):S20–S30. [PubMed] [Google Scholar]
- Fenton WS, Blyler CR, Heinssen RK. (1997). Determinants of medication compliance in schizophrenia: empirical and clinical findings. Schizophr Bull 23:637–651. [DOI] [PubMed] [Google Scholar]
- Fujikawa M, Togo T, Yoshimi A, Fujita J, Nomoto M, Kamijo A, et al. (2008). Evaluation of subjective treatment satisfaction with antipsychotics in schizophrenia patients. Prog Neuropsychopharmacol Biol Psychiatry 32:755–760. [DOI] [PubMed] [Google Scholar]
- Gharabawi GM, Greenspan A, Rupnow MF, Kosik-Gonzalez C, Bossie CA, Zhu Y, et al. (2006). Reduction in psychotic symptoms as a predictor of patient satisfaction with antipsychotic medication in schizophrenia: data from a randomized double-blind trial. BMC Psychiatry 6:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopal S, Vijapurkar U, Lim P, Morozova M, Eerdekens M, Hough D. (2011). A 52-week open-label study of the safety and tolerability of paliperidone palmitate in patients with schizophrenia. J Psychopharmacol 25:685–697. [DOI] [PubMed] [Google Scholar]
- Hargarter L, Cherubin P, Bergmans P, Keim S, Rancans E, Bez Y, et al. (2014). Intramuscular long-acting paliperidone palmitate in acute patients with schizophrenia unsuccessfully treated with oral antipsychotics. Prog Neuropsychopharmacol Biol Psychiatry 58C:1–7. [DOI] [PubMed] [Google Scholar]
- Hofer A, Kemmler G, Eder U, Edlinger M, Hummer M, Fleischhacker WW. (2004). Quality of life in schizophrenia: the impact of psychopathology, attitude toward medication, and side effects. J Clin Psychiatry 65:932–939. [PubMed] [Google Scholar]
- Hough D, Gopal S, Vijapurkar U, Lim P, Morozova M, Eerdekens M. (2010). Paliperidone palmitate maintenance treatment in delaying the time-to-relapse in patients with schizophrenia: a randomized, double-blind, placebo-controlled study. Schizophr Res 116:107–117. [DOI] [PubMed] [Google Scholar]
- Kalali A. (1999). Patient satisfaction with, and acceptability of, atypical antipsychotics. Curr Med Res Opin 15:135–137. [DOI] [PubMed] [Google Scholar]
- Kane J. (2001). Progress defined – short-term efficacy, long-term effectiveness. Int Clin Psychopharmacol 16 (Suppl 1):S1–S8. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, et al. (2005). Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 353:1209–1223. [DOI] [PubMed] [Google Scholar]
- Lindenmayer JP, Jarboe K, Bossie CA, Zhu Y, Mehnert A, Lasser R. (2005). Minimal injection site pain and high patient satisfaction during treatment with long-acting risperidone. Int Clin Psychopharmacol 20:213–221. [DOI] [PubMed] [Google Scholar]
- Mahmoud RA, Englehart LM, Janagap CC, Oster G, Ollendorf D. (1997). Risperidone vs. conventional antipsychotics: a prospective randomized naturalistic effectiveness trial of outcomes in chronic schizophrenia. 36th Annual Meeting of the American College of Neuropsychopharmacology; 8–12 December; Waikoloa, HI.
- Mihajlovic G, Jovanovic-Mihajlovic N, Radmanovic B, Radonjic K, Djukic-Dejanovic S, Jankovic S, et al. (2011). Quality of life of schizophrenic patients treated with haloperidol depot and injection preparation of long-lasting risperidone. Srp Arh Celok Lek 139 (Suppl 1):S36–S40. [PubMed] [Google Scholar]
- Mohamed S, Rosenheck R, McEvoy J, Swartz M, Stroup S, Lieberman JA. (2009). Cross-sectional and longitudinal relationships between insight and attitudes toward medication and clinical outcomes in chronic schizophrenia. Schizophr Bull 35:336–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrallah H, Morosini P, Gagnon DD. (2008). Reliability, validity and ability to detect change of the Personal and Social Performance scale in patients with stable schizophrenia. Psychiatry Res 161:213–224. [DOI] [PubMed] [Google Scholar]
- Nick B, Vauth R, Braendel D, Riecher-Rössler A, The Swiss StoRMi Investigators Group (2006). Symptom control, functioning and satisfaction among Swiss patients treated with risperidone long-acting injectable. Int J Psychiatry Clin Pract 10:174–181. [DOI] [PubMed] [Google Scholar]
- Pandina GJ, Lindenmayer JP, Lull J, Lim P, Gopal S, Herben V, et al. (2010). A randomized, placebo-controlled study to assess the efficacy and safety of 3 doses of paliperidone palmitate in adults with acutely exacerbated schizophrenia. J Clin Psychopharmacol 30:235–244. [DOI] [PubMed] [Google Scholar]
- Parellada E, Andrezina R, Milanova V, Glue P, Masiak M, Turner MS, et al. (2005). Patients in the early phases of schizophrenia and schizoaffective disorders effectively treated with risperidone long-acting injectable. J Psychopharmacol 19 (Suppl 5):S5–S14. [DOI] [PubMed] [Google Scholar]
- Park H, Bae SM, Ryoo JH, Kim SI, Lim WJ. (2009). Attitudes of Korean psychiatrists toward treatment long-acting injectable antipsychotic. J Korean Neuropsychiatr Assoc 48:182–189. [Google Scholar]
- Potkin SG, Gharabawi GM, Greenspan AJ, Mahmoud R, Kosik-Gonzalez C, Rupnow MFT, et al. (2006). A double-blind comparison of risperidone, quetiapine and placebo in patients with schizophrenia experiencing an acute exacerbation requiring hospitalization. Schizophr Res 85:254–265. [DOI] [PubMed] [Google Scholar]
- Robinson D, Woerner MG, Alvir JM, Bilder R, Goldman R, Geisler S, et al. (1999). Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry 56:241–247. [DOI] [PubMed] [Google Scholar]
- Rofail D, Gray R, Gournay K. (2005). The development and internal consistency of the satisfaction with Antipsychotic Medication Scale. Psychol Med 35:1063–1072. [DOI] [PubMed] [Google Scholar]
- Samtani MN, Gopal S, Gassmann-Mayer C, Alphs L, Palumbo JM. (2011). Dosing and switching strategies for paliperidone palmitate: based on population pharmacokinetic modelling and clinical trial data. CNS Drugs 25:829–845. [DOI] [PubMed] [Google Scholar]


