Abstract
Production of excessive levels of reactive oxygen species (ROS) in the vascular endothelium is a common pathogenic pathway in many dangerous conditions, including acute lung injury, ischemia-reperfusion, and inflammation. Ineffective delivery of antioxidants to the endothelium limits their utility for management of these conditions. In this study, we devised a novel translational antioxidant intervention targeted to the vascular endothelium using PEG-liposomes loaded with EUK-134 (EUK), a potent superoxide dismutase/catalase mimetic. EUK loaded into antibody-coated liposomes (size 197.8±4.5 nm diameter, PDI 0.179±0.066) exerted partial activity in the intact carrier, while full activity was recovered upon liposome disruption. For targeting we used antibodies (Abs) to platelet-endothelial cell adhesion molecule (PECAM-1). Both streptavidin-biotin and SATA/SMCC conjugation chemistries provided binding of 125-150 Ab molecules per liposome. Ab/EUK/liposomes, but not IgG/EUK/liposomes: i) bound to endothelial cells and inhibited cytokine-induced inflammatory activation in vitro; and, ii) accumulated in lungs after intravascular injection, providing >60% protection against pulmonary edema in endotoxin-challenged mice (vs <6% protection afforded by IgG/liposome/EUK counterpart). Since the design elements of this drug delivery system are already in clinical use (PEG-liposomes, antibodies, SATA/SMCC conjugation), it is an attractive candidate for translational interventions using antioxidant molecules such as EUK and other clinically acceptable drugs.
Keywords: targeted drug delivery, endothelial targeting, liposomes, inflammation, antioxidant therapy, antioxidant enzyme mimetic
Graphical Abstract
Introduction
Reactive oxygen species (ROS) play a central role in oxidative stress both as injurious and pro-inflammatory signaling molecules. Antioxidants may mitigate some forms of mild chronic oxidative stress [1, 2], but their benefits have not been proven in clinical settings associated with acute vascular oxidative stress, such as stroke, inflammation, ischemia/reperfusion, and acute lung injury (ALI). These pathologies, which are all characterized by unacceptably high morbidity and mortality, require more effective antioxidant interventions [3-5].
There are several mechanisms by which ROS appear to contribute to the pathogenesis of these critical illnesses. Activated leukocytes release ROS in the milieu that can cause tissue damage [6]. Administration of antioxidant formulations, including antioxidant enzymes (AOEs) modified with PEG or PEG-containing pluronics and N-acetyl cysteine (NAC)-loaded liposomes, has been shown to mitigate some of the harmful effects of extracellular ROS [7-9]. However, ROS are also produced in intracellular compartments of the endothelial cells lining blood vessels in response to inflammatory mediators and other damage-associated signals [10, 11]. In particular, ROS produced in the endosomes activate the NF-κB-mediated signaling pathway leading to endothelial activation and dysfunction, as manifested by expression of inducible adhesion molecules (e.g., VCAM-1) [12], enhanced permeability [13], and loss of the anti-thrombotic phenotype [14], aggravating tissue injury and propagating the vicious cycle of vascular oxidative stress and inflammation [15].
It is unlikely that untargeted carriers would provide antioxidants with access to ROS in the intracellular endothelial compartments, which may play an even more prominent role in the pathogenesis of relevant human illnesses [12]. To deliver antioxidants to these sites, we and others have devised a means of vascular immunotargeting of protein conjugates and nanocarriers [16-20]. In particular, conjugating antioxidants or their carriers to ligands of the endothelial cell surface determinant Platelet Endothelial Cell Adhesion Molecule, PECAM-1, represents an attractive strategy [16, 17]. Anti-PECAM targeted conjugates bind to the endothelium and are internalized via the noncanonical CAM-mediated endocytic pathway [21-23]. Further, AOEs conjugated with anti-PECAM, but not with control IgG, have been shown to 1) enter endosomes in vitro and in vivo [22, 24, 25], 2) quench endothelial ROS [12, 22, 26], and 3) alleviate lung ischemia-reperfusion [18, 27], vascular oxidative stress [28], and angiotensin-induced vasoconstriction in vivo [27]. In particular, PECAM-targeted superoxide dismutase (SOD) has been shown to quench endosomal superoxide and modulate inflammatory signaling [12].
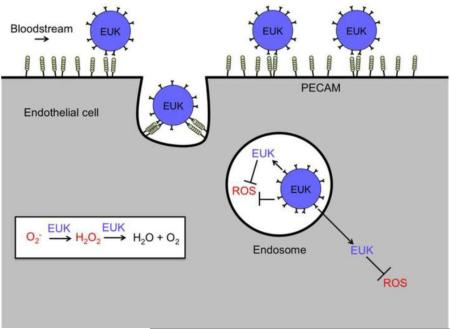
Ab/AOE conjugates may find clinical utility in some acute situations, in particular, organ transplantation, a scenario in which localization of Ab/AOE within a graft will limit potential systemic side effects [29]. Nevertheless, clinical translation of enzyme conjugates faces challenges typical of biotherapeutics. Replacing enzyme therapeutics with small non-immunogenic molecules may expedite clinical translation of targeted antioxidants. This manuscript describes the development of a PECAM-targeted liposomal system loaded with EUK-134, a small molecule SOD/Catalase mimetic (Figure 1) [30, 31].
Figure 1. Endothelial targeting of EUK-134 loaded liposomes coated with anti-PECAM.
EUK-134 (denoted as EUK) catalyzes the sequential degradation of superoxide to H2O2 to H2O and O2. Targeted immunoliposomes bind to and are internalized by endothelial cells. Endosomal ROS may be quenched by EUK-134 both inside the liposomes and following release. Free EUK-134 may distribute throughout the cell, quenching ROS in diverse cellular compartments.
This compound has the advantages of both a catalytic mechanism of action, typical of AOEs, combined with immunological neutrality and resistance to proteolytic degradation, typical of small molecules. Further, the dual SOD- and catalase-like activity may allow for elimination of not only superoxide but also the produced H2O2. Rather than attempting a problematic conjugation of EUK-134 directly to anti-PECAM, we elected to employ PEGylated liposomes, an extensively well-characterized, uniform, biocompatible nanocarrier already used clinically. Loading in carriers may also help to surmount obstacles associated with intravenous delivery of the drug, including solubility [32], possible loss of manganese due to interaction with chelators [33], and reduced activity with time [34]. Here we report the first (to our knowledge) successful attempt of endothelial targeting of a liposomal antioxidant enzyme mimetic providing tangible therapeutic advantages in vitro and in animal models of oxidative stress.
Materials and Methods
Reagents
EUK-134 (chloro[[2,2′-[1,2-ethanediylbis[(nitrilo-κN)methylidyne]]bis[6-methoxyphenolato-κO]]]-manganese) was purchased from Cayman Chemicals (Ann Arbor, MI). DPPC (1,2-dipalmitoyl-sn-glycero-3-phosphocholine), PG (L-α-phosphatidylglycerol), cholesterol, DSPE-PEG(2000) biotin (1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[biotinyl(polyethylene glycol)-2000]), and DSPE-PEG(2000) maleimide (1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[maleimide(polyethylene glycol)-2000]) were from Avanti Polar Lipids (Alabaster, AL). PC (L-α-Phosphatidylcholine), lipopolysaccharide (LPS, from E. coli B4), and methanol were purchased from Sigma-Aldrich (St. Louis, MO). Succinimidyl 4-[N-maleimidiomethyl] cyclohexane-1-carboxylate (SMCC) and N-succinimidyl-S-acetylthioacetate (SATA) were from Thermo Scientific Pierce (Rockford, IL) Bovine serum albumin (BSA) was from Fischer Scientific (Pittsburg, PA). Mouse anti-PECAM MEC13.3 was purchased from BD Bioscience (San Jose, CA), and monoclonal antibody (mAb 62) against human anti-PECAM was provided by Dr. Marian Nakada (Centoor; Malvern, PA). Whole molecule rat IgG was from Rockland Immunochemicals (Gilbertsville, PA). Streptavidin (SA) was from Calbiochem (San Diego, CA).
Cell culture
Human umbilical vein endothelial cells (HUVECs) were purchased at first passage from Lonza Walkersville (Walkersville, MD), and were grown in Falcon tissue culture flasks (BD Biosciences, San Jose, CA) coated with 1% gelatin (Sigma-Aldrich) in EGM-BulletKit media (Lonza Walkersville) containing 10% v/v fetal bovine serum (FBS). All studies were performed with passage 5 cells in a confluent state (105 cells/cm2).
Protein iodination
Throughout the experiments, proteins (IgG or BSA) were labeled with Na-125I (Perkin Elmer, Boston, MA) using iodination beads as instructed by the manufacturer (Thermo Scientific Pierce). Unbound iodine was removed using Zeba desalting columns (Thermo Scientific Pierce). The extent of radiolabeling was measured using a standard trichloroacetic (TCA) assay. A 2 μl aliquot of labeled antibody, 1 ml 3% BSA, and 200 μl TCA were mixed and allowed to sit at room temperature for 15 min. Following a 15 min centrifugation (4°C, 2300 g), the amount of free iodine in the supernatant was quantified using a Wizard2 2470 gamma counter (PerkinElmer; Waltham, MA).
Liposome preparation
Liposomes were prepared using a thin-film hydration method followed by extrusion. Briefly, 50 μl of DPPC (73.4 mg/ml), 18.4 μl of PC (100 mg/ml), 74 μl of PG (10 mg/ml), 50 μl of cholesterol (11.6 mg/ml), and 64.4 μl of DSPE-PEG(2000)-biotin or DSPE-PEG(2000)-maleimide were combined in a glass tube, and the solvent allowed to evaporate overnight. Where appropriate, EUK-134 (10 mg/ml solution in methanol) was added to this initial solution. The thin films were hydrated with 500 μl PBS or saline by agitation, followed by three freeze-thaw cycles using liquid nitrogen and a 50°C water bath (Branson 1510, Branson Ultrasonics Corporation; Woodbury, CT). Liposomes were subsequently extruded 10 times through 200 nm polycarbonate filters (Mini-Extruder, Avanti Polar Lipids).
Antibody-SATA (Ab-SATA) modification
SATA (20 mM, in DMSO) was added to the Ab in a 10-fold molar excess for 30 min at room temperature in order to introduce ~1 sulfhydryl group per Ab. Unreacted SATA was removed using a Zeba desalting column (Pierce Biotechnology). N-hydroxylamine (0.5 M) was added at a 10:1 volume ratio in order to achieve deprotection of the acetylated sulfhydryls, and the final solution was again filtered through a desalting column. Actual volumes used varied according to the individual preparation.
Antibody-SA (Ab-SA) conjugate preparation
Ab was modified with SATA as described above but using a 6:1 molar ratio SATA:Ab. In a parallel reaction, SMCC (45.8 mM, in DMF) was used to introduce stable maleimide groups onto SA (6 mg/ml) using a 20-fold molar excess at room temperature for 1 h. Again, products were passed through desalting columns for the removal of unreacted components. Abs were then conjugated to activated SA using a 2:1 molar ratio Ab:SA in a 1 h reaction on ice. Actual volumes used varied according to the individual preparations.
Surface coating of liposomes with modified antibodies
Liposomes prepared with DSPE-PEG-maleimide and DSPE-PEG-biotin were coated with Ab-SATA and Ab-SA, respectively. Liposomes and modified antibodies were combined and slowly rotated for up to 1 h at room temperature. Free materials (lipids, drug, and protein) were removed by ultracentrifugation at 28 k RCF and 4°C for 60 min (Sorvall WX80 Ultra Series Ultracentrifuge, Thermo Scientific; Waltham, MA). Binding efficiency was measured by radiotracing a 10% substitution of 125I-IgG-SATA or 125I-IgG-SA. For comparison, liposomes were passed through a Sepharose CL-4B column (GE Healthcare Life Sciences; Piscataway, NJ).
Analytical Methods
Size and zeta potential measurements were obtained following a 100-fold dilution in DI H2O using a 90Plus Particle Size Analyzer (Brookhaven Instruments, Holtsville, NY) and Zetasizer Nano S (Malvern Instruments, Worcestershire, UK), respectively. All UV measurements were performed on a Varian Cary® 50 Bio UV-Visible Spectrophotomer (Agilent Technologies, Santa Clara, CA). EUK-134 concentration was calculated based on a standard curve obtained at 328 nm from 1-50 μg/ml in methanol. Encapsulation efficiency was calculated as loaded drug/added drug × 100. Measurements were obtained for initial liposome preparations, purified liposomes, and supernatants to confirm ~100% drug recovery.
The SOD-like activity of EUK-134 was determined using a cytochrome C reduction assay. Briefly, xanthine and xanthine oxidase are combined to produce superoxide anion with cytochrome C acting as an indicating scavenger that competes with EUK-134. The working solution (0.6 ml) contained 50 mM phosphate buffer (pH 7.8), 0.1 mM EDTA, 20 μM cytochrome C, and 50 μM xanthine. Reaction was initiated by the addition of 10 μl 0.2 U/ml xanthine oxidase, and the absorbance was monitored at 550 nm. One unit of SOD-like activity was defined as the amount of EUK-134 required to inhibit the rate of reduction of cytochrome C by 50%. The catalase-like activity of the drug was determined using a H2O2 degradation assay. PBS-buffered 5 mM H2O2 was added to a quartz cuvette, and the decay in absorbance at 242 nm measured upon addition of EUK-134. One unit of catalase-like activity was defined as the amount of EUK-134 required to decompose 1.0 μmole of H2O2 per minute at pH 7.0 at 25 °C. In both cases, calibration curves were prepared for use in evaluating drug loading, and calculations performed as described above with the UV measurements. Additionally, comparisons were obtained between intact liposomes (diluted in PBS) and ruptured liposomes (diluted in methanol).
Drug release
Purified IgG-coated liposomes substituted with 10% 125I-IgG (1.5 ml) were loaded to 10k MWCO Slide-a-lyzer dialysis cassettes (0.1-0.5 ml, Thermo Scientific) and dialyzed against 500 ml PBS (10 mM, pH 7.4) at 37°C. At each time point, ~50 μl were removed from inside the cassette for measurement of remaining EUK-134. To correct for any dilution, the 125I-IgG activity of a 10 μl aliquot was also measured at each time point and compared to the initial starting activity.
Binding of liposomes to endothelial cells
For these preliminary cell culture studies, antibodies were conjugated to the liposome surface through a biotin-streptavidin linkage and comparisons made between targeted anti-PECAM and control IgG coated liposomes. Cold antibodies were doped with 10% 125I-IgG-SA for radiotracing. Use of this doping technique allows quantitative measurement of binding to cells while minimizing the chance of false positive results, such as might occur due to binding of detached Ab-SA if that was labeled directly.Confluent HUVECs were pretreated with 3% BSA in complete media for 1 h at 37°C. Cells were then incubated with increasing quantities of EUK-loaded liposomes for 30 min at 37°C. The number of liposomes added was roughly estimated based on the size and determined density (1.5 g/ml, with drug and Ab coating). After the incubation, media was removed, and cells were lysed (1% Triton X100 in 1 N NaOH). The radioactivity in both supernatants and cell lysates was measured. Particle number bound per cell calculations were derived on the basis of a liposome concentration of 6.5×1012 particles/ml and an EC density of 105 cells/well on a 24 well plate.
Liposome biodistribution
Animal experiments were performed according to the protocol approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. For liposome tracing, 10% of the total surface antibody coating was again substituted with 125I-IgG. Comparisons were made between anti-PECAM/liposome/EUK and control IgG/liposome/EUK. 200 μl aliquots of 3.2 mg total lipid at 2000 CPM/μl were injected intravenously in ketamine/xylazine anesthetized (100/10 mg/kg) C57BL/6 male mice (The Jackson Laboratory, Bar Harbor, ME). One hour later, organs were harvested, rinsed, blotted dry, and weighed. Radioactivity was measured, and the results used in calculating the tissue biodistribution (% injected dose/gram).
In vitro VCAM expression
HUVECs in 24 well plates were used at confluency (~105 cells/well). Cells were pretreated with 3% BSA in complete media for 1 h at 37°C. Media was then removed and replaced with either anti-PECAM/liposome/EUK or IgG/liposome/EUK in media; control wells received media alone. Media was removed again, and cells challenged with TNF (10 ng/ml in media). Media was replaced in control wells. Following a 5 h TNF exposure, media was removed and cells lysed in 100 μl sample buffer for sodium dodecyl sulfate polyacrylamide gel electrophoresis. Samples were placed at 100°C for 5 minutes and then stored at 4°C until use. Cell proteins were subjected to a 4-15% gradient gel (Biorad Laboratories, Hercules, CA) and transferred to a PVDF membrane (Millipore, Billerica, MA) for Western blotting. The membrane was subsequently blocked for 1 h with 3% nonfat dry milk in TBS-T (100 mM Tris, pH 7.6; 150 mM NaCl; and 0.1% Tween 20), followed by incubations with primary and secondary antibodies for VCAM and actin. The blot was detected using ECL Plus reagents (GE Healthcare, New York, NY). Quantification of blots was performed using standard densitometry methods (Biorad Fluor-SM, Biorad Laboratories, Hercules, CA). VCAM expression was normalized to actin. Results were expressed as the percent of protection, with untreated cells representing 100% protection and TNF-treated cells 0% protection.
In vivo VCAM expression
To induce acute pulmonary injury, lipopolysaccharide (LPS) was administered intratracheally to C57BL/6 male mice at 1 mg/kg in 100 μl sterile PBS, either with or without EUK liposomal pre-treatment 15 minutes prior. Twenty-four hours after challenge, lungs were harvested and perfused. Samples were frozen in liquid nitrogen and stored at −80°C until use. For VCAM detection, lungs were first homogenized in 1 ml of a protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO) in PBS for 6 min with a 5 mm steel ball (TissueLyser II, Qiagen, Valencia, CA). 50 μl of lysis buffer (10% SDS, 10% Triton X-100) were added, and samples were rotated for 1 h at 4°C. Each sample was then sonicated for ~1 min and centrifuged for 10 min at 16 k RCF and 4°C. The supernatant was removed, diluted 1:2 in sample buffer, and analyzed for VCAM expression as described above for cell lysates.
BSA Leakage
C57BL/6 male mice were injected intravenously with EUK-loaded liposomes coated with either anti-PECAM or control IgG, blank liposomes coated with IgG, or saline. Ten minutes later, mice received a 100 μl injection of 125I-BSA (3000-4000 CPM/μl). Five minutes following the BSA injection, LPS was administered intratracheally (1 mg/kg in 100 μl sterile PBS). Lungs (perfused) and blood were collected at 24 h for radioactivity measurement. Lung/blood ratios were calculated for comparison.
Results and Discussion
Despite the data supporting the role of oxidative stress in numerous vascular maladies, neither small molecule antioxidants, such as NAC, vitamin E, and β-carotene, nor AOEs have proven significantly efficacious in clinical trials [35-38]. Use of nanocarrier drug delivery systems (e.g., PEGylation, liposomes, nanozymes) may extend the circulation lifetime of these compounds, particularly AOEs, and improve quenching of extracellular ROS [7-9]. However, this may not be sufficient for treating acute vascular oxidative stress, which appears to be highly associated with endothelial ROS. Accordingly, in this study we designed endothelial-targeted liposomes carrying a potent SOD/catalase mimetic, EUK-134. PEGylated liposomes represent a desirable carrier based on their small size, uniformity, biocompatibility, and clinical acceptability. Further, use of a mimetic that displays similar catalytic activity to AOEs but with enhanced stability and non-immunogenicity may extend the therapeutic utility of this technology.
Liposome characterization
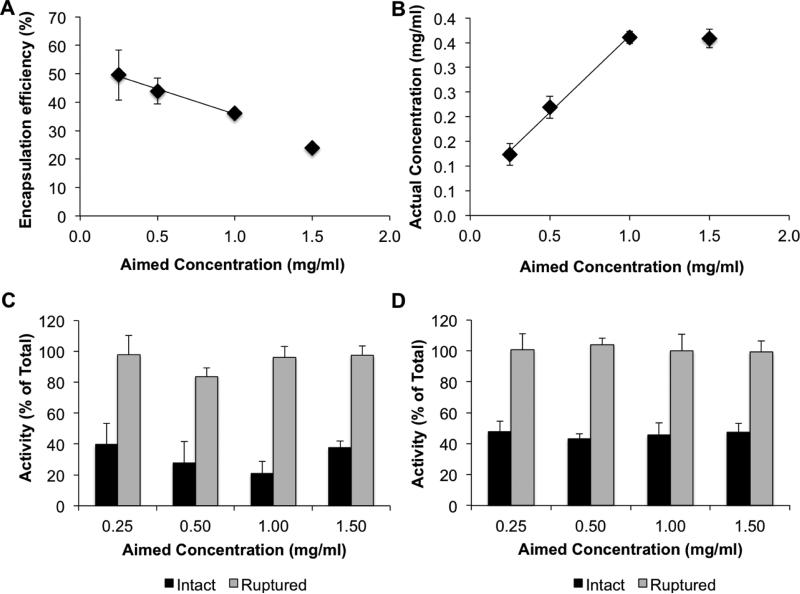
Dynamic light scattering (DLS) measurements indicated that the size of liposomes prior to antibody coating was consistently ~180 nm, independent of the amount of drug loaded (Table 1). At aimed EUK-134 concentrations close to 1 mg/ml, all samples exhibited polydispersity indices (PDI) < 0.1, typical of highly homogenous formulations. Up to this concentration, the amount of loaded drug increased linearly with added drug, with an apparent maximum at ~0.4 mg/ml (Figure 2). This was true, independent of the method of purification. Ultracentrifugation data shown below has been affirmed using gel purification (encapsulation efficiency of 36% for an aimed concentration of 1.00 mg/ml). Attempts to further increase drug loading were not successful and led to loss of homogeneity of the liposomes, likely due to destabilization of the phospholipid bilayer.
Table 1.
Effect of added drug on size, polydispersity, and encapsulation efficiency of liposomes (as determined by ultracentrifugation, mean±SD)
| Aimed Concentration (mg/ml) | Size (nm) | Polydispersity Index (PDI) | Encapsulation Efficiency (%) | ||
|---|---|---|---|---|---|
| Abs328 | SOD-Like Activity | Catalase-Like Activity | |||
| 0.25 | 178.9±3.1 | 0.078±0.037 | 49.56±8.87 | 45.13±10.19 | 47.92±4.25 |
| 0.50 | 176.6±6.0 | 0.050±0.044 | 43.88±4.51 | 45.92±3.41 | 43.22±1.06 |
| 1.00 | 179.0±2.3 | 0.083±0.014 | 36.05±1.19 | 41.43±3.67 | 39.66±4.57 |
| 1.50 | 178.2±2.8 | 0.165±0.014 | 23.89±1.23 | 24.62±2.22 | 40.43±4.32 |
Figure 2. Drug loading and activity.
Effect of added drug on (A) encapsulation efficiency, (B) loaded drug concentration, (C) SOD-like activity, and (D) catalase-like activity. Mean±SD.
Activity of EUK-134 in liposomes
Initial characterization studies confirmed that EUK-134 had SOD-like activity, corresponding to 3483±228 units/mg, which is similar to that previously reported [31]. The catalase-like activity of EUK-134 was approximately 303±26 units/mg. Standard curves could be obtained for both types of activity to allow additional measurements of drug loading in the liposomes. By both SOD- and catalase-like activity, calculated encapsulation efficiency was similar to that observed with Abs328 measurements (Table 1).
As expected, EUK-134 exerted partial activity in intact liposomes (Figure 2C-D) and regained full activity upon liposome destruction. This is likely due to limited ROS diffusion through the phospholipid bilayer, which is more impeded for superoxide than H2O2 (the catalase-like activity of encapsulated EUK-134 was reduced by ~50% vs free drug, whereas SOD-like activity was more affected). As it is known that superoxide does not easily pass through lipid membranes, this result was relatively unsurprising. In fact, the presence of any SOD-like activity in the intact particles is likely indicative of some drug residing at or close to the liposome surface. It is also possible that drug is being released from the particles, and the free drug is being detected. However, as the activity assays only take several minutes, free drug is not expected to be a major contributor. The reduction in H2O2 degradation is more interesting as it should pass through the membranes relatively easily. It is possible that EUK-134 aligns within the lipids such that its active site is inaccessible. Importantly, however, full activity was recovered upon disruption of the lipid bilayer. Therefore, as the particles are degraded in vivo or as the drug leaks out of the membrane, EUK-134 should still be able to sufficiently degrade superoxide or H2O2.
Drug release
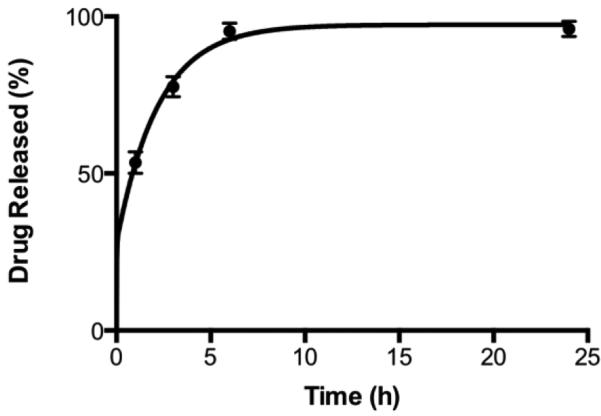
The release of EUK-134 from the liposomes was rapid, reaching ~95% within 6 hours. While too rapid for some indications (e.g., cancer), this is a realistic time scale for treating acute inflammatory conditions. Since targeting provides very rapid pulmonary localization of the liposomes (discussed below), premature release of the drug within the circulation is of minimal concern. Further, because the drug is an antioxidant and not a cytotoxic agent, any release that would occur in the circulation is not likely to elicit adverse effects.
Surface coating of liposomes with antibodies
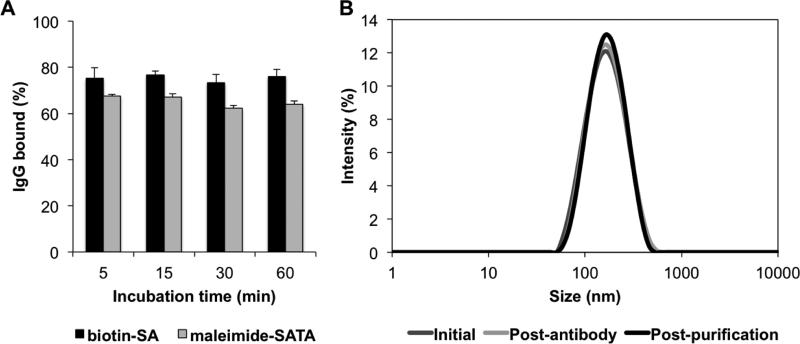
Both streptavidin-biotin and maleimide conjugation chemistries provided similar surface coupling of antibodies within a few minutes (Figure 4). The overall average for streptavidin-mediated coupling was 150.5±2.9 IgG molecules per liposome when determined by ultracentrifugation, while maleimide-mediated coupling resulted in just slightly lower coverage at 130.6±5.1.
Figure 4. Antibody binding.
(A) Percent of IgG binding as a function of incubation time as measured by 125I-IgG using ultracentrifugation. Comparisons are made between the biotin-SA and SMCC/SATA chemistries. (B) Representative DLS curves for liposomes pre- and post-antibody and post-purification. Mean±SD.
The effect of incubation time was negligible. Although not unexpected, this validation is important. Use of the high affinity biotin-streptavidin linkage is a popular technique for adding targeting agents to the surface of nanocarriers, but SMCC/SATA chemistry is more amenable to translation into the clinical domain because it lacks the potential immune complications associated with streptavidin use.
Antibody conjugation led to a size increase of ~20 nm to around 200 nm, corresponding to the size of the IgG molecules bound to the liposomes (Table 2). Importantly, no remarkable change was observed in liposome homogeneity (PDI<0.2). Purification of the particles by ultracentrifugation resulted in no further changes in size or PDI. DLS distribution curves (Figure 4, intensity distribution shown) confirmed the absence of large, micron-sized aggregates. Zeta potential was slightly negative (−5 mV) and did not change with antibody binding or purification.
Table 2.
Effect of antibody coating procedure on the size, polydispersity, and zeta potential of liposomes (Mean±SD)
| Liposome | Size (nm) | Polydispersity Index | Zeta Potential |
|---|---|---|---|
| Initial | 181.2±8.4 | 0.074±0.090 | −4.46±1.09 |
| Antibody-coated | 205.4±7.8 | 0.195±0.004 | −5.02±1.44 |
| Post-reconstitution | 197.8±4.5 | 0.179±0.066 | −4.78±0.96 |
Targeting and anti-inflammatory effect of liposomes in vitro
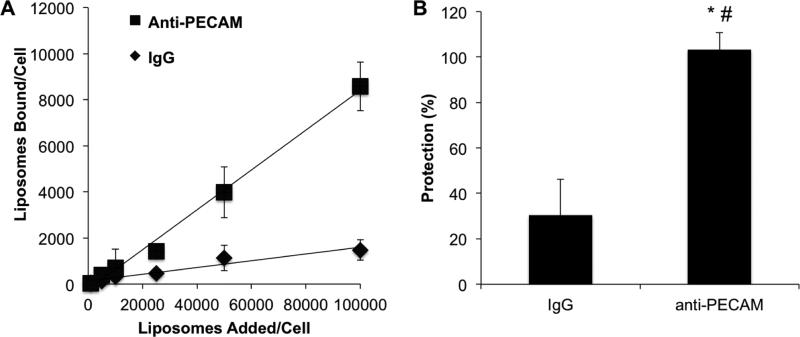
Initial characterization of the targeting ability of EUK/liposomes was performed using culture of endothelial cells (HUVECs). Anti-PECAM/EUK/liposomes but not control IgG/EUK/liposomes, bound to endothelial cells in a dose-dependent fashion, at maximal dose providing delivery of almost 10,000 liposomes per cell within 30 min (Figure 5A).
Figure 5. Endothelial targeting and anti-inflammatory effect of liposomes in vitro.
(A) HUVEC binding of anti-PECAM/EUK/liposome versus IgG/EUK/liposome following a 30 min incubation. Mean±SD (B) Protection against pro-inflammatory endothelial activation as assessed by VCAM upregulation. HUVECs were pretreated with either anti-PECAM/EUK/liposome or IgG/EUK/liposome 30 minutes prior to a 5 hour challenge with TNF (5 μg/ml). Resulting cell lysates were measured for VCAM and actin by western blot. VCAM expression is normalized to actin. Data is shown as % protection, with untreated cells representing 100% protection and TNF-treated cells 0% protection. Mean±SEM, *p<0.05 anti-PECAM/EUK/liposome versus LPS-treatment, #p<0.05 anti-PECAM/EUK/liposome versus control IgG/EUK/liposome
Correspondingly, targeting EUK/liposomes to endothelial cells markedly enhanced their functional effect in this experimental model. Pretreatment with anti-PECAM/EUK/liposomes completely eliminated stimulation of VCAM synthesis (100% protection) induced by the cytokine TNF (Figure 5B). In contrast, IgG/EUK/liposomes provided ~30% protection, clearly inferior to the effect of the targeted antioxidant.
The latter outcome is likely a result of non-specific uptake, which is a rather common artifact of experiments involving incubation of untargeted nanoparticles with cultivated cells under static conditions. First, static endothelial cells grown in reduced serum levels are known to take up components of their milieu more eagerly than their vascular counterparts. Second, non-targeted nanoparticles are rapidly eliminated from the bloodstream, whereas in vitro cells are exposed to a stable high concentration of liposomes. From this standpoint, in vivo experiments represent a profoundly more adequate model system (see below).
Endothelial targeting of liposomes in vivo
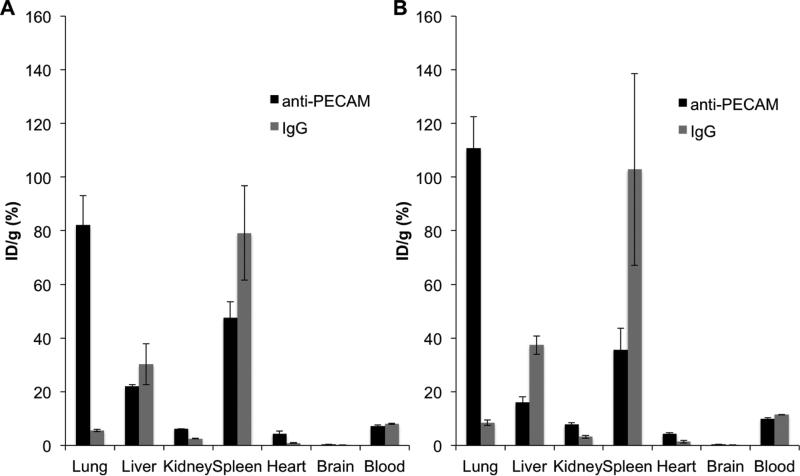
The endothelial targeting of EUK/liposomes was next tested in intact mice. The pulmonary vasculature represents ~25-30% of the total vascular surface and receives more than half of the total cardiac blood output (i.e., whole venous blood from the right ventricle plus a fraction of arterial blood from the left ventricle via the bronchial circulation), in contrast to other organs that share arterial output. Therefore, pulmonary endothelium is the preferential site for accumulation of endothelial-targeted materials, such as anti-PECAM conjugates and nanoparticles [12, 22]. Accordingly, we characterized the endothelial targeting by measuring the level of pulmonary accumulation of anti-PECAM/EUK/liposomes versus IgG/EUK/liposomes, using the same radioisotope tracing approach as in cell culture studies.
One hour after IV injection in mice, anti-PECAM/EUK/liposomes, but not IgG/EUK/liposomes, accumulated in the lungs regardless of the particular chemistry used for conjugation (Figure 6). In fact, the extent of specific pulmonary uptake of anti-PECAM/EUK/liposome was so high (~15 times over the level of IgG/liposomes) that their uptake in the liver and spleen was markedly reduced compared with that of non-specific IgG/EUK/liposome. Data is expressed as the percent of injected dose/gram, which allows the total percent (and in some cases, the percent associated with a specific organ) to reach levels higher than 100%.
Figure 6. In vivo endothelial targeting.
Comparison of the in vivo biodistribution of anti-PECAM/liposome/EUK versus IgG/liposome/EUK as measured by 125I-IgG using two different antibody conjugation chemistries: (A) biotin-SA and (B) maleimide-SATA. Data is expressed as the percent injected dose (ID)/gram. Mean±SD.
Of note, targeting (i.e., ratio of uptake of targeted vs non-targeted liposomes) was markedly more specific in vivo vs in cell culture. This phenomenon has been reported previously [39] and is likely a result of decreased nonspecific accumulation in vivo. In animal studies, liposomes not securely bound to the endothelium via an affinity linkage are likely to be detached from cells by hemodynamic forces and eliminated from the circulation.
Anti-inflammatory effects of anti-PECAM/liposome/EUK in animals
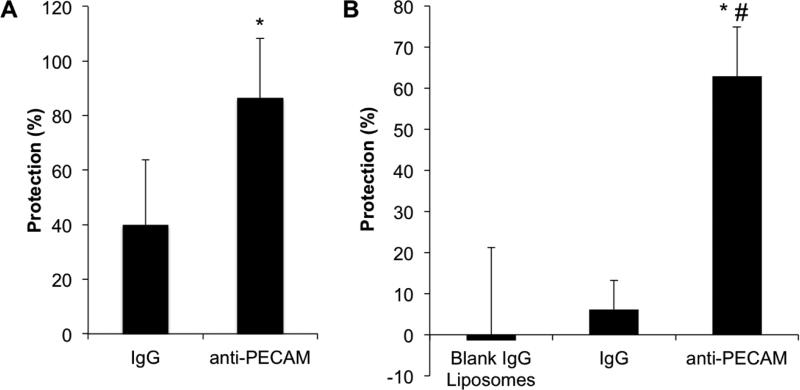
Finally, we tested the anti-inflammatory effect of targeted EUK/liposomes in vivo. In a model of endotoxin (LPS) induced acute pulmonary inflammation, anti-PECAM/EUK/liposomes, but not IgG/EUK/liposomes mitigated expression of VCAM-1 in the lung (Figure 7A). Therefore, this in vivo result, recapitulating the in vitro data (Figure 5B), implied that endothelial targeting augments the anti-inflammatory effects of non-enzymatic antioxidant interventions in vivo. Since VCAM-1 is both a marker of inflammation and a pro-inflammatory entity involved in leukocyte adhesion, this data supports the idea that endothelial targeting can enhance the anti-inflammatory effects of antioxidant interventions.
Figure 7. Anti-inflammatory effects of anti-PECAM/EUK/liposome in vivo.
(A) Mice were injected with liposomes 15 min prior to receiving an intra-tracheal LPS challenge (1 mg/kg). Lungs were perfused and harvested 24 hours later. Lung homogenates were measured for VCAM and actin by western blot. VCAM expression is normalized to actin. Data is shown as % protection, with naïve animals representing 100% protection and LPS-treated animals 0% protection. Mean±SEM (B) Mice were injected with liposomes and 125I-BSA 15 and 5 min, respectively, prior to intra-tracheal LPS challenge (1 mg/kg). Lungs and blood were collected 6 h later and assessed for radioactivity. Data is expressed as % protection in reference to positive (LPS) and negative (saline) controls. Mean±SD, *p<0.05 anti-PECAM/EUK/liposomes versus LPS-treatment, #p<0.05 anti-PECAM/EUK/liposomes versus control IgG/EUK/liposomes.
In order to appraise the potential therapeutic benefits of this effect, we measured pulmonary vascular edema caused by LPS challenge using deposition in the lungs of intravenously injected 125I-BSA as a marker of vascular leakiness (Figure 7B). In this study, anti-PECAM/EUK/liposome but not its IgG counterpart, markedly mitigated pulmonary edema, indicating that this targeted antioxidant intervention may provide tangible therapeutic advantages.
Based on this data, it appears that endothelial-targeted liposomes loaded with AOE mimetics may be useful for modulating the endothelial inflammatory response. Comparison with other small molecule antioxidants (e.g., NAC) and AOE mimetics may be valuable, and studies in other models of human vascular oxidative stress and inflammation are warranted. Considering the ability of liposomes to load both hydrophobic drugs (such as EUK-134, in the lipid bilayer) and hydrophilic drugs (in the aqueous core), scenarios could also be envisioned where loading of complementary therapeutic agents might be beneficial.
Conclusions
Anti-PECAM liposomes loaded with EUK-134, an SOD/catalase mimetic, were successfully prepared and characterized. Both in vitro and in vivo studies confirmed enhanced targeting to the endothelium as compared to control IgG coated liposomes. Preliminary studies, including both VCAM expression and BSA leakage into the lungs, provided evidence that anti-PECAM/EUK/liposome may be useful for alleviating acute pulmonary inflammation.
Supplementary Material
Figure 3. Drug release.
Release of EUK-134 from liposomes at 37°C under sink conditions. Mean±SD.
Acknowledgements
This work was supported by NIH NHLBI grants HL087036 and HL073940. MH acknowledges financial support from the University of Pennsylvania Hematology T32 Training Grant (Grant number T32 HL07439).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Christofidou-Solomidou M, Muzykantov VR. Antioxidant Strategies in Respiratory Medicine. Treatments in Respiratory Medicine. 2006;5:47–78. doi: 10.2165/00151829-200605010-00004. [DOI] [PubMed] [Google Scholar]
- 2.Muzykantov VR. Targeting of superoxide dismutase and catalase to vascular endothelium. Journal of Controlled Release. 2001;71:1–21. doi: 10.1016/s0168-3659(01)00215-2. [DOI] [PubMed] [Google Scholar]
- 3.Fisher AB. Redox signaling across cell membranes. Antioxidants and Redox Signaling. 2009;11:1349–1356. doi: 10.1089/ars.2008.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Janssen-Heininger YMW, Mossman BT, Heintz NH, Forman HJ, Kalyanaraman B, Finkel T, Stamler JS, Rhee SG, van der Vliet A. Redox-based regulation of signal transduction: Principles, pitfalls, and promises. Free Radical Biology and Medicine. 2008;45:1–17. doi: 10.1016/j.freeradbiomed.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Vliet A. NADPH oxidases in lung biology and pathology: Host defense enzymes, and more. Free Radical Biology and Medicine. 2008;44:938–955. doi: 10.1016/j.freeradbiomed.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conner EM, Grisham MB. Inflammation, free radicals, and antioxidants. Nutrition. 1996;12:274–277. doi: 10.1016/s0899-9007(96)00000-8. [DOI] [PubMed] [Google Scholar]
- 7.Mitsopoulos P, Omri A, Alipour M, Vermeulen N, Smith MG, Suntres ZE. Effectiveness of liposomal-N-acetylcysteine against LPS-induced lung injuries in rodents. International Journal of Pharmaceutics. 2008;363:106–111. doi: 10.1016/j.ijpharm.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 8.Supinski GS, Callahan LA. Polyethylene Glycol–Superoxide Dismutase Prevents Endotoxin-induced Cardiac Dysfunction. American Journal of Respiratory and Critical Care Medicine. 2006;173:1240–1247. doi: 10.1164/rccm.200410-1346OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manickam DS, Brynskikh AM, Kopanic JL, Sorgen PL, Klyachko NL, Batrakova EV, Bronich TK, Kabanov AV. Well-defined cross-linked antioxidant nanozymes for treatment of ischemic brain injury. Journal of Controlled Release. 2012;162:636–645. doi: 10.1016/j.jconrel.2012.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas SR, Witting PK, Drummond GR. Redox control of endothelial function and dysfunction: molecular mechanisms and therapeutic opportunities. Antioxidants and Redox Signaling. 2008;10:1713–1765. doi: 10.1089/ars.2008.2027. [DOI] [PubMed] [Google Scholar]
- 11.Guzik TJ, Harrison DG. Vascular NADPH oxidases as drug targets for novel antioxidant strategies. Drug Discovery Today. 2006;11:524–533. doi: 10.1016/j.drudis.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Shuvaev VV, Han J, Yu KJ, Huang S, Hawkins BJ, Madesh M, Nakada M, Muzykantov VR. PECAM-targeted delivery of SOD inhibits endothelial inflammatory response. FASEB Journal. 2011;25:348–357. doi: 10.1096/fj.10-169789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han J, Shuvaev VV, Muzykantov VR. Catalase and SOD conjugated with PECAM antibody distinctly alleviate abnormal endothelial permeability caused by exogenous ROS and vascular endothelial growth factor. Journal of Pharmacology and Experimental Therapeutics. 2011 doi: 10.1124/jpet.111.180620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding B-S, Hong N, Christofidou-Solomidou M, Gottstein C, Albelda SM, Cines DB, Fisher AB, Muzykantov VR. Anchoring Fusion Thrombomodulin to the Endothelial Lumen Protects against Injury-induced Lung Thrombosis and Inflammation. American Journal of Respiratory and Critical Care Medicine. 2009;180:247–256. doi: 10.1164/rccm.200809-1433OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shuvaev VV, Muzykantov VR. Targeted modulation of reactive oxygen species in the vascular endothelium. Journal of Controlled Release. 2011;153:56–63. doi: 10.1016/j.jconrel.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding BS, Dziubla T, Shuvaev VV, Muro S, Muzykantov VR. Advanced drug delivery systems that target the vascular endothelium. Molecular Interventions. 2006;6:98–112. doi: 10.1124/mi.6.2.7. [DOI] [PubMed] [Google Scholar]
- 17.Muro S, Muzykantov VR. Targeting of Antioxidant and Anti-Thrombotic Drugs to Endothelial Cell Adhesion Molecules. Current Pharmaceutical Design. 2005;11:2383–2401. doi: 10.2174/1381612054367274. [DOI] [PubMed] [Google Scholar]
- 18.Nowak K, Weih S, Metzger R, Albrecht RF, Post S, Hohenberger P, Gebhard M-M, Danilov SM. Immunotargeting of catalase to lung endothelium via anti-angiotensin-converting enzyme antibodies attenuates ischemia-reperfusion injury of the lung in vivo. American Journal of Physiology - Lung Cellular and Molecular Physiology. 2007;293:L162–L169. doi: 10.1152/ajplung.00001.2007. [DOI] [PubMed] [Google Scholar]
- 19.Maruyama K, Holmberg E, Kennel SJ, Klibanov A, Torchilin VP, Huang L. Characterization of in vivo immunoliposome targeting to pulmonary endothelium. Journal of Pharmaceutical Sciences. 1990;79:978–984. doi: 10.1002/jps.2600791107. [DOI] [PubMed] [Google Scholar]
- 20.Adrian JE, Morselt HW, Suss R, Barnert S, Kok JW, Asgeirsdottir SA, Ruiters MH, Molema G, Kamps JA. Targeted SAINT-O-Somes for improved intracellular delivery of siRNA and cytotoxic drugs into endothelial cells. J Control Release. 2010;144:341–349. doi: 10.1016/j.jconrel.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Han J, Zern BJ, Shuvaev VV, Davies PF, Muro S, Muzykantov V. Acute and Chronic Shear Stress Differently Regulate Endothelial Internalization of Nanocarriers Targeted to Platelet-Endothelial Cell Adhesion Molecule-1. ACS Nano. 2012;6:8824–8836. doi: 10.1021/nn302687n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muzykantov VR, Christofidou-Solomidou M, Balyasnikova I, Harshaw DW, Schultz L, Fisher AB, Albelda SM. Streptavidin facilitates internalization and pulmonary targeting of an anti-endothelial cell antibody (platelet-endothelial cell adhesion molecule 1): A strategy for vascular immunotargeting of drugs. Proceedings of the National Academy of Sciences. 1999;96:2379–2384. doi: 10.1073/pnas.96.5.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muro S, Wiewrodt R, Thomas A, Koniaris L, Albelda SM, Muzykantov VR, Koval M. A novel endocytic pathway induced by clustering endothelial ICAM-1 or PECAM-1. Journal of Cell Science. 2003;116:1599–1609. doi: 10.1242/jcs.00367. [DOI] [PubMed] [Google Scholar]
- 24.Muzykantov VR, Atochina EN, Kuo A, Barnathan ES, Notarfrancesco K, Shuman H, Dodia C, Fisher AB. Endothelial cells internalize monoclonal antibody to angiotensin-converting enzyme. American Journal of Physiology - Lung Cellular and Molecular Physiology. 1996;270:L704–L713. doi: 10.1152/ajplung.1996.270.5.L704. [DOI] [PubMed] [Google Scholar]
- 25.Muzykantov VR, Atochina EN, Ischiropoulos H, Danilov SM, Fisher AB. Immunotargeting of antioxidant enzyme to the pulmonary endothelium. Proceedings of the National Academy of Sciences. 1996;93:5213–5218. doi: 10.1073/pnas.93.11.5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shuvaev VV, Tliba S, Nakada M, Albelda SM, Muzykantov VR. Platelet-Endothelial Cell Adhesion Molecule-1-Directed Endothelial Targeting of Superoxide Dismutase Alleviates Oxidative Stress Caused by Either Extracellular or Intracellular Superoxide. Journal of Pharmacology and Experimental Therapeutics. 2007;323:450–457. doi: 10.1124/jpet.107.127126. [DOI] [PubMed] [Google Scholar]
- 27.Shuvaev VV, Christofidou-Solomidou M, Bhora F, Laude K, Cai H, Dikalov S, Arguiri E, Solomides CC, Albelda SM, Harrison DG, Muzykantov VR. Targeted Detoxification of Selected Reactive Oxygen Species in the Vascular Endothelium. Journal of Pharmacology and Experimental Therapeutics. 2009;331:404–411. doi: 10.1124/jpet.109.156877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christofidou-Solomidou M, Scherpereel A, Wiewrodt R, Ng K, Sweitzer T, Arguiri E, Shuvaev V, Solomides CC, Albelda SM, Muzykantov VR. PECAM-directed delivery of catalase to endothelium protects against pulmonary vascular oxidative stress. American Journal of Physiology - Lung Cellular and Molecular Physiology. 2003;285:L283–L292. doi: 10.1152/ajplung.00021.2003. [DOI] [PubMed] [Google Scholar]
- 29.Kozower BD, Christofidou-Solomidou M, Sweitzer TD, Muro S, Buerk DG, Solomides CC, Albelda SM, Patterson GA, Muzykantov VR. Immunotargeting of catalase to the pulmonary endothelium alleviates oxidative stress and reduces acute lung transplantation injury. Nature Biotechnology. 2003;21:392–398. doi: 10.1038/nbt806. [DOI] [PubMed] [Google Scholar]
- 30.Rong Y, Doctrow SR, Tocco G, Baudry M. EUK-134, a synthetic superoxide dismutase and catalase mimetic, prevents oxidative stress and attenuates kainate-induced neuropathology. Proceedings of the National Academy of Sciences. 1999;96:9897–9902. doi: 10.1073/pnas.96.17.9897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baker K, Marcus CB, Huffman K, Kruk H, Malfroy B, Doctrow SR. Synthetic Combined Superoxide Dismutase/Catalase Mimetics Are Protective as a Delayed Treatment in a Rat Stroke Model: A Key Role for Reactive Oxygen Species in Ischemic Brain Injury. Journal of Pharmacology and Experimental Therapeutics. 1998;284:215–221. [PubMed] [Google Scholar]
- 32.Riley DP. Functional Mimics of Superoxide Dismutase Enzymes as Therapeutic Agents. Chemical Reviews. 1999;99:2573–2588. doi: 10.1021/cr980432g. [DOI] [PubMed] [Google Scholar]
- 33.Batinic-Haberle I, Reboucas JS, Spasojevic I. Superoxide dismutase mimics: chemistry, pharmacology, and therapeutic potential. Antioxidants and Redox Signaling. 2010;13:877–918. doi: 10.1089/ars.2009.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park W, Lim D. Effect of the oligo(ethylene glycol) group on the antioxidant activity of manganese salen complexes. Bioorganic & Medicinal Chemistry Letters. 2009;19:614–617. doi: 10.1016/j.bmcl.2008.12.063. [DOI] [PubMed] [Google Scholar]
- 35.Bernard GR, Wheeler AP, Arons MM, Morris PE, Paz HL, Russell JA, Wright PE. A trial of antioxidants N-acetylcysteine and procysteine in ARDS. The Antioxidant in ARDS Study Group. Chest. 1997;112:164–172. doi: 10.1378/chest.112.1.164. [DOI] [PubMed] [Google Scholar]
- 36.Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P. Vitamin E Supplementation and Cardiovascular Events in High-Risk Patients. New England Journal of Medicine. 2000;342:154–160. doi: 10.1056/NEJM200001203420302. [DOI] [PubMed] [Google Scholar]
- 37.Hennekens CH, Buring JE, Manson JE, Stampfer M, Rosner B, Cook NR, Belanger C, LaMotte F, Gaziano JM, Ridker PM, Willett W, Peto R. Lack of Effect of Long-Term Supplementation with Beta Carotene on the Incidence of Malignant Neoplasms and Cardiovascular Disease. New England Journal of Medicine. 1996;334:1145–1149. doi: 10.1056/NEJM199605023341801. [DOI] [PubMed] [Google Scholar]
- 38.Flaherty JT, Pitt B, Gruber JW, Heuser RR, Rothbaum DA, Burwell LR, George BS, Kereiakes DJ, Deitchman D, Gustafson N. Recombinant human superoxide dismutase (h-SOD) fails to improve recovery of ventricular function in patients undergoing coronary angioplasty for acute myocardial infarction. Circulation. 1994;89:1982–1991. doi: 10.1161/01.cir.89.5.1982. [DOI] [PubMed] [Google Scholar]
- 39.Hood ED, Greineder CF, Dodia C, Han J, Mesaros C, Shuvaev VV, Blair IA, Fisher AB, Muzykantov VR. Antioxidant protection by PECAM-targeted delivery of a novel NADPH-oxidase inhibitor to the endothelium in vitro and in vivo. Journal of Controlled Release. 2012;163:161–169. doi: 10.1016/j.jconrel.2012.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.